Abstract
Background/Aims
Laparoscopy is important in staging pancreatic cancer, but false negatives remain problematic. Making tumors fluorescent has the potential to improve the accuracy of staging laparoscopy.
Methodology
Orthotopic and carcinomatosis models of pancreatic cancer were established with BxPC-3 human pancreatic cancer cells in nude mice. Alexa488-anti-CEA conjugates were injected via tail vein 24 hours prior to laparoscopy. Mice were examined under bright field laparoscopic (BL) and fluorescence laparoscopic (FL) modes. Outcomes measured included time to identification of primary tumor for the orthotopic model and number of metastases identified within 2 minutes for the carcinomatosis model.
Results
FL enabled more rapid and accurate identification and localization of primary tumors and metastases than BL. Using BL took statistically significantly longer time than FL. More metastatic lesions were detected and localized under FL compared to BL and with greater accuracy, with sensitivities of 96% vs. 40%, respectively, when compared to control. FL was sensitive enough to detect metastatic lesions <1mm.
Conclusions
The use of fluorescence laparoscopy with tumors labeled with fluorophore-conjugated anti-CEA antibody permits rapid detection and accurate localization of primary and metastatic pancreatic cancer in an orthotopic model. The results of the present report demonstrate the future clinical potential of fluorescence laparoscopy.
Keywords: Pancreatic Cancer, CEA, Staging, Laparoscopy, Fluorescence
INTRODUCTION
Increasing the capability to visualize tumor margins or to identify small metastatic nodules can significantly impact patient treatment and outcomes. This is particularly relevant for pancreatic adenocarcinoma, a lethal disease often under-staged based on current techniques for staging laparoscopy and usually incompletely resected at the time of surgery (1,2). Improvements in both laparoscopy and resection of pancreatic adenocarcinoma should lead to better survival rates.
Targeting techniques developed to fluorescently label native tumors are multiple and include activatable probes that rely on tumor-specific enzymatic activity (3), replication-competent viruses engineered to express the green fluorescent protein (GFP) in the presence of activated telomerase (4,5) and antigen-specific antibodies that can be directly conjugated to a fluorescent probe (6,7), as well as tumor-targeting fluorescent nano particles (8,9). Of these methods, monoclonal antibodies are in clinical use for diagnosis and therapy (10,11). In the case of pancreatic cancer, specific antigens that can be targeted by monoclonal antibodies include CA19-9 and carcinoembryonic antigen (CEA). CEA is expressed in up to 98% of pancreatic cancers and is often clinically utilized as a tumor marker for pre-operative staging and post-therapy surveillance (12,13).
We demonstrated the tumor-targeting capability of anti-CEA antibodies in a previous study using fluorophore-conjugated anti-CEA antibody for intraoperative tumor visualization in an orthotopic model of pancreatic cancer (14). In the above study, the pancreatic tumors and their metastases became visible when examined with fluorescence imaging, affording greater and more accurate detection of tumor burden.
The application of these results for laparoscopy would be an important benefit for pancreatic cancer patients, since currently, standard bright field laparoscopy has significant false-negative problems (15). Toward this goal, we have recently developed a fluorescence laparoscope that permits facile and rapid identification and localization of GFP-expressing primary and metastatic tumors while maintaining adequate visualization of the surrounding tissue for proper surgical orientation and navigation (16,17).
The aim of the present study was to investigate whether fluorophore-conjugated anti-CEA antibody could improve detection of both primary and metastatic deposits in an orthotopic mouse model of pancreatic cancer labeled with fluorophore-conjugated anti-CEA antibodies.
METHODOLOGY
Cell culture
Human BxPC-3 pancreatic cancer cells were maintained in RPMI (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), penicillin/streptomycin (Gibco-BRL), sodium pyruvate (Gibco-BRL), sodium bicarbonate (Cellgro, Manassas, VA, USA), L-glutamine (Gibco-BRL), and minimal essential medium non-essential amino acids (Gibco-BRL). Cells were incubated at 37°C with 5% carbon dioxide.
Antibody conjugation
Monoclonal antibody specific for CEA was purchased from Biodesign International (Saco, ME, USA). The antibody was labeled with the AlexaFluor 488 Protein Labeling Kit (Molecular Probes Inc., Eugene, OR, USA), according to the manufacturer’s instructions. Briefly, the monoclonal antibody was reconstituted at 1mg/mL in 0.1M sodium bicarbonate; 100µL of the 1mg/mL solution was added to the reactive dye and allowed to incubate for 1 hour at room temperature, then overnight at 4°C. The conjugated antibody was then separated from the remaining unconjugated dye on a purification column by centrifugation. Antibody and dye concentrations in the final sample were determined using spectrophotometric absorbance.
