Abstract
Objective
To evaluate associations between Lipoprotein-associated phospholipase A2 (Lp-PLA2) mass and activity with risk of dementia and its subtypes.
Methods
Analysis were completed on 3,320 participants of the Cardiovascular Health Study (CHS), a population-based longitudinal study of community-dwelling adults age ≥ 65 years followed for an average of 5.4 years. Baseline serum Lp-PLA2 mass was measured using a sandwich enzyme immunoassay and Lp-PLA2 activity utilized a tritiated-platelet activating factor activity assay. Cox proportional hazards regression assessed the relative risk of incident dementia with higher baseline Lp-PLA2 adjusting for demographics, cardiovascular disease (CVD) and risk factors, inflammation markers and apolipoprotein E (APOE) genotype.
Results
Each standard deviation higher Lp-PLA2 mass and activity were related to increased risk of dementia (fully adjusted HR:1.11 per SD, 95% CI:1.00-1.24 for mass; HR:1.12 per SD, 95% CI:1.00-1.26 for activity). Persons in the highest quartile of Lp-PLA2 mass were 50% more likely to develop dementia than those in the lowest quartile in adjusted models (HR: 1.49; 95% CI: 1.08-2.06). Among dementia subtypes, the risk of AD was increased two-fold in the highest compared to lowest quartile of Lp-PLA2 mass (adjusted HR:1.98, 95% CI:1.22-3.21). Results were attenuated in models of mixed dementia and VaD. Lp-PLA2 activity also doubled the risk of mixed dementia in the highest compared to lowest quartile (HR:2.21, 95% CI:1.12-4.373).
Interpretation
These data support Lp-PLA2 as a risk factor for dementia independent of CVD and its risk factors. Further study is required to clarify the role of Lp-PLA2-related mechanisms in dementia subtypes.
Keywords: Lp-PLA2, dementia, Alzheimer’s disease, cardiovascular risk factors
INTRODUCTION
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an enzyme that is part of the phospholpase A2 superfamily. Plasma Lp-PLA2 is produced by hematopoietic lineage cells including macrophages (1). In the plasma, 80% of Lp-PLA2 is bound to low-density lipoprotein (LDL) whereas less than 20% is associated with high-density lipoprotein (HDL) (2). Lp-PLA2 hydrolyzes oxidized LDL, forming lysophosphatidylcholine and oxidized nonesterified fatty acids, which are inflammatory molecules (3). However, Lp-PLA2 can also hydrolyze platelet activating factor, which is involved in activating platelets, monocytes, and macrophages (4). Higher Lp-PLA2 is associated with risk of coronary heart disease and stroke independent of traditional cardiovascular risk factors (5-9). A meta-analysis involving over 79,000 individuals reported modest increases of up to 15% for coronary heart disease, stroke and vascular mortality related to 1 SD higher Lp-PLA2 mass and activity (10). As cardiovascular and cerebrovascular risk factors may increase the risk of developing dementia and Alzheimer’s disease (AD) (11,12), Lp-PLA2 may also be associated with risk of developing dementia.
Two epidemiological studies evaluated the association between Lp-PLA2 activity or mass and dementia. In a case-cohort analysis of the Rotterdam Study, RS, (13), individuals aged 55 years and over within the highest quartile of Lp-PLA2 activity had a greater than 70% higher risk of developing dementia (fully adjusted HR: 1.74, 95% CI: 1.07-2.83) compared to individuals in the lowest quartile. The effect estimate was greater for vascular dementia (VaD, HR = 2.02 for highest quartile relative to lowest; CI 0.59–6.88) than for AD (HR = 1.38; CI 0.82–2.34). Lp-PLA2 mass was not associated with dementia or AD in the Framingham Heart Study, FHS (Dementia, age and sex adjusted HR=0.98 per SD increase; 95% CI 0.84-1.15) (14).
We sought to extend these findings in the Cardiovascular Health Study (CHS), a population-based prospective cohort study of cardiovascular risk factors in 5,888 community-dwelling older adults (15). Objectives included evaluating both Lp-PLA2 mass and activity in association with dementia and subtypes AD and VaD. We hypothesized that participants free of dementia at baseline with higher levels of plasma Lp-PLA2 would have a greater risk of developing dementia during follow-up. The CHS study provides a larger sample than prior studies, greater number of incident dementia cases, and evaluation of both Lp-PLA2 mass and activity in relation to dementia and its subtypes.
METHODS
Study Sample
The Cardiovascular Health Study (CHS) is a multi-site prospective cohort study of 5,888 adults age 65 years and older designed to evaluate risk factors for heart disease and stroke (15). Enrollment was initiated in 1989/90 utilizing Medicare beneficiary files from four US communities: Forsyth county, NC: Sacramento country, CA; Washington country, MD: and Pittsburgh, PA (16). In 1989/90, 5,201 participants were enrolled supplemented with 687 African Americans in 1992/3. This analysis included follow up of up to 10 annual clinic visits, at which extensive information on cardiovascular risk factors were collected including demographics, medical history, health behaviors, psychosocial measures, anthropometry, and blood pressure, Cognition was evaluated via the Modified Mini-Mental State Exam (3MSE) from the first follow-up onward and the Digit Symbol Substitution test was collected all years. Carotid ultrasound was performed several times over the study period as was a fasting phlebotomy for analysis of serum lipids, glucose/insulin, and inflammatory markers. DNA was extracted and apoliprotein E (APOE) genotype was assessed for participants providing a genetic consent. Institutional Review Board (IRB) approvals were received at all study sites.
Ascertainment of Dementia
We ascertained dementia and its subtypes in the CHS Cognition Study (17). Enrollment included 3,602 CHS participants who were non-demented at the 1992/3 examination and who had completed both cerebral magnetic resonance imaging and the Modified Mini-Mental Status Exam during that clinic visit. A standardized protocol was developed for new data collection and classification of dementia across the four sites (18). Retrospective evaluation of all CHS data was supplemented with a battery of neuropyschiatric tests on individuals alive and consenting to the study; medical records review, physician questionnaires, and participant/informant interviews for deceased were collected on other participants unable to come into the clinic. A committee of neurologists and psychiatrists reviewed all data and classified dementia by year according to DSM-IV criteria (APA-DSM IV) (19). MRIs were viewed to classify dementia subtypes of AD using the National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer Disease and Related Disorders Association (NINDS-ADRDA) criteria (20) and VaD using the State of California Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC) criteria (21). As both subtypes were classified independently, we coded participants with both probable/possible AD and VaD as having “mixed” dementia. Follow-up of dementia status occurred from the 1992/93 clinic visit until 1998/99 for an average of 5.4 years of follow-up.
