Abstract
Background
Compounds targeting somatostatin-receptor-type-2 (SSTR2) are useful for small bowel (SBNET) and pancreatic neuroendocrine tumor (PNET) imaging and treatment. We recently characterized expression of 13 cell-surface receptor genes in SBNETs and PNETs, identifying three drug targets (GIPR, OXTR, and OPRK1). This study set out to characterize expression of this gene panel in the less-common neuroendocrine tumors of the stomach and duodenum (GDNETs).
Methods
Primary tumors and adjacent normal tissue were collected at surgery, RNA was extracted, and expression of 13 target genes was determined by quantitative-PCR. Expression was normalized to GAPDH and POLR2A internal control genes. Expression relative to normal tissue (ddCT) and absolute expression (dCT) were calculated. Wilcoxon tests compared median expression with false discovery rate correction for multiple comparisons.
Results
Gene expression was similar in 2 gastric and 7 duodenal tumors, and these were analyzed together. Like SBNETs (n=63) and PNETs (n=51), GDNETs showed significant overexpression compared to normal tissue of BRS3, GIPR, GRM1, GPR113, OPRK1, and SSTR2 (p<0.05 for all). Of these, SSTR2 had the highest absolute expression in GDNETs (median dCT 4.0). Absolute expression of BRS3, GRM1, GPR113, and OPRK1 was significantly lower than SSTR2 in GDNETs (p<0.05 for all), while expression of GIPR was similar to SSTR2 (median 4.3, p=0.4).
Conclusions
As in SBNETs and PNETs, GIPR shows absolute expression close to SSTR2, but has greater overexpression relative to normal tissue (21.1 vs. 3.5-fold overexpression). We conclude that GIPR could provide an improved signal-to-noise ratio for imaging versus SSTR2, and represents a promising novel therapeutic target in GDNETs.
Keywords: Neuroendocrine tumors, Small bowel, Pancreatic, Gastric, Duodenal, Gastric inhibitory polypeptide receptor, somatostatin receptor, octreotide, gene expression, GIPR
Introduction
Duodenal neuroendocrine tumors constitute one of the rarest types of neuroendocrine tumors (NETs), with an annual incidence of 0.19 per 100,000 in the United States, representing approximately 4% of all NETs(1, 2). Gastric neuroendocrine tumors have an incidence of 0.30 per 100,000, and their numbers have increased dramatically over time due to the proliferation of upper endoscopy and possibly the use of acid suppressive medications(1, 3, 4). Although most GDNET tumors are small, amenable to endoscopic resection, and rarely metastatic, a subpopulation exists of generally larger and more aggressive tumors requiring a more extensive staging evaluation, operative treatment, and often postoperative medical therapy(3).
Staging, preoperative planning, and medical treatment of other types of NETs exploits high expression of the somatostatin receptor in these tumors(5, 6). Molecules such as octreotide, which binds principally to the somatostatin-receptor-type- 2 (SSTR2), can decrease symptoms from hormone overproduction syndromes and reduce tumor progression in midgut NET patients(7). Linking somatostatin analogues to radioligands such as 111In or 68Ga allows them to accumulate selectively at the tumor, permitting radiographic visualization of tumor tissues(8–10). In peptide receptor radionuclide therapy (PRRT), somatostatin analogues linked to higher-energy isotopes (90Y or 177Lu) can actually kill tumor cells(11). Yet even with the success of these tests and therapies in some patients, not all NETs express somatostatin receptors, and not all patients respond to octreotide(9, 12–14).
To build on the success of somatostatin-based NET treatments, and respond to the dilemma of patients in whom they fail, our group used G protein-coupled receptor (GPCR) and exon-expression microarrays to find new therapeutic targets, determining expression of 384 genes in a small number of initial tumor samples(15), and validating expression of a panel of 13 genes in over 100 SBNETs and PNETs(15–17). In these studies, OPRK1 and OXTR in SBNETs and GIPR in both SBNETs and PNETs, emerged as potentially useful receptors. These three genes displayed absolute expression similar to SSTR2, while having significantly higher expression in tumors relative to normal tissues. This suggested that imaging strategies directed at these receptors might display improved signal-to-background characteristics, and treatments might have fewer effects on non-cancerous tissue.
In GDNETs, the optimal use of somatostatin-directed therapies is unclear. Due to GDNETs’ rarity, most studies of somatostatin-based therapies do not include them, or analyze them along with other tumor types(4). To inform the use of peptide receptor-directed imaging and therapy in GDNETs, and assess potential new therapeutic targets, we set out to determine expression levels of this 13-gene panel in the rare and poorly-studied population of GDNETs and compared these results to our previous findings in SBNETs and PNETs.
