Abstract
The postnatal mammary gland develops extensively through cycles of proliferation, branching, involution and remodeling. We review recent advances made in the field of stress signaling pathways and its roles in mammary gland organogenesis, how they contribute to normal organ specification and homeostasis and how its subversion by oncogenes leads to cancer. We analyze stress signaling in mammary gland biology taking into account the interrelationship with the extracellular matrix and adhesion signaling during morphogenesis. By integrating the information gathered from in vivo and three dimensional in vitro organogenesis studies, we review the novel contribution of p38SAPK, c-Jun NH2-terminal kinase and PKR-like endoplasmic reticulum kinase (PERK) signaling pathways to the timely activation of cell death, correct establishment of polarity and growth arrest and autophagy, respectively. We also review the evidence supporting that the activation of the aforementioned stress kinases maintain breast acinar structures as part of a tumor suppressive program and that its deregulation is commonplace during breast cancer initiation.
Keywords: stress, anoikis, mammary gland, ECM, breast cancer, autophagy
INTRODUCTION
Mammary gland and acinar morphogenesis
Mammary gland (MG) morphogenesis proceeds by expansion of the terminal end buds (highly proliferative bulbous structures) that result in the formation of tree-like structures made of interconnected hollow ducts and alveoli that are essential for the secretion and delivery of the milk. Increasing evidence supports the role of stress signaling pathways in the physiological development of MG tissue.1–4 In this review, we focus on the endoplasmic reticulum kinase PERK (PKR-like endoplasmic reticulum kinase) and the stress-activated kinases p38 and c-Jun NH2-terminal kinase (JNK), which have been shown to have important roles during MG development.5–8 Analysis of signaling pathways regulating MG development and pathogenesis have been fueled by the advent of three dimensional (3D) organotypic cell culture assays that not only mimic conditions close to physiological state but also allow the biochemical analysis of mechanisms underlying breast acinar formation.9 More importantly, the relevance of the 3D cell culture assay to the field of MG development relies on its amenability in functional studies of genes regulating complex processes, such as cell–cell and cell-extracellular matrix (ECM) engagement, apico-basal polarity formation and cell fate determination.
The mammary gland ECM
The MG microenvironment is composed of multiple cell types (that is, endothelial cells, fibroblasts, myofibroblasts and macrophages) and the ECM (that is, laminin, collagen and fibronectin). The ECM serves not only as scaffolding but also for cell fate determination10 by regulating signal transduction. In fact, the ECM provides the essential support for cell proliferation, migration and differentiation and contributes actively both to MG development and tumorigenesis. The MG ECM is constantly remodeled during development by ECM metalloproteinases (MMPs) and plasminogen activators, and defects in the control of this remodeling can favor tumorigenesis.11–13 The latter can be illustrated by the abnormal deposition of collagen-I in the stroma of transgenic mice with mutations in MMPs leading to increased mammary tumorigenesis and metastasis.10,14 Alternatively, fibronectin fragments in the MG microenvironment can promote motility and invasion by inducing MMP2 activity in human breast cancer cell lines.10 When combined, fibronectin and collagen can align in ‘cell-rail’ substrates suitable for the binding of highly motile transformed cells and thereby promoting breast cancer metastasis.15 Another contribution of the ECM to MG morphogenesis is exemplified by the basement membrane, a specialized ECM structure composed of sheet-like matrices that are closely attached to epithelial cells. The mammary epithelial cell–basement membrane interaction regulates normal lumen morphogenesis by promoting both cavitation and hollowing of MG ducts and alveoli. During cavitation, loosely attached mammary epithelial cells within the body of terminal end buds (TEBs) are removed by apoptosis.16 This process was successfully recapitulated in 3D cultures,17 where initially filled mammary acinar structures achieved lumen formation through clearing of centrally located cells that lack attachment through a process similar to anoikis.18 Alternatively, during hollowing, a cluster of MECs guided by cell polarity dynamics assembles into a hollow tubular structure with lumen created by exocytosis and membrane separation without apoptosis.19 More importantly, by providing the apico-basal polarization and the survival cues to the mammary epithelium, basement membrane can regulate hollowing and cavitation during lumen formation. This is evidenced by the inverse correlation between polarization efficiency and apoptosis depending on the ECM substrates.19,20