Measurement of Lp-PLA2
Baseline fasting plasma samples (at enrollment into CHS 1989/90) stored at the CHS repository were analyzed to determine levels of Lp-PLA2 mass and activity. Plasma Lp-PLA2 mass was measured at the University of Vermont using a sandwich enzyme immunoassay (ELISA second generation PLAC Test; diaDexus Inc, South San Francisco, CA) and Lp-PLA2 activity was measured at GlaxoSmithKline (Research Triangle, NC) utilized a tritiated-platelet activating factor activity assay. The interassay coefficients of variation were 6.3% and 7.5% for Lp-PLA2 mass and activity, respectively (22).
Data Utilized in the Analysis
Laboratory results of baseline Lp-PLA2 mass and activity, outcomes of dementia, AD, mixed dementia, and VaD, and other covariates collected at the 1992/93 clinic visit (time of entry into the CHS Cognition cohort) were included for analysis. Race was self-reported in CHS according to the following categories: White, Black, American Indian/Alaskan Native, Asian/Pacific Islander, or other. Height and weight were measured in person at this exam and body mass index (BMI) was calculated as weight (kg)/height (m)2. Diabetes status was determined based on American Diabetes Association criteria of fasting glucose concentration greater than or equal to 7·0 mmol/L, and impaired fasting glucose (IFG) is defined as fasting glucose between 5·8 mmol/L and 6·9 mmol/L (23). Hypertension was defined as systolic blood pressure above 140 or diastolic over 90 mm/Hg; borderline was calculated as systolic between 130-140 or diastolic between 80-90 mm Hg. Smoking status was self-reported (current, previous or never). History of myocardial infarction (MI), stroke and congestive heart failure (CHF) included adjudicated prevalence of the specific condition prior to baseline and until the 1992/93 exam (24,25). Use of lipid-lowering medications was determined from an inventory transcribed from prescription medications brought into the clinic at each visit (26). Intima-medial thickness (IMT) was determined using B-mode carotid ultrasound of the common and internal carotid arteries (27). The ankle-brachial index was assessed by Doppler and computed with a result of less than 0.90 indicating peripheral arterial disease (28). Analyses of fasting insulin, glucose, C-reactive protein and interleukin-6 were completed centrally at the CHS Central Laboratory at the University of Vermont (29).
Statistical Analysis
Bivariate statistics were produced as means/standard deviations for continuous and counts/percent for categorical variables by quartile of Lp-PLA2 mass and activity. Analysis of variance or chi-square tests evaluated group differences as appropriate. Cox proportional hazards regression assessed time to dementia, AD, mixed dementia and VaD over follow-up in separate models with Lp-PLA2 mass and activity included as a continuous variable (per standard deviation) and as quartiles. Censoring occurred at onset of dementia as determined by the neurology adjudication committee, death, or end of follow-up. Models were adjusted hierarchically for demographics (age, race, gender and years of education), demographics plus cardiovascular disease and risk factors (history of hypertension, diabetes, CHD, stroke, smoking status, pack years of cigarettes smoked, body mass index, alcohol use, common carotid intima medial thickness (IMT), internal carotid IMT, C-reactive protein (CRP), Interleukin 6 (IL-6), total and LDL cholesterol, triglycerides and use of lipid-lowering drugs); and for demographics, cardiovascular (CVD) risk factors plus number of APOE ε4 alleles. Hazard ratios (HR), 95% confidence intervals (CI), and p-values as well as p-for-trend across quartiles were presented for all associations. Tests of interactions between Lp-PLA2 mass and activity with age, gender and presence of the APOE ε4 allele were conducted to determine effect modification with these variables. We also completed generalized additive models to test for non-linearity of the associations between the Lp-PLA2 measures and dementia outcomes. The Statistical Package for the Social Sciences, version 16.0, and STATA version 11.1 were used to analyze data for this study.
RESULTS
There were 3,320 CHS participants with measurements of both dementia and Lp-PLA2 mass, and 3,315 with both dementia and Lp-PLA2 activity. A total of 470 cases of incident dementia, 241 AD (without VaD), 146 mixed dementia (AD and VaD) and 61 VaD (without AD) had both Lp-PLA2 mass and activity measured. Twenty-two cases of other dementia subtypes (including Parkinson’s disease dementia and Lewy-body dementia) were included in incident dementia analysis; in models of AD or VaD, these cases were censored at time of dementia onset. Both biomarkers were normally distributed with a mean of 341 (SD 117) ng/ml and 39.4 (SD 13.0) nmol/min/mL for Lp-PLA2 mass and activity, respectively. Mean baseline age was 71.9 years (SD 4.8), 59% were female, and 85% were Caucasian.
A number of bivariate relationships were found in common with both Lp-PLA2 mass (Table 1) and activity (Table 2) including gender, race, education, common and internal IMT, total cholesterol, LDL and trigycerides. CVD risk factors related to Lp-PLA2 mass but not activity included age, BMI, and use of any lipid-lowering medication. Variables related to Lp-PLA2 activity but not mass were prevalence of diabetes and hypertension, CRP, and presence of the APOE ε4 allele. Use of tobacco, alcohol, baseline history of stroke, and IL-6 did not differ by Lp-PLA2 mass or activity.
Table 1.
