Abstract
Purpose
Early and late effects of cancer treatment are of increasing concern with growing survivor populations, but relevant data are sparse. We sought to determine the prevalence and hazard ratio of such effects in breast cancer cases.
Patients and Methods
Women with invasive breast cancer and women with no cancer history recruited for a cancer research cohort completed a mailed questionnaire at a median of 10 years post-diagnosis or matched reference year (for the women without cancer). Reported medical conditions including lymphedema, osteopenia, osteoporosis, or heart disease (congestive heart failure, myocardial infarction, coronary heart disease) were assessed in relation to breast cancer therapy and time since diagnosis using Cox regression. The proportion of women currently receiving treatment for these conditions was calculated.
Results
Study participants included 2535 women with breast cancer and 2428 women without cancer (response rates 66.0% and 50.4%, respectively) Women with breast cancer had an increased risk of lymphedema (Hazard ratio (HR) 8.6; 95% confidence interval (CI) 6.3-11.6), osteopenia (HR 2.1; 95% CI 1.8-2.4), and osteoporosis (HR 1.5; 95% CI 1.2-1.9) but not heart disease, as compared to women without cancer Hazard ratios varied by treatment and time since diagnosis. Overall, 49.3% of breast cancer cases reported at least one medical condition, and at 10 or more years post-diagnosis, 37.7% were currently receiving condition-related treatment.
Conclusions
Responses from survivors a decade following cancer diagnosis demonstrate substantial treatment-related morbidity, and emphasize the need for continued medical surveillance and follow-up care into the second decade post diagnosis.
Keywords: Breast Neoplasms, Lymphedema, Osteoporosis, Heart Disease, Chemotherapy, Radiotherapy
Introduction
Early and late effects of breast cancer diagnosis and treatment have received increasing attention with the advent of therapies that extend survival, resulting in a growing population of breast cancer survivors at risk. In the U.S., approximately 3.0 million women were living with the sequelae of breast cancer diagnosis as of early 2012.[1] However, there is a paucity of data that describes the relative risk of treatment-related adverse effects among breast cancer survivors, as well as the time course of such outcomes. Such information is vital for physicians overseeing the care of survivors, particularly as many transfer from oversight of oncologists to primary care doctors at five or more years following diagnosis. Among the most commonly reported treatment-related effects are lymphedema, bone loss, and cardiac disease, which can occur either during treatment (early effects) or following it (late).[2]
Lymphedema is often a relatively early effect of breast cancer treatment. However, elevated risk may persist throughout much of a woman’s lifetime. Women who have undergone axillary lymph node dissection (ALND) to determine the extent of cancer spread have a higher lymphedema risk than those who undergo only a sentinel lymph node biopsy (SLNB).[3] Lymphedema risk may also be increased among women who receive radiotherapy directed to axillary nodes [4] or anthracycline or other chemotherapy [3,4], which are closely associated with ALND. Few studies have followed women for longer than 5-6 years to understand the time course and persistence of the condition.
Late effects commonly arise as a consequence of breast cancer treatment by chemotherapy, radiotherapy, or endocrine therapy. Several breast cancer therapies result in bone loss, leading to an increase in risk of osteopenia, osteoporosis, or bone fracture, usually at least 3-5 years following diagnosis [5,6]. Chemotherapy, premature ovarian failure (from chemotherapy or surgery), tamoxifen (if premenopausal) and aromatase inhibitors can all lead to decrease in bone mineral density (BMD)[7]. However, limited data exist regarding the long-term impact on bone health.
Women who receive specific therapies for breast cancer may also have an elevated risk of early and late cardiac toxicities. Women treated with chemotherapy regimens containing anthracycline[8] or targeted therapies for Her2-positive tumors[9] have an increased risk of cardiac disease or death, but persistence of elevated risk post-therapy is unclear[10,11]. While historically women who received radiotherapy for left-sided breast cancer have had an excess risk of cardiac disease, therapeutic advances that decreased cardiac dose are believed to have substantially reduced this risk,[12,13] although longer-term follow-up data are lacking.
Prior studies addressing common effects of breast cancer treatment have sometimes consisted of surveys of women who survived at least three to five years,[4,11,14-16] of which one included a non-cancer comparison group to assess hazard ratios. Other studies, predominantly clinical trials and analyses of medical claims, have measured the prevalence of such effects prior to treatment and reported new events during the trial,[3,5,6,17,12,13,18-22] sometimes comparing outcomes to those of breast cancer cases who received the alternate treatment. Some prior studies have been limited by small sample sizes or short follow up. We sought to determine the prevalence of such effects and to estimate hazard ratios for them among 2535 women with invasive breast cancer and a comparison group of 2428 women with no history of cancer followed for a median of 10 years in the Cancer Genetics Network (CGN).
Methods
Design, Setting and Patients
We examined the relative risk of developing four health conditions associated with prior invasive breast cancer treatment among women enrolled in the Cancer Genetics Network (CGN), a national registry of individuals with a personal or family history of cancer (predominantly breast, prostate, colorectal, and melanoma) established by the National Cancer Institute in 1998 [23]. The CGN enrolled 26,953 volunteer participants at 14 academic research centers across the U.S., the majority from 1998-2000. Institutional Review Boards at each center approved the study, and participants provided informed consent for long-term follow-up. Participants were ascertained both from local and state tumor registries, and from high risk cancer clinics. Some centers also recruited cases’ unaffected family members. Only female participants diagnosed with invasive breast cancer between 1990 and most recent follow-up (2009-2011) or who had never had a cancer diagnosis (except non-melanoma skin cancer) referred to as “women without cancer” were included in this analysis.