Animal care
Female athymic nu/nu nude mice were maintained in a barrier facility on high-efficiency particulate air filtered racks. The animals were fed with autoclaved laboratory rodent diet (Teckland LM-485; Western ResearchProducts, Orange Co., CA, USA). All surgical procedures were performed under anesthesia with an intramuscular injection of 100µL of a mixture of 100mg/kg ketamine and 10mg/kg xylazine. Euthanasia was achieved by 100% carbon dioxide inhalation, followed by cervical dislocation. All animal studies were approved by the UCSD Institutional Animal Care and Use Committee (IACUC) and conducted in accordance with the principles and procedures outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Animals.
Orthotopic model
BxPC-3 cells were harvested by trypsinization and washed three times with serum-free medium. Viability was verified to be greater than 95% using the Vi-Cell XR automated cell viability analyzer (Beckman Coulter, Brea, CA, USA). The cells were re-suspended at concentrations of 1 million cells per 10µL serum-free medium. Orthotopic implantation was performed in the following manner: a 6–10mm transverse incision was made on the left flank of the mouse through the skin and peritoneum and the tail of the pancreas was then exposed through this incision 1×106 cells in 10µL saline were injected into the pancreatic tail, which was subsequently returned into the abdomen. The incision was closed in two layers using 6.0 Ethibond non-absorbable sutures (Ethicon Inc., Somerville, NJ, USA).
Carcinomatosis model
BxPC-3 cells were resuspended at concentrations of 1×106 cells per 100µL of serum-free medium and placed on ice before intra-peritoneal (IP) administration which was performed by injecting 1×106 cells directly into the peritoneal cavity of 6-week-old female nude mice using a 27-gauge needle.
Fluorescence laparoscopy
As previously described, a standard laparoscopic system was modified in the following manner to achieve fluorescence laparoscopy: the excitation light source, a 300W Xenon lamp (Stryker, Kalamazoo, MI, USA) was filtered by a 480nm interference short-pass filter that was placed at the end of the optical fiber, which delivered the light to the laparoscope (16). A glass emission filter with peak emission wavelength in the GFP range was placed between the laparoscope and the camera. A Multi-Cam 310C camera (UVP, Upland, CA, USA) was used to allow variable exposure time and gain setting in the controlling software (VisionWorks LS, UVP).
Mouse laparoscopy
Under anesthesia and sterile conditions, a 3mm trocar was gently introduced into the abdominal cavity at an angle to avoid injury to the underlying bowel. The trocar was secured to the abdominal wall with a suture and connected to the insufflation tubing of the laparoscopic tower. Insufflation was set to a final pressure of 2mmHg. A 3mm laparoscope (Karl-Storz GmbH & Co., Tuttlingen, Germany) was introduced through the trocar to perform standard staging laparoscopy under either the BL or FL mode. Due to the limitations of the small size of the mice, no other ports were placed. At termination of laparoscopy, the mice were sacrificed and their abdominal cavities exposed for fluorescence imaging with the OV100 small animal and imaging system (Olympus, Tokyo, Japan). Tumors were then collected for histological evaluation when possible.
Experimental designs
Orthotopic model
Four weeks following orthotopic implantation of BxPC-3 pancreatic cancer cells, three mice each received an injection of 75µg anti-CEA-Alexa 488 conjugates via tail vein, 24 hours prior to laparoscopy. The dose and timing of the injection were derived from our previous in vivo study of mouse imaging (14). Mice were prepared for laparoscopy as described above. For each mouse, up to four surgical residents (subjects) blinded to the location of the tumor were asked to perform standard laparoscopy to identify the primary tumor under either BL or FL. Time to tumor detection was then recorded. Subjects were randomized to examine each mouse under only one light mode. Identified lesions were harvested for histological evaluation.