Selected characteristics of study participants by quartile of Lp-PLA2 mass in 3,320 participants of the Cardiovascular Health Study
| Lp-PLA2 Mass (ng/ml) | ||||||
|---|---|---|---|---|---|---|
| < 257.3 | 257.3-326.6 | 326.7-404.8 | ≥ 404.9 | Total | ||
| N | 843 | 857 | 826 | 794 | 3320 | |
|
| ||||||
| Characteristic | N(%) or Mean(SD) |
N(%) or Mean(SD) |
N(%) or Mean(SD) |
N(%) or Mean(SD) |
N(%) or Mean(SD) |
p- value* |
|
| ||||||
| Age (years) | 71.5 (4.6) | 71.8 (4.8) | 71.9 (4.6) | 72.4 (5.1) | 71.9 (4.8) | 0.001 |
| Sex | ||||||
| Female | 550 (65.2) | 540 (63.0) | 455 (55.1) | 423 (53.3) | 1968 (59.3) | <0.001 |
| Male | 293 (34.8) | 317 (37.0) | 371 (44.9) | 371 (46.7) | 1352 (40.7) | |
| Race | ||||||
| Caucasian | 633 (75.1) | 720 (84.0) | 738 (89.3) | 736 (92.7) | 2827 (85.2) | <0.001 |
| Other | 210 (24.9) | 137 (16.0) | 88 (10.7) | 58 (7.3) | 493 (14.8) | |
| Education | ||||||
| LT High School | 173 (20.5) | 195 (22.8) | 192 (23.3) | 234 (29.5) | 794 (24.0) | 0.002 |
| High School/GED | 246 (29.2) | 235 (27.5) | 251 (30.5) | 222 (28.0) | 954 (28.8) | |
| Some College | 203 (24.1) | 211 (24.6) | 203 (24.6) | 178 (22.4) | 795 (24.0) | |
| College/Post | ||||||
| Graduate | 220 (26.1) | 215 (25.1) | 178 (21.6) | 159 (20.1) | 772 (23.3) | |
| Body Mass Index | 26.7 (4.7) | 26.4 (4.4) | 26.8 (4.2) | 26.2 (4.1) | 26.5 (4.4) | 0.02 |
| Diabetes Status** | ||||||
| Normal | 607 (72.1) | 649 (76.1) | 616 (74.8) | 575 (72.5) | 2447 (73.9) | 0.41 |
| IFG | 113 (13.4) | 97 (11.4) | 109 (13.2) | 111 (14.0) | 430 (13.0) | |
| Definite Diabetes | 122 (14.5) | 107 (12.5) | 98 (11.9) | 107 (13.5) | 434 (13.1) | |
| Hypertension Status | ||||||
| Normal | 403 (47.9) | 386 (45.1) | 358 (43.3) | 356 (44.8) | 1503 (45.3) | 0.58 |
| Borderline | 105 (12.5) | 120 (14.0) | 122 (14.8) | 118 (14.9) | 465 (14.0) | |
| Definite | 333 (39.6) | 350 (40.9) | 346 (41.9) | 320 (40.3) | 1349 (40.7) | |
| Smoking Status | ||||||
| Never | 422 (50.2) | 418 (48.8) | 382 (46.2) | 352 (44.4) | 1574 (47.5) | 0.12 |
| Former | 341 (40.5) | 348 (40.6) | 358 (43.3) | 338 (42.6) | 1385 (41.8) | |
| Current | 78 (9.3) | 91 (10.6) | 86 (10.4) | 103 (13.0) | 358 (10.8) | |
|
Alcohol Use (drinks per week) mean |
2.9 (7.3) | 2.6 (6.3) | 2.9 (6.6) | 2.2 (5.3) | 2.6 (6.4) | 0.07 |
|
History of CHD
(Baseline) |
||||||
| No CHD | 718 (85.2) | 724 (84.5) | 686 (83.1) | 657 (82.7) | 2785 (83.9) | 0.49 |
| Prevalent CHD | 125 (14.8) | 133 (15.5) | 140 (16.9) | 137 (17.3) | 535 (16.1) | |
| History of Stroke (Baseline) | ||||||
| Incident | 826 (98.0) | 835 (97.4) | 805 (97.5) | 762 (96.0) | 3228 (97.2) | 0.08 |
| Prevalent | 17 (2.0) | 22 (2.6) | 21 (2.5) | 32 (4.0) | 92 (9.8) | |
|
Common Carotid
IMT (mm) |
1.03 (0.18) | 1.04 (0.20) | 1.05 (0.21) | 1.06 (0.22) | 1.04 (0.20) | 0.03 |
|
Internal Carotid IMT (mm) |
1.32 (0.52) | 1.35 (0.52) | 1.40 (0.54) | 1.45 (0.56) | 1.38 (0.54) | <0.001 |
|
C-Reactive Protein
(mg/L) |
3.34 (6.69) | 3.06 (4.47) | 3.02 (6.52) | 3.19 (5.54) | 3.15 (5.87) | 0.68 |
| Interleukin-6 (pg/ml) | 1.97 (2.28) | 2.08 (1.75) | 1.96 (1.40) | 2.04 (1.41) | 2.01 (1.75) | 0.48 |
| APOE ε4 Allele | ||||||
| No ε4 Allele | 590 (76.6) | 602 (76.2) | 565 (75.7) | 547 (74.8) | 2304 (75.9) | 0.95 |
| 1 ε4 Allele | 171 (22.2) | 177 (22.4) | 173 (23.2) | 172 (23.5) | 693 (22.8) | |
| 2 ε4 Alleles | 9 (1.2) | 11 (1.4) | 8 (1.1) | 12 (1.6) | 40 (1.3) | |
|
Any Lipid Lowering
Drug |
||||||
| No | 777 (92.3) | 808 (94.4) | 786 (95.2) | 770 (97.1) | 3141 (94.7) | <0.001 |
| Yes | 65 (7.7) | 48 (5.6) | 40 (4.8) | 23 (2.9) | 176 (5.3) | |
| Cholesterol (mg/dl) | 200 (36) | 210 (36) | 215 (37) | 222 (42) | 212 (38) | <0.001 |
| LDL (mg/dl) | 118 (32) | 127 (33) | 134 (33) | 142 (37) | 130 (35) | <0.001 |
| Triglyceride (mg/dl) | 131 (66) | 134 (69) | 142 (68) | 137 (69) | 136 (68) | 0.008 |
p-values for chi-square tests or analysis of variance are shown for categorical and continuous variables, respectively.
Table 2.
Selected characteristics of study participants by quartile of Lp-PLA2 activity in 3,315 participants of the Cardiovascular Health Study.
| Lp-PLA2 Activity (nmols/min/ml) | ||||||
|---|---|---|---|---|---|---|
| <30.4 | 30.4-37.7 | 37.8-46.5 | ≥46.6 | Total | ||
| N | 840 | 844 | 808 | 823 | 3315 | |
|
| ||||||
| Characteristic | N(%) or Mean(SD) |
N(%) or Mean(SD) |
N(%) or Mean(SD) |
N(%) or Mean(SD) |
N(%) or Mean(SD) |
p- value |
|
| ||||||
| Age (years) | 71.8 (4.9) | 72.0 (4.9) | 71.7 (4.5) | 72.0 (4.8) | 71.9 (4.8) | 0.52 |
| Sex | ||||||
| Female | 626 (74.5) | 550 (65.2) | 425 (52.6) | 366 (44.5) | 1967 (59.3) | <0.001 |
| Male | 214 (25.5) | 294 (34.8) | 383 (47.4) | 457 (55.5) | 1348 (40.7) | |
| Race | ||||||
| Caucasian | 616 (73.3) | 707 (83.8) | 720 (89.1) | 779 (94.7) | 2822 (85.1) | <0.001 |