At entry to the registry, participants completed a baseline questionnaire (in-person, via telephone interview or mail), providing information on socio-demographic characteristics, personal cancer history, and cancer risk factors. Participants were contacted annually or bi-annually to update baseline information. In 2009-2010, the annual survey included questions regarding common risk factors for both cancers and late effects (smoking, body mass index (BMI), menopausal status, and menopausal hormone use) and cancer-related medical history. Respondents were asked to indicate whether they had received surgery, radiotherapy, chemotherapy or hormonal therapy for their cancer, and hormonal therapy was defined as including tamoxifen and aromatase inhibitors.
Medical Condition Outcomes
Outcomes of interest included participants’ self-report of lifetime history of particular medical conditions. Respondents were asked to indicate whether they had ever had the condition, and the age at which it was first identified. The list of conditions included lymphedema (defined as “arm or leg swelling”), osteopenia (“mild decrease in bone density”), osteoporosis (“severe decrease in bone density”), and three heart conditions (separate questions regarding “heart attack”, “congestive heart failure”, or “coronary heart disease (also called angina or coronary artery disease)”). Participants indicated whether each condition was diagnosed by “my doctor”, “myself” or “other healthcare professional” by checking all that apply. In these analyses, lymphedema was restricted to diagnoses by a doctor or other health care professional, as 20% of reported lymphedema was self-diagnosed; 99% or more of other conditions were diagnosed by a health professional.
Statistical Analysis
Invasive breast cancer diagnosis was the exposure of interest. Follow-up for cancer cases began at date of invasive breast cancer diagnosis. To equalize follow-up time, women with no history of cancer were assigned a reference year, frequency-matched to case diagnosis year, as a comparable starting point for follow-up. Participants who first developed a given health condition before their cancer diagnosis or reference year were excluded from analyses for that health condition (left censored).
Cox proportional hazards models were used to assess the relationship between treatment of invasive breast cancer and subsequent development of each health condition. Time-to-event variables indicated either the age at diagnosis for each condition, or if unaffected, the age at follow-up. Women entered the analysis at cancer diagnosis or reference age, and exited at age of onset of each condition (“event”) or at completion of follow-up (“censored” at date of questionnaire completion). Using age as the “time” variable in Cox regression ensures that all estimates are t adjusted very finely for age, as only women of the same age are compared in each risk set. Cox models that utilized women without cancer as the reference group did not allow statistical control for other treatments received, because such women had not received treatment. Thus, Cox models were also fit among breast cancer cases only. Women missing data on a particular exposure or outcome were omitted from that specific analysis. For women missing age at menopause, a potential confounder, those who were age 52 or older in the year prior to diagnosis/reference were assigned ‘postmenopausal’ status at age 52 (n=124; 2.5% of participants), and those who were younger were given a missing value indicator (n=45; 1% of participants) All analyses were conducted using SAS software (v.9, Cary, N.C.). Analyses were adjusted for potential confounders including race (white, black, other), Hispanic ethnicity, education (at least some college vs. less), and BMI (quartiles). Current and past smoking, menopausal status, and current and past use of menopausal hormone therapies were adjusted for as time-dependent covariates, with women coded as ‘zero’ at ages when they were unexposed, switching to a code of ‘one” at ages when exposed. . Such adjustment accounts for treatment-induced menopause. Estimates of disease prevalence among women without cancer were adjusted to the age distribution of the breast cancer cases.
To examine temporal changes in the risk of health conditions with time since onset of follow-up, separate Cox regression models were fit for five-year intervals following diagnosis/reference year (0-4 years, 5-9 years, and 10+ years). Participant ages at entry and exit from specified five-year interval were calculated. The hazard ratio o for development of the health condition within the interval, comparing breast cancer cases and women without cancer, adjusted for potential confounders, was estimated. Participants who developed the health condition before their age at entry into specified five-year intervals are excluded from models for that interval and subsequent intervals. Due to use of time-dependent covariates, the proportional hazards assumption could not be assessed, although the change in risk with time since diagnosis is addressed in the temporal models.
Results
Median follow-up time for the 2535 women with an invasive breast cancer diagnosis and 2429 women with no history of cancer (“women without cancer”) who completed the 2009-2010 questionnaire (Response rates 66.0% and 50.4%, respectively; Consort diagram Figure 1) was 10 years (Interquartile range 9-12 years). Compared with original CGN participants, those who completed that questionnaire were on average slightly younger, more educated, and were more likely to report white, Asian, and non-Hispanic background, less likely to smoke, and more likely to have first- and second-degree relatives affected with breast cancer than CGN participants who did not complete the questionnaire.
Figure 1.
Among participants included in this analysis, women with breast cancer were comparable to women without cancer in race and ethnicity, but the latter women tended to be younger and more likely to have a breast cancer family history than the cases (12.8% were relatives of breast cancer cases) (Table 1). In addition, of 1220 women with cancer who were premenopausal at diagnosis, 685 (56.1%) became menopausal within two years following diagnosis, compared with 194 of 1494 premenopausal women without cancer (14.8% after age-adjustment to the distribution of the cases). Of the 685 premenopausal breast cancer cases who became menopausal, 132 received an oophorectomy within two years of diagnosis. Of the remaining 553 who did not receive an oophorectomy, 399 reported treatment with chemotherapy.