Stress signaling in normal and cancer tissues
Cellular (oxidative stress, ER stress and DNA-replication stress) and environmental (starvation, hyper-/hypo-osmosis, ionizing radiation and hypoxia/ischemia) stress can affect the tissue homeostasis, which can be operationally defined as an equilibrium between the net growth and cell death rates.21 Cells have evolutionary conserved pathways that allow them to sense and cope with stress damages that may compromise cellular functions. When a certain threshold of damage or energy depletion is reached, intracellular sensors and transducers will trigger a cascade of reactions termed the cellular stress response (CSR).22 Interestingly, the same CSR signals that promote cell survival can also induce cell death when normal homeostasis restoration is not guaranteed (that is, the p53 pathway and autophagy induction). We will focus on the transducers and sensors of stress signaling JNK, p38 and PERK, respectively, as novel integrators of stress signals, and discuss how these signals are turned into cell fate decisions critical for both the normal MG morphogenesis and breast cancer progression.
The initial response to stress is the attenuation or full impairment of cellular growth and proliferation, which precludes mutagenic or catastrophic events.23 Concordantly, the activation of cell-cycle checkpoints that monitor cell integrity and proper completion of cellular processes emerges as a key component of the CSR.24–26 This can be illustrated by the activation of p38α/β and the PERK-eukaryotic initiation factor 2 (eIF2α) pathways. On the one hand, the p38α/β kinase functions as a component of the spindle assembly checkpoint27 and the G1 checkpoint, the latter by inducing cyclin-dependent kinase inhibitors or downregulating G1-exit cyclins.28 Therefore, the activation of p38α/β prevents propagation of genetically damaged cells. On the other hand, under stressful conditions imposed by nutritional deprivation, intracellular ATP levels decrease and misfolded proteins accumulate in the ER lumen. As a result, the chaperone BiP/Grp78 is recruited to assist client protein folding and releases PERK from its monomeric inhibited state. This leads to PERK dimerization/oligomerization and phosphorylation of the translation initiation factor eIF2α at Ser51. This phosphorylation inhibits the ability of its activator eIF2B to bind the initiator tRNA (tRNAi Met) to the 40S ribosome, thereby attenuating global translation initiation but selectively inducing the translation of proteins that promote G0 arrest (that is, p21),29 induce antioxidant responses (that is, activating transcription factor 4 (ATF4)) and stabilize proteins (that is, BIP) to reestablish ER folding capacity. Additionally, as part of the global CSR, heat shock proteins are also induced and many of them function as molecular chaperones.30 Other chaperones, might function as transcription factors, like HSF1 that can translocate to the nucleus, oligomerize and bind to DNA in response to environmental stress.31,32 Alternatively, p53 is also induced in response to the DNA damage33 triggered by CSR. Its apoptotic role is vital for tissue integrity by removing cells with genomic instability to avoid accumulation of threatening mutations.
The ability of CSR to promote tolerance against stressful microenvironments and evading cell death also explains why cancer cells can co-opt normal CSR to override apoptosis. For instance, toxic intracellular concentration of reactive oxygen species (ROS) as a result of hypoxic conditions and metabolic stress can be rewired to favor tumor cell survival via stabilization of hypoxia-inducible factor-134 and degradation of phosphatase and tensin homolog (PTEN).35 In transformed MECs, this is achieved by blocking the activation of the pro-apoptotic p38α signaling pathway in response to ROS production.36 Similarly, by selective inactivation of p38 and its downstream transcription factor target MyoD, human rhabdomyosarcoma cells are able to persist in an undifferentiated and malignant state.37 Thus, co-option or ablation of CSR pathways may serve the ultimate purpose of evading prolonged growth arrest, differentiation, senescence and apoptosis.