Carcinomatosis model
Two weeks following IP injection of BxPC-3 cells, 10 mice received a tail vein injection of 75µg anti-CEA-Alexa 488 conjugates 24 hours prior to laparoscopy. Mice were prepared for laparoscopy as described above. For each mouse, between three and five surgical residents (subjects) were given 2 minutes to identify as many lesions under BL or FL as possible. To standardize the procedure, they were instructed to examine all four quadrants of the peritoneal cavity in a systematic way. For this part of the study, subjects first examined each mouse under BL and they then repeated the diagnostic laparoscopy with FL. All identified lesions, under either light mode, were harvested, when possible, for histology.
Tissue histology
At necropsy, all identified lesions were collected when possible for histology with hematoxylin and eosin (H&E) staining. Small tumor foci observed during in vivo fluorescence laparoscopic imaging were localized post mortem in fresh ex vivo organ blocks using both bright field and fluorescence imaging. Fresh tissues were fixed in Bouin’s solution and regions of interest embedded in paraffin prior to sectioning and staining with H&E for standard light microscopy. H&E-stained permanent sections were examined using an Olympus BX41 microscope equipped with a Micropublisher 3.3 RTV camera (QImaging, Surrey, B.C., Canada). All images were acquired using QCapture software (QImaging) without post-acquisition processing. For a wide field view of tissue sections, composites of overlapping images were assembled using Power Point.
Data processing and statistical analysis
Images obtained during laparoscopy were not processed in any way. Representative frames are presented. Histology images were processed for brightness and contrast using Photoshop Element 4 (Adobe Systems Inc., San Jose, CA, USA). Statistical analysis was carried out in R (version 2.12.0) using two-sided tests at the 5% significance level. Proportions were compared using Fisher’s exact test. A mixed effects model was used to compare light modes (BF vs. FL) where light mode and mouse are both treated as fixed effects and a random technician effect was included to take into account the correlation between the outcomes performed by the same technician. The outcome, time to identify primary tumor, was log transformed to improve model fitting. Residual plots were made for model diagnostics.
RESULTS
Primary tumor detection
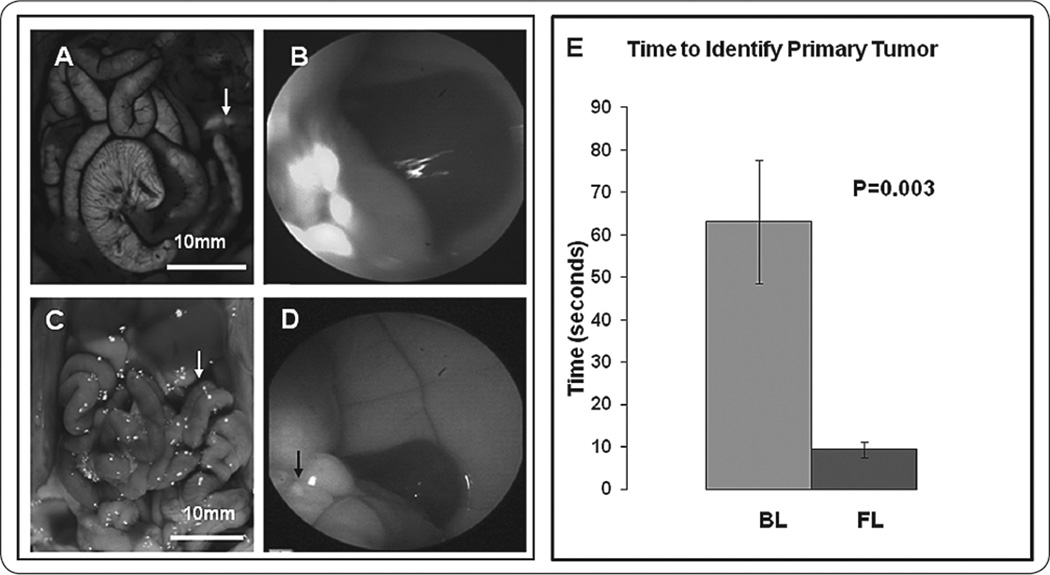
Time to correctly identify the primary tumor was measured for each subject. FL enabled rapid detection of brightly fluorescent tumors that were challenging to identify with BL (Figure 1A–D). The average time±SEM to identification of primary tumor under FL was 9.45s±1.81 vs. 63.1s±13.94 under BL (Figure 1E). Using BL takes statistically significantly longer time than FL (p<0.0001, fold change and 95% CI for BL vs. FL: 8.12 (4.54, 14.52)); mouse effect and technician effect were both taken into account.
FIGURE 1.
Time to identify primary tumor in the body of the pancreas. Images under fluorescence and bright field laparoscopy demonstrating the primary tumor in the body of the pancreas. The two images on the left (A,C) are OV-100 positive control images for comparison with laparoscopic images on the right (B,D) under fluorescence (top) and bright field (bottom). The primary pancreatic tumor was more easily detected under fluorescence compared to bright field. (E) Under bright field laparoscopy (BL), the average duration was 63.1±13.94 seconds vs. 9.45±1.81 seconds under fluorescence laparoscopy (FL) (p=0.003).
Visualization of metastases
Overall, a total of 109 tumor deposits were established in the 10 experimental animals. One hundred and five of these lesions were ultimately detected between all subjects under FL (cumulative sensitivity 96.3%), whereas only 44 were picked up under BL (cumulative sensitivity 40.4%; p<0.001). There were 24 false-positive lesions identified under BL and 10 false-positive identifications for FL. Thus the positive predictive value for an identified lesion was 44/(44+24)=35.3% for BL and was 105/(105+10)=91.3% for FL (p<0.001). Although subjects varied in their ability to correctly identify lesions, the FL mode was superior for all subjects. Across subjects, FL enabled positive identification of 2.5 to 5 times as many lesions as BL, with individual detection rates ranging from 64% to 100% for the FL mode and 13% to 35% for the BL mode. For these same subjects, the median number of false positives identified was 1 (range 0 to 3) for the FL mode and was 2.5 (range 0 to 5) for the BL mode.
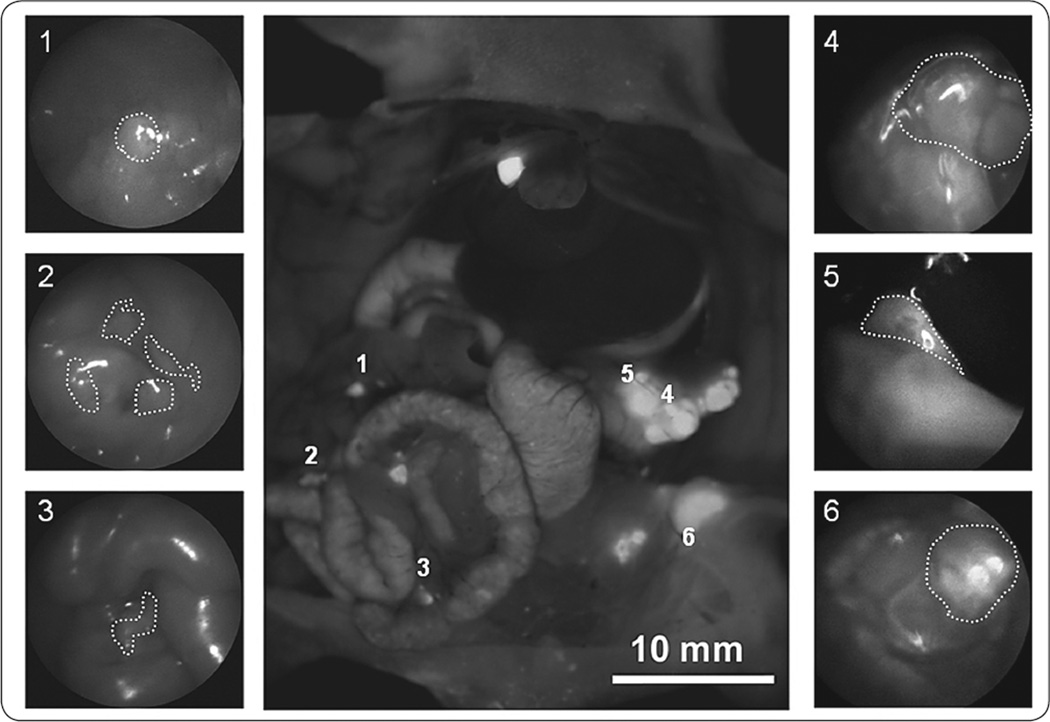
Figure 2 illustrates the advantages that FL offers: while larger lesions (areas 4 and 5) may be easily detected regardless of the light mode, smaller or more deeply located deposits were often detected by FL and missed by BL (areas 2 and 3). Surgical residents were much more likely to erroneously identify normal tissue, such as parts of the stomach, cecum or mesentery as malignant lesions under BL compared to FL (24 vs. 10). The false-positive findings in the FL group, on the other hand, consisted primarily of tissues that express some degree of auto-fluorescence, such as the gallbladder (3/10) or urinary bladder (4/10).
FIGURE 2.
Use of fluorescence laparoscopy to identify primary and metastatic lesions. The center image is a positive control OV-100 image for comparison with BL and FL. The surrounding images, labeled 1–6, are representative FL images of primary and metastatic pancreatic tumor lesions. The numbers in the upper left corner of each outer panel corresponds with the numbered lesion in the centered OV-100 panel.
Post-laparoscopy analysis
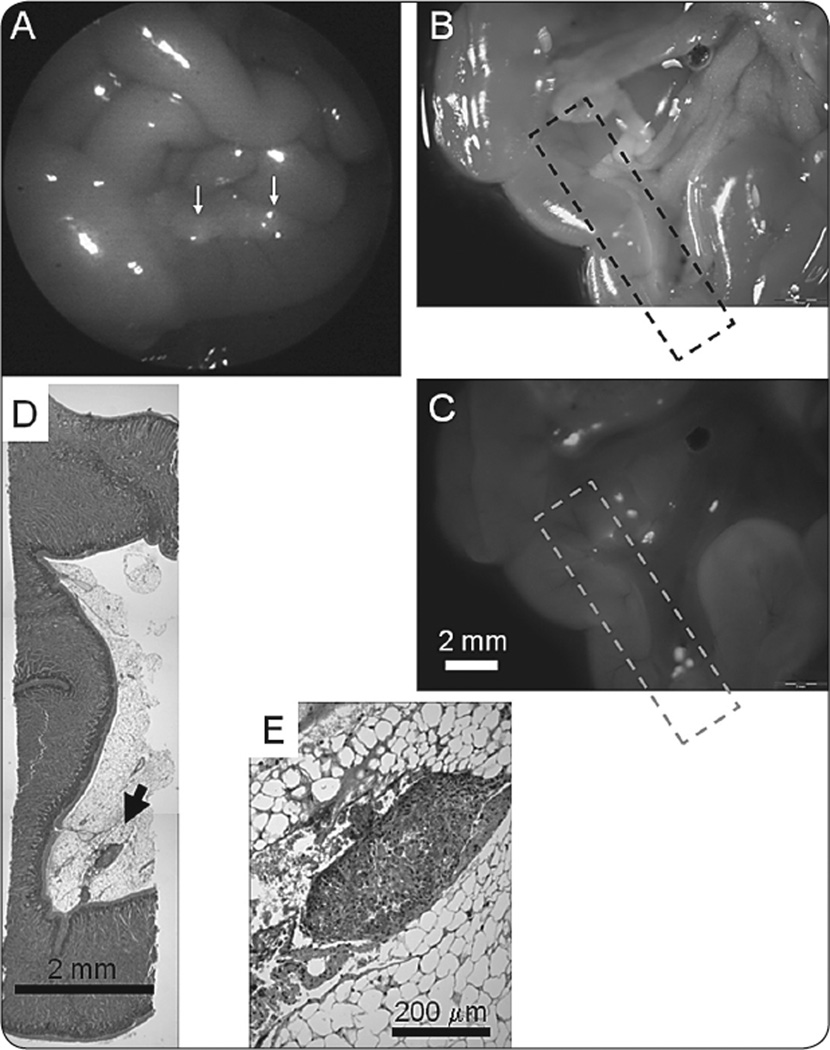
Upon termination of laparoscopy, the mice were sacrificed and their abdominal cavities were exposed and imaged with the OV-100 Small Animal Imaging System using a GFP filter. These images served as a control against which to compare the laparoscopic findings. After imaging, all identified lesions under both light modes were collected when possible and verified histologically. There was no discrepancy of tissue histology between pathological review and OV-100 images (Figure 3).
FIGURE 3.
Histological confirmation of tumors visualized by FL. (A) Small fluorescent foci on the peritoneal surface of the bowel (white arrows) visualized during fluorescence laparoscopy. (B,C) Ex vivo visualization of small tumor foci, previously seen during fluorescence laparoscopic imaging using bright field (B) and fluorescence (C) light. The hatched box indicates the segment of tissue analyzed by histology (scale bar=2mm) (D). Composite image showing small focus of tumor (arrow) lodged within the bowel mesentery, corresponding to the fluorescent focus seen within the hatched box in panel (C). This paraffin section was cut in a plane parallel to the images shown in panels (B) and (C) (scale bar=2mm). (E) High magnification view of tumor focus indicated in panel (D) (scale bar=200µm).
DISCUSSION
Kelly et al. described a series of 115 patients with pancreatic adenocarcinoma who underwent a diagnostic laparoscopy; of these, 62 had negative findings and went on to receive R0 resection. Eleven of these 62 patients had peritoneal washings that were negative for cytology but positive for CEA by reverse transcription-polymerase chain reaction (RT-PCR). All of these patients developed early peritoneal and overall disease recurrence (18). These results illustrate the value of CEA as a staging tool and underscore the need for improvements in current staging laparoscopy.
In the present study, we took advantage of the high sensitivity and specificity of CEA for fluorescence laparoscopy. The concept of fluorescence laparoscopy for tumor detection has grown more popular in recent years. Winer et al. used the near-infrared (NIR) fluorescent properties of methylene blue (MB) to create a tumor contrast in order to localize and resect small tumors and occult metastases of insulinomas (19). The MB dose required for imaging was consistent with current clinical use of the drug, making it clinically translatable. However, the optimal viewing period to allow adequate signal-to-background ratios was limited to an hour. In order to accurately interpret MB NIR fluorescent signals in the abdomen, an understanding of normal MB elimination routes, which involves the small bowel, extrahepatic bile ducts and ureter along with the pancreas, would be required. Other studies have likewise been limited by the lack of specificity of the targeting molecules (20–22).
Our laboratory has previously established that fluorophore-conjugated anti-CEA antibody, when injected intravenously in a mouse bearing human pancreatic cancer, enabled more accurate imaging of tumor deposits after laparotomy and enhanced the ability to resect them under fluorescence guidance, compared to unlabeled tumors (14). In subsequent studies, we showed that fluorescence laparoscopy could identify tumors pre-labeled with GFP before implantation (16,17).
In the current study, we aimed to expand this concept utilizing a fluorescence laparoscope developed to simultaneously allow facile and rapid identification of tumors labeled in situ with fluorescent anti-CEA antibodies. Our results here confirm that anti-CEA antibody delivers its fluorophore to pancreatic tumors in a targeted and specific way, enabling more rapid and accurate detection of both primary and metastatic lesions under fluorescence laparoscopy. Our results demonstrate the clinical potential of our approach. The main utility of this novel technology would be to improve upon the detection of low-volume metastases that could not be visualized with conventional bright field laparoscopy.
There are some limitations to our study. The biggest limitation is that FL followed BL such that subjects identified more lesions on the second viewing. However, subjects also made fewer false positive identifications using FL. Additionally, the setting of a time limited experiment in these studies is somewhat artificial. Finally, surgical residents might make more false positive mistakes than experienced surgeons.
The use of FL resulted in significantly shorter time to identification of a primary tumor when compared to BL in an orthotopic model of pancreatic cancer. More importantly, FL carried a cumulative 96% sensitivity rate for metastatic tumor detection compared to a rate of only 40% for BL. At the same time, FL also greatly diminished the number of false positive lesions identified on BL. The results of the present study support a clinical role for fluorophore-conjugated anti-CEA antibody in the staging and possibly surgical resection of pancreatic adenocarcinoma.
CONCLUSIONS
We have demonstrated the utility of fluorescence laparoscopy and fluorophore-conjugated anti-CEA labeled tumors for rapid and accurate detection of primary and metastatic lesions of CEA-expressing pancreatic cancer in an orthotopic model of human pancreatic cancer. The novel fluorescence laparoscopy method described here was a significant improvement over standard laparoscopy. CEA offers the advantage of being widely expressed in many gastrointestinal cancers, including pancreatic cancer. Fluorophore-conjugated anti-CEA antibody can serve as a novel intraoperative method not only to improve the staging of pancreatic cancer but also possibly to facilitate the complete resection of primary and metastatic tumors.
ACKNOWLEDGEMENTS
Work supported in part by grants from the National Cancer Institute CA142669 and CA132971 (to M.B. and AntiCancer, Inc) and T32 training grant CA121938 (to H.S. T.C.).
REFERENCES
- 1.Jimenez RE, Warshaw AL, Rattner DW, et al. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409–414. doi: 10.1001/archsurg.135.4.409. (discussion 414-405). [DOI] [PubMed] [Google Scholar]
- 2.Verbeke CS. Resection margins and R1 rates in pancreatic cancer--are we there yet? Histopathology. 2008;52:787–796. doi: 10.1111/j.1365-2559.2007.02935.x. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen QT, Olson ES, Aguilera TA, et al. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishimoto H, Kojima T, Watanabe Y, et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med. 2006;12:1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto H, Zhao M, Hayashi K, et al. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc Natl Acad Sci USA. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McElroy M, Kaushal S, Luiken GA, et al. Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg. 2008;32:1057–1066. doi: 10.1007/s00268-007-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urano Y, Asanuma D, Hama Y, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15:104–109. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu CM, Kaushal S, Tran Cao HS, et al. Half-antibody functionalized lipid-polymer hybrid nanoparticles for targeted drug delivery to carcinoembryonic antigen presenting pancreatic cancer cells. Mol Pharm. 2010;7:914–920. doi: 10.1021/mp900316a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erten A, Wrasidlo W, Scadeng M, et al. Magnetic resonance and fluorescence imaging of doxorubicin-loaded nanoparticles using a novel in vivo model. Nanomedicine. 2010;6:797–807. doi: 10.1016/j.nano.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6:349–356. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 11.Waldmann TA, Levy R, Coller BS. Emerging Therapies: Spectrum of Applications of Monoclonal Antibody Therapy. Hematology Am Soc Hematol Educ Program. 2000:394–408. doi: 10.1182/asheducation-2000.1.394. [DOI] [PubMed] [Google Scholar]
- 12.Albers GH, Fleuren G, Escribano MJ, Nap M. Immunohistochemistry of CEA in the human pancreas during development, in the adult, chronic pancreatitis and pancreatic adenocarcinoma. Am J Clin Pathol. 1988;90:17–22. doi: 10.1093/ajcp/90.1.17. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi K, Enjoji M, Tsuneyoshi M. Pancreatoduodenal carcinoma: a clinicopathologic study of 304 patients and immunohistochemical observation for CEA and CA19-9. J Surg Oncol. 1991;47:148–154. doi: 10.1002/jso.2930470303. [DOI] [PubMed] [Google Scholar]
- 14.Kaushal S, McElroy MK, Luiken GA, et al. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg. 2008;12:1938–1950. doi: 10.1007/s11605-008-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlon KC, Dougherty E, Klimstra DS, et al. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223:134–140. doi: 10.1097/00000658-199602000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran Cao HS, Kaushal S, Lee C, et al. Fluorescence laparoscopy imaging of pancreatic tumor progression in an orthotopic mouse model. Surg Endosc. 2011;25:48–54. doi: 10.1007/s00464-010-1127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran Cao HS, Kaushal S, Menen RS, et al. Submillimeter-resolution fluorescence laparoscopy of pancreatic cancer in a carcinomatosis mouse model visualizes metastases not seen with standard laparoscopy. J Laparoendosc Adv Surg Tech A. 2011;21:485–489. doi: 10.1089/lap.2011.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly KJ, Wong J, Gladdy R, et al. Prognostic impact of RT-PCRbased detection of peritoneal micrometastases in patients with pancreatic cancer undergoing curative resection. Ann Surg Oncol. 2009;16:3333–3339. doi: 10.1245/s10434-009-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winer JH, Choi HS, Gibbs-Strauss SL, et al. Intraoperative localization of insulinoma and normal pancreas using invisible near-infrared fluorescent light. Ann Surg Oncol. 2010;17:1094–1100. doi: 10.1245/s10434-009-0868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiles BM, Bhargava A, Adusumilli PS, et al. The replication-competent oncolytic herpes simplex mutant virus NV1066 is effective in the treatment of esophageal cancer. Surgery. 2003;134:357–364. doi: 10.1067/msy.2003.244. [DOI] [PubMed] [Google Scholar]
- 21.Gahlen J, Prosst RL, Pietschmann M, et al. Laparoscopic fluorescence diagnosis for intraabdominal fluorescence targeting of peritoneal carcinosis experimental studies. Ann Surg. 2002;235:252–260. doi: 10.1097/00000658-200202000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loning M, Diddens H, Kupker W, Diedrich K, Huttmann G. Laparoscopic fluorescence detection of ovarian carcinoma metastases using 5-aminolevulinic acid-induced protoporphyrin IX. Cancer. 2004;100:1650–1656. doi: 10.1002/cncr.20155. [DOI] [PubMed] [Google Scholar]