Materials and Methods
Patients and Clinical Data
Patients undergoing surgery for abdominal NETs at a single center since 2005 were enrolled under an Institutional Review Board-approved protocol with full informed consent. Clinical and pathologic information for these patients was reviewed and included in the Iowa Neuroendocrine Tumor Registry Database(18). Tumor and adjacent normal tissues were collected at the time of surgery and maintained in RNALater solution (Life Technologies, Grand Island, NY). GDNETs included neuroendocrine tumors of the stomach and duodenum. Ampullary NETs were not included.
Target genes and quantitative-PCR (qPCR)
The target gene panel was selected from pilot experiments with GPCR and exon-expression microarrays, and evaluated in a large number of SBNETs and PNETs(15–17). Target genes, primers, and qPCR methods were as described(17). The target gene panel included somatostatin-receptor- type-2 (SSTR2), adenosine-A1 receptor (ADORA1), bombesin-like receptor 3 (BRS3), dopamine receptor D1 (DRD1), gastric inhibitory polypeptide receptor (GIPR), G protein-coupled receptor 98 (GPR98), G protein-coupled receptor 113 (GPR113), glutamate receptor metabotropic 1 (GRM1), meprin-A-beta receptor (MEP1B), mucin-13 cell-surface-associated protein (MUC13), opioid receptor kappa 1 (OPRK1), oxytocin receptor (OXTR), and secretin receptor (SCTR). Internal control genes included glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and polymerase (RNA) II polypeptide-A (POLR2A). Total RNA from tumor and adjacent normal tissue samples was reverse-transcribed to cDNA and expression of target genes was determined by qPCR in triplicate using the 7900 HT-Fast RT-PCR System (Applied Biosystems, Grand Island, NY).
Data Analysis
Mean threshold cycles (Ct) were calculated and normalized to internal control genes to give dCT. Expression in tumor tissues relative to adjacent normal tissue was determined by the ddCT method (ddCT = Tumor(dCT) – Normal(dCT)). Fold changes were determined as 2(−ddCT). Median dCT and ddCT gene expression levels were compared by Wilcoxon Rank-Sum and Sign-Rank tests. All P values are Benjamini-Hochberg false discovery rate-adjusted to correct for multiple comparisons(19). Statistical analyses were performed with R v. 3.0.1 (Vienna, Austria).
Results
Patient and Tumor Characteristics
Patients with gastric (n=2) and duodenal (n=7) NETs were included, and results compared to patients with SBNETs (n=63) and PNETs (n=51). Gene expression in the gastric NETs was found to be similar to that in duodenal tumors, and these were analyzed together (GDNETs, n=9). Median age at surgery for GDNET patients was 57.3 (range 52.6–70.9). Median progression-free survival and overall survival were not yet reached. Preoperative laboratory values were available for most patients. The most common abnormal preoperative hormonal markers were elevated chromogranin A (in 5 of 7 who were screened), serotonin (4/8), and pancreastatin (4/8). One duodenal tumor was recognized as a gastrinoma, with a preoperative gastrin level of 828 pg/mL. All duodenal tumors were located in the first or second portions of the duodenum. Mean primary tumor size was 3.3cm (range 0.7–6.8), and was not significantly different between gastric and duodenal NETs (p=0.4). Two gastric and 2 of 7 duodenal NETs were associated with liver metastases, with the remaining 5 tumors limited to the primary site. One gastric tumor was high-grade and one duodenal tumor was intermediate-grade, while the other 7 were low-grade. None of the patients had a recognized familial syndrome.