JNK AND MAMMARY GLAND MORPHOGENESIS
The JNK belongs to a subgroup of protein kinases that are activated primarily by extracellular (DNA-damage heat shock, inflammatory cytokines, mitogens and hormones) and cellular (metabolic or DNA damage) stress stimuli. The JNKSAPK itself is activated by upstream mitogen-activated protein kinase kinases (MKKs), including MKK4 (SEK1) and MKK7 (SEK2), and is able to integrate various signaling pathways governing cellular processes that run the gamut from cell proliferation to differentiation and apoptosis to polarity. It was clear initially from the animal experiments that the outcome of JNK activation would depend not only on the cell type but also on the context of signal activation as well. For instance, JNK1−/− and JNK2−/− mice showed a lack of cell death in the hindbrain in contrast to the massive apoptosis observed in the forebrain.38 This and other examples of the multifaceted character of JNKSAPK signaling39,40 hints on its role as an integrator, rather than executor, of diverse inputs needed to fine-tune cellular responses. Importantly, the JNK pathway is altered in breast cancer, as MAP2K4, which co-activates the JNK1/p38SAPK, is mutated and inactivated in ~12% of human luminal subtype breast tumors.41 This argues that perhaps early during tumor development inactivation of these signals can favor progression by affecting basic functions of the normal mammary tissue morphogenesis program.
JNKSAPK and normal mammary gland formation
As described previously, JNKSAPK can integrate multiple signal inputs from cytokines and hormones, including glucocorticoid. In fact, fast activation of JNKSAPK by glucocorticoid was previously reported in lymphoid and neuronal cells studies.42–45 Glucocorticoid signaling is critically required to induce milk protein gene expression, such as whey acidic protein and, to a lesser extent, casein.46,47 Further, glucocorticoid signaling has a fundamental role in the formation of cell–cell tight junction needed to establish a correct polarity for luminal secretion.48 Murtagh et al.49 have shown that integration of glucocorticoid and JNK signaling regulates mammary acinar integrity. In organotypic 3D cultures, JNK signaling was shown to be required for the establishment of apico-basal polarity and proper localization of tight and adherent junction proteins of MECs incubated with hydrocortisone. Importantly, abrogation of the JNKSAPK signaling activity with SP600125 inhibitor disrupted apicolateral and basal polarity of the 3D acini structures in a manner reminiscent to lack of glucocorticoid stimulation. It also established the JNKSAPK regulation of the AP1 transcriptional pathway at a downstream level of the glucorcorticoid-induced differentiation and growth arrest of MECs, as it was shown to be required for p21 induction and cyclin D1 repression in mature mammary acini grown in 3D culture. Taken together, these data argue that JNKSAPK integrates differentiation and growth arrest signals initiated by glucocorticoid receptors to ensure proper acini formation during MG morphogenesis.
Loss of JNKSAPK induces tumorigenesis in mouse model of breast cancer
The findings using monolayer and organotypic culture systems described above suggest that a disorder in the JNKSAPK signaling may predispose the MG to tumor development. Cellurale et al.50,51 have supported this idea in an animal study of the breast epithelium development. Using conditional gene ablation of JNK1 and JNK2 in mice, they found in the primary cell culture that MECs deficient for JNK1/2 incorporated more bromodeoxyuridine and showed enhance migration and invasion. Although there were no major changes in morphology or cell type differentiation detected in the monolayer cell culture, JNK deficiency alone did result in greater mammary branching morphogenesis in organoid cultures. The findings were corroborated by in vivo transplantation assays. Furthermore, gene expression analysis showed a decrease expression of tissue inhibitors of metalloproteases as seen in the aggressive basal subtype of breast carcinoma. This could lead to an increase in matrix metalloproteinases (MMPs) activity and explain the enhanced invasiveness and branching morphogenesis seen in JNK-deficient MECs. Enhanced proteolysis, which on its own can induce mammary tumorigenesis52,53 may cooperate with reduced stress signaling via JNK to accelerate progression. Taken together, these findings suggested that loss of JNKSAPK signaling might not perturb breast luminal cell differentiation per se but could promote a phenotypic switch to a more aggressive basal subtype of breast cancer in the context of other tumorigenic insults during disease progression. This idea was confirmed by Cellurale et al.,50 showing that loss of JNK1/2 lead to cancer formation by de-differentiating tumors toward a more invasive basal-like subtype in a KRas/Trp53 mouse model of breast cancer. These findings correlate well with the results of glucocorticoid study in 3D cultures by Murtagh et al.,49 and point out that the JNKSAPK signaling is a key integrator of polarity, differentiation and proliferation signals required for MG integrity. Interestingly, Cellurale et al.,50 also found an incomplete luminal clearance in JNK-deficient MGs, which could be attributed to JNK pro-apoptotic signaling or its role in maintaining polarity and differentiation of the mammary epithelium as a whole.
PERK AND P38 AS NOVEL STRESS-ACTIVATED REGULATORS OF MAMMARY GLAND DEVELOPMENT
The ER stress kinase PERK
Cells have developed specific mechanisms devoted to sense, adapt and overcome stressful conditions.54 Regardless the nature of the stress, the outcome is frequently the accumulation of misfolded proteins in the ER. In particular, the ER kinase PERK has a critical role in sensing and transducing signals to cope with ER insults. Further, PERK has an essential role in tissues with high secretion demand such as the pancreas, liver or MG due that most secreted and transmembrane proteins fold and mature in the ER lumen.7,55–57 PERK phosphorylation is a hallmark of the unfolded protein response, which promotes a transient growth arrest and, initially, survival responses.58 Once the protein load at the ER is reestablished then growth and proliferation can resume. However, persistent unresolved stress can push PERK signaling to induce cell death.59–62 Interestingly, PERK downstream targets ATF4 and GADD15363 are regulated during MG development, suggesting that ER stress signaling might have a role in mammary epithelium development.
PERK functions during mammary gland development and carcinogenesis
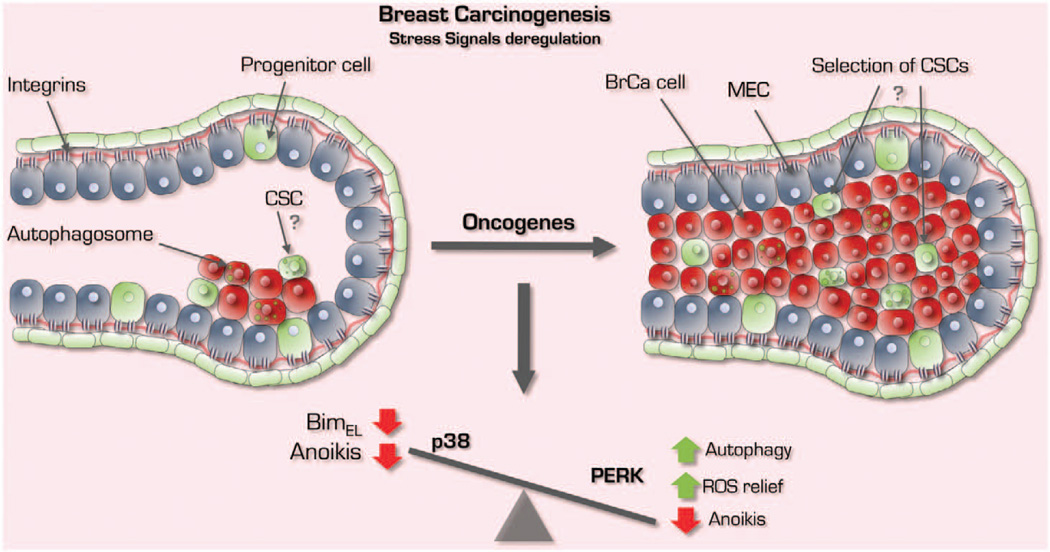
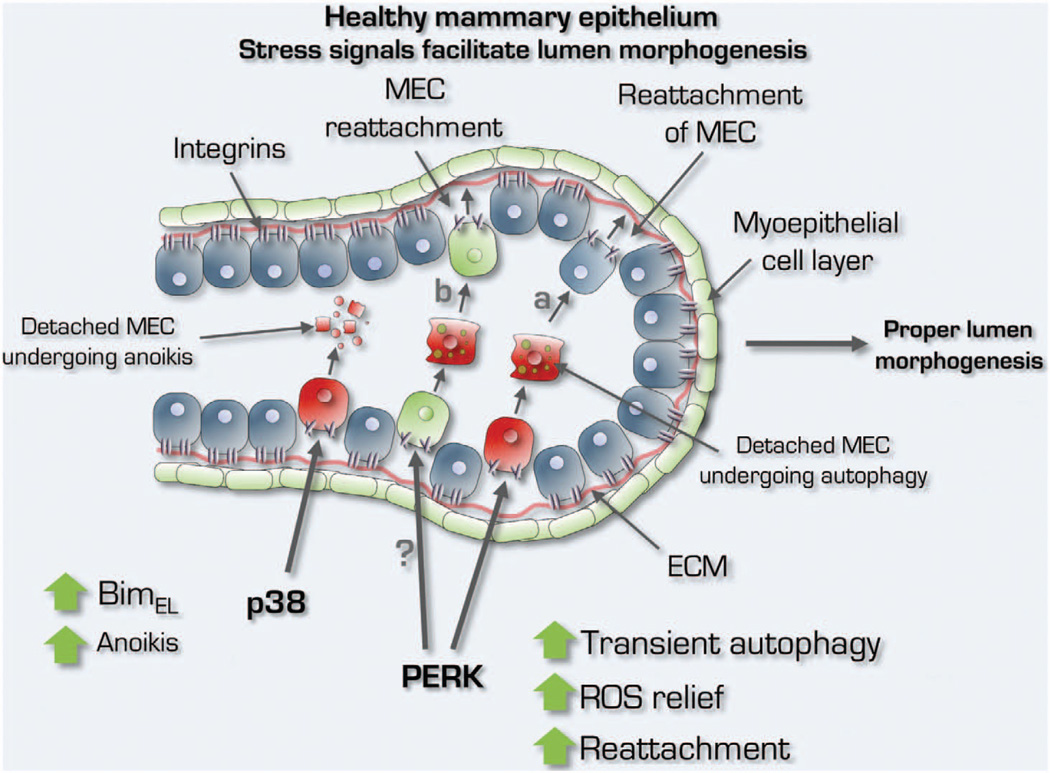
PERK is a key regulator in secretory glands.7,55–57 For instance, PERK is highly expressed in mouse pancreas, organ with active protein secretion55 and regulates lipogenesis during MG morphogenesis.7 Mouse models of breast development, using both