| Other* | 224 (26.7) | 137 (16.2) | 88 (10.9) | 44 (5.3) | 493 (14.9) | |
| Education | ||||||
| LT High School | 179 (21.4) | 184 (21.8) | 188 (23.3) | 242 (29.4) | 793 (24.0) | 0.005 |
| High School/GED | 239 (28.6) | 241 (28.6) | 242 (30.0) | 232 (28.2) | 954 (28.8) | |
| Some College | 207 (24.7) | 206 (24.4) | 197 (24.4) | 185 (22.5) | 795 (24.0) | |
| College/Post | ||||||
| Graduate | 212 (25.3) | 213 (25.2) | 180 (22.3) | 163 (19.8) | 768 (23.2) | |
| Body Mass Index | 26.5 (4.8) | 26.3 (4.4) | 26.5 (4.2) | 26.6 (4.1) | 26.5 (4.4) | 0.6 |
| Diabetes Status** | ||||||
| Normal | 638 (76.1) | 660 (78.6) | 586 (72.6) | 559 (68.1) | 2443 (73.9) | <0.001 |
| IFG | 102 (12.2) | 83 (9.9) | 109 (13.5) | 136 (16.6) | 430 (13.0) | |
| Definite Diabetes | 98 (11.7) | 97 (11.5) | 112 (13.9) | 126 (15.3) | 433 (13.1) | |
| Hypertension Status | ||||||
| Normal | 376 (44.8) | 419 (49.6) | 336 (41.6) | 370 (45.1) | 1501 (45.3) | 0.04 |
| Borderline | 116 (13.8) | 113 (13.4) | 111 (13.7) | 124 (15.1) | 464 (14.0) | |
| Definite | 347 (41.4) | 312 (37.0) | 361 (44.7) | 327 (39.8) | 1347 (40.7) | |
| Smoking Status | ||||||
| Never | 418 (49.9) | 406 (48.2) | 375 (46.4) | 372 (45.2) | 1571 (47.4) | 0.28 |
| Former | 323 (38.5) | 346 (41.0) | 355 (43.9) | 358 (43.5) | 1382 (41.7) | |
| Current | 97 (11.6) | 91 (10.8) | 78 (9.7) | 93 (11.3) | 359 (10.8) | |
|
Alcohol Use (drinks
per week) mean |
2.7 (6.5) | 2.8 (6.7) | 2.7 (6.4) | 2.4 (6.0) | 2.7 (6.4) | 0.7 |
|
History of CHD
(Baseliine) |
||||||
| Incident | 736 (87.6) | 725 (85.9) | 651 (80.6) | 669 (81.3) | 2781 (83.9) | <0.001 |
| Prevalent | 104 (12.4) | 119 (14.1) | 157 (19.4) | 154(18.7) | 534 (16.1) | |
| History of Stroke (Baseline) | ||||||
| Incident | 824 (98.1) | 819 (97.0) | 780 (96.5) | 800 (97.2) | 3223 (97.2) | 0.27 |
| Prevalent | 16 (1.9) | 25 (3.0) | 28 (3.5) | 23 (2.8) | 92 (2.8) | |
|
Common Carotid IMT
(mm) |
1.02 (0.18) | 1.03 (0.19) | 1.06 (0.21) | 1.06 (0.21) | 1.04 (0.20) | <0.001 |
|
Internal Carotid IMT
(mm) |
1.28 (0.49) | 1.34 (0.52) | 1.44 (0.57) | 1.46 (0.55) | 1.38 (0.54) | <0.001 |
| C-Reactive Protein | 3.70 (7.11) | 3.01 (5.34) | 3.09 (5.38) | 2.80 (5.41) | 3.15 (5.85) | 0.01 |
| Interleukin-6 (pg/ml) | 1.96 (1.71) | 2.01 (2.20) | 2.08 (1.66) | 2.01 (1.32) | 2.01 (1.75) | 0.64 |
| APOE ε4 Allele | ||||||
| No ε4 Allele | 625 (81.2) | 585 (76.1) | 546 (74.4) | 544 (71.8) | 2334 (75.9) | 0.001 |
| 1 ε4 Allele | 138 (17.9) | 175 (22.8) | 174 (23.7) | 204 (26.9) | 700 (22.8) | |
| 2 ε4 Alleles | 7 (0.9) | 9 (1.2) | 14 (1.9) | 10 (1.3) | 41 (1.3) | |
| Any Lipid Lowering Drug | ||||||
| No | 801 (95.4) | 792 (94.1) | 767 (94.9) | 776 (94.4) | 3136 (94.7) | 0.65 |
| Yes | 39 (4.6) | 50 (5.9) | 41 (5.1) | 46 (5.6) | 176 (5.3) | |
| Cholesterol (mg/dl) | 197 (33) | 209 (36) | 215 (38) | 225 (41) | 211 (38) | <0.001 |
| LDL (mg/dl) | 111 (29) | 128 (32) | 135 (33) | 145 (36) | 130 (35) | <0.001 |
| Triglycerides | 120 (66) | 127 (60) | 140 (66) | 158 (74) | 136 (68) | <0.001 |
p-values for chi-square tests or analysis of variance are shown for categorical and continuous variables, respectively.
A significant association was found between Lp-PLA2 mass and incidence of dementia (Table 3). For each standard deviation of Lp-PLA2 mass measured as a continuous variable, risk of dementia was increased 12% when adjusted for demographics (HR: 1.12, 95% CI: 1.03-1.22). The relationship remained when adjustments were made for CVD risk factors (HR: 1.14, 95% CI: 1.04-1.26) and for number of APOE ε4 alleles (HR: 1.11, 95% CI: 1.00-1.24). When categorized into quartiles, risk of dementia was increased by about 50% in the highest quartile relative to the lowest quartile in all models (i.e. HR: 1.49, 95% CI: 1.08-2.06 in the fully adjusted model). Associations between continuous measures of Lp-PLA2 mass and dementia subtypes were similar to those found with dementia with point estimates between 1.11 and 1.24 and mostly of borderline significance. In the fully adjusted model, participants in the highest quartile of Lp-PLA2 mass (>404 ng/ml) were at a two-fold increased risk of AD compared to those in the lowest quartile (< 258 ng.ml; HR: 1.98, 95% CI: 1.22-3.21). While no associations were found between Lp-PLA2 mass and mixed dementia, an increased risk of VaD was found only in the demographically adjusted quartile model (HR: 2.08, 95% CI: 0.99-4.39). Results of generalized additive models to test for non-linearity adjusted for age, race, gender and education found that Lp-PLA2 mass did not deviate from a linear association with dementia outcomes (p > 0.13).
Table 3.
Risk of incident dementia1 and type of dementia by total and quartiles of Lp-PLA2 mass (ng/ml) in 3315 participants of the CHS Cognition Study using Cox Proportional Hazards Regression.
| N Dementia |
Adjusted for Demographics1 |
Adjusted for CVD/Risk Factors2 |
Adjusted for CVD Risk Factors and APOE ε43 |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Yes/No | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | p | ||
|
| ||||||||
| Dementia | Lp-PLA2 mass (ng/ml) per SD |
470/2845 | 1.12 (1.03-1.22) | .01 | 1.14 (1.04-1.26) | .007 | 1.11 (1.00-1.24) | .04 |
|
| ||||||||
| Lp-PLA2 | .02 | .03 | .09 | |||||
| < 257.3 | 91/751 | 1.00 (reference) | --- | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| 257.3-326.6 | 126/730 | 1.34 (1.02-1.76) | .03 | 1.28 (0.95-1.72) | .10 | 1.26 (0.92-1.73) | .16 | |
| 326.7-404.8 | 113/711 | 1.24 (0.93-1.64) | .14 | 1.16 (0.85-1.59) | .34 | 1.15 (0.82-1.60) | .42 | |
| > 404.9 | 140/653 | 1.53 (1.16-2.00) | .002 | 1.55 (1.14-2.10) | .005 | 1.49 (1.08-2.06) | .02 | |
| p for trend | .007 | .01 | .04 | |||||
|
| ||||||||
| Alzheimer’s dementia |
Lp-PLA2 mass (ng/ml) per SD |
241/3074 | 1.13 (1.00-1.28) | .04 | 1.16 (1.02-1.33) | .03 | 1.12 (0.97-1.30) | .13 |
|
| ||||||||
| Lp-PLA2 | .02 | .01 | .04 | |||||
| < 257.3 | 41/801 | 1.00 (reference) | --- | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| 257.3-326.6 | 67/789 | 1.59 (1.07-2.34) | .02 | 1.80 (1.17-2.75) | .007 | 1.82 (1.14-2.92) | .01 | |
| 326.7-404.8 | 59/765 | 1.47 (0.98-2.20) | .06 | 1.55 (0.99-2.43) | .06 | 1.59 (0.97-2.61) | .07 | |
| > 404.9 | 74/719 | 1.85 (1.25-2.73) | .002 | 2.13 (1.38-3.28) | .001 | 1.98 (1.22-3.21) | .006 | |
| p for trend | .006 | .005 | .02 | |||||
|
| ||||||||
| Mixed Dementia (AD+VaD)5 |
Lp-PLA2 mass (ng/ml) per SD |
146/3169 | 1.16 (0.99-1.4) | .06 | 1.24 (1.05-1.47) | .01 | 1.18 (0.98-1.42) | .07 |
| Lp-PLA2 mass | .40 | .15 | .31 | |||||
| < 257.3 | 29/813 | 1.00 (reference) | --- | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| 257.3-326.6 | 41/815 | 1.33 (0.82-2.14) | .24 | 1.13 (0.67-1.89) | .66 | 1.00 (0.58-1.73) | 1.0 | |
| 326.7-404.8 | 32/792 | 1.06 (0.64-1.76) | .83 | 0.82 (0.46-1.45) | .49 | 0.79 (0.43-1.46) | .45 | |
| > 404.9 | 44/749 | 1.41 (0.87-2.27) | .17 | 1.46 (0.87-2.46) | .15 | 1.34 (0.77-2.32) | .30 | |
| p for trend | .30 | .24 | .47 | |||||
|
| ||||||||
| Vascular Dementia (VaD)6 Only |
Lp-PLA2 mass (ng/ml) per SD |
61/3254 | 1.23 (0.98-1.55) | .08 | 1.11 (0.84-1.46) | .46 | 1.09 (0.81-1.46) | .58 |
|
| ||||||||
| Lp-PLA2 mass | .20 | .68 | .64 | |||||
| < 257.3 | 11/831 | 1.00 (reference) | --- | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| 257.3-326.6 | 13/843 | 1.19 (0.53-2.67) | .67 | 0.90 (0.37-2.22) | .83 | 1.05 (0.42-2.65) | .92 | |
| 326.7-404.8 | 15/809 | 1.39 (0.63-3.06) | .42 | 1.25 (0.53-2.93) | .61 | 1.35 (0.55-3.30) | .51 | |
| > 404.9 | 22/771 | 2.08 (0.99-4.39) | .05 | 1.45 (0.62-3.40) | .40 | 1.66 (0.68-4.07) | .27 | |
| p for trend | .04 | .30 | .24 | |||||
Adjusted for age, race, gender and years of education.
Adjusted for demographics plus history of hypertension, diabetes, CHD, stroke, smoking status, pack years of cigarettes smoked, body mass index, alcohol use, common carotid intima medial thickness (IMT), internal carotid IMT, C-reactive protein, Interleukin 6, total and LDL cholesterol, triglycerides and use of lipid-lowering drugs.
Adjusted for demographics, cardiovascular (CVD) risk factors plus number of APOE ε4 alleles.
AD using NINCDS-ADRDA criteria with no ADDTC criteria VaD.
AD using NINCDS-ADRDA criteria and VaD using ADDTC criteria.
VaD using ADDTC criteria with no NINCDS-ADRDA AD.
Similar to mass, Lp-PLA2 activity modeled as a continuous variable was associated with a significantly increased risk of dementia adjusted for demographics (HR: 1.15, 95% CI: 1.02-1.26) and for CVD risk factors (HR: 1.18, 95% CI: 1.06-1.30) although the relationship was attenuated in the fully-adjusted model (HR: 1.12, 95% CI: 1.00-1.26). Lp-PLA2 activity in the highest compared to lowest quartile was associated with a 43% higher risk of dementia (HR: 1.43, 95% CI: 1.03-1.98) in the CVD risk factor-adjusted model although this association was no longer significant after adjusting for the number of APOE ε4 alleles. High levels of Lp-PLA2 activity were associated with an increased risk of mixed dementia. Participants in the highest quartile compared to those in the lowest had a two-fold increased risk of mixed dementia adjusted for demographics (HR: 1.98, 95% CI: 1.19-3.30), CVD risk factors (HR: 2.11, 95% CI: 1.14-3.89) and number of APOE ε4 alleles (HR: 2.21, 95% CI: 1.12-4.37). Results of generalized additive models to test for non-linearity adjusted for age, race, gender and education found that Lp-PLA2 activity did not deviate from a linear association with dementia outcomes (p > 0.21).
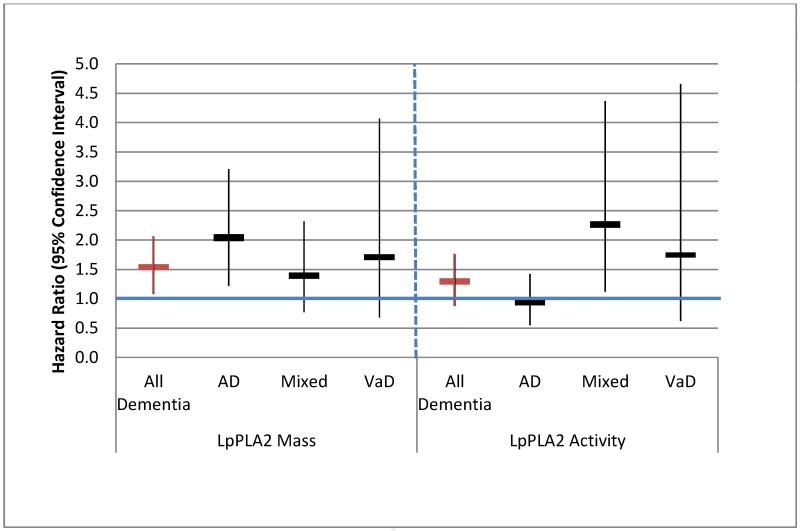
A summary of all associations for both mass and activity for the fully adjusted model is provided in Figure 1. Examination of confidence intervals shows that no important differences can be distinguished between the two Lp-PLA2 assays and dementia and its subtypes with the exception of AD. No interactions between Lp-PLA2 mass or activity with dementia outcomes by age, gender or presence of an APOE ε4 allele were found (p>0.20).
Figure 1.
Risk of dementia and subtypes Alzheimer’s (AD), vascular (VaD) and mixed dementia, by level of Lp-PLA2 mass and activity in fully adjusted models*; hazard ratios and 95% confidence intervals are shown comparing the highest to lowest quartile.
* Models adjusted for age, race, gender, years of education, history of hypertension, diabetes, CHD, stroke, smoking status, pack years of cigarettes smoked, body mass index, alcohol use, common carotid intima medial thickness (IMT), internal carotid IMT, C-reactive protein, Interleukin 6, total and LDL cholesterol, triglycerides, use of lipid-lowering drug, and ApoE genotype.
DISCUSSION
In this large prospective cohort study, higher levels of Lp-PLA2 mass and activity were associated with risk of dementia after accounting for several cardiovascular and other factors although models were attenuated when the APOE ε4 allele was added to the model. While persons in the highest quartile of Lp-PLA2 mass had a two-fold increased risk of AD (i.e. without VaD) compared to those in the lowest quartile, a similar association (twice the risk) was found between Lp-PLA2 activity and those classified with mixed dementia (i.e. both AD and VaD). This is the third study to our knowledge that has evaluated associations between Lp-PLA2 measures and dementia; all three were longitudinal designs. The results obtained by the FHS, which did not identify an association of Lp-PLA2 mass with dementia or AD, albeit with a smaller sample size (14), contrast with results of the RS. Because of the contradictory outcomes of these two studies, additional data was essential in order to obtain clarity on the association. The current study expands the results of the RS and FHS by investigation of both mass and activity in a larger sample with more cases of dementia including subtypes.
Results for dementia found here are similar to those reported for Lp-PLA2 activity by the RS (13), while they differ from those in the FHS which did not find associations between Lp-PLA2 mass and dementia or AD (14). All three studies are similar in that they utilize data from prospective cohorts, all participants are adults over the age of 55 (mean age within 8 years), and gender distributions are similar. Sample size, however, differed by study with CHS having the largest sample (3,320) compared to 1,742 in the RS and 840 in the FHS. The number of incident dementia cases also varied by study (470 in CHS, 77 in RS and 159 in FHS). Both of these factors may well have affected the power to determine associations. A comparison of the Lp-PLA2 measurements found that while mean activity was similar between CHS and RS (39.4 vs 44.5 ng/ml, respectively), Lp-PLA2 mass was much higher in CHS [341 (SD 117) ng/ml] than in FHS [268 (SD 88) ng/ml] in FHS. The lower level of inflammation in FHS participants may have been a factor in the lack of associations should there be a threshold in Lp-PLA2 mass for detecting associations with dementia. Finally, while average follow-up time was similar for CHS and RS (5.4 and 5.7 years, respectively), dementia was ascertained an average of 13 years after the Lp-PLA2 measurements were made in FHS. While longer follow-up allows for accrual of more cases, it is possible that, for associations such as inflammation which change over time, outcomes further from the study baseline may be affected by longitudinal changes of the biomarker resulting in weaker associations. This effect has been discussed in a previous study using CHS data (30). Related to this, it is possible that over longer periods of time, destabilizing atherosclerosis may lead to higher inflammation over time that would not be captured in the FHS analysis. As such, the shorter follow-up may reflect less measurement error in the analysis. Some of these issues, or others not identified here, may have played a role in differences found between the CHS, RS and FHS results.
The value of Lp-PLA2 as a marker of coronary heart disease and stroke has been established both in the CHS (31,32) and many other studies including a comprehensive meta-analysis (10). In fact, Lp-PLA2 may be a better marker for CVD than LDL and dense LDL due to its role in plaque inflammation and stability (33,34). It has been argued that its clinical utility, especially in conjunction with other markers of subclinical CVD and inflammation, may be important as a proxy for evaluating therapeutic response (35).
While Lp-PLA2 is known to be related to inflammation, vascular risk, and APOE genotype, more research is required to clarify potential mechanisms for the relationship of Lp-PLA2 and dementia. The associations found here by subtype are unique to this study and provide information of potential value in the role of Lp-PLA2 and AD. Many studies including CHS have reported associations of CVD and its risk factors with dementia (28). Thus, it follows that a biomarker for CVD may be found to increase the risk of dementia based on influences of vascular disease and inflammation on cognition. A potential pathway linking vascular disease with dementia could involve an increased risk of cerebral microbleeds. In the FHS, while no overall association was found between Lp-PLA2 and total or lobar cerebral microbleeds, an interaction with the APOE genotype revealed an increased risk in carriers of either the ε2 or ε4 allele (37). While it is possible that error or lack of precision in classifying dementia subtype, especially in evaluation of vascular disease in the AD cases, may be influencing the associations reported here, the potential for a new marker to identify dementia risk beyond those known for CVD is a significant finding. The null/weak relationship with VaD most likely was impacted by low number of these events. We observed an association of Lp-PLA2 with APOE genotype and APOE partly confounded the association of Lp-PLA2 with dementia. This is supported by recent evidence showing an association between Lp-PLA2 activity and APOE genotype (38,39). More more work in this area is needed, however, as a recent study in Japan reported that presence of the null activity polymorphism of the Lp-PLA2 gene was not associated with AD (36).
In this study Lp-PLA2 was associated with AD, both with and without a concurrent diagnosis of VaD, independent of CVD and inflammatory risk factors, raising the possibility that Lp-PLA2 may be a risk factor for Alzheimer/dementia-related pathology. Many of the CHS participants in this cohort, mean age 72 years, may already have had AD pathology as the development of amyloid plaques can begin decades before symptoms of AD are evident. However, it is also possible that Lp-PLA2 is actually a risk factor for another comorbid condition that lowers the threshold of dementia in people who already have some brain pathology.
Strengths of this study include the population-based and prospective design of the CHS cohort, the large sample size and number of incident dementia cases, and the standardized methodology for collecting all data including participant characteristics, assays for Lp-PLA2 mass and activity, and dementia outcomes. Several limitations should be mentioned. These include the potential for misclassification of dementia subtype, low number of participants classified with VaD (no AD), and inability to capture residual confounding that may be present. It should also be cautioned that while this study has presented associations between Lp-PLA and the clinical syndrome of dementia/AD, these data do not necessarily address underlying pathologies as risk factors may be different. Generalization of results to other ethnicities is also limited due to the low number of non-whites in the CHS cohort.
CONCLUSION
Increased levels of Lp-PLA2, measured as both mass and activity, were associated with increased risk of dementia independent of several cardiovascular, inflammatory and other factors. Here we have shown that Lp-PLA2 may also be a valuable predictor of AD, either with or without concurrent VaD, adding to its importance as either a risk factor or biomarker for AD. As dementia and AD are major public health problems worldwide, the need to identify persons at increased risk of developing dementia and who may benefit more from preventive interventions is critical. Understanding the role that Lp-PLA2 may play in the etiology of cognitive dysfunction or its utility beyond other biomarkers to detect preclinical stages of dementia may be vital in the battle against this devastating disease and its consequences.
LpPLA2 mass and activity were both related to an increased risk of dementia in the Cardiovascular Health Study.
The risk of Alzheimer’s dementia was increased two-fold in the highest compared to lowest quartile of Lp-PLA2 mass.
Lp-PLA2 activity doubled the risk of mixed dementia (AD and vascular dementia) in the highest compared to lowest quartile.
These associations were independent of demographics, cardiovascular disease and its risk factors.
These data add to the literature reporting conflicting results of the association between LpPLA2 and dementia.
Table 4.
Risk of incident dementia1 and type of dementia by total and quartile of Lp-PLA2 activity in 3310 participants of the CHS Cognition Study using Cox Proportional Hazards Regression.
| N Dementia |
Adjusted for Demographics1 |
Adjusted for CVD/Risk Factors2 |
Adjusted for CVD Risk Factors and APOE ε43 |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Yes/No | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
|
| ||||||||
| Dementia | Lp-PLA2 activity per SD (nmol/min/mLl) |
470/2840 | 1.15 (1.01-1.26) | .002 | 1.18 (1.06-1.30) | .002 | 1.12 (1.00-1.26) | .06 |
|
| ||||||||
| Lp-PLA2 activity | .06 | .01 | .06 | |||||
| <30.4 | 111/726 | 1.00 (reference) | --- | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| 30.4-37.7 | 115/729 | 1.00 (0.77-1.30) | .99 | 1.03 (0.76-1.38) | .87 | 0.89 (0.65-1.23) | .48 | |
| 37.8-46.5 | 104/703 | 1.01 (0.76-1.32) | .97 | 0.90 (0.65-1.24) | .51 | 0.84 (0.60-1.18) | .31 | |
| ≥46.6 | 140/682 | 1.33 (1.02-1.73) | .03 | 1.43 (1.03-1.98) | .03 | 1.24 (0.88-1.76) | .23 | |
| p for trend | .04 | .07 | .25 | |||||
|
| ||||||||
| Alzheimer’s dementia | Lp-PLA2 activity per SD (nmol/min/mLl) |
241/3069 | 1.10 (0.96-1.24) | .16 | 1.15 (0.99-1.33) | .06 | 1.08 (0.92-1.28) | .33 |
|
| ||||||||
| Lp-PLA2 activity | .33 | .23 | .04 | |||||
| <30.4 | 67/770 | 1.00 (reference) | --- | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| 30.4-37.7 | 59/785 | 0.87 (0.61-1.24) | .44 | 0.98 (0.59-1.31) | .53 | 0.60 (0.39-0.94) | .03 | |
| 37.8-46.5 | 48/759 | 0.80 (0.55-1.16) | .24 | 0.80 (0.51-1.23) | .30 | 0.60 (0.38-0.96) | .03 | |
| ≥46.6 | 67/755 | 1.09 (0.76-1.56) | .63 | 1.19 (0.77-1.86) | .44 | 0.89 (0.55-1.42) | .61 | |
| p for trend | .74 | .57 | .69 | |||||
|
| ||||||||
| Mixed Dementia (AD+VaD)5 |
Lp-PLA2 activity per SD (nmol/min/mLl) |
146/3164 | 1.25 (1.07-1.45) | .004 | 1.25 (1.05-1.49) | .01 | 1.20 (0.98-1.46) | .08 |
| Lp-PLA2 activity | .07 | .09 | .15 | |||||
| <30.4 | 25/812 | 1.00 (reference) | --- | 1.00 (reference) | --- | 1.00 (reference) | --- | |
| 30.4-37.7 | 38/806 | 1.44 (0.87-2.40) | .16 | 1.42 (0.81-2.49) | .22 | 1.64 (0.88-3.06) | .12 | |
| 37.8-46.5 | 36/771 | 1.55 (0.92-2.61) | .10 | 1.33 (0.73-2.42) | .35 | 1.67 (0.87-3.19) | .12 | |
| ≥46.6 | 47/775 | 1.98 (1.19-3.30) | .008 | 2.11 (1.14-3.89) | .02 | 2.21 (1.12-4.37) | .02 | |
|
| ||||||||
| p for trend | .009 | .02 | .02 | |||||
|
| ||||||||
| Vascular Dementia (VaD)6 Only |
Lp-PLA2 activity per SD (nmol/min/mLl) |
61/3249 | 1.24 (0.99-1.56) | .06 | 1.18 (0.88-1.60) | .26 | 1.15 (0.84-1.58) | .38 |
|
| ||||||||
| Lp-PLA2 activity | .30 | .32 | .42 | |||||
| <30.4 | 13/824 | 1.00 (reference) | 1.00 (reference) | --- | 1.00 (reference) | --- | ||
| 30.4-37.7 | 14/830 | 1.02 (0.48-2.20) | .95 | 1.18 (0.49-2.82) | .71 | 1.35 (0.54-3.33) | .52 | |
| 37.8-46.5 | 12/795 | 0.91 (0.41-2.04) | .82 | 0.71 (0.27-1.90) | .50 | 0.86 (0.31-2.36) | .77 | |
| ≥46.6 | 22/800 | 1.65 (0.79-3.45) | .18 | 1.56 (0.60-4.05) | .36 | 1.70 (0.62-4.66) | .30 | |
| p for trend | .19 | .57 | .55 | |||||
Adjusted for age, race, gender and years of education.
Adjusted for demographics plus history of hypertension, diabetes, CHD, stroke, smoking status, pack years of cigarettes smoked, body mass index, alcohol use, common carotid intima medial thickness (IMT), internal carotid IMT, C-reactive protein, Interleukin 6, total and LDL cholesterol, triglycerides and use of lipid-lowering drugs.
Adjusted for demographics, cardiovascular (CVD) risk factors plus number of APOE ε4 alleles.
AD using NINCDS-ADRDA criteria with no ADDTC criteria VaD.
AD using NINCDS-ADRDA criteria and VaD using ADDTC criteria.
VaD using ADDTC criteria with no NINCDS-ADRDA AD.
Acknowledgement
This research was supported by contracts HHSN268201200036C, N01HC85239, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85085, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by grants R01AG15928 and AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org. A grant from GlaxoSmithKline (GSK) provided analytic support for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest
Dr. Koro is an employee at GlaxoSmithKline which provided funding for this project. Dr. Irizarry was an employee of GlaxoSmithKline at the time this work was conducted but his current affiliation is Medical - Neurosciences, Eli Lilly and Company, Indianapolis, IN. Dr. Fitzpatrick received funding for analysis of this project from GlaxoSmithKline. Drs. Cushman and Jenny have research funding from diaDexus, San Francisco, CA.
REFERENCES
- 1.Asano K, Okamoto S, Fukunaga K, et al. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochemical and Biophysical Research Communications. 1999;261:511–514. doi: 10.1006/bbrc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 2.Caslake MJ, Packard CJ, Suckling KE, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis. 2000;150:413–419. doi: 10.1016/s0021-9150(99)00406-2. [DOI] [PubMed] [Google Scholar]
- 3.MacPhee CH, Moores KE, Boyd HF, et al. Lipoproteinassociated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338:479–487. [PMC free article] [PubMed] [Google Scholar]
- 4.Tselepis AD, Chapman MJ. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atherosclerosis. 2002;(Suppl 3):57–68. doi: 10.1016/s1567-5688(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 5.Packard CJ, O’Reilly DS, Caslake MJ, et al. West of Scotland Coronary Prevention Study Group Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 7.Caslake MJ, Packard CJ. Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol. 2003;14:347–352. doi: 10.1097/00041433-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Rader DJ. Inflammatory markers of coronary risk. N Engl J Med. 2000;343:1179–1182. doi: 10.1056/NEJM200010193431609. [DOI] [PubMed] [Google Scholar]
- 9.Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 10.The Lp-PLA2 Studies Collaboration Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–44. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a Risk Factor for Dementia and Cognitive Decline: A Systematic Review of Prospective Studies With Meta-Analysis. American Journal of Geriatric Psych. 2008;16(5) doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 12.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia--A double edged sword. Ageing Research Reviews. 2009 Apr;8(2):61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Van Oijen M, van der Mer IM, Hofman A, Witteman JCM, Koudstaal PJ, Breteler MMB. Lipoprotein-Associated Phospholipase A2 Is Associated with Risk of Dementia. Ann Neurol. 2006;59:139–144. doi: 10.1002/ana.20721. [DOI] [PubMed] [Google Scholar]
- 14.van Himbergen TM, Beiser AS, Ai M, Seshadri S, Otokozawa S, Au R, Thongtang N, Wolf PA, Schaefer EJ. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Arch Neurol. 2012;69:594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–66. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 17.Lopez OL, Kuller LH, Fitzpatrick AL, Ives D, Becker JT, Beauchamp N. Evaluations of dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick AL, Kuller LH, Ives D, Lopez OL, Jagust W, Breitner J, Beauchamp N, Lyketsos C, Dulberg C. Incidence and Prevalence of Dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 19.APA . DSM-IV: Diagnostic and Statistic Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- 20.McKhann G, Drachman DA, Folstein MF, Katzman R, Price DL, Stadlan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Solomon C, Jenny NS, et al. Lipoprotein-associated phospholipase A(2) and risk of congestive heart failure in older adults: the Cardiovascular Health Study. Circ Heart Fail. 2009 Sep;2(5):429–436. doi: 10.1161/CIRCHEARTFAILURE.108.839613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wahl PW, Savage PJ, Psaty BM, Orfanrd TJ, Robbins JA, Tracy RP. Diabetes in older adults: comparison of 1997 American Diabetes Association classification of diabetes mellitus with 1985 WHO classification. Lancet. 1998;352:1012–5. doi: 10.1016/S0140-6736(98)04055-0. [DOI] [PubMed] [Google Scholar]
- 24.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–77. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 25.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG. Surveillance and ascertainment of cardiovascular events. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 26.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M, The Cardiovascular Health Study Collaborative Research Group Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. J Clin Epidemiol. 1992 Jun;45(6):683–92. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 27.O’Leary DH, Polak JF, Wolfson SK, et al. The Cardiovascular Health Study CHS Collaborative Research Group Use of sonography to evaluate carotid atherosclerosis in the elderly. Stroke. 1991;22:1155–63. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in The Cardiovascular Health Study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 29.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clinical Chemistry. 1995;41:264–270. [PubMed] [Google Scholar]
- 30.Jenny NS, Yanez ND, Psaty BM, Kuller LH, Hirsch CH, Tracy RP. Inflammation Biomarkers and Near-Term Death in Older Men. Am J Epidemiol. 2007;165:684–95. doi: 10.1093/aje/kwk057. [DOI] [PubMed] [Google Scholar]
- 31.Furberg CD, Nelson JJ, Solomon C, Cushman M, Jenny NS, Psaty BM. Distribution and correlates of lipoprotein-associated phospholipase A2 in an elderly cohort: the Cardiovascular Health Study. J Am Geriatr Soc. 2008 May;56(5):792–9. doi: 10.1111/j.1532-5415.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 32.Jenny NS, Solomon C, Cushman M, Tracy RP, Nelson JJ, Psaty BM, Furberg CD. Lipoprotein-associated phospholipase A(2) (Lp-PLA(2)) and risk of cardiovascular disease in older adults: results from the Cardiovascular Health Study. Atherosclerosis. 2010;209:528–32. doi: 10.1016/j.atherosclerosis.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenstein A, Izkhakov E. Lipoprotein associated phospholipase A2. Harefuah. 2011;150:136–40. [PubMed] [Google Scholar]
- 34.Corson MA, Jones PH, Davidson MH. Review of the evidence for the clinical utility of lipoprotein-associated phospholipase A2 as a cardiovascular risk marker. Am J Cardiol. 2008;101(suppl):41F–50F. doi: 10.1016/j.amjcard.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Dadu RT, Nambi V, Ballantyne CM. Developing and assessing cardiovascular biomarkers. Translational Research. 2012:1–13. doi: 10.1016/j.trsl.2012.01.003. e-copy prior to publication. [DOI] [PubMed] [Google Scholar]
- 36.Koshy B, Miyashita A, St Jean P, Stirnadel H, Kaise T, Rubio JP, Mooser V, Kuwano R, Irizarry MC. Genetic Deficiency of Plasma Lipoprotein-Associated Phospholipase A2 (PLA2G7 V297F Null Mutation) and Risk of Alzheimer’s Disease in Japan. J Alzheimers Dis. 2010;21:775–780. doi: 10.3233/JAD-2010-100513. [DOI] [PubMed] [Google Scholar]
- 37.Romero JR, Preis SR, Beiser AS, et al. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham Heart Study. Stroke. 2012 Nov;43(11):3091–4. doi: 10.1161/STROKEAHA.112.656744. doi: 10.1161/STROKEAHA.112.656744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drenos F, Talmud PJ, Casas JP, Smeeth L, Palmen J, Humphries SE, Hingorani AD. Integrated associations of genotypes with multiple blood biomarkers linked to coronary heart disease risk. Hum Mol Genet. 2009;18:2305–2316. doi: 10.1093/hmg/ddp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suchindran S, Rivedal D, Guyton JR, Milledge T, Gao X, Benjamin A, Rowell J, Ginsburg GS, McCarthy JJ. Genome-wide association study of Lp-PLA2 activity and mass in the Framingham Heart Study. PLoS Genet. 2010;6:e1000928. doi: 10.1371/journal.pgen.1000928. doi:10.1371/journal.pgen.1000928. [DOI] [PMC free article] [PubMed] [Google Scholar]