Table 1. Demographic characteristics of invasive breast cancer cases (n=2535) and women without cancer (n=2428) followed through 2009-2010 in the Cancer Genetics Network.
| Breast Cancer Cases n (%) |
Women without Cancer n (%) |
||
|---|---|---|---|
| Characteristic | |||
| Race: | |||
| White | 2195 (90.0) | 2002 (93.7) | |
| Black | 145 (6.0) | 94 (4.4) | |
| Other | 98 (4.0) | 40 (1.9) | |
| Missing | 97 | 292 | |
| Ethnicity: | |||
| Hispanic | 186 (8.0) | 166 (8.1) | |
| Non-Hispanic | 2132 (92.0) | 1882 (91.9) | |
| Missing | 217 | 380 | |
|
Age at Diagnosis/
Reference Age: |
|||
| < 30 | 42 (1.7) | 316 (13.0) | |
| 30-39 | 320 (12.6) | 549 (22.6) | |
| 40-49 | 857 (33.8) | 712 (29.3) | |
| 50-59 | 720 (28.4) | 486 (20.0) | |
| 60-69 | 428 (16.9) | 250 (10.3) | |
| 70+ | 168 (6.6) | 115 (4.8) | |
|
First –Degree Family History of Breast or
Ovarian Cancer: | |||
| No | 1409 (55.6) | 844 (34.8) | |
| Yes | 1126 (44.4) | 1584 (65.2) | |
|
Menopausal Status (Year Prior to
Diagnosis/Reference Age) | |||
| Pre/Perimenopausal | 1220 (50.7) | 1494 (63.9) | |
| Postmenopausal | 1184 (49.3) | 855 (36.1) | |
| Menopausal age unknown | 105 | 64 | |
| Missing | 26 | 26 | |
| Time since diagnosis or reference year: | |||
| < 5 yrs | 52 (2.0) | 41 (1.7) | |
| 5-9 yrs | 623 (24.6) | 614 (25.3) | |
| 10-14 yrs | 1582 (62.4) | 1492 (61.4) | |
| 15 + yrs | 278 (11.0) | 281 (11.6) | |
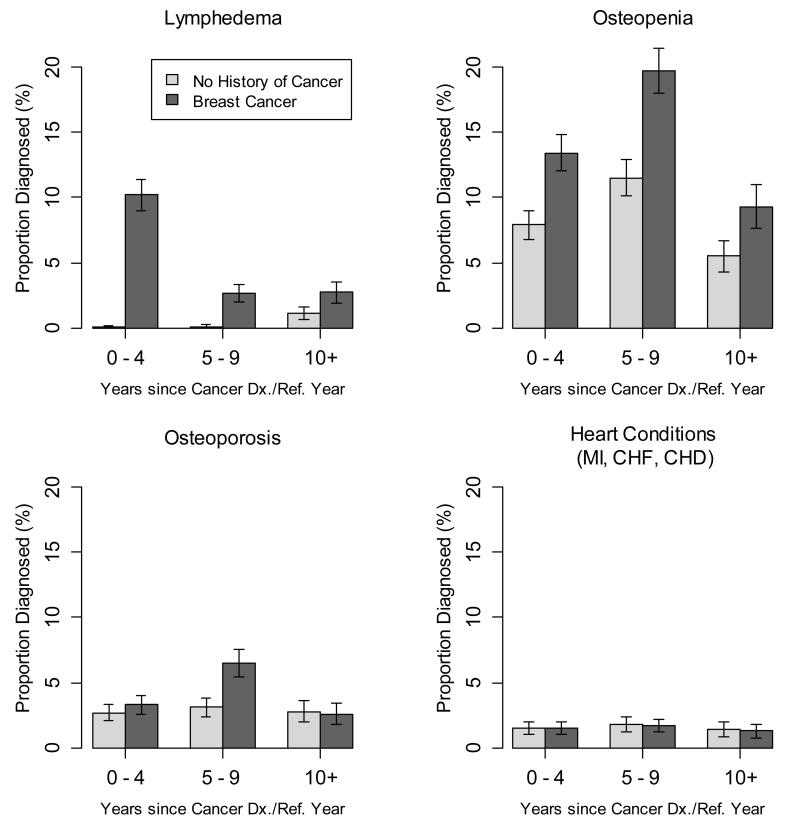
Women who developed lymphedema did so at a median of 2.0 years of follow-up. As expected, women with invasive breast cancer had an increased risk for lymphedema (Table 2). When compared to women without cancer, cases treated with chemotherapy, radiation, or endocrine therapy had a 9-10-fold increased lymphedema risk (Table 3). Among cases, risk was 1.3 -1.4-fold higher among those treated with radiation or endocrine therapies, compared with those who were not (Table 3). The increase in lymphedema risk associated with breast cancer was highest during the initial years following diagnosis, and risk remained elevated at 5-9 and and 10 or more years of follow-up (Table 4). A lymphedema diagnosis was reported by 14.1% of breast cancer cases overall, with the proportion differing by follow-up time (Figure 2), and among those followed for 10 or more years after cancer diagnosis, 7.0% were currently being treated for the condition (data not shown).
Table 2. Lymphedema, osteopenia, osteoporosis and heart disease reported by women with invasive breast cancer, compared to women without cancer.