immunohistochemical staining for 4-Hydroxynonenal and immunoblot for S100A7 (psoriasin), which measures lipid peroxidation and abnormal squamous differentiation, respectively, as a readout for ROS-induced stress revealed that ROS-induced damage or signaling may be key to regulate MG cavitation.64 However, as luminal clearance is a late event and detachment from the basal lamina triggers ROS production within minutes, how does the inner acinar cell population temporally survive? A clue was provided with the finding that PERK is phosphorylated soon after detachment and activates a transcriptional antioxidant program driven by ATF4 and CHOP.8,65 This signal provides a relief to detached cells of the inner cell population of the mammary acinus. This is a physiological and tightly regulated process whereby epithelial cells need this survival license to move and proliferate dynamically in a nascent acinus before differentiation. Avoiding ROS or becoming insensitive to its apoptotic signals may be too risky for MG integrity. When detachment-induced ROS is inhibited by antioxidants66 or PERK activation via inducible systems,8 anoikis is greatly reduced leading to the formation of hyperplastic and lumen-filled structures reminiscent of early breast cancer lesions (that is, atypical ductal hyperplasia or ductal carcinoma in situ) (Figure 2). These studies provided insight into the physiological link between ROS induction and lumen maintenance, which is critical to preserve a normal architecture during glandular morphogenesis (Figure 1).
Figure 2.
Inactivation of p38 signaling and aberrant regulation of PERK foster early breast cancer progression. In the tumorigenic setting, migratory and less differentiated cells abrogate p38 signaling and co-opt PERK signaling to maximize survival capacity via continuous autophagy induction and an antioxidant response. As a result, transformed cells are simultaneously refractory to apoptosis and more tolerant to oxidative stress in scenarios where adhesion signaling is lacking. Together, this deregulated stress response may give rise to the lumen-filled structures seen in DCIS and could explain how cancer stem cells (CSCs) are selected and expanded.
Figure 1.
The role of p38 and PERK signaling in normal mammary gland biology. Physiological activation of p38 and PERK signaling by cell detachment governs mammary epithelial cell (MEC) lumen integrity that is lost during cancer initiation. In healthy MGs, whereas lumen formation is favored by p38SAPK- and BimEL-mediated apoptosis, PERK activation is required for cell survival to allow transient movement of MECs (a) within the luminal layer and/or hypothetically for the protection of progenitors and stem cells (b) that detach from the ECM within a plastic epithelium during ductal/acinar morphogenesis. The protective function of PERK is via a timed and tightly regulated ‘survival license’ during which autophagy and antioxidant gene induction protect ECM-detached cells from anoikis.
Finally, multiple studies have shown opposing roles for PERK, as it could either prevent5,56,67,68 or promote proliferation/survival of mammary epithelial cells.7,8,67,69,70 This may be due to a dual cellular function that can be rendered intrinsically by PERK. As we previously mentioned, PERK acts as a stress-induced checkpoint that promotes growth arrest and survival via cyclin D1 synthesis inhibition, upregulation of ATF4 and an antioxidant response.71,72 However, PERK was shown to have an anti-proliferative role, in fact we confirmed that chronic activation of PERK-eIF2α can also lead to apoptosis.68 All these observation suggests that competing function of PERK during normal mammary morphogenesis can elicit different outcomes in tumorigenesis (Figures 1 and 2).
Growth-suppressive and antioxidant responses mediated by PERK prevent tumor growth
Observations in the late ‘70s showed that MEFs in suspension (that is, devoid of attachment to the ECM) exhibited translational repression.73,74 In contrast, fibroblasts bound to fibronectin stimulated translation initiation.75 Altogether this suggests that loss of ECM signals and adhesion can impinge on pathways regulating translation initiation, like PERK, and affect MG development. Accordingly, ECM-detached MECs robustly activate p-eIF2α upstream kinase, PERK.5 Strikingly, other eIF2α kinases, such as GCN2 and PKR remained unresponsive in suspension suggesting that PERK is a specific integrator of adhesion signaling and translational repression. Notably, PERK could inhibit proliferation and tumor formation both in 3D in vitro and in vivo animal models.5 This was not due to PERK ability to induce cell death but due to the inhibition of proliferation in ECM adherent cells. Clear evidence of the role of PERK in inducing growth arrest was revealed in studies by Brewer et al.,71 where they found that overexpression of PERK inhibited cyclin D1 synthesis. This was associated with increased phosphorylation of eIF2α and correlated with cell-cycle arrest, suggesting that PERK serves as a major link between ER stress and cell-cycle withdrawal. In agreement, Liang et al.,56 found that PERK inhibited cell growth in a renal cystogenesis model. Polycystin-2, a non-selective Ca2+ release channel localized on the ER membrane, commonly shows an inhibitory mutation in renal cystogenesis resulting in hyper-proliferation and dedifferentiation. In this study, they demonstrate that polycystin-2 regulates negatively cell proliferation through activation of the PERK-eIF2α signaling pathway. Additionally, findings by Bobrovnikova-Marjon et al.,67 suggest a tumor suppressive role of PERK via its antioxidant function. Aged mice lacking PERK were more prone to develop Her2+ lesions when compared with controls with intact PERK. Altogether, these studies suggest that PERK via its growth-suppressive and antioxidant functions can prevent or delay tumor formation in secretory tissues.
PERK supports cell survival during early carcinoma progression
Tumors have to overcome bioenergetic and biosynthetic demands of increased cell proliferation. For instance, beyond a critical volume of 2mm3, oxygen, growth factors and nutrients have difficulty to diffuse to the tumor cells located in the center of the lesion, resulting in hypoxia. To resolve this restriction, tumors have hijacked CSR signals aimed to the generation of neo-vasculature to provide a strategic advantage during tumorigenesis. Bi et al.76 found that PERK-eIF2α signaling abrogation impairs cell survival under extreme hypoxia. Remarkably, they found that PERK mounts an angiogenic response aimed to promote survival under hypoxic conditions. Tumors derived from PERK-knockout MEFs are smaller and show less angiogenesis than wt counterpart tumors.76 Supporting these observations, PERK was also found to promote a tumor microenvironment that favors the formation of functional micro-vessels.69 This is achieved due to PERK ability to specifically enhance translation efficiency of several proangiogenic mRNAs (that is, vascular endothelial growth factor A, adrenomedullin 2 and heme oxygenase-1) during the course of hypoxic stress. Thus, the proangiogenic function of PERK might enable tumor cells with an adaptive advantage to hypoxic stress.
Alternatively, to circumvent the metabolic restriction for proliferation tumor cells might follow the autophagy pathway. In fact, tumors in where the unfolded protein response is triggered due to hypoxia, autophagy is activated to promote survival.77 Interestingly, autophagy is induced in non-transformed mammary epithelial cells and protects them from anoikis.8,78 Further analysis in breast cancer tumors showed that autophagy markers are easily detected in ductal carcinoma in situ lesions from human patients.8,65 However, the scenario here is more complex, because defective autophagy can also propel tumorigenesis.79 Beclin 1, the mammalian homolog of yeast ATG6, is monoallelically deleted in human breast cancer.80 In agreement, autophagy inhibition induces p62 accumulation, which is sufficient to promote tumorigenesis.81 However, it seems clear that PERK-induced autophagy has a survival role than can be co-opted by tumors.8,70,82–84
Emerging evidence indicates that during a period of time after cell detachment, MECs actually activate survival pathways.85 It is thought that this mechanism allows them to survive brief changes in partial or full loss of adhesion before attaching again to the ECM.8,65 This might constitute a safeguard mechanism to protect progenitors or stem cells from anoikis, and thus guaranteeing the survival of a sub-population of pluripotent cells with the ability to repopulate the mammary tissue in physiological processes such as involution. In order to survive in suspension, PERK is activated in MECs to induce autophagy and promote the expression of antioxidant genes from the glutathione (a reducing agent that prevents ROS-mediated damage) pathway.8,65 Thus, commitment to anoikis is not triggered immediately after detachment but only after the balance of competing pro-survival and pro-apoptotic signals is tipped toward the latter, most commonly if ECM attachment does not occur in a specific time frame. This fate decision occurs after a progressive decrease in ATP levels and accumulation of toxic ROS that reach a certain threshold that commits the cell to anoikis.
But, what happens if signals propagated by proteins-like oncogenes co-opt adaptation pathways and make them permanent? By studying ER stress signaling during acinar mammary morphogenesis, it was observed that sustained activation of PERK promoted the survival of luminal cells by engaging antioxidant and pro-autophagic responses. The outcome was luminal cell accumulation in the center of the acinar structures resembling ductal carcinoma in situ lesions. When this mechanism was tested in human breast cancer lesions such as ductal carcinoma in situ, