Abstract
Objectives
Dental implant abutments are fundamental prosthetic components within dentistry that require optimal biocompatibility. The primary aim of this cross-sectional study was to preliminarily assess differences in the pro-inflammatory cytokine and bone metabolism mediator protein expression in the peri-implant crevicular fluid adjacent to transmucosal abutments.
Material and Methods
Abutments were fabricated from either titanium or zirconia in patients previously receiving single-tooth implant therapy. All subjects sampled in this study had an identical implant system and implant-abutment connection. Participants (n=46) had an average time of clinical function for 22 months (6.2–72.8 months, ±SD 17.0 months) and received a clinical and radiographic exam of the implant site at the time of peri-implant crevicular fluid (PICF) sampling using a paper strip-based sampling technique. Cytokine, chemokine, and bone metabolism mediator quantities (picograms/30 s) were determined using a commercial 22-multiplexed fluorescent bead-based immunoassay instrument. A total of 19 pro-inflammatory cytokines and 7 bone metabolism mediators were evaluated.
Results
Multivariable analyses provided no evidence of a group (titanium or zirconia), gender, or age effect with regard to the expression of pro-inflammatory mediators evaluated. Significant (p=0.022) differences were observed for the bone-mediator leptin, with titanium abutments demonstrating significantly elevated levels in comparison to zirconia. Osteopontin demonstrated a significant (p=0.0044) correlation with age of the subjects.
Conclusions
No significant differences in pro-inflammatory cytokine or bone metabolism mediator profiles were observed biochemically, with the exception of leptin, for the abutment biomaterials of titanium or zirconia The molecular PICF findings support the observed clinical biocompatibility of both titanium and zirconia abutments.
Keywords: Biomaterial(s), Cytokine(s), Titanium, Zirconium-oxide, Oral Implants/Implantology, Prosthetic Dentistry/Prosthodontics, Peri-implant mucosa, Dental abutment, Clinical research, Clinical trials, Patient centered outcomes
Introduction
Requisite to the long-term stability of an implant-supported restoration is the use of prosthetic materials possessing optimal biomechanical and biocompatible characteristics (Norton, 2002; Ratner, 2001; Stanford, 1999; Steigenga et al., 2003).
An implant’s transmucosal abutment highlights these requirements, as it must span three distinct interfaces. Apically, the abutment must possess a stable mechanical connection to the implant that imparts rigid fixation. As the abutment exits the implant platform, its external transition zone is juxtaposed by the connective tissue and epithelium of the peri-implant mucosa, necessitating biological compatibility. The abutment complex generally terminates in the mechanical retention of a coronal restoration via either a luting agent (cement-retained prosthesis) or direct apposition of restorative materials to the abutment surface (screw-retained prosthesis).
Therefore, the ideal transmucosal abutment should, as a result of its material properties, contribute to long-term homeostasis of the peri-implant mucosal microenvironment. This can be achieved via the suppression or downregulation of pro-inflammatory mediators (e.g., cytokines, chemokines, and bone markers) that may be released in a paracrine fashion by cells of the peri-implant mucosa in response to a given abutment biomaterial.
To date, analyses via PICF sampling techniques, such as paramagnetic bead (Lowney et al., 1995) and filter paper strip collection (Fiorellini et al., 2000) have been instrumental in determining the role that biomarkers play in the pathogenesis and progression of peri-implant mucositis and peri-implantitis. Investigators have evaluated various conditional parameters as they relate to PICF dynamics, including comparison of samples obtained from healthy dental implants and natural teeth (Nowzari et al., 2008; Nowzari et al., 2012), effects of de novo plaque-induced peri-implant mucositis (Salvi et al., 2012; Schierano et al., 2008), established peri-implant mucositis (Petkovic et al., 2010), peri-implantitis (Javed et al., 2011; Murata et al., 2002; Severino, Napimoga, & de Lima Pereira, 2011), influence of genotypic characteristics (Andreiotelli et al., 2008; Lachmann et al., 2007), localization of the prosthetic microgap (Boynuegri et al., 2012), and effects of smoking (Ataoglu et al., 2002). Consistently, these studies have demonstrated that the elevation of pro-inflammatory mediators can be quantified under conditions associated with localized acute or chronic inflammation.
The aim of this cross-sectional study was to utilize a paper strip-based peri-implant crevicular fluid (PICF) sampling technique to evaluate the influence that transmucosal abutment biomaterials of either titanium or zirconia, which have been in situ for greater than six months, have in the expression of specific pro-inflammatory cytokines and bone-mediators.
Materials and Methods
Subject Population
Subjects who had either previously undergone single-tooth implant replacement therapy as part of a randomized controlled clinical trial (NCT00607022) conducted at the Craniofacial Clinical Research Center, College of Dentistry, The University of Iowa or who were treated as private patients in the authors’ intramural practices were recruited for this study (NCT01870349). The study protocol was approved by the University of Iowa Institutional Review Board (IRB ID#200911729) and informed consent was obtained from each subject. Inclusion criteria included the following: 1) presence of a single-tooth implant restoration bounded by natural teeth in stable occlusion, 2) pre-fabricated or computer aided design and computer aided manufactured (CAD/CAM) transmucosal abutments of either commercially-pure titanium dioxide or zirconium dioxide, 3) a minimum of 6 months of clinical function in situ. Exclusion criteria included subjects who were pregnant, immunosuppressed, diabetic, currently using tobacco, currently abusing drugs or alcohol, currently using or had a previous history of bisphosphonate use, or were taking systemic anti-inflammatory medications. All patients enrolled had bone-level implants of a single manufacturer (Astra Tech Osseospeed, Astra Tech AB, Mölndal, Sweden).
Clinical Procedures
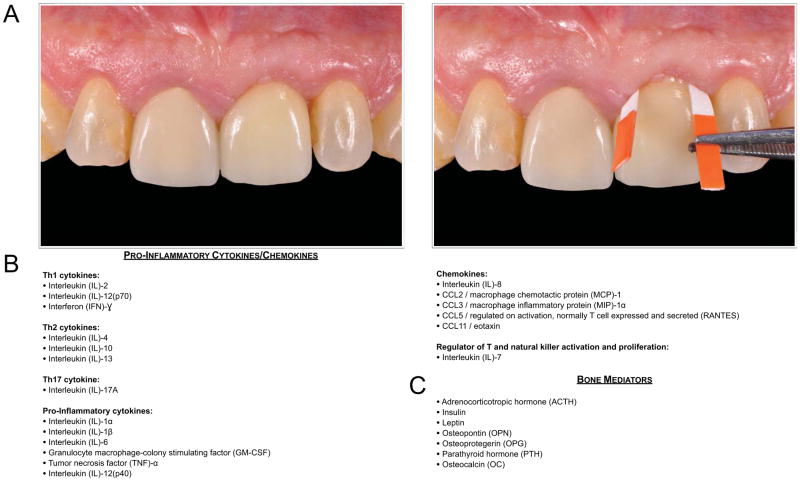
All participants received a clinical and radiographic exam of the implant site that was sampled. Clinical and radiographic parameters such as implant mobility, presence or absence of supragingival plaque, and interproximal bone levels were assessed. Peri-apical radiographs, utilizing a paralleling technique, were obtained from each implant fixture to evaluate the marginal bone level compared to baseline at the time of definitive prosthesis delivery. Each participant’s implant site was isolated with cotton rolls, and light air was applied over the site to eliminate ambient salivary contamination of the PICF sample. Sampling occured for 30 seconds at four distinct sites (mesio-buccal, disto-buccal, mesio-lingual, disto-lingual) by one clinician under loupe magnification. The paper strip (PerioPaper Strips, Oraflow Inc., Smithtown, NY, USA) was inserted with cotton forceps into the gingival crevice until mild resistance was felt (Figure 1A). Upon termination of sampling, gingival crevicular fluid volumes were immediately quantified for each strip using the Periotron 8000 Instrument (Oraflow, Inc., Smithtown, NY, USA), which was calibrated using known volumes of buffer. In cases of visible contamination with blood, the strip was discarded. Each strip was then placed in an empty sterile microcentrifuge tube (Seal Rite, USA Scientific Inc., Ocala, FL, USA) and frozen at −80°C.
Figure 1.
Figure 1A: Clinical example of a paper strip sampling technique at an implant-supported restoration to quantify PICF pro-inflammatory cytokine and bone-mediator protein expression adjacent to an abutment of either titanium or zirconia. Paper strips were positioned at four line angles of the implant restoration sampled for all subjects.
Figure 1B: Classification of pro-inflammatory cytokines/chemokines assessed by PICF sampling and quantified using a multiplexed fluorescent bead-based immunoassay instrument.
Figure 1C: Bone metabolism mediators assessed by PICF sampling and quantified using a multiplexed fluorescent bead-based immunoassay instrument.
Determination of Cytokine and Bone Metabolism Mediator Amounts
Cytokine and bone metabolism mediator quantities (pg/30 s) were determined using a commercial 22-multiplexed fluorescent bead-based immunoassay (Millipore, Billerica, MA) and the Luminex 100 IS Instrument (Luminex, Austin, TX, USA). Two specific multiplex kits were utilized. The first (Kit MPXHCYTO-60K-22, Milliplex MAP Human cytokine/Chemokine Immunoassay, Millipore, Billerica, MA) was capable of detecting specific pro-inflammatory cytokines and chemokines (Figure 1B). The second multiplex kit (Kit HBN1B-51K-07, Milliplex MAP Human Bone Panel 1B Immunoassay, Millipore, Billerica, MA) was capable of detecting pertinent mediators of bone metabolism (Figure 1C).
Four PICF samples per patient were warmed from −80°C on ice. Each sample was resuspended in 75 μl cold 0.01 M PBS, pH 7.2 and protease inhibitor (Complete Mini, protease inhibitor cocktail tablets, Roche Applied Science, IN, USA). Samples were vortex mixed for 10 s and placed on a shaker for 20 min. at 4°C. The tubes were centrifuged for 5 min. at 3,220 g (4,000 rpm) to pellet the strip, plaque, and cellular debris. The contents of each of the four samples per patient were removed and pooled into a single microcentrifuge tube (Seal Rite, USA Scientific Inc., Ocala, FL, USA).
For the assay, 25 μl aliquots of 0.01M PBS, pH 7.2 containing PICF samples were incubated with anti-human multi-cytokine beads for 4°C for 18 h. Unbound antigen was removed by filtration. Anti-human multi-cytokine biotin reporter was added, and reactions were incubated at room temperature for 1.5 h in the dark. Streptavidin-phycoerythrin was then added, and the plates were incubated at room temperature for an additional 30 min. Stop solution was added, and the plates read in a plate reader (Luminex 100 IS, Luminex, Austin, TX, USA). Cytokine quantities in each sample were extrapolated based on standards utilizing Beadview software (Millipore, Billerica, MA).
Statistical Analysis
Biostatisticians were masked to the implant abutment biomaterial designations (masked as “A” and “B”) during analysis. The Wilcoxon-Mann-Whitney test was employed to assess the difference in distribution between the two groups (titanium dioxide or zirconia) for each cytokine/mediator of bone metabolism, as well as for age and gender. Gender distribution in the two groups was compared using the Fisher exact test. The Spearman rank correlation was used to assess the possibility of a relationship with age for each cytokine. Rank-based regression models were used to adjust for possible effects of age and gender for both cytokine and mediator of bone metabolism levels. This rank transformation approach was used due to the difficulty in identifying a suitable normalizing transformation to conform to regression model assumptions (Conover & Iman, 1981). The Holm method was used to adjust for 24 multiple comparisons (associated with each of the 24 cytokine and bone metabolism mediator variables) in conjunction with an overall 0.05 level of Type I error (Holm, 1979). All p-values cited are the original values before considering multiple testing adjustment. Nonparametric confidence intervals for the differences between group means were constructed according to the T3 method of Zhou and Dinh (2005), who demonstrated via simulation studies that this method yielded results quite similar to those obtained by bootstrapping.
Results
Group, Age, and Gender Distribution
Of the 46 patients, 20 males and 26 females aged 23–77 years old (mean = 61.5 years old, ±SD 12.1 years) were sampled. Subjects in this study had an average time of function for nearly two years (22 months, range= 6.2–72.8, ±SD 17.0 months) at the time of sampling. The two treatment groups did not differ significantly in terms of age (p=0.16, Wilcoxon-Mann-Whitney test) or gender (p=0.23, Fisher’s exact test). The median age was 62 years old in the zirconia group (masked as “A”) and 66 years old in the titanium group (masked as “B”). The two treatment groups differed significantly in the distribution of maxillary sextants sampled, but did not differ significantly for mandibular sextants sampled (Table 1). 40 of the 46 (87.0%) subjects had cement-retained crowns, 6 (13.0%) subjects exhibited screw-retained restorations. 18 of the 46 (39.1%) subjects were restored with high-noble alloy porcelain fused to metal restorations, and the remaining 28 subjects (60.9%) were restored with monolithic lithium disilicate ceramic.
Table 1.
Group, age, gender, and anatomic site distribution of the 46 subjects sampled. No significant differences (p<0.05) were found between groups for age or gender. There were significant group differences in the distribution of sites sampled for the maxillary arch, but no significant differences in the mandibular arch.
| Zirconia | Titanium | P-value | |
|---|---|---|---|
| Gender | |||
| Female | 15 | 11 | 0.23 |
| Male | 12 | 8 | |
| Age (years) | |||
| Range | 23–75 | 39–77 | 0.16 |
| Median | 62 | 66 | |
| Mean ±SD | 59 ±13.3 | 65 ±9.7 | |
| Sextant Sites Sampled | |||
| Maxillary Right | 9 | 6 | 0.05 (for sextant) |
| Maxillary Anterior | 10 | 1 | |
| Maxillary Left | 5 | 7 | |
| Mandibular Right | 1 | 1 | 0.99 (for sextant) |
| Mandibular Anterior | 1 | 3 | |
| Mandibular Left | 1 | 1 |
Clinical Findings
All implant sites sampled for PICF exhibited clinical stability of both the implant fixture and coronal restoration. No clinical evidence of peri-implant mucositis was present based on visual assessment. The mean presence of supragingival plaque was 8.7% per patient. Each patient sampled demonstrated radiographic stability (visual assessment) of the interproximal bone levels on both mesial and distal aspects of the implant fixture, when compared with baseline periapical radiographs made at the time of prosthesis delivery.
Pro-Inflammatory Cytokine/Chemokine Quantification
Of the 19 total pro-inflammatory cytokines evaluated, one (Eotaxin) had concentrations designated as “below detectable levels” for 26 of the 46 samples (56.5%), with 16 such designations in the ‘zirconia’ group and 10 in the ‘titanium’ group. Therefore, this specific cytokine was omitted from further analysis. The remaining 18 cytokines evaluated (Table 2) demonstrated no significant group difference based on abutment biomaterial using the Wilcoxon-Mann-Whitney Test (p>0.05 in all instances). Of the 18 cytokines evaluated, a suggestive result was identified for IL-13 (p=0.074), which demonstrated slightly elevated levels in the titanium group as compared to the zirconia group. All other cytokine levels demonstrated significance probabilities that were ≥ 0.28.
Table 2.
Cytokine and bone metabolism mediator quantities (picograms/30 seconds) for titanium and zirconia abutments. (*Leptin levels were significantly elevated (p=0.022) in patients with titanium abutments, as compared with patients with zirconia abutments after adjustment for multiple comparisons).
| Zirconium Oxide | Titanium | ||||||
|---|---|---|---|---|---|---|---|
| Mean (pg/30s) | Median (pg/30s) | Std. Dev. | Mean (pg/30s) | Median (pg/30s) | Std. Dev. | ||
|
|
|||||||
| Cytokine/Chemokine | IL-2 | 2.35 | 2.27 | 0.21 | 2.39 | 2.40 | 0.22 |
| IL-12(p70) | 3.30 | 3.36 | 0.34 | 3.25 | 3.20 | 0.30 | |
| IFN-γ | 3.57 | 3.57 | 0.32 | 3.80 | 3.57 | 1.11 | |
| IL-4 | 11.76 | 11.10 | 1.57 | 11.32 | 11.10 | 1.23 | |
| IL-10 | 6.14 | 5.65 | 2.15 | 7.76 | 4.68 | 7.93 | |
| IL-13 | 6.22 | 6.22 | 1.06 | 5.71 | 5.58 | 1.41 | |
| IL-17A | 2.92 | 2.79 | 0.64 | 3.03 | 3.04 | 0.57 | |
| IL-1α | 742.37 | 599.00 | 462.97 | 766.32 | 643 | 897.28 | |
| IL-1β | 14.03 | 9.95 | 14.68 | 46.19 | 9.27 | 83.03 | |
| IL-6 | 4.94 | 4.08 | 3.73 | 8.31 | 4.35 | 8.55 | |
| GM-CSF | 3.75 | 3.63 | 0.64 | 4.09 | 3.34 | 1.76 | |
| TNF-α | 5.73 | 4.89 | 2.66 | 5.49 | 3.68 | 3.11 | |
| IL-12(p40) | 10.32 | 10.20 | 1.40 | 10.42 | 10.00 | 1.85 | |
| IL-8 | 472.69 | 412.00 | 291.63 | 699.93 | 514.00 | 724.33 | |
| CCL2/MCP-1 | 13.60 | 11.60 | 10.80 | 15.88 | 13.50 | 11.04 | |
| CCL3/MIP-1α | 31.06 | 27.50 | 12.19 | 32.74 | 23.90 | 15.73 | |
| CCL5/RANTES | 7.82 | 6.14 | 3.89 | 7.85 | 6.14 | 3.66 | |
| IL-7 | 7.78 | 7.69 | 0.89 | 8.08 | 7.69 | 1.25 | |
|
|
|||||||
| Bone Mediator | ACTH | 2.02 | 2.03 | 0.46 | 2.79 | 2.23 | 1.63 |
| Insulin | 147.81 | 149.00 | 10.40 | 165.47 | 157 | 35.05 | |
| Leptin | 34.91* | 32.60 | 8.32 | 45.27* | 36.90 | 17.97 | |
| OPG | 33.51 | 33.00 | 14.11 | 42.99 | 29.50 | 26.15 | |
| OPN | 95.17 | 97.50 | 14.57 | 114.93 | 109.00 | 39.11 | |
| PTH | 5.89 | 5.67 | 1.62 | 8.79 | 7.08 | 5.73 | |
There was no evidence of a gender difference for any of these 18 cytokines, based on comparison of the males and females using the Wilcoxon-Mann-Whitney Test (p>0.13 in all instances).
There was no evidence of an increasing or decreasing relationship with subject age for 17 of these 18 cytokines, based on the Spearman rank procedure (p>0.05 in all instances). The single exception was for the positive association IL-12-p40, with r=0.32 (p=0.028).
For the two cytokines with suggestive results, multiple regression modeling was used to assess the effects of group while adjusting for possible gender and age effects. There was no evidence of a group effect for either IL-12-p40 (p=0.86) or IL-13 (p=0.10) after adjustment for covariates, and the overall model including group, age and gender was non-significant for both IL-12-p40 (p=0.38) and IL-13 (p=0.13). Since there was no evidence of any other such relationship with group, gender, or age, multivariable modeling approaches jointly considering the potential effects of these potential covariates were not considered for the remaining cytokines.
Bone Metabolism Mediator Quantification
Of the seven bone metabolism mediators evaluated (Table 2), the majority (43/46) of the subjects had OC determinations that were below detectable levels. No other mediators of bone metabolism produced values that were too low to detect. Analysis of the effect of treatment group revealed significant differences for leptin (p=0.026; Wilcoxon Rank Sum), and the suggestion of treatment group differences with respect to ACTH (p=0.051), PTH (p=0.064), and insulin (p=0.053) using the same analysis method. None of these results would be considered significant after multiple comparisons adjustment; all tests represented in Table 2 were included in the multiple comparisons adjustment.
Median leptin levels were 35.5 pg/30s for the ‘zirconium-oxide’ group and 36.2 pg/30 s for the ‘titanium’ group. The 95% nonparametric confidence limits (Zhou and Dinh, 2005) for the difference in the means for leptin (Mean for Titanium – Mean for Zirconium Oxide) were (3.53 pg/30s – 25.72 pg/30s). The analogous 95% confidence limits for the other three bone mediators, all in pg/30s, were as follows: ACTH (0.18 – 2.16), insulin (5.03 – 47.54), and PTH (0.84 – 7.76).
Rank-based regression modeling provided no evidence of age or gender relationships, yet leptin levels were significantly higher in the ‘titanium’ group (p=0.022) (Figure 2A). The same rank-based regression models were applied to ACTH (p=0.051), PTH (p=0.064), and insulin (p=0.056), and while similar suggestions of a treatment group difference existed, none of these four bone metabolism mediators was considered statistically significant after adjustment for multiple comparisons.
Figure 2.
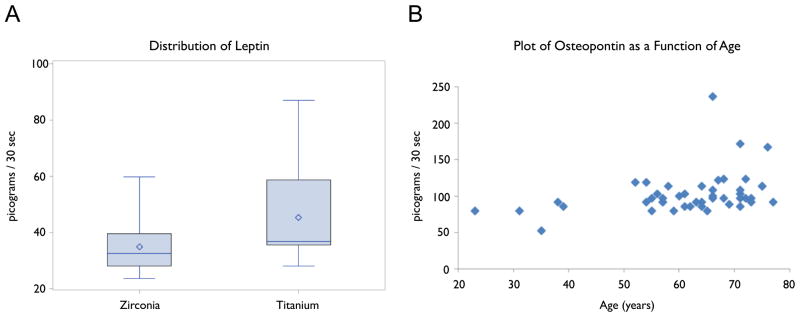
Figure 2A: Box plot depiction of leptin distribution between zirconia and titanium abutment groups sampled in picograms/30 seconds.
Figure 2B: Scatter plot of osteopontin (OPN) as a function of age. OPN was significantly correlated with age after adjustment for multiple comparisons.
Analyses using the Wilcoxon Rank Sum test provided evidence for gender differences in ACTH (p=0.016) and PTH (p=0.032). There was some suggestion of a gender difference for insulin (p=0.094); males tended to have higher values for all three of these mediators. None would be considered significant after adjustment for multiple comparisons.
Analyses using the Spearman Rank Correlation procedure provided evidence of an increasing relationship with age for OPN (r=0.41, p=0.0044) and suggested one for PTH (r=0.29, p=0.10). No other bone metabolism mediator displayed evidence of an age effect. The relationship with age for OPN was significant after adjustment for multiple comparisons (Figure 2B).
Discussion
This cross-sectional study compared for the first time in human subjects the expression of pro-inflammatory cytokine/chemokine and bone metabolism mediators in PICF samples obtained from the sulcus around transmucosal abutments fabricated from either titanium or zirconia, and found no evidence of significant differences between the two biomaterials.
The PICF data obtained in the present study contrasts with the histological findings of a recent study(Degidi et al., 2006), where inflammatory profiles of peri-implant soft tissues adjacent to either commercially-pure titanium or experimental zirconia healing abutments were evaluated in five human subjects after six months of healing. This study reported significant elevations in pro-inflammatory infiltrates (lymphocytes, plasma cells, and histiocytes), as well as increased expression of vascular endothelial growth factor (VEGF) and nitric oxide synthase (NOS) isoforms 1 and 3 adjacent to titanium healing abutments, as compared to zirconia abutments. In contrast, the PICF data reported here was based on a larger (albeit modest) sample population (46 subjects as compared to 5) and evaluated a larger breadth of mediators (24 mediators as compared to 8). The patient population in the present study was recalled retrospectively, and whereas the aforementioned study had patients placed on strict hygiene maintenance programs, the present study did not have controlled oral hygiene protocols in place, reflecting more clinically prevalent conditions. Additionally, the abutment biomaterials evaluated here were in occlusal function and supporting a coronal restoration, whereas Degidi’s study evaluated healing abutment biomaterials that were directly exposed to the oral environment.
All subjects sampled in this study had an identical implant system (Astra Tech Osseospeed, Astra Tech AB, Mölndal, Sweden) and implant-abutment connection, thus serving as an important calibration element for controlling the influence of the implant-abutment interface as a source of differential pro-inflammatory profiles (Machtei, Oved-Peleg, & Peled, 2006). Additionally, neither age nor gender of the subject had a significant effect on the expression of pro-inflammatory cytokines/chemokines.
Regarding established bone metabolism mediators, titanium abutments did demonstrate significantly elevated levels of leptin, a 16 kDa protein that is primarily produced by adipocytes (Zhang et al., 1994) and has been demonstrated to induce osteoblastogenesis and inhibit osteoclastogenesis (Coen, 2004; Zeadin et al., 2012). Leptin has been determined to have a central immunomodulatory role that regulates the host response to inflammatory stimuli by up regulating pro-inflammatory cytokine production and macrophage phagocytosis (Ahima & Flier, 2000). Within the craniofacial domain, Johnson & Serio evaluated leptin for its possible role in periodontal disease progression and found that leptin concentrations were greatest in solubilized gingival biopsies of healthy gingiva (non-hemorrhagic gingiva with ≤3.0mm sulcus), and least in gingival biopsies of inflamed gingiva (hemorrhagic gingiva with >3.0mm sulcus) (Johnson & Serio, 2001). Consistent with this trend, recent findings have subsequently demonstrated that there exists a strong negative correlation between gingival crevicular fluid (GCF) leptin concentration and periodontal disease progression, yet a strong positive correlation exists between serum leptin concentration and periodontal disease progression (Karthikeyan & Pradeep, 2007). Speculation regarding the possible protective role that leptin may have in healthy periodontal homeostasis can be substantiated by the fact that heavy smoking in patients with chronic periodontitis has been shown to significantly lower GCF leptin levels as compared to a similar non-smoking cohort (Bozkurt et al., 2006), potentially pointing to leptin dysregulation by smoking as a comorbidity for periodontal disease progression. Thus, an image of leptin as a “guardian” of a healthy periodontium via regulation of pro-inflammatory cytokines locally in GCF is emerging, but further investigation is necessary to elucidate the specific mechanism by which such regulation occurs. Interestingly however, the PICF analysis completed in the current study failed to demonstrate significant differences of other evaluated bone metabolism mediators based on abutment material. Whether elevated leptin levels seen in this study are suggestive of a specific biomaterial-related regulation of peri-implant mucosa or bone-metabolism, or whether leptin levels were elevated due to systemic conditions (e.g., obesity (Thomas, 2003)) in significantly greater abundance in the titanium-group remains inconclusive. Body Mass Index values were not collected from this study population, thus preventing further covariate analysis that may have provided more insight to answer such questions.
Interestingly, OPN levels displayed a significantly positive correlation with the age of the subject, but displayed no differences based on abutment material. OPN, a Ca2+-binding, glycosylated phosphoprotein expressed by both osteoblasts as well as osteoclasts, has been shown to be an important regulator of bone resorption, remodeling, mineralization, and osteoporosis (Shapses et al., 2003). OPN has also been characterized in GCF, and has been shown to increase in concentration with progression of periodontal disease (Kido et al., 2001). Additionally, findings of a significant positive correlation between GCF and plasma concentrations of OPN with periodontal disease progression, as well as the ability to reduce plasma and GCF OPN levels with non-surgical therapy (scaling and root-planning) (Sharma & Pradeep, 2007) points to OPN as a biomarker of clinical progression of periodontal disease. The results of the current study point to a trend of elevated OPN levels in PICF based on age. To the authors’ best knowledge, this is the first report of a correlation of PICF OPN levels based on chronological age of a patient. However, it has been reported in a recent study of pre- and postmenopausal women that serum OPN levels had a significant positive correlation with age, and served as a significant statistical risk factor in postmenopausal women for developing osteoporosis (Chang et al., 2010). Further investigation is warranted to determine if GCF and serum OPN levels have a direct positive correlation based on age, as they appear to have in periodontal disease progression. Thus, it can be speculated that the trend of elevated OPN expression seen in the PICF of older subjects, who are more at risk for age-associated osteopenia or osteoporosis, strengthens the notion that GCF/PICF OPN levels reflect systemic regulation of bone metabolism, as compared to local regulation attributable to abutment biomaterial. The authors acknowledge that further work is necessary to validate this finding, but if confirmed, would substantiate the ability of PICF to act as a reporter of certain systemic processes.
The PICF analysis completed in this study substantiates biochemically what has been found clinically in human trials comparing the clinical performance of titanium and zirconia abutments. Recent results of a five-year, randomized controlled clinical trial (Zembic et al., 2013) evaluating the survival, as well as technical and biological complication rates of customized zirconia and titanium abutments revealed no statistical or clinically relevant difference between the five-year survival or complication rates of the two abutment materials. Additional clinical studies of shorter duration (1–3 years follow-up) have also demonstrated favorable results for pre-fabricated and customized zirconia abutments (Canullo, 2007; Nothdurft & Pospiech, 2010), when compared to the gold-standard of titanium abutments. The PICF results reported here contribute to a growing body of evidence demonstrating equal biocompatibility of these two abutment materials.
Several limitations were present in the current study. The primary limitation was the relatively modest sample size (46 patients). While this is more robust than other aforementioned studies, future investigations would benefit from larger sample populations that could foster the identification of biomarker differences between groups, which may have not been perceived for this study cohort. The investigators specifically chose to limit the study population in the present study to those individuals that had an identical implant-abutment configuration, thus removing variability at this important prosthetic interface. Second, the present study was cross-sectional in nature. This only allows for the determination of the association between the evaluated mediators and peri-implant health status with no possibility of establishing inferences of causality or long-term peri-implant tissue stability in the presence of certain biomarkers. The authors acknowledge this limitation, and recognize the benefit that a prospective, randomized, longitudinal study would offer. However, it is noteworthy that the present study represents an initial effort to establish and characterize differences in expression of pro-inflammatory and bone metabolism mediators around these two abutment biomaterials. Third, probing depths (PD) and bleeding on probing (BOP) were not recorded at the time of PICF sampling. The primary clinical parameters assessed at the time of sampling were presence or absence of supragingival plaque and radiographic interproximal bone levels relative to prosthesis insertion. None of the subjects sampled exhibited evidence of radiographic marginal bone loss, and supragingival plaque levels were low (8.7%). Future investigations would benefit from further clinical analyses such as Gingival Index (GI), PD, BOP at the time of PICF sampling, so that cytokine expression could be better correlated with routine clinical assessments.
Taken together, the PICF sampling results of this cross-sectional clinical trial did not demonstrate significant differences in pro-inflammatory cytokine or bone metabolism mediator profiles, with the exception of leptin, for the abutment biomaterials of titanium or zirconia. Further studies on the role of Body Mass Index and the production of pro-inflammatory mediators adjacent to dental implants would help to elucidate leptin’s role, if any, in the peri-implant microenvironment.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) grant UL1 RR024979 and Astra Tech AB, Mölndal, Sweden.
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ahima RS, Flier JS. Leptin. Annual Review of Physiology. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Andreiotelli M, Koutayas SO, Madianos PN, Strub JR. Relationship between interleukin-1 genotype and peri-implantitis: A literature review. Quintessence international (Berlin, Germany: 1985) 2008;39:289–298. [PubMed] [Google Scholar]
- Ataoglu H, Alptekin NO, Haliloglu S, Gursel M, Ataoglu T, Serpek B, Durmus E. Interleukin-1beta, tumor necrosis factor-alpha levels and neutrophil elastase activity in peri-implant crevicular fluid. Clinical oral implants research. 2002;13:470–476. doi: 10.1034/j.1600-0501.2002.130505.x. [DOI] [PubMed] [Google Scholar]
- Boynuegri AD, Yalim M, Nemli SK, Erguder BI, Gokalp P. Effect of different localizations of microgap on clinical parameters and inflammatory cytokines in peri-implant crevicular fluid: A prospective comparative study. Clinical oral investigations. 2012;16:353–361. doi: 10.1007/s00784-010-0497-4. [DOI] [PubMed] [Google Scholar]
- Bozkurt FY, Yetkin Ay Z, Sutcu R, Delibas N, Demirel R. Gingival crevicular fluid leptin levels in periodontitis patients with long-term and heavy smoking. Journal of periodontology. 2006;77:634–640. doi: 10.1902/jop.2006.050277. [DOI] [PubMed] [Google Scholar]
- Canullo L. Clinical outcome study of customized zirconia abutments for single-implant restorations. The International journal of prosthodontics. 2007;20:489–493. [PubMed] [Google Scholar]
- Chang IC, Chiang TI, Yeh KT, Lee H, Cheng YW. Increased serum osteopontin is a risk factor for osteoporosis in menopausal women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:1401–1409. doi: 10.1007/s00198-009-1107-7. [DOI] [PubMed] [Google Scholar]
- Coen G. Leptin and bone metabolism. Journal of nephrology. 2004;17:187–189. [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. The American Statistician. 1981;35:124–129. [Google Scholar]
- Degidi M, Artese L, Scarano A, Perrotti V, Gehrke P, Piattelli A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. Journal of periodontology. 2006;77:73–80. doi: 10.1902/jop.2006.77.1.73. [DOI] [PubMed] [Google Scholar]
- Fiorellini JP, Nevins ML, Sekler J, Chung A, Oringer RJ. Correlation of peri-implant health and aspartate aminotransferase levels: A cross-sectional clinical study. The International journal of oral & maxillofacial implants. 2000;15:500–504. [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. 1979;6:65–70. [Google Scholar]
- Javed F, Al-Hezaimi K, Salameh Z, Almas K, Romanos GE. Proinflammatory cytokines in the crevicular fluid of patients with peri-implantitis. Cytokine. 2011;53:8–12. doi: 10.1016/j.cyto.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Johnson RB, Serio FG. Leptin within healthy and diseased human gingiva. Journal of periodontology. 2001;72:1254–1257. doi: 10.1902/jop.2000.72.9.1254. [DOI] [PubMed] [Google Scholar]
- Karthikeyan BV, Pradeep AR. Gingival crevicular fluid and serum leptin: Their relationship to periodontal health and disease. Journal of clinical periodontology. 2007;34:467–472. doi: 10.1111/j.1600-051X.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- Kido J, Nakamura T, Asahara Y, Sawa T, Kohri K, Nagata T. Osteopontin in gingival crevicular fluid. Journal of periodontal research. 2001;36:328–333. doi: 10.1034/j.1600-0765.2001.360509.x. [DOI] [PubMed] [Google Scholar]
- Lachmann S, Kimmerle-Muller E, Axmann D, Scheideler L, Weber H, Haas R. Associations between peri-implant crevicular fluid volume, concentrations of crevicular inflammatory mediators, and composite IL-1A -889 and IL-1B +3954 genotype. A cross-sectional study on implant recall patients with and without clinical signs of peri-implantitis. Clinical oral implants research. 2007;18:212–223. doi: 10.1111/j.1600-0501.2006.01322.x. [DOI] [PubMed] [Google Scholar]
- Lowney JJ, Norton LA, Shafer DM, Rossomando EF. Orthodontic forces increase tumor necrosis factor alpha in the human gingival sulcus. American Journal of Orthodontics and Dentofacial Orthopedics: Official Publication of the American Association of Orthodontists, its Constituent Societies, and the American Board of Orthodontics. 1995;108:519–524. doi: 10.1016/s0889-5406(95)70052-8. [DOI] [PubMed] [Google Scholar]
- Machtei EE, Oved-Peleg E, Peled M. Comparison of clinical, radiographic and immunological parameters of teeth and different dental implant platforms. Clinical oral implants research. 2006;17:658–665. doi: 10.1111/j.1600-0501.2006.01282.x. [DOI] [PubMed] [Google Scholar]
- Murata M, Tatsumi J, Kato Y, Suda S, Nunokawa Y, Kobayashi Y, Takeda H, Araki H, Shin K, Okuda K, Miyata T, Yoshie H. Osteocalcin, deoxypyridinoline and interleukin-1beta in peri-implant crevicular fluid of patients with peri-implantitis. Clinical oral implants research. 2002;13:637–643. doi: 10.1034/j.1600-0501.2002.130610.x. [DOI] [PubMed] [Google Scholar]
- Norton MR. Understanding the intimate relationship between biomechanics and optimal clinical performance: Application of implant design. Compendium of continuing education in dentistry (Jamesburg, NJ: 1995) 2002;23:21–25. [PubMed] [Google Scholar]
- Nothdurft F, Pospiech P. Prefabricated zirconium dioxide implant abutments for single-tooth replacement in the posterior region: Evaluation of peri-implant tissues and superstructures after 12 months of function. Clinical oral implants research. 2010;21:857–865. doi: 10.1111/j.1600-0501.2009.01899.x. [DOI] [PubMed] [Google Scholar]
- Nowzari H, Botero JE, DeGiacomo M, Villacres MC, Rich SK. Microbiology and cytokine levels around healthy dental implants and teeth. Clinical implant dentistry and related research. 2008;10:166–173. doi: 10.1111/j.1708-8208.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- Nowzari H, Phamduong S, Botero JE, Villacres MC, Rich SK. The profile of inflammatory cytokines in gingival crevicular fluid around healthy osseointegrated implants. Clinical implant dentistry and related research. 2012;14:546–552. doi: 10.1111/j.1708-8208.2010.00299.x. [DOI] [PubMed] [Google Scholar]
- Petkovic AB, Matic SM, Stamatovic NV, Vojvodic DV, Todorovic TM, Lazic ZR, Kozomara RJ. Proinflammatory cytokines (IL-1beta and TNF-alpha) and chemokines (IL-8 and MIP-1alpha) as markers of peri-implant tissue condition. International journal of oral and maxillofacial surgery. 2010;39:478–485. doi: 10.1016/j.ijom.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Ratner BD. Replacing and renewing: Synthetic materials, biomimetics, and tissue engineering in implant dentistry. Journal of dental education. 2001;65:1340–1347. [PubMed] [Google Scholar]
- Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clinical oral implants research. 2012;23:182–190. doi: 10.1111/j.1600-0501.2011.02220.x. [DOI] [PubMed] [Google Scholar]
- Schierano G, Pejrone G, Brusco P, Trombetta A, Martinasso G, Preti G, Canuto RA. TNF-alpha TGF-beta2 and IL-1beta levels in gingival and peri-implant crevicular fluid before and after de novo plaque accumulation. Journal of clinical periodontology. 2008;35:532–538. doi: 10.1111/j.1600-051X.2008.01224.x. [DOI] [PubMed] [Google Scholar]
- Severino VO, Napimoga MH, de Lima Pereira SA. Expression of IL-6, IL-10, IL-17 and IL-8 in the peri-implant crevicular fluid of patients with peri-implantitis. Archives of Oral Biology. 2011;56:823–828. doi: 10.1016/j.archoralbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Shapses SA, Cifuentes M, Spevak L, Chowdhury H, Brittingham J, Boskey AL, Denhardt DT. Osteopontin facilitates bone resorption, decreasing bone mineral crystallinity and content during calcium deficiency. Calcified tissue international. 2003;73:86–92. doi: 10.1007/s00223-002-1090-x. [DOI] [PubMed] [Google Scholar]
- Sharma CG, Pradeep AR. Plasma and crevicular fluid osteopontin levels in periodontal health and disease. Journal of periodontal research. 2007;42:450–455. doi: 10.1111/j.1600-0765.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Stanford CM. Biomechanical and functional behavior of implants. Advances in Dental Research. 1999;13:88–92. doi: 10.1177/08959374990130012101. [DOI] [PubMed] [Google Scholar]
- Steigenga JT, al-Shammari KF, Nociti FH, Misch CE, Wang HL. Dental implant design and its relationship to long-term implant success. Implant dentistry. 2003;12:306–317. doi: 10.1097/01.id.0000091140.76130.a1. [DOI] [PubMed] [Google Scholar]
- Thomas T. Leptin: A potential mediator for protective effects of fat mass on bone tissue. Joint, bone, spine: revue du rhumatisme. 2003;70:18–21. doi: 10.1016/s1297-319x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Zeadin MG, Butcher MK, Shaughnessy SG, Werstuck GH. Leptin promotes osteoblast differentiation and mineralization of primary cultures of vascular smooth muscle cells by inhibiting glycogen synthase kinase (GSK)-3beta. Biochemical and biophysical research communications. 2012;425:924–930. doi: 10.1016/j.bbrc.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Zembic A, Bosch A, Jung RE, Hammerle CH, Sailer I. Five-year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single-implant crowns in canine and posterior regions. Clinical oral implants research. 2013;24:384–390. doi: 10.1111/clr.12044. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhou XH, Dinh P. Nonparametric confidence intervals for the one- and two-sample problems. Biostatistics. 2005;6:187–200. doi: 10.1093/biostatistics/kxi002. [DOI] [PubMed] [Google Scholar]