| Exposure: | Age-Adjusted Proportion Diagnosed |
Yes n (%) |
Noa n (%) |
Missing n |
Hazard Ratiob (95% CI) c |
|---|---|---|---|---|---|
| Lymphedema | |||||
| Breast Cancer | 14.1% | 349 (86) | 2080 (47.6) | 39 | 8.6 (6.3-11.6) |
| Women without Cancer | 2.5% | 57 (14) | 2287 (52.4) | 27 | 1.0 |
| Osteopenia | |||||
| Breast Cancer | 34.8% | 804 (67.6) | 1505 (44.6) | 66 | 2.1 (1.8-2.4) |
| Women without Cancer | 19.3% | 385 (32.4) | 1866 (55.4) | 55 | 1.0 |
| Osteoporosis | |||||
| Breast Cancer | 11.3% | 268 (66.7) | 2114 (49.0) | 66 | 1.5 (1.2-1.9) |
| Women without Cancer | 6.9% | 134 (33.3) | 2197 (51.0) | 47 | 1.0 |
| Myocardial Infarction, Congestive Heart Failure, or Coronarv Heart Disease | |||||
| Breast Cancer | 4.0% | 100 (58.8) | 2397 (50.8) | 13 | 1.0 (0.7-1.3) |
| Women without Cancer | 3.9% | 70 (41.2) | 2321 (49.2) | 11 | 1.0 |
The number of women included in each analysis differs due to deletion of those who developed a particular medical condition prior to diagnosis or reference date
Adjusted for age, race/ethnicity, education, and body mass index. Menopausal status, smoking, and menopausal hormone use were accounted for as time-dependent variables: Women were coded as ‘0’ until the age they became exposed, and then as “1” for the ages that they were exposed
CI = Confidence Interval
Table 3. Lymphedema, osteopenia, osteoporosis and heart disease reported by women with invasive breast cancer, in comparison to women without cancer, according to treatment received.
| Exposure | Yes n (%) |
No n (%) |
Missing n |
Among all Womena HR (95% CI)c |
Among Breast Cancer Casesb HR (95% CI)c |
|---|---|---|---|---|---|
| Lymphedema | |||||
| Breast Cancer: | |||||
| Chemotherapy | 210 (57.1) | 1000 (24.4) | 18 | 9.3 (6.8-12.8) | 1.1 (0.9-1.4) |
| No Chemotherapy | 101 (27.4) | 810 (19.8) | 6 | 7.3 (5.1-10.4) | 1.0 |
| Missing | 38 | 270 | 15 | ||
| Women without Cancer | 57 (15.5) | 2287 (55.8) | 27 | 1.0 | |
| Breast Cancer: | |||||
| Radiation | 213 (57.9) | 1150 (28) | 12 | 9.3 (6.7-12.8) | 1.3 (1.0-1.6) |
| No Radiation Missing | 98 (26.6) 38 | 677 (16.4) 253 | 12 15 | 7.3 (5.1-10.4) | 1.0 |
| Women without Cancer | 57 (15.5) | 2287 (55.6) | 27 | 1.0 | |
| Breast Cancer: | Lymphedema | ||||
| Endocrine therapy | 197 (53.7) | 1080 (26.5) | 17 | 10.1 (7.2-13.9) | 1.4 (1.1-1.8) |
| No Endocrine therapy | 113 (30.8) | 711 (17.4) | 5 | 7.3 (5.2-10.2) | 1.0 |
| Missing | 39 | 289 | 17 | ||
| Women without Cancer | 57 (15.5) | 2287 (56.1) | 27 | 1.0 | |
| Osteopenia | |||||
| Breast Cancer: | |||||
| Chemotherapy | 426 (39.1) | 733 (23.1) | 32 | 2.1 (1.8-3.2) | 1.0 (0.8-1.2) |
| No Chemotherapy | 279 (25.6) | 579 (18.2) | 28 | 2.0 (1.7-2.3) | 1.0 |
| Missing | 99 | 193 | 6 | ||
| Women without Cancer | 385 (35.3) | 1866 (58.7) | 55 | 1.0 | |
| Breast Cancer: | |||||
| Radiation | 453 (41.3) | 838 (26.4) | 47 | 2.1 (1.8-2.4) | 0.9 (0.8-1.1) |
| No Radiation | 260 (23.7) | 475 (14.9) | 14 | 2.1 (1.8-2.5) | 1.0 |
| Missing | 91 | 192 | 5 | ||
| Women without Cancer | 385 (35.1) | 1866 (58.7) | 55 | 1.0 | |
| Osteopenia | |||||
| Breast Cancer: | |||||
| Endocrine therapy | 483 (44.4) | 727 (23.1) | 36 | 2.4 (2.0-2.7) | 1.5 (1.2-1.7) |
| No Endocrine therapy | 221 (20.3) | 559 (17.7) | 21 | 1.7 (1.4-2.0) | 1.0 |
| Missing | 100 | 219 | 9 | ||
| Women without Cancer | 385 (35.3) | 1866 (59.2) | 55 | 1.0 | |
| Osteoporosis | |||||
| Breast Cancer: | |||||
| Chemotherapy | 130 (35.3) | 1070 (26.5) | 32 | 1.5 (1.2-2.0) | 1.0 (0.7-1.3) |
| No Chemotherapy | 104 (28.3) | 773 (19.1) | 26 | 1.5 (1.2-1.9) | 1.0 |
| Missing | 34 | 271 | 8 | ||
| Women without Cancer | 134 (36.4) | 2197 (54.4) | 47 | 1.0 | |
| Breast Cancer: | |||||
| Radiation | 154 (41.7) | 1173 (29.0) | 43 | 1.6 (1.2-2.0) | 1.2 (0.9-1.5) |
| No Radiation | 81 (22.0) | 681 (16.8) | 19 | 1.4 (1.0-1.8) | 1.0 |
| Missing | 33 | 260 | 4 | ||
| Women without Cancer | 134 (36.3) | 2197 (54.2) | 47 | 1.0 | |
| Osteoporosis | |||||
| Breast Cancer | |||||
| Endocrine therapy | 144 (39.5) | 1106 (27.6) | 36 | 1.5 (1.2-1.9) | 1.0 (0.8-1.4) |
| No Endocrine therapy | 87 (23.8) | 712 (17.7) | 22 | 1.5 (1.1-2.0) | 1.0 |
| Missing | 37 | 296 | 8 | ||
| Women without Cancer | 134 (36.7) | 2197 (54.7) | 47 | 1.0 | |
|
Myocardial Infarction,
Congestive Heart Failure, or Coronary Heart Disease | |||||
| Breast Cancer: | |||||
| Chemotherapy | 48 (28.8) | 1193 (27.1) | 10 | 1.2 (0.8-1.7) | 1.4 (0.9-2.2) |
| No Chemotherapy | 43 (26.7) | 892 (20.2) | 2 | 0.8 (0.5-1.2) | 1.0 |
| Missing | 9 | 312 | 1 | ||
| Women without Cancer | 70 (43.5) | 2321 (52.7) | 11 | 1.0 | |
| Breast Cancer: | |||||
| Radiation | 51 (31.7) | 1347 (30.5) | 6 | 0.9 (0.6-1.3) | 0.7 (0.5-1.0) |
| No Radiation | 40 (24.8) | 752 (17.0) | 6 | 1.2 (0.8-1.8) | 1.0 |
| Missing | 9 | 298 | 1 | ||
| Women without Cancer | 70 (43.5) | 2321 (52.5) | 11 | 1.0 | |
|
Myocardial Infarction,
Congestive Heart Failure, or Coronary Heart Disease | |||||
| Breast Cancer: | |||||
| Endocrine therapy | 54 (29.8) | 1253 (28.6) | 9 | 1.0 (0.7-1.4) | 1.0 (0.7-1.6) |
| No Endocrine therapy | 31 (21.7) | 811 (18.5) | 2 | 0.9 (0.6-1.4) | 1.0 |
| Missing | 15 | 333 | 2 | ||
| Women without Cancer | 70 (48.5) | 2321 (52.9) | 11 | 1.0 | |
Adjusted for race, ethnicity, education, and body mass index. Menopausal status, menopausal hormone use and smoking were accounted for as time-dependent covariates: Women were coded as ‘0’ until the age they became exposed, and as “1” for the ages exposed. Risk estimates could not be adjusted for other therapies, as women without cancer had not been treated.
Restricted to breast cancer cases only; thus also adjusted for other therapies.
HR - Hazard Ratio; CI - Confidence Interval
Table 4. Lymphedema, osteopenia, osteoporosis and heart disease reported by invasive breast cancer cases, in comparison to women without cancer, according to time since invasive breast cancer diagnosis or reference date.
| Time Interval since Diagnosis/Reference (yrs): |
Women Under Observationa |
Yes n (%) |
No n (%) |
Missingb n |
Hazard Ratioc (95% CI)d |
|---|---|---|---|---|---|
| First Lymphedema Diagnosis by a Health Professional During Time Interval | |||||
| 0-4 | |||||
| Breast Cancer | 2454 | 250 (94.3) | 2204 (48.5) | 39 | 18.3 (10.7-31.3) |
|
Women without
Cancer |
2358 | 15 ( 5.7) | 2343 (51.5) | 27 | 1.0 |
| 5-9: | |||||
| Breast Cancer | 2101 | 56 (70.9) | 2045 (47.3) | 26 | 2.3 (1.4-3.9) |
|
Women without
Cancer |
2301 | 23 (29.1) | 2278 (52.7) | 25 | 1.0 |
|
First Lymphedema Diagnosis By a Health
Professional During Time Interval | |||||
| 10+ | |||||
| Breast Cancer | 1509 | 41 (69.5) | 1468 (46.9) | 17 | 1.9 (1.0-3.4) |
|
Women without
Cancer |
1683 | 18 (30.5) | 1665 (53.1) | 18 | 1.0 |
| First Reported Occurrence of Osteopenia During Time Interval | |||||
| 0-4 : | |||||
| Breast Cancer | 2309 | 309 (70.4) | 2000 (48.5) | 66 | 1.5 (1.2-1.9) |
|
Women without
Cancer |
2251 | 130 (29.6) | 2121 (51.5) | 55 | 1.0 |
| 5-9: | |||||
| Breast Cancer | 1964 | 386 (67.6) | 1578 (45.3) | 63 | 1.6 (1.4-2.0) |
|
Women without
Cancer |
2092 | 185 (32.4) | 1907 (54.7) | 54 | 1.0 |
| First Reported Occurrence of Osteopenia During Time Interval | |||||
| 10+: | |||||
| Breast Cancer | 1176 | 109 (60.9) | 1067 (44.0) | 45 | 1.2 (0.9-1.7) |
| Women without Cancer | 1426 | 70 (39.1) | 1356 (66.0) | 37 | 1.0 |
| First Reported Occurrence of Osteoporosis During Time Interval | |||||
| 0-4 : | |||||
| Breast Cancer | 2382 | 79 (63.7) | 2303 (50.2) | 66 | 1.1 (0.7-1.6) |
| Women without Cancer | 2331 | 45 (36.3) | 2286 (49.8) | 47 | 1.0 |
| 5-9: | |||||
| Breast Cancer | 2256 | 147 (72.7) | 2109 (49.0) | 64 | 1.8 (1.3-2.4) |
| Women without Cancer | 2250 | 55 (27.3) | 2195 (51.0) | 38 | 1.0 |
| First Reported Occurrence of Osteoporosis During Time Interval | |||||
| 10+: | |||||
| Breast Cancer | 1585 | 42 (55.3) | 1543 (49.0) | 50 | 0.8 (0.5-1.2) |
|
Women without
Cancer |
1643 | 34 (44.7) | 1609 (51.0) | 36 | 1.0 |
|
First Reported Occurrence of Myocardial Infarction, Congestive Heart Failure, or
Coronary Heart Disease During Time Interval | |||||
| 0-4 : | |||||
| Breast Cancer | 2497 | 37 (60.7) | 2460 (51.0) | 13 | 1.2 (0.7-2.1) |
| Women without Cancer | 2391 | 24 (39.3) | 2367 (49.0) | 11 | 1.0 |
| 5-9: | |||||
| Breast Cancer | 2410 | 41 (57.8) | 2369 (50.8) | 12 | 0.8 (0.5-1.4) |
| Women without Cancer | 2329 | 30 (42.2) | 2299 (49.2) | 10 | 1.0 |
|
First Reported Occurrence of Myocardial Infarction,
Congestive Heart Failure, or Coronary Heart Disease During Time Interval | |||||
| 10+: | |||||
| Breast Cancer | 1772 | 22 (57.9) | 1750 (50.7) | 9 | 1.1 (0.5-2.2) |
|
Women without
Cancer |
1718 | 16 (42.1) | 1702 (49.3) | 8 | 1.0 |
Counts of women under observation at the start of each time interval. Each successive time interval has fewer women under observation, as those who had an event in a previous interval and those who did not have longer follow-up drop out.
No women had missing data for time interval
Adjusted for age, race, ethnicity, education, and body mass index. Menopausal status, menopausal hormone use and smoking were accounted for as time-dependent covariates: Women were coded as ‘0’ until the age they became exposed, and as “1” for the ages exposed
CI - Confidence Interval
Figure 2.
Medical conditions diagnosed in women with invasive breast cancer and women without cancer, by time since diagnosis or reference year (Proportion diagnosed and 95% confidence interval)*. Abbreviations: Dx, diagnosis; Ref, reference; MI, myocardial infarction; CHF, congestive heart failure; CHD, coronary heart disease
*All proportions in women without cancer were adjusted to the age distribution of women with cancer.
Women who developed osteopenia reported a diagnosis at a median of 6.0 years of follow-up, and the median time to osteoporosis was also 6.0 years, Women diagnosed with invasive breast cancer were 1.5-2.1 times more likely to develop osteopenia or osteoporosis than women without cancer (Table 2). Among women with breast cancer, and adjusted for other therapies received, osteopenia risk was 1.5-fold higher for those treated with endocrine therapy, in comparison with women with breast cancer who did not receive endocrine therapy (Table 3). Breast cancer cases had a greater risk of osteopenia than women without cancer for 0-4 and 5-9 years following diagnosis, and a higher osteoporosis risk between 5-9 years following diagnosis (Table 4). Overall, 34.8% of women with breast cancer reported osteopenia and 11.3% osteoporosis, and among those completing 10 years of follow-up, 31.8% were currently receiving treatment for bone loss.
Of women who developed any of three included heart conditions, the first occurred at a median of 6.0 years of follow-up. Compared to women without cancer, women with invasive breast cancer did not have an increased risk for developing one or more of the three heart conditions considered (myocardial infarction, congestive heart failure, or coronary heart disease) (Table 2). Those treated with chemotherapy had a non-significant 1.4-fold higher risk of these conditions, compared with cases who were not (Table 3). Risk was not elevated during any specific post-diagnosis time interval (Table 4). Overall, 4% of breast cancer cases reported at least one of three forms of cardiac disease, and a similar proportion reported current treatment at 10 or more years of follow-up.
In total, among those who were followed for 10 or more years post-diagnosis, 37.7% of breast cancer cases were currently receiving treatment for either lymphedema, osteopenia, osteoporosis, or one of the forms of cardiac disease.
Discussion
At a median of 10 years follow-up, we observed that women with breast cancer had up to an 8.6-fold greater risk of medical conditions commonly associated with cancer treatment, compared with women without cancer. The increased risk differed by therapy received and by time since diagnosis. Most relationships were elevated in comparisons restricted to breast cancer patients only, thus the increased risks were not attributable to a generally higher risk of particular conditions among women with breast cancer in comparison to women without cancer. Our findings may be useful in survivorship care planning, and can inform clinical practice regarding the prevalence and long-term persistence of elevated risk.
Our results should be interpreted in light of the strengths and weaknesses of the study. Women with breast cancer retrospectively reported medical conditions at a median of a decade following diagnosis. Their responses are those of cancer survivors, thus may be underestimates, and are more likely to be representative of earlier stage disease. CGN participants were recruited from local and state-based cancer registries, cancer genetics clinics, and families of participant cases, and only a fraction were included in assessment of late effects, as in other studies.[14,11]. Breast cancer cases included in our study are more likely to be less than age 50, compared with average breast cancer cases, and that younger age should be taken into account in interpretation of the results. In addition, women treated for cancer and known to have an increased risk of particular early or late effects may receive heightened surveillance for those conditions, and that surveillance may not have been comparable for women without cancer, therefore our risk estimates may be artificially elevated. Further, breast cancer risk factors may contribute to some of the outcomes we assessed, and their overrepresentation in the case population must be considered: Increased estrogen-related risk factors in cases suggest that they were at lower risk of bone loss at the onset of follow-up than non-cases, and the likely higher BMI among postmenopausal breast cancer cases suggests that they had a greater risk of cardiac-related diseases than women without cancer. Although factors potentially related to increased surveillance (education), estrogen exposure (menopausal status, menopausal hormone therapy, BMI, smoking), and cardiac disease risk factors (menopausal status, smoking, BMI) were adjusted for in the analysis, it is likely that some residual confounding remained. A potential study limitation is lack of detailed information regarding tumor characteristics and treatment regimens. In previous studies, agreement between self-reports of cancer therapy received or chronic diseases diagnosed has generally been high when compared with medical records.[24-27] Study strengths include the large size (4963 total participants), the extended follow-up, with 1850 women with breast cancer followed for 10 or more years post-diagnosis, and the availability of data from an untreated comparison group, recruited similarly to cases.
In our study, relative risk of lymphedema among women with breast cancer was highest in the first 5 years following diagnosis, and remained elevated at 5-9 and 10 or more years of follow-up, indicating the need for persistent surveillance and continued care of lymphedema among survivors. Lymphedema most often occurs early, with up to 62% of women eventually affected demonstrating symptoms within the year following diagnosis, [28] and 77% within three years.[15] The point prevalence of lymphedema was 16% among women who underwent ALND vs. 5% among those who received SLNB at 5 years follow-up in one study.[16] In another series, the cumulative incidence of lymphedema was 42% within 5 years of diagnosis.[28] Choice of statistical measures and of lymphedema case definition may contribute to the range of findings. Overall, 14.1% of women with breast cancer in our cohort reported lymphedema diagnosed by a physician or health care provider, but we did not have information to stratify by ALND receipt. In analyses restricted to breast cancer cases, women who received radiation or endocrine therapies had a slightly elevated risk of lymphedema as in previous studies,[4,3] although it is unclear whether the association is independent of that with ALND.
Although the increased risk of bone loss with breast cancer therapies has been previously described, our results suggest continued vigilance regarding bone effects up to 10 years subsequent to breast cancer diagnosis. Importantly, among women with breast cancer who completed 10 or more years of follow-up, 31.8% were currently receiving treatment for bone loss, indicating a long-term continued need for follow-up care among breast cancer survivors. In previous investigations, an elevated risk of bone loss has been evident among breast cancer cases treated with hormonal blockade agents for estrogen receptor (ER) positive tumors, but most had relatively short follow-up. Tamoxifen therapy, which prevents ER binding, was associated with a 1.4-1.5% annual reduction in lumbar BMD in premenopausal women in two studies, [18,19] but 1.2% annual BMD gain in postmenopausal women in another.[18] Aromatase inhibitor (AI) therapies, which decrease estrogen synthesis, are usually directed only to postmenopausal ER-positive women, and reduce BMD in that group.[20,21] In one study, women treated with AIs had a significant increase in osteoporosis diagnosis at 3 years compared with placebo (5.2% vs. 3.1%),[5] and in another, the incidence of osteoporosis in treated women was 6% at 5 years, compared with 0% at baseline.[6] In our study, 34.8% of women with breast cancer reported osteopenia and 11.3% osteoporosis, consistent with longer follow-up, and adding to the understanding of the persistence of elevated risk.
In our study, women with breast cancer who received any type of chemotherapy have a non-significant 1.4-fold increased risk of cardiac disease compared to those that did not, comparable to that observed in other studies.[22,29] In a number of investigations, women treated for breast cancer with anthracycline-based chemotherapy have had a higher cardiac disease risk.[22,29] However, women treated with non-anthracycline chemotherapy in some studies have also had an elevated risk of adverse cardiac effects either comparable to (both 1.4-fold)[29] or somewhat less than (1.3-fold vs. 2.5-fold)[22] that of anthracycline. Trastuzumab treatment, also associated with cardiotoxicity, was approved for first-line therapy in 2006, thus it is unlikely that many participants received it.
Late cardiac effects of breast cancer treatment also have been observed among a subset of women who received radiotherapy. Women treated prior to 1990 for left vs. right-sided breast cancer had a 2.7-3.1-fold higher risk of coronary artery disease at a median of 12 years following diagnosis,[30] and a 1.3 – 1.6 fold[13,31,32] increased cardiovascular mortality at 15 or more years of follow-up. We did not identify a greater risk of cardiac disease among women who received radiotherapy. Decreased radiation dose to the heart tissue in more recently treated women, as well as lack of cancer laterality information and follow-up beyond 15 years, may have limited our ability to do so. In this study, 4% of women with breast cancer reported at least one of three cardiac conditions, a proportion similar to that in other studies.[22,29] We collected information on three severe forms of cardiac disease but more detailed assessment of cardiac outcomes may be needed to capture the full range of therapy effects.
At least one of the four medical conditions evaluated was reported by 49.3% of breast cancer cases during follow-up, and 9.4% reported more than one. In addition, at 10 or more years after cancer diagnosis, 37.7% were currently receiving treatment for these conditions. Our results demonstrate substantial treatment-related morbidity in women with breast cancer, and highlight the need for continued delivery of high-quality care to treated women into the second decade post-diagnosis.
Acknowledgement of Research Support
This study was supported by grants U01CA078284, U24CA078134, U24CA078142, U24CA078146, U24CA078148, U24CA078156, U24CA078157, U24CA078164, U24CA078174, and contract HHSN2612007440000C from the National Cancer Institute.
Footnotes
Supplemental survey response rate:
Women with breast cancer: 2535/(5338-732-766) = 66%
Women without cancer: 2429/(5966-224-931) = 50%
References
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. doi:10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Dictionary of Cancer Terms. 2013 Sep 14; Accessed at http://www.cancer.gov/dictionary?cdrid=390292.
- 3.Norman SA, Localio AR, Kallan MJ, Weber AL, Torpey HA, Potashnik SL, Miller LT, Fox KR, DeMichele A, Solin LJ. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2734–2746. doi: 10.1158/1055-9965.EPI-09-1245. doi:10.1158/1055-9965.EPI-09-1245 1055-9965.EPI-09-1245 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women’s Health Study. Breast Cancer Res Treat. 2011;130(3):981–991. doi: 10.1007/s10549-011-1667-z. doi:10.1007/s10549-011-1667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Pater JL, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Tu D. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26(12):1948–1955. doi: 10.1200/JCO.2007.11.6798. doi:10.1200/JCO.2007.11.6798 JCO.2007.11.6798 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26(7):1051–1057. doi: 10.1200/JCO.2007.11.0726. doi:10.1200/JCO.2007.11.0726 26/7/1051 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Winters-Stone KM, Schwartz AL, Hayes SC, Fabian CJ, Campbell KL. A prospective model of care for breast cancer rehabilitation: bone health and arthralgias. Cancer. 2012;118(8 Suppl):2288–2299. doi: 10.1002/cncr.27465. doi:10.1002/cncr.27465. [DOI] [PubMed] [Google Scholar]
- 8.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. doi:10.1186/1471-2407-10-337 1471-2407-10-337 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell SD, Blackwell KL, Lawrence J, Pippen JE, Jr., Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28(21):3416–3421. doi: 10.1200/JCO.2009.23.6950. doi:JCO.2009.23.6950 [pii] 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 10.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive Heart Failure in Older Women Treated With Adjuvant Anthracycline Chemotherapy for Breast Cancer. Journal of Clinical Oncology. 2007;25(25):3808–3815. doi: 10.1200/JCO.2006.10.4976. doi:10.1200/jco.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 11.Ganz PA, Hussey MA, Moinpour CM, Unger JM, Hutchins LF, Dakhil SR, Giguere JK, Goodwin JW, Martino S, Albain KS. Late cardiac effects of adjuvant chemotherapy in breast cancer survivors treated on Southwest Oncology Group protocol s8897. J Clin Oncol. 2008;26(8):1223–1230. doi: 10.1200/JCO.2007.11.8877. doi:JCO.2007.11.8877 [pii] 10.1200/JCO.2007.11.8877. [DOI] [PubMed] [Google Scholar]
- 12.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97(6):419–424. doi: 10.1093/jnci/dji067. doi:97/6/419 [pii] 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–565. doi: 10.1016/S1470-2045(05)70251-5. doi:S1470-2045(05)70251-5 [pii] 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 14.Bonneterre J, Roche H, Kerbrat P, Fumoleau P, Goudier MJ, Fargeot P, Montcuquet P, Clavere P, Barats JC, Monnier A, Veyret C, Datchary J, Van Praagh I, Chapelle-Marcillac I. Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French adjuvant study group. J Clin Oncol. 2004;22(15):3070–3079. doi: 10.1200/JCO.2004.03.098. doi:10.1200/JCO.2004.03.098 22/15/3070 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. doi:10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–5219. doi: 10.1200/JCO.2008.16.3725. doi:10.1200/JCO.2008.16.3725 JCO.2008.16.3725 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25(25):3808–3815. doi: 10.1200/JCO.2006.10.4976. doi:JCO.2006.10.4976 [pii] 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 18.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. Journal of Clinical Oncology. 1996;14(1):78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 19.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006;24(4):675–680. doi: 10.1200/JCO.2005.02.3515. doi:24/4/675 [pii] 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 20.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. doi:S0140673604176666 [pii] 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 21.Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, Bliss JM, Coombes RC. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8(2):119–127. doi: 10.1016/S1470-2045(07)70003-7. doi:S1470-2045(07)70003-7 [pii] 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 22.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23(34):8597–8605. doi: 10.1200/JCO.2005.02.5841. doi:23/34/8597 [pii] 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 23.Anton-Culver H, Ziogas A, Bowen D, Finkelstein D, Griffin C, Hanson J, Isaacs C, Kasten-Sportes C, Mineau G, Nadkarni P, Rimer B, Schildkraut J, Strong L, Weber B, Winn D, Hiatt R, Nayfield S. The Cancer Genetics Network: recruitment results and pilot studies. Community Genet. 2003;6(3):171–177. doi: 10.1159/000078165. doi:78165 78165 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Gupta V, Gu K, Chen Z, Lu W, Shu XO, Zheng Y. Concordance of self-reported and medical chart information on cancer diagnosis and treatment. BMC Med Res Methodol. 2011;11:72. doi: 10.1186/1471-2288-11-72. doi:10.1186/1471-2288-11-72 1471-2288-11-72 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips KA, Milne RL, Buys S, Friedlander ML, Ward JH, McCredie MR, Giles GG, Hopper JL. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. J Clin Oncol. 2005;23(21):4679–4686. doi: 10.1200/JCO.2005.03.002. doi:JCO.2005.03.002 [pii] 10.1200/JCO.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. doi:S0895-4356(04)00113-1 [pii] 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 28.Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, Miller LT, DeMichele A, Solin LJ. Lymphedema in Breast Cancer Survivors: Incidence, Degree, Time Course, Treatment, and Symptoms. Journal of Clinical Oncology. 2009;27(3):390–397. doi: 10.1200/JCO.2008.17.9291. doi:10.1200/jco.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–1305. doi: 10.1093/jnci/djs317. doi:10.1093/jnci/djs317 djs317 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, Solin LJ. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24(25):4100–4106. doi: 10.1200/JCO.2005.05.1037. doi:JCO.2005.05.1037 [pii] 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 31.Bouillon K, Haddy N, Delaloge S, Garbay JR, Garsi JP, Brindel P, Mousannif A, Le MG, Labbe M, Arriagada R, Jougla E, Chavaudra J, Diallo I, Rubino C, de Vathaire F. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol. 2011;57(4):445–452. doi: 10.1016/j.jacc.2010.08.638. doi:10.1016/j.jacc.2010.08.638 S0735-1097(10)04485-2 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Moller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer. 2007;7:9. doi: 10.1186/1471-2407-7-9. doi:1471-2407-7-9 [pii] 10.1186/1471-2407-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]