Abstract
In the present study, hydroxyapatite (HA) was successfully grafted to carboxylated carbon nanotubes (CNTs) and graphene nanosheets. The HA grafted CNTs and HA-graphene nanosheets were characterized using FT-IR, TGA, SEM and X-ray diffraction. The HA grafted CNTs and graphene nanosheets (CNTs-HA and Gr-HA) were further used to examine the proliferation and differentiation rate of temperature-sensitive human fetal osteoblastic cell line (hFOB 1.19). Total protein assays and western blot analysis of osteocalcin expression were used as indicators of cell proliferation and differentiation. Results indicated that hFOB 1.19 cells proliferate and differentiate well in treatment media containing CNTs-HA and graphene-HA. Both CNTs-HA and graphene-HA could be promising nanomaterials for use as scaffolds in bone tissue engineering.
Keywords: Hydroxyapatite, Carbon nanotubes, Graphene, hFOB 1.19 cells, Bioceramic
1. Introduction
Hydroxyapatite (Ca10(PO4)6(OH))2 (HA) is a bioceramic material often used for clinical bone grafting and implantation. HA has the ability to bond chemically with living bone tissue because of its chemical, compositional, biological, and crystal structure which are similar to native apatite in the human skeleton. Furthermore, the bioactivity and biocompatibility of HA enable osteoblast adhesion and proliferation. However, brittle HA is fragile in tension and offers low fracture toughness in comparison with natural bone. This drawback can be substantially minimized by strengthening and toughening HA with carbon nanomaterials while keeping its bioactivity [1].
Recently, carbon nanomaterials have attracted considerable attention due to their unique properties and wide range of applications [2-5]. Among the carbon nanomaterials, graphene (GR) is a new member with extraordinary electrical, thermal, and mechanical properties that have sparked current interest in materials science [6-8].Carbon nanotube on the otherhand have extensively been studied since its discovery. Its biocompatibility and bioactivity have also been tested and documented [9].
Till date, there are contradictory reports on the biomedical and biocompatibility of nanomaterials (particularly CNTs) due to its toxicity. However, the toxicity of CNTs has been shown to be reduced through chemical functionalization or coating with substances like polymers, hydroxyapatite or collagen [7 - 8, 10]. Zanello et al (2006) reported the mineralization of bone cells on chemically functionalized Single Walled Carbon nanotubes (SWCNTs) with HA. This being the first study on the potential use of SWCNTs as scaffold for bone growth [11]. Balani et al.(2007) also applied CNTs in HA coating using plasma spraying to improve the fracture toughness (by 56%) and enhance crystallinity (by 27%). The CNTs reinforced HA coating was further used to culture human osteoblast hFOB 1.19 cells to reveal its biocompatibility with living cells. Unrestricted growth of human osteoblast hFOB 1.19 cells has been observed near the CNTs regions assisted by CNTs surfaces to promote cell growth and proliferation [11-13].
Herein we report the fabrication of CNTs-HA and Gr-HA, and characterization, including the proliferation and differentiation of bone cells (hFOB 1.19) in the media containing these nanocomposites.
2. Materials and Method
All the reagents were purchased from Aldrich and used without further purification unless otherwise noted. All the aqueous solutions were prepared with ultrapure water obtained from Milli-Q Plus system (Millipore).
2.1. Synthesis of CNTs-COOH and Graphene Nanosheet
CNTs were functionalized as described before by refluxing in a 3:1 mixture of concentrated sulfuric acid and nitric acid under stirring at 70°C for 24hrs, followed by centrifugation and repeated washings with distilled water [9]. Graphene nanosheet was prepared by ethylenediamine (EDA) reduction of Graphite oxide (GO) which was prepared from graphite powder according to the Hummers and Offeman method [14].
2.2. In-situ deposition of HA over CNT-COOH and graphene nanosheets
The CNTs-HA and Gr-HA were prepared as earlier reported [9, 15].The GR nanosheets(50mg) and carboxylated CNTs (200 mg) were separately dispersed in 50 mL deionized water (DI) water by sonication for 5 mins. To this, an aqueous solution of Ca(OH)2 (0.01mol L−1) was added and the suspension was stirred under ambient conditions for 1 hr. Then pH of the suspension was adjusted to ≈9 by dropwise addition of H3PO4 under constant stirring. The resulting HA functionalized nanomaterials (Gr-HA and CNTs-HA) were centrifuged, washed with DI water and dried under vacuum at 40 °C for 7h (Schemes S1 and S2).
2.3. Characterization
FT-IR spectra were recorded using Thermo-Nicolet IR 2000 spectrometer, TGA was performed with a TGA Q500 instrument under nitrogen environment at a heating rate of 10°C/min. Powder XRD patterns were recorded on scintag X-ray diffractometer (PAD X), equipped with Cu Kα photon source (45kV, 40mA) at scanning rate of 3°/min. SEM measurements were carried out a JEOLJXA-8900 microscope
2.4. Human Fetal Osteoblast Cell Culture
Human fetal osteoblastic cells (hFOB 1.19) were cultured in a 1:1 mixture of Dulbecco's Modified Eagle's Medium (DMEM) and F-12 Medium (Kaighn's Modification of Ham's F-12 Medium; ATCC, Manassas, VA, USA). The culture medium was supplemented with 10% Fetal Bovine serum (FBS), 0.3mg/ml G418 (geneticin) and 1% Penicillin/Streptomycin (ATCC, Manassas, VA, USA). The hFOB 1.19 cells were plated in a six-well plate at a concentration of 1.0×105 cells/well. The cells were allowed to reach 50-80% confluency after which the Gr-HA and CNTs-HA were added in duplicate to the cell culture medium in 200 and 400 ng/ml dosages. Control cells were grown in cell culture medium alone. The plates were incubated at 34°C and 5% CO2. The day of plating was considered as day 0 of the experiment and the treatment medium renewed every 2-3 days.
2.5. Cellular proliferation and differentiation
The fetal osteoblastic cell line, hFOB 1.19 cells were cultured for 12 days at 34°C. However, at day 3, 6, 9 and 12, proliferating cells were assayed by to determine total protein quantification and osteocalcin expression. In addition, after proliferation for 6 days, one half of the samples were placed in another incubator at 39°C to stimulate differentiation. On day 9 and 12, cells stimulated to differentiate were harvested and the total protein quantification and osteocalcin expression were also assayed cells and compared to cells continue proliferating at 34°C.
2.6. Total protein analysis
The hFOB 1.19 cells were collected on day 3, 6, 9 and 12, and washed with Phosphate Buffered Saline (PBS) after removal of treatment medium and lysed by scrapping in 500μL celLytic per well (10 mM Tris Cl, 0.2% NP-40, and 2 mM phenylmethylsulfonyl fluoride; Sigma Aldrich, USA). The extracted solution was centrifuged and the supernatant stored at −20°C. The total protein quantification of the supernatant was determined by Bradford Protein Assay (Bio-Rad Laboratory Inc., Hercules, CA, USA). Bovine Serum Albumin (BSA) standards with concentrations of 0, 1, 2.5, 5, 10, 20 and 40 μg/ml and the unknown protein were placed in a 96 well plate using the 300μl microplate assay protocol published by BIO-RAD DC Protein assay kit. The absorbance was measured at 595nm with a Spectra Max microplate reader (SpectraMax M2, Molecular Devices Corporation, Sunnyvale, CA, USA) and SoftMax Pros software (SoftMax Pro 5, Molecular Devices Corporation, Sunnyvale, CA, USA).
2.7. Osteocalcin Expression
Osteocalcin expression was determined in both proliferating and differentiating cells collected on 3, 6, 9, and 12 days using Western blot analysis as published in ABCAM western blotting protocol. The molecular imager ChemiDoc XRS system (ChemiDoc XRS, BIO-RAD, Philadelphia, PA, USA) was used to capture the images signifying osteocalcin expression.
3. Results and Discussion
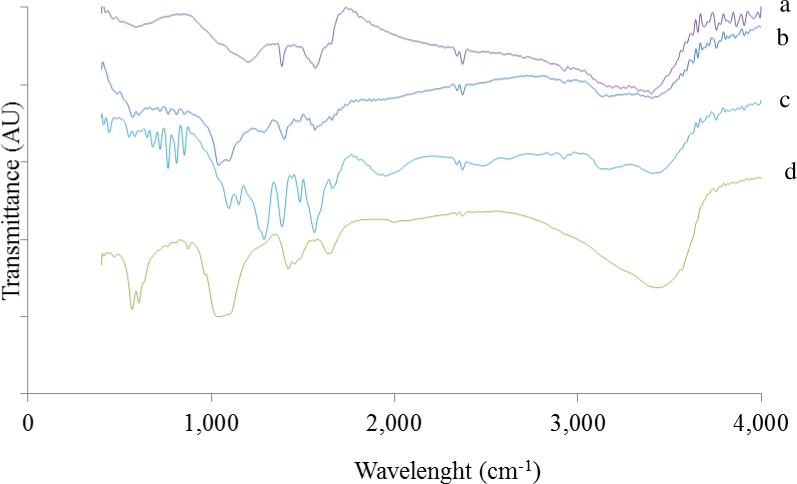
The FTIR spectrum of graphene (Fig. 1a) shows small O-H stretching vibrations peak at about 3500 cm−1. The band at about 1720 cm−1 is attributed to the stretching vibrations of C=O. These suggests the presence of oxygen containing functional groups on the surface of graphene nanosheets after reduction of GO. In addition, a new band appeared in the spectrum of GR at 1509 cm−1, which is ascribed to the skeletal vibration of Gr nanosheets[15]. These conformational changes demonstrate the successful reduction of GO to Gr in presence of EDA. The FTIR spectra of CNTs-HA and Gr-HA (Fig. 1b and 1c) showed all the characteristic bands of HA. In addition to these, a weak band at 1630 cm−1 was observed owing to C-C stretching of CNTs. Another weak band at 1720 cm−1 corresponds to C = O, which indicates the existence of carboxylic groups on the surface of CNTs [9, 15].
Fig. 1.
FTIR of (a) Gr (b) CNTs-HA (c) Gr-HA (d) HA
The FTIR spectrum of hydroxyapatite (Fig. 1d) displayed well resolved characteristic absorption band of natural HA with bands at 565 and 604 cm−1 attributed to P-O bending of phosphate group while the band at 1034 cm−1 is assigned to O-P-O phosphate ions of hydroxyl site. The bands at 3430 and 1640 cm−1 were attributed to stretching and bending vibrations of hydroxyl (-OH) group. The band at 1459 cm−1 was owing to the vibrational mode of carbonate ion [9].
The TGA analysis (Fig. 2e) of graphite oxide (GO) shows a major mass reduction at ~200 °C which was caused by pyrolysis of the oxygen-containing functional groups, generating CO, CO2 and steam [19]. The mass of GO slowly further decreased up to 600°C. Graphene (Fig. 2d) however, showed an enhanced thermal stability due to the partial removal of oxygen-containing functional groups by EDA reduction. Both Gr-HA (Fig. 2c) and CNTs-HA (Fig. 2b) show slight weight loss between 360 and 700°C attributed to the combustion of carbon [16].
Fig. 2.
Thermogravimetric analysis (TGA) of (a) CNTs (b) CNTs-HA (c) Gr-HA (d) Gr (e) GO
To further characterize the materials, X-ray diffraction analysis was carried out. For CNTs-HA, the diffraction peaks (Fig. 3a) observed at 26.3° and 42.6° were assigned to (0 0 2) and (1 0 0) reflections of CNTs [9]. Two principal diffraction peaks were displayed at 2 values of 25.9° and 32°, these were attributed to (0 0 2) and (2 1 1) reflections of HA, respectively. The remaining peaks at 39.8°, 46.7°, 49.4°, 53.2° and 63.5° were assigned to (3 1 0), (2 2 2), (2 1 3), (0 0 4) and (3 0 4) reflections of HA, respectively [9]. The pattern of GO (Fig. 6a) displayed a characteristic peak at 2θ value of 13.2°, corresponding to (0 0 1) reflection of stacked GO nanosheets [16]. After reduction of GO with EDA, two distinctive peaks at 26.4° and 44.4° appeared in the pattern of GR (Fig. 3b) corresponding to (0 0 2) and (1 0 0) reflections of Gr. The principal peaks appeared at 27.5°and 33.4° for Gr-HA (Fig. 3c) were attributed to (0 0 2) and (2 1 1) reflections of HA, respectively [9]. Hence, the XRD pattern of Gr-HA and CNTs-HA further reveal the successful grafting of HA onto functionalized CNTs and Graphene nanosheets.
Fig. 3.
Powder XRD of XRD of (a) GO (b) Gr (c) Gr-HA (d) CNT-HA (e) CNTs
Fig. 6.
Comparison of total protein in hFOB cells cultured at permissible temperature of 34°C to cells stimulated to differentiate at 39°C after (a) day 9 (b) day 12 of incubation in treatment media containing different concentrations of Gr-HA.
The SEM images further evident the successful grafting of HA on graphene (Fig. 4a) and CNTs (Fig. 4b). The loading of HA on the surfaces of both materials is clearly detectable with the integral shapes of the CNTs and graphene been preserved. The rough surface of CNTs-HA and Gr-HA is ideal for the better adhesion of cells [9].
Fig. 4.
High magnification SEM of (a) CNTs-HA (b) Gr-HA
The hFOB 1.19 cells are very sensitive to subtle change in temperature, cells cultured at 33.5 [16]or 37 °C [17] have been found to show rapid proliferation and hence, protein synthesis. However, when the cells are switched to 39.5°C, there is slow-down in proliferation and the cells start differentiating [16-17]. Differentiation of hFOB cells is accompanied by continuous expression of markers such as osteocalcin, Alkaline Phosphatase (AP) e.t.c.[17] The proliferation and differentiation of hFOB cells at different dosages of Gr-HA and CNTs-HA were studied for 12 days at 34 and 39°C by quantification of total protein and analysis of osteocalcin expression. Similar to control cells, hFOB cells cultured at 34°C and different dosages of Gr-HA (Fig. 5a) showed increase in total protein with time but peaked at day 9. An overall increase in total protein with time was also observed for cells cultured in CNTs-HA (Fig. 5b) .The overall increase in the quantity of protein in both Gr-HA and CNTs-HA treated hFOB cells suggest the ability of hFOB 1.19 cells to proliferate in the treatment medium. The hFOB cells stimulated to differentiate at 39°C after 6 day of incubation at 34°C also displayed an increase in total protein with time, similar to proliferating cells at 34°C. As expected, comparison of total protein in hFOB cells cultured at permissible temperature of 34°C was considerably lower than those stimulated to differentiate at 39°C in both Gr-HA (Fig. 6) and CNTs-HA (Fig. 7). This signifies that hFOB 1.19 cells are very sensitive to subtle change in temperature and differentiation of these cells is stimulated at restrictive temperature of 39°C.
Fig. 5.
Protein in hFOB cells at 34°C and different concentrations of (a) Gr-HA and (b) CNTs-HA .
Fig. 7.
Comparison of total protein in hFOB cells cultured at permissible temperature of 34°C to cells stimulated to differentiate at 39°C after (a) day 9 (b) day 12 of incubation in treatment media containing different concentrations of CNTs-HA.
Expression of osteocalcin in proliferating cells at 34°C and cells stimulated to differentiation at 39°C was also studied by Western blot analysis of hFOB cells cultured at 34°C for 12 days (Fig. 8). For proper analysis, the intensity of osteocalcin expressed in each cell culture was quantified using Image Lab software and the results are shown in Figs. 9-11. Typically, osteoblast shows gradual increase in osteocalcin expression and peaks during mineralization stage of their development. However, these result shows early expression of osteocalcin after third day of incubation in both control cells and cells cultured in at different concentrations of Gr-HA (Fig. 9a) and CNTs-HA (Fig. 9b) at 34°C. Before observing a notable increase in expression after 12 day of incubation, a continuous decline in quantity of osteocalcin was observed up to ninth day in all treatment groups including control cells. This early expression of osteocalcin in all treatment groups implies early maturation of osteoblast in the treatment media because osteocalcin is a marker of mature osteoblast [12, 18-23].
Fig. 8.
Western blot analysis of Osteocalcin expression and GAPDH in hFOB cells cultured at 34°C in (a) CNT-HA (b) Gr-HA
Fig. 9.
Osteocalcin expression in hFOB cells cultured at 34°C and different concentrations of (a) Gr-HA and (b) CNTs-HA.
Fig. 11.
Comparison of osteocalcin expression in hFOB cells cultured at permissible temperature of 34C to cells stimulated to differentiate at 39C after (a) day 9 (b) day 12 of incubation in treatment media containing different concentrations of CNTs-HA.
Comparing the cells cultured at 34°C to those of stimulated after 6 day of incubation (Figs. 10 and 11) showed lower osteocalcin expression in the cells stimulated to differentiate at 39°C. Surprisingly, the cells cultured in 400ng/mL of Gr-HA at 39°C showed higher osteocalcin expression compared to the cells left to continue proliferating at 34°C. Failure of the control cells to follow the same trend observed in the cells cultured in 400ng/ml of Gr-HA, which suggests that osteocalcin might not be the proper marker of differentiation. Other proteins such as Alkaline Phosphatase (AP), osteopontin might be the proper marker for differentiating cells, that will be the task of our future work. However, the observed trend in total protein (Figs. 5-8) is in accordance with the relationship between proliferating and differentiating of cells. Overall, the total protein analysis indicates the subtle change in incubation temperature from 34 to 39°C stimulated the differentiation of hFOB cells in the treatment media. The hFOB 1.19 cells have also been reported to display the reduction in proliferation at restrictive temperature of 39°C which is indicative of differentiation of these cells [17]. Hence, the observed reduction in the total protein at 39°C indicates that hFOB 1.19 cells are very sensitive to subtle change in temperature.
Fig. 10.
Comparison of osteocalcin expression in hFOB cells cultured at permissible temperature of 34°C to cells stimulated to differentiate at 39°C after (a) day 9 (b) day 12 of incubation in treatment media containing different concentrations of Gr-HA.
To date, few studies have been done on biocompatibility of graphene but there are contradictory reports on the biocompatibility of CNTs. Some studies have shown that CNTs inhibit proliferation and cell viability of macrophages [24-27], keratinocytes [28] and human peripheral blood lymphocytes (HPBL) [29]. A strong relationship between concentration of CNTs and cytotoxicity was observed [29-32]. Conversely, CNTs have been shown to have no toxic effects on cells. Wang et al. (2007) provided the first in vivo testing evidence that pure bulk CNTs are not a strongly inflammatory substance and have no toxicity for bone regeneration[8,12]. Chemically functionalized CNTs have been used successfully as substrates for neuronal growth [19, 20, 22]. Collagen-CNTs composite materials sustained high smooth muscle cell viability [17] and CNTs in suspension in the culture medium were incorporated into the cell cytoplasm by macrophages and leukemia cells without affecting the cell population growth [29-30].
Bone cell proliferation has also been reported on several carbon nanomaterials composites including those prepared by coating substrates such as collagen, hydroxyapatite or polymers [12]. These nanocomposite materials (CNTs-HA and Gr-HA) at different concentrations of 200ng/ml and 400ng/ml were further used to study the proliferation and differentiation of a temperature-sensitive human fetal osteoblastic cell line (hFOB 1.19) at 34°C and 39°C. Total protein assays and western blot analysis of osteocalcin expression were used as indicators of cell proliferation and differentiation. The hFOB 1.19 cell line cultured at 34°C was found to proliferate with time in culture containing different concentrations of nanocomposite materials (CNTs-HA and Gr-HA) as indicated by increase in the quantity of total protein produced by the cells. This result indicates that the Gr-HA and CNTs-HA nanocomposites could support the proliferation of hFOB 1.19 cells. In addition, hFOB cells stimulated to differentiate at 39°C, displayed a reduction in the total protein indicating inhibition or slow down of proliferation rate at 39°C. This is consistent with the reciprocal relationship between proliferation and differentiation [20]. Harris et. al (1995), also found similar results with hFOB 1.19 cells cultured at 33.5°C and 39.5°C [16-17].
It has been reported that inhibition of proliferation upregulates the expression of genes (such as alkaline phosphatase, osteopontin, osteocalcin) during developmental sequence of some osteoblast up to the stage where mineralization initiates [21-23]. In vivo and in vitro study of mineralization of the extracellular matrix of osteocalcin (bone Gla protein) has been reported [23]. This study however, does not indicate upregulation of osteocalcin when proliferation is inhibited in hFOB 1.19 cell lines. Instead, we observed early expression of osteocalcin protein in proliferating cells followed by continuous decline until ninth day of incubation in all treatment groups including control cells. Differentiating cells also display lower osteocalcin expression when compared to proliferating cells. This is in contrast to the expected greater osteocalcin expression in cells stimulated at 39°C. Further, the expression of osteocalcin in osteoblast depends on various factors. Several modification of culture conditions of fetal osteoblast demonstrate the dependence of osteocalcin level on formation of mineralized extra cellular matrix [23] which is composed primarily of hydroxyapatite. Osteoblasts from more mature bone or post natal derived cells have also exhibited osteocalcin expression in non-mineralizing cultures [19, 33].
4. Conclusions
Both CNTs-HA and Gr-HA are promising composite for scaffolds fabrication in bone tissue engineering as these are able to support in proliferation and differentiation of hFOB cells. However, for in vivo applications, careful consideration of the biocompatibility and toxicity of CNTs and graphene nanosheet is important, which is part of our future work.
Supplementary Material
Highlights.
Successful grafting of hydroxyapatite on CNTs and graphene
CNTs-HA and Gr-HA were used in proliferation of hFOB 1.19 cells
CNTs-HA and Gr-HA could be promising in bone tissue engineering
Acknowledgments
The authors acknowledge the support from NIH-NIGMS grant #1SC3GM086245, NIH-NIGMS, Welch foundation and Cooperative Agricultural Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang S, Kong B, Jung D, Baek Y, Han C, Oh S, Jung H. Nanoscale. 2011;3:1361–1373. doi: 10.1039/c0nr00855a. [DOI] [PubMed] [Google Scholar]
- 2.Neelgund GM, Oki A. Appl. Catal. A: Gen. 2011;399:154–158. doi: 10.1016/j.apcata.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neelgund GM, Oki A. Applied Catalysis, B: Environmental. 2011;110:99–107. doi: 10.1016/j.apcatb.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelgund GM, Oki A. J. Nanosci. Nanotechnol. 2011;11:3621–3629. doi: 10.1166/jnn.2011.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geim AK, Novoselov KS. Nat. Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 6.Rao CV, Reddy ALM, Ishikawa Y, Ajayan PM. Carbon. 2011;49:931–936. [Google Scholar]
- 7.Verdejo R, Jell G, Safinia L, Bismarck A, Stevens MM, Shaffer MS. J Biomed Mater Res A. 2009;88:65–73. doi: 10.1002/jbm.a.31698. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Watari F, Omori M, Liao S, Zhu Y, Yokoyama A, Uo M, Kimura H, Ohkubo A. J Biomed Mater Res B Appl Biomater. 2007;82:223–230. doi: 10.1002/jbm.b.30724. [DOI] [PubMed] [Google Scholar]
- 9.Neelgund GM, Olurode Kehinde, Luo Zhiping, Oki A. Mat. Sci and Engr C. 2011;31:1477–1881. doi: 10.1016/j.msec.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macdonal RA, Laurenzi BF, Viswanathan G, Ajayan PM, Stegemann JP. J. Biomed. Mater. Res. A. 2005;74:489–496. doi: 10.1002/jbm.a.30386. [DOI] [PubMed] [Google Scholar]
- 11.Zanello L, Zhao B, Hu H, Haddon RC. Nano Lett. 2006;6:562–567. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]
- 12.Balani K, Anderson R, Laha T, Andara M, Tercero J, Crumpler E, Agarwal A. Biomaterials. 2007;28:618–624. doi: 10.1016/j.biomaterials.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Meyer JC, Geim AK, Katsnelson MI, Novoselov KS, Booth TJ, Roth S. Nature. 2007;446:60. doi: 10.1038/nature05545. [DOI] [PubMed] [Google Scholar]
- 14.Hummers WS, Offeman RE. J. Am. Chem. Soc. 1958;80:1339–1341. [Google Scholar]
- 15.Neelgund GM, Oki A, Luo Z. Materials Research Bulletin. 2013;48:175–179. doi: 10.1016/j.materresbull.2012.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris SA, Enger RJ, Riggs BL, Spelsberg TC. J. Bone Miner. Res. 1995;10:178–186. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- 17.Eun-Young C, Tae H, Jihyun H, Ji EK, Sun HL, Hyun WK, Sang OK. J of Mater. Chem. 2010;20:1907–1912. [Google Scholar]
- 18.Donahue HJ, Li Z, Zhou Z, Yellowley CE. Am J Cell Physiol. 2000;278:C315–C322. doi: 10.1152/ajpcell.2000.278.2.C315. [DOI] [PubMed] [Google Scholar]
- 19.Eliason MT, Sunden EO, Cannon AH, Graham S, Garcia AJ, King WP. J Biomed Mater Res A. 2008;86:996–1001. doi: 10.1002/jbm.a.31697. [DOI] [PubMed] [Google Scholar]
- 20.Hauschka PV, Lian JB, Cole DEC, Gundberg CM. Physiol. Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 21.Lian JB, Stein GS, Berdanier CD, Hargrove JL, editors. CRC Press; Boca Raton, FL, USA: 1993. pp. 391–429. [Google Scholar]
- 22.Lian JB, Stein GS. Ital. J. Min. Elect. Metab. 1993;7:175–183. [Google Scholar]
- 23.Lain JB, Stein GS. Iowa Orthoped. J. 1995;15:118–140. [PMC free article] [PubMed] [Google Scholar]
- 24.P Lynch M, Stein JS, Lian JB. J. Bone Min. Res. 1994;9:S352–S355. [Google Scholar]
- 25.Murr LE. Int. J. Environ. Res. Public Health. 2008;5:321–336. doi: 10.3390/ijerph5050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Environ. Sci. Technol. 2005;39:1378–83. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 27.Murugan AV, Muraliganth T, Manthiram A. Chem. Mater. 2009;21:5004–5006. [Google Scholar]
- 28.Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, Rice-Ficht AC, Ramesh GT. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB. J. Am. Chem. Soc. 2004;126:15638–15639. doi: 10.1021/ja0466311. [DOI] [PubMed] [Google Scholar]
- 30.Shi Kam NW, Jessop TC, Wender PA, Dai H. J. Am. Chem. Soc. 2004;126:6850 – 6851. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Yokoyama A, Liao S, Omori M, Zhu Y, Uo M, Akasaka T, Watari F. Mater. Sci. Eng. C. 2007;7:1082–1086. [Google Scholar]
- 32.Tejral G, Redy P, Havel J. J. Appl. Biomed. 2009;7:1–13. [Google Scholar]
- 33.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilning L, S Tassinari M, B Kennedy M, Pockwinse S, Lian JB, Stein GS. J. Cell. Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.