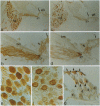
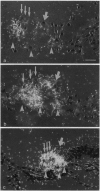
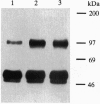
Abstract
Here we show that the mature cochlear neurons are a rich source of acidic fibroblast growth factor (aFGF), which is expressed in the neuronal circuitry consisting of afferent and efferent innervation. The site of action of neuronal aFGF is likely to reside in the organ of Corti, where one of the four known FGF receptor (FGFR) tyrosine kinases--namely, FGFR-3 mRNA--is expressed. Following acoustic overstimulation, known to cause damage to the organ of Corti, a rapid up-regulation of FGFR-3 is evident in this sensory epithelium, at both mRNA and protein levels. The present results provide in vivo evidence for aFGF being a sensory neuron-derived, anterogradely transported factor that may exert trophic effects on a peripheral target tissue. In this sensory system, aFGF, rather than being a neurotrophic factor, seems to promote maintenance of the integrity of the organ of Corti. In addition, aFGF, released from the traumatized nerve endings, may be one of the first signals initiating protective recovery and repair processes following damaging auditory stimuli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamis A. P., Meklir B., Joyce N. C. In situ injury-induced release of basic-fibroblast growth factor from corneal epithelial cells. Am J Pathol. 1991 Nov;139(5):961–967. [PMC free article] [PubMed] [Google Scholar]
- Baird A. Fibroblast growth factors: activities and significance of non-neurotrophin neurotrophic growth factors. Curr Opin Neurobiol. 1994 Feb;4(1):78–86. doi: 10.1016/0959-4388(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Canlon B., Borg E., Flock A. Protection against noise trauma by pre-exposure to a low level acoustic stimulus. Hear Res. 1988 Jul 15;34(2):197–200. doi: 10.1016/0378-5955(88)90107-4. [DOI] [PubMed] [Google Scholar]
- Canlon B., Brundin L., Flock A. Acoustic stimulation causes tonotopic alterations in the length of isolated outer hair cells from guinea pig hearing organ. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7033–7035. doi: 10.1073/pnas.85.18.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. H., Pettersson R. F. Human acidic fibroblast growth factor overexpressed in insect cells is not secreted into the medium. Growth Factors. 1990;3(1):1–13. doi: 10.3109/08977199009037497. [DOI] [PubMed] [Google Scholar]
- Cao Y., Pettersson R. F. Release and subcellular localization of acidic fibroblast growth factor expressed to high levels in HeLa cells. Growth Factors. 1993;8(4):277–290. doi: 10.3109/08977199308991573. [DOI] [PubMed] [Google Scholar]
- Chellaiah A. T., McEwen D. G., Werner S., Xu J., Ornitz D. M. Fibroblast growth factor receptor (FGFR) 3. Alternative splicing in immunoglobulin-like domain III creates a receptor highly specific for acidic FGF/FGF-1. J Biol Chem. 1994 Apr 15;269(15):11620–11627. [PubMed] [Google Scholar]
- Cotanche D. A., Lee K. H. Regeneration of hair cells in the vestibulocochlear system of birds and mammals. Curr Opin Neurobiol. 1994 Aug;4(4):509–514. doi: 10.1016/0959-4388(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Elde R., Cao Y. H., Cintra A., Brelje T. C., Pelto-Huikko M., Junttila T., Fuxe K., Pettersson R. F., Hökfelt T. Prominent expression of acidic fibroblast growth factor in motor and sensory neurons. Neuron. 1991 Sep;7(3):349–364. doi: 10.1016/0896-6273(91)90288-b. [DOI] [PubMed] [Google Scholar]
- Hawkins J. E., Jr Comparative otopathology: aging, noise, and ototoxic drugs. Adv Otorhinolaryngol. 1973;20:125–141. doi: 10.1159/000393093. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Williams L. T. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Luo L., Koutnouyan H., Baird A., Ryan A. F. Acidic and basic FGF mRNA expression in the adult and developing rat cochlea. Hear Res. 1993 Sep;69(1-2):182–193. doi: 10.1016/0378-5955(93)90106-b. [DOI] [PubMed] [Google Scholar]
- McNeil P. L. Cellular and molecular adaptations to injurious mechanical stress. Trends Cell Biol. 1993 Sep;3(9):302–307. doi: 10.1016/0962-8924(93)90012-p. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Muthukrishnan L., Warder E., D'Amore P. A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989 Aug;109(2):811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesch A., Hartmann E., Rohde K., Rubartelli A., Sitia R., Rapoport T. A. A novel pathway for secretory proteins? Trends Biochem Sci. 1990 Mar;15(3):86–88. doi: 10.1016/0968-0004(90)90186-f. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M., Lim R., Hicklin D. J., Cotman C. W. Early release of glia maturation factor and acidic fibroblast growth factor after rat brain injury. Neurosci Lett. 1988 Apr 12;86(3):361–365. doi: 10.1016/0304-3940(88)90511-3. [DOI] [PubMed] [Google Scholar]
- Oellig C., Pirvola U., Taylor L., Elde R., Hökfelt T., Pettersson R. F. Acidic FGF and FGF receptors are specifically expressed in neurons of developing and adult rat dorsal root ganglia. Eur J Neurosci. 1995 May 1;7(5):863–874. doi: 10.1111/j.1460-9568.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Oesterle E. C., Dallos P. Intracellular recordings from supporting cells in the guinea pig cochlea: DC potentials. J Neurophysiol. 1990 Aug;64(2):617–636. doi: 10.1152/jn.1990.64.2.617. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Leder P. Ligand specificity and heparin dependence of fibroblast growth factor receptors 1 and 3. J Biol Chem. 1992 Aug 15;267(23):16305–16311. [PubMed] [Google Scholar]
- Ornitz D. M., Yayon A., Flanagan J. G., Svahn C. M., Levi E., Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992 Jan;12(1):240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A., Givol D., Yayon A., Yarden Y., Lonai P. Developmental expression of two murine fibroblast growth factor receptors, flg and bek. Development. 1991 Dec;113(4):1419–1434. doi: 10.1242/dev.113.4.1419. [DOI] [PubMed] [Google Scholar]
- Partanen J., Mäkelä T. P., Eerola E., Korhonen J., Hirvonen H., Claesson-Welsh L., Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991 Jun;10(6):1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Ornitz D., Werner S., Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993 Feb;155(2):423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- Puel J. L., Bobbin R. P., Fallon M. The active process is affected first by intense sound exposure. Hear Res. 1988 Dec;37(1):53–63. doi: 10.1016/0378-5955(88)90077-9. [DOI] [PubMed] [Google Scholar]
- Raphael Y., Altschuler R. A. Scar formation after drug-induced cochlear insult. Hear Res. 1991 Feb;51(2):173–183. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- Saito H., Kasayama S., Kouhara H., Matsumoto K., Sato B. Up-regulation of fibroblast growth factor (FGF) receptor mRNA levels by basic FGF or testosterone in androgen-sensitive mouse mammary tumor cells. Biochem Biophys Res Commun. 1991 Jan 15;174(1):136–141. doi: 10.1016/0006-291x(91)90496-t. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta Otolaryngol. 1971 Feb-Mar;71(2):166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- Stock A., Kuzis K., Woodward W. R., Nishi R., Eckenstein F. P. Localization of acidic fibroblast growth factor in specific subcortical neuronal populations. J Neurosci. 1992 Dec;12(12):4688–4700. doi: 10.1523/JNEUROSCI.12-12-04688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Fitzpatrick S. Purification and characterization of acidic fibroblast growth factor from bovine brain. Proc Natl Acad Sci U S A. 1984 Jan;81(2):357–361. doi: 10.1073/pnas.81.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J., Pirvola U., Moshnyakov M., Palgi J., Arumäe U., Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993 Feb;65(1-2):69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]