Relative Gene Expression
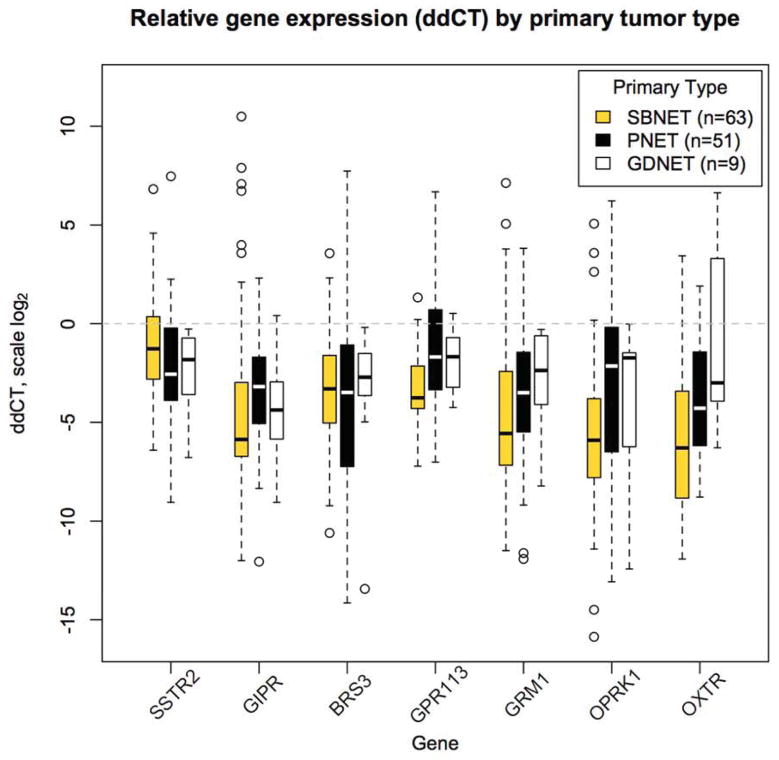
We previously demonstrated that eight of the thirteen genes in our panel have significantly higher expression in primary SBNETs and PNETs relative to adjacent normal tissue(17). To determine whether GDNETs also overexpress these genes, their relative gene expression was quantified by ddCT (Table 1). For both ddCT and dCT expression, lower numeric values indicate higher expression. GDNET expression of the somatostatin-receptor-type-2 (SSTR2) was significantly greater than in adjacent normal tissue (median ddCT −1.8), and corresponded to 3.5-fold overexpression. Five other genes, GIPR, BRS3, GPR113, GRM1, and OPRK1 showed significant overexpression relative to normal tissues (Figure 1). Two genes that were overexpressed in SBNETs and PNETs, OXTR and GPR98, had expression in GDNETs that was not significantly different from that of normal tissue. Among the overexpressed genes, the gastric inhibitory polypeptide receptor (GIPR) had the largest median overexpression compared to normal tissue, at 21.1-fold. The only gene with a greater fold-overexpression was GPR98, but due to more variability in its expression, its difference from normal tissue did not reach significance (p=0.07). These results indicate that in addition to SSTR2, these 5 genes and particularly GIPR, represent potential therapeutic targets in GDNETs due to their higher expression in primary tumor compared to normal tissues.
Table 1.
Relative gene expression in primary tumors by ddCT identifies 5 genes with significantly higher expression in GDNETs compared to adjacent normal tissue. IQR: Interquartile range. P values are false-discovery rate adjusted.
| Primary Tumor Relative Expression Median ddCT (IQR) | Median GDNET Fold Change | P value GDNET ddCT | |||
|---|---|---|---|---|---|
| Gene | SBNET (n=63) | PNET (n=51) | GDNET (n=9) | vs. Normal | vs. Normal |
| SSTR2 | −1.2 (−2.8, 0.4) | −2.6 (−3.9, −0.3) | −1.8 (−3.4, −0.8) | 3.5 | 0.013 |
| GIPR | −5.8 (−6.7, −3.0) | −3.2 (−5.0, −1.8) | −4.4 (−5.7, −3.1) | 21.1 | 0.02 |
| BRS3 | −3.3 (−5.0, −1.6) | −3.5 (−7.2, −1.2) | −2.7 (−3.6, −1.5) | 6.5 | 0.013 |
| GPR113 | −3.8 (−4.3, −2.1) | −1.7 (−3.3, 0.6) | −1.7 (−3.1, −0.7) | 3.2 | 0.02 |
| GRM1 | −5.5 (−7.2, −2.4) | −3.5 (−5.5, −1.4) | −2.4 (−3.9, −0.9) | 5.3 | 0.013 |
| OPRK1 | −5.9 (−7.8, −3.9) | −2.1 (−6.4, −0.3) | −1.7 (−6.2, −1.5) | 3.2 | 0.013 |
| ADORA1 | 0.0 (−1.3, 0.9) | 2.9 (0.2, 6.0) | −0.7 (−3.0, 0.5) | 1.6 | 0.52 |
| DRD1 | 0.2 (−1.2, 1.3) | −0.3 (−1.4, 0.8) | −0.6 (−0.9, 0.3) | 1.5 | 0.82 |
| GPR98 | −5.0 (−6.5, −3.0) | −1.2 (−2.2, 0.2) | −4.5 (−6.1, −0.1) | 22.6 | 0.07 |
| MEP1B | 2.1 (0.1, 4.3) | −3.7 (−8.3, −1.3) | 3.8 (−1.0, 5.1) | −13.9 | 0.81 |
| MUC13 | 0.6 (−0.9, 2.4) | −3.1 (−5.2, −0.9) | 1.3 (−1.0, 2.9) | −2.5 | 0.81 |
| OXTR | −6.3 (−8.8, −3.4) | −4.3 (−6.1, −1.5) | −3.0 (−3.9, 3.3) | 8.0 | 0.85 |
| SCTR | 1.3 (0.3, 2.2) | 4.8 (1.8, 6.8) | 0.0 (−1.3, 2.4) | 1.0 | 0.85 |
Figure 1.
Expression in primary tumors relative to adjacent normal tissue (ddCT) for selected genes, shown by tumor type. Lower ddCT indicates higher expression on log scale. Boxes show interquartile range (IQR), whiskers show 1.5*IQR, open circles show outliers, bar shows median. Dotted line at zero indicates expression equal to normal tissue. All except OXTR in GDNETs have significant overexpression in tumor compared to normal tissues. GIPR shows the greatest median overexpression in GDNETs.
Absolute Gene Expression
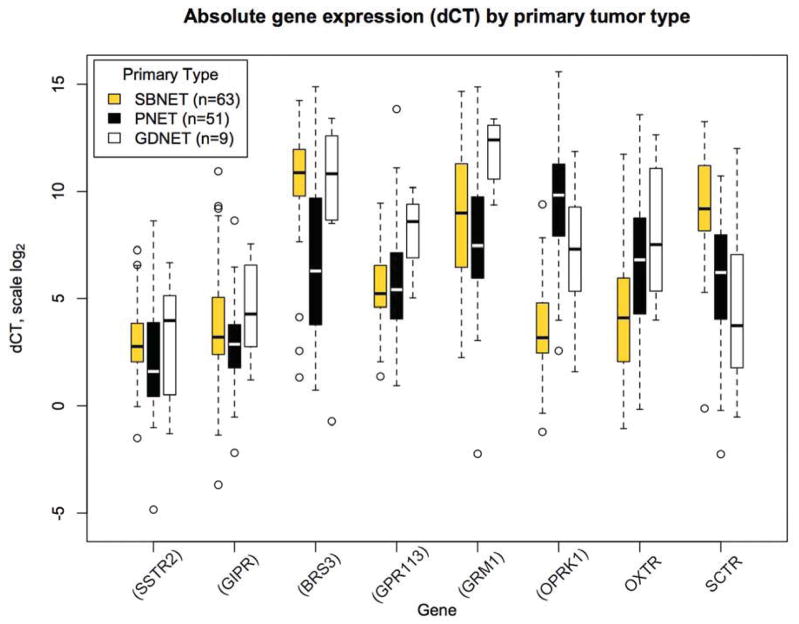
Although 8 genes showed significant overexpression relative to normal tissue in SBNETs and PNETs, most had absolute expression in tumors that was much lower than that of SSTR2, making them less attractive targets(17). To evaluate whether the genes overexpressed in GDNETs had absolute expression consistent with therapeutic utility, we next examined their absolute expression normalized to internal control genes (dCT) (Table 2). Among the six genes overexpressed in GDNETs relative to normal tissues, SSTR2 had the highest absolute expression (lowest median dCT, 4.0). MUC13 and SCTR both had higher absolute expression than SSTR2 (median dCT 0.6 and 3.7), but as neither was overexpressed relative to normal tissue, they would likely offer minimal specificity to tumor tissue as therapeutic targets. Four of the GDNET-overexpressed genes (BRS3, GPR113, GRM1, and OPRK1) had expression levels significantly below that of SSTR2, with median dCTs corresponding to expression 111, 24, 239, and 10-fold lower than SSTR2, respectively (Figure 2). GIPR, however, had a median dCT of 4.3, which was not significantly different from that of SSTR2 (median dCT 4.0, p=0.38 vs. GIPR). As in SBNETs and PNETs, the high absolute GIPR expression was similar to that of SSTR2, with greater overexpression relative to normal tissues, indicating that GIPR represents an excellent potential therapeutic target in GDNETs.
Table 2.
Absolute gene expression in primary tumors (dCT) shows that of genes with overexpression relative to normal tissue, only GIPR has expression comparable to SSTR2. IQR: Interquartile range. Bolded SBNET and PNET dCT values indicate expression significantly different from GDNETs (p<0.05). P values show comparison of GDNET genes to GDNET SSTR2 expression and are false-discovery rate adjusted.
| Primary Tumor Absolute Expression Median dCT (IQR) | P value GDNET dCT vs. SSTR2 | |||
|---|---|---|---|---|
| Gene | SBNET (n=63) | PNET (n=51) | GDNET (n=9) | |
| SSTR2 | 2.8 (2.0, 3.8) | 1.6 (0.4, 3.9) | 4.0 (1.0, 5.1) | -- |
| GIPR | 3.2 (2.4, 5.0) | 2.9 (1.8, 3.8) | 4.3 (2.8, 6.3) | 0.38 |
| BRS3 | 10.9 (9.8, 12.0) | 6.3 (3.8, 9.7) | 10.8 (9.0, 12.2) | 0.0022 |
| GPR113 | 5.2 (4.6, 6.5) | 5.4 (4.1, 7.1) | 8.6 (6.9, 9.3) | 0.00052 |
| GRM1 | 9.0 (6.5, 11.2) | 7.5 (5.6, 9.8) | 11.9 (10.9, 13.0) | 0.00013 |
| OPRK1 | 3.2 (2.5, 4.8) | 9.8 (7.9, 11.3) | 7.3 (5.5, 9.1) | 0.010 |
| ADORA1 | 8.9 (8.2, 9.7) | 9.1 (7.3, 10.9) | 9.4 (6.5, 10.8) | 0.0058 |
| DRD1 | 7.1 (5.8, 8.4) | 5.6 (4.0, 8.0) | 7.4 (7.0, 8.4) | 0.00045 |
| GPR98 | 7.0 (6.0, 8.6) | 6.8 (6.0, 7.4) | 8.0 (6.5, 10.5) | 0.0050 |
| MEP1B | 3.3 (1.5, 5.5) | 5.4 (2.7, 8.0) | 6.1 (5.3, 5.1) | 0.047 |
| MUC13 | 0.2 (−0.4, 2.3) | 0.8 (−0.5, 2.9) | 0.6 (0.2, 1.7) | 0.34 |
| OXTR | 4.1 (2.0, 6.0) | 6.8 (4.4, 8.7) | 7.5 (5.3, 11.1) | 0.0071 |
| SCTR | 9.2 (8.2, 11.2) | 6.2 (4.1, 7.9) | 3.7 (1.9, 6.6) | 0.53 |
Figure 2.
Absolute gene expression for selected genes normalized to internal control genes (dCT). In GDNETs, SSTR2 has high absolute expression with similar expression of GIPR. Lower dCT indicates higher expression on log scale. Boxes show interquartile range (IQR), whiskers show 1.5*IQR, open circles show outliers, bar shows median. Genes in parentheses have expression significantly higher in GDNETs relative to normal tissue by ddCT.
Comparison to SBNET and PNETs
The tissues in which GDNETs arise share a foregut embryologic origin with pancreatic tissue, but these hollow viscera may be more functionally similar to small bowel. To investigate whether GDNET expression patterns for this gene panel correspond to those of SBNETs or PNETs, we compared their gene expression to that of GDNETs (Table 2, Figure 2). Among four genes showing significantly different expression between SBNETs and PNETs, (BRS3, OPRK1, OXTR, and SCTR)(17), GDNET gene expression more closely resembled that of PNETs. GDNET BRS3 expression was closer to SBNETs than PNETs (median dCT of 10.8 in GDNETs vs. 10.9 in SBNETs and 6.3 in PNETs, p=0.82 and 0.09), whereas GDNET expression of OPRK1, OXTR, and SCTR was significantly different than SBNET expression of these genes (p<0.01 for all), and similar to that of PNETs. Expression of GRM1 and GPR113 was significantly lower in GDNETs (higher dCT) than in either SBNETs or PNETs (p<0.05 for both). Most notably, however, GDNETs expressed SSTR2 and GIPR at levels similar to SBNETs and PNETs (p>0.15 for all). These results suggest that GDNETs might demonstrate a more PNET-like foregut neuroendocrine tumor gene expression signature, while low GRM1 and GPR113 expression could serve as a useful marker for distinguishing GDNETs from SBNETs or PNETs.
Discussion
Duodenal and gastric neuroendocrine tumors (GDNETs) that require operative treatment are a rare subset of all GDNETs for which optimal management remains uncertain. The present study demonstrates that GDNETs express SSTR2 at levels similar to SBNETs and PNETs, supporting the potential efficacy of somatostatin receptor-directed diagnostics and therapies in these tumors. That GDNETs also highly express GIPR, but with greater overexpression compared to background tissues, suggests that as in SBNETs and PNETs, GIPR is a potential target for imaging and therapy in these tumors.
Both gastric and duodenal neuroendocrine tumors more often present at an earlier stage than SBNETs and PNETs, but a high-risk subpopulation requiring more aggressive treatment exists. Analysis of the Surveillance, Epidemiology, and End Results (SEER) database revealed that 76% of gastric and 81% of duodenal NETs present as localized tumors (rather than regional or distant), while only 29% of SBNETs and 14% of PNETs are localized at presentation(1). Outcomes for GDNETs overall were likewise excellent, with reported median survival times of 124 and 99 months for gastric and duodenal tumors, respectively(1). Yet, despite these seemingly favorable characteristics, SEER results mask heterogeneity within the GDNET population. Although most gastric NETs are Type I or II, which are smaller, have low rates of nodal metastasis, and can often be managed endoscopically, type III tumors demonstrate higher rates of regional and distant metastasis (>50%) that more closely resemble the behavior of SBNET and PNETs(4). A similar situation exists in duodenal NETs, which have high rates of metastasis (40–60%) after exclusion of the many small, lower-risk tumors that SEER includes(3). More aggressive GDNET tumors require a more extensive imaging and staging workup, and operative management is usually warranted(4). With their higher incidence of metastasis, these patients are also likely to benefit from medical therapy.
The patients in the present cohort are not representative of all gastric and duodenal NETs. Our study included only patients whose preoperative assessment determined that they required major surgery to treat their tumors, and as such they are drawn from the subgroup of larger, higher-risk GDNETs. A recent SEER analysis identified 1,360 duodenal NETs between 1988 and 2009, with 787 undergoing resection. Of these, only 25% had any lymph nodes removed, and the median primary tumor size was 1.0cm(2). In our cohort, patients underwent more extensive surgery with 78% having lymph nodes removed, and had larger tumors, with all but 1 primary tumor larger than 1.2cm.
Analyzing this population is advantageous for a study of GDNET therapeutic targets, because patients with higher-risk GDNETs are those for whom novel therapies offer the greatest clinical relevance. How to evaluate and treat high-risk GDNET patients is unclear. Due to their rarity, many studies of somatostatin receptor-based imaging and treatment do not include GDNETs, or combine them with other tumor types(7, 9, 20–22). Current guidelines recommend using somatostatin receptor-based diagnostic and therapeutic strategies, but rely largely on analogy to pancreatic and midgut NETs, or on small case series(4, 23). Despite these limitations, somatostatin-based imaging is recommended to localize primary tumors and metastases from gastric NETs in Type III and some type II tumors, and in duodenal NETs to identify metastases(4). Similarly, octreotide is recommended for control of symptoms in functional GDNETs, and PRRT can be considered for advanced disease with positive somatostatin-based imaging, although no prospective studies address the GDNET population specifically(4, 23).
Previous investigations of somatostatin receptor expression have included GDNETs, but not in great enough numbers for independent analysis. Studies of SSTR2-directed immunohistochemistry (IHC) included 1 gastric and 0 duodenal NETs of 89 total, while another included 6 gastric and 2 duodenal NETs out of 44(24, 25). These demonstrated positive SSTR2 staining by IHC in most GDNET tissues, but were not quantitative. Studies of SSTR2 expression by qPCR included 1 duodenal and 2 gastric NETs out of 32 total tumors(26), and 1 duodenal out of 34 total(27). One of these reported high SSTR2 expression in its three GDNETs, in agreement with our findings(26). In gene expression studies, large sample sizes help to minimize the impact of variability on determining true expression levels(16, 17). Although the present study’s group of 9 GDNETs is not as large as would be ideal, given their rarity, it is a comparatively large sample size of these neoplasms. Our finding of high SSTR2 expression, in terms of both absolute expression and relative to normal tissues, provides direct evidence specific to this population supporting the use of SSTR2-directed therapies and imaging.
For patients not responding to SSTR2-directed interventions, the development of additional peptide-receptor targets for NET imaging and therapy has been pursued for some time(28). GIPR, the gastric inhibitory polypeptide receptor (also known as the glucose-dependent insulinotropic polypeptide receptor), binds gastric inhibitory polypeptide (GIP), a 42-amino acid peptide secreted by the K cells of the duodenum in response to glucose(29). GIP binding induces insulin release in the pancreas, and its role has been extensively studied in relation to diabetes, but evidence increasingly shows additional roles in cell proliferation and differentiation, beta cell survival, and bone homeostasis(29, 30).
More recently it has been recognized that neuroendocrine tumors express GIPR. Our own group determined that SBNETs and PNETs express GIPR mRNA at levels far above that of normal small bowel and pancreatic tissue, and that this expression was similar to that of SSTR2(17). Waser et al. investigated protein expression of SSTR2 and GIPR by autoradiography, finding high GIPR density in a range of neuroendocrine tissues, including PNETs, SBNETs, and bronchial NETs, with lower expression in other cancer types(31). Human medullary thyroid cancers also express GIPR(32). Interestingly, although no GDNET tumor samples were available in their studies for comparison, Waser et al. reported that no GIPR expression was detectable in normal stomach, duodenum, or ileum(31). While we were able to detect GIPR mRNA in all of these normal tissues, expression levels were far below those in the corresponding NETs (13-fold lower GIPR in normal small bowel and pancreas (17), 21-fold lower GIPR in normal stomach and duodenum). One weakness of our earlier study was that because our design measures mRNA rather than protein expression, it remained uncertain whether GIPR protein was truly overexpressed in tissues. Waser et al.’s protein measurement results(31), showing high GIPR in NETs and low or undetectable GIPR protein in normal tissues, follow the same pattern of our results with mRNA, suggesting a good correlation of mRNA to protein expression results for GIPR in these tumors.
The present study therefore extends findings of high GIPR expression to GDNETs. As in SBNETs and PNETs, absolute GIPR expression by dCT was similar to that of SSTR2. At the same time, GIPR overexpression compared to normal tissues by ddCT was many times higher than that of SSTR2 (21-fold overexpression for GIPR vs. 3.5-fold for SSTR2). Peptide receptor-directed imaging and therapy, which depends on high expression of the target receptor in neoplastic tissues and low expression in normal background tissues, may therefore be more effective if radioligands binding GIPR are used to image these gastric and duodenal NETs as opposed to ligands binding SSTR2. Results from Lacroix et al. demonstrated successful imaging of a GIPR-overexpressing adrenal tumor using a radiolabeled GIP molecule, supporting the feasibility of GIP-based imaging(33). Currently, development of improved GIP-like ligands for more widespread application in treatment of both diabetes and neuroendocrine tumors is ongoing(29, 34, 35). When suitable GIPR ligands become available, clinical trials will likely focus on the more common SBNETs and PNETs, however, these results provide a biochemical rationale for their application to GDNETs as well.
This 13-gene panel identified a foregut-like expression profile for GDNETs. Of 4 genes differentially expressed between SBNETs and PNETs, GDNETs had a pattern of expression significantly different than SBNETs and closer to PNETs, possibly reflecting their common foregut embryologic origin. Confirmation of this expression signature will require accrual of a larger number of GDNET tumors, but whether foregut NET gene expression similarities will translate into similar efficacy with PNET-targeted therapies, such as mTOR inhibitors or sunitinib, is an untested but intriguing avenue of study.
That GPR113 and GRM1 were expressed at significantly lower levels in GDNETs compared to both SBNETs and PNETs suggests that these genes might inform a unique gene expression signature for distinguishing GDNETs. We have shown that genes differentially expressed between SBNETs and PNETs can be used to distinguish their liver metastases with greater than 94% accuracy(36), with potential implications for diagnosis of NETs of unknown primary site. While NETs of unknown primary are less likely to arise from gastric or duodenal NETs due to both their rarity and visibility on endoscopy(37), it remains possible that incorporation of GPR113 and GRM1 as GDNET-specific genes could improve the performance of a gene expression classifier for determining primary NET sites from biopsies of metastases. Confirmation of such utility will require study of additional GDNET specimens.
In summary, we have shown that GDNETs express GIPR at levels similar to the current clinical standard, SSTR2. Compared to normal tissues, however, GDNETs overexpress GIPR more than 17-fold more than SSTR2. Taken together, we conclude that these results support the use of SSTR2-directed strategies in high-risk GDNETs, and that ligands directed against the GIPR-receptor represent promising targets for imaging and treatment of these tumors.
Acknowledgments
We gratefully acknowledge our patients for their participation.
Supported by NIH 5T32#CA148062-03 (SKS, JEM, JCC)
Footnotes
Author Contributions: Concept and design: SKS, JCC, MSO, TMO, JRH; Analysis and Interpretation: SKS; Data Collection: SKS, JEM, JCC, DW; Writing: SKS, JEM; Critical Revision: MSO, TMO, JRH; Obtaining Funding: JRH
Accepted for Presentation at the 9th Annual Academic Surgical Congress in San Diego, CA, on February 4, 2014
None of the authors has any potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Randle RW, Ahmed S, Newman NA, et al. Clinical Outcomes for Neuroendocrine Tumors of the Duodenum and Ampulla of Vater:A Population-Based Study. J Gastrointest Surg. 2013 doi: 10.1007/s11605-013-2365-4. [DOI] [PubMed] [Google Scholar]
- 3.O’Toole D, Delle Fave G, Jensen RT. Gastric and duodenal neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:719–735. doi: 10.1016/j.bpg.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Delle Fave G, Kwekkeboom DJ, Van Cutsem E, et al. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology. 2012;95:74–87. doi: 10.1159/000335595. [DOI] [PubMed] [Google Scholar]
- 5.Hofland LJ, Lamberts SW. Somatostatin receptor subtype expression in human tumors. Ann Oncol. 2001;12 (Suppl 2):S31–36. doi: 10.1093/annonc/12.suppl_2.s31. [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 7.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 8.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 9.Kwekkeboom DJ, Kam BL, van Essen M, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–73. doi: 10.1677/ERC-09-0078. [DOI] [PubMed] [Google Scholar]
- 10.Dahdaleh FS, Lorenzen A, Rajput M, et al. The value of preoperative imaging in small bowel neuroendocrine tumors. Ann Surg Oncol. 2013;20:1912–1917. doi: 10.1245/s10434-012-2836-y. [DOI] [PubMed] [Google Scholar]
- 11.Zaknun JJ, Bodei L, Mueller-Brand J, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jais P, Terris B, Ruszniewski P, et al. Somatostatin receptor subtype gene expression in human endocrine gastroentero-pancreatic tumours. Eur J Clin Invest. 1997;27:639–644. doi: 10.1046/j.1365-2362.1997.1740719.x. [DOI] [PubMed] [Google Scholar]
- 13.Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 14.Sherman SK, Howe JR. Translational research in endocrine surgery. Surg Oncol Clin N Am. 2013;22:857–884. doi: 10.1016/j.soc.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr JC, Boese EA, Spanheimer PM, et al. Differentiation of small bowel and pancreatic neuroendocrine tumors by gene-expression profiling. Surgery. 2012;152:998–1007. doi: 10.1016/j.surg.2012.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr JC, Sherman SK, Wang D, et al. Overexpression of membrane proteins in primary and metastatic gastrointestinal neuroendocrine tumors. Ann Surg Oncol. 2013;20 (Suppl 3):739–746. doi: 10.1245/s10434-013-3318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman SK, Carr JC, Wang D, et al. Gastric inhibitory polypeptide receptor (GIPR) is a promising target for imaging and therapy in neuroendocrine tumors. Surgery. 2013;154:1206–1214. doi: 10.1016/j.surg.2013.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahdaleh FS, Calva-Cerqueira D, Carr JC, et al. Comparison of clinicopathologic factors in 122 patients with resected pancreatic and ileal neuroendocrine tumors from a single institution. Ann Surg Oncol. 2012;19:966–972. doi: 10.1245/s10434-011-1997-4. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 20.Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 21.Wolin EM, Hu K, Hughes G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a long-acting release (LAR) formulation of pasireotide (SOM230) in patients with gastroenteropancreatic neuroendocrine tumors: results from a randomized, multicenter, open-label, phase I study. Cancer Chemother Pharmacol. 2013;72:387–395. doi: 10.1007/s00280-013-2202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 23.Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 24.Korner M, Waser B, Schonbrunn A, et al. Somatostatin receptor subtype 2A immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting. Am J Surg Pathol. 2012;36:242–252. doi: 10.1097/PAS.0b013e31823d07f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sclafani F, Carnaghi C, Di Tommaso L, et al. Detection of somatostatin receptor subtypes 2 and 5 by somatostatin receptor scintigraphy and immunohistochemistry: clinical implications in the diagnostic and therapeutic management of gastroenteropancreatic neuroendocrine tumors. Tumori. 2011;97:620–628. doi: 10.1177/030089161109700514. [DOI] [PubMed] [Google Scholar]
- 26.Mizutani G, Nakanishi Y, Watanabe N, et al. Expression of Somatostatin Receptor (SSTR) Subtypes (SSTR-1, 2A, 3, 4 and 5) in Neuroendocrine Tumors Using Real-time RT-PCR Method and Immunohistochemistry. Acta Histochem Cytochem. 2012;45:167–176. doi: 10.1267/ahc.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Toole D, Saveanu A, Couvelard A, et al. The analysis of quantitative expression of somatostatin and dopamine receptors in gastro-entero-pancreatic tumours opens new therapeutic strategies. Eur J Endocrinol. 2006;155:849–857. doi: 10.1530/eje.1.02307. [DOI] [PubMed] [Google Scholar]
- 28.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 29.Irwin N, Flatt PR. Therapeutic potential for GIP receptor agonists and antagonists. Best Pract Res Clin Endocrinol Metab. 2009;23:499–512. doi: 10.1016/j.beem.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Prabakaran D, Wang B, Feuerstein JD, et al. Glucose-dependent insulinotropic polypeptide stimulates the proliferation of colorectal cancer cells. Regul Pept. 2010;163:74–80. doi: 10.1016/j.regpep.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waser B, Rehmann R, Sanchez C, et al. Glucose-dependent insulinotropic polypeptide receptors in most gastroenteropancreatic and bronchial neuroendocrine tumors. J Clin Endocrinol Metab. 2012;97:482–488. doi: 10.1210/jc.2011-2454. [DOI] [PubMed] [Google Scholar]
- 32.Waser B, Beetschen K, Pellegata NS, et al. Incretin receptors in non-neoplastic and neoplastic thyroid C cells in rodents and humans: relevance for incretin-based diabetes therapy. Neuroendocrinology. 2011;94:291–301. doi: 10.1159/000330447. [DOI] [PubMed] [Google Scholar]
- 33.Lacroix A, Bolte E, Tremblay J, et al. Gastric inhibitory polypeptide-dependent cortisol hypersecretion--a new cause of Cushing’s syndrome. N Engl J Med. 1992;327:974–980. doi: 10.1056/NEJM199210013271402. [DOI] [PubMed] [Google Scholar]
- 34.Reubi JC. Old and new peptide receptor targets in cancer: future directions. Recent Results Cancer Res. 2013;194:567–576. doi: 10.1007/978-3-642-27994-2_34. [DOI] [PubMed] [Google Scholar]
- 35.Kerr BD, Irwin N, Flatt PR, et al. Prolonged GIP receptor activation using stable mini-PEGylated GIP improves glucose homeostasis and beta-cell function in age-related glucose intolerance. Peptides. 2009;30:219–225. doi: 10.1016/j.peptides.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Sherman SK, Carr JC, Wang D, et al. Gene expression in neuroendocrine tumor liver metastases accurately distinguishes between pancreas and small bowel primary tumors. J Am Coll Surg. 2013;217:S129. [Google Scholar]
- 37.Wang SC, Parekh JR, Zuraek MB, et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch Surg. 2010;145:276–280. doi: 10.1001/archsurg.2010.10. [DOI] [PubMed] [Google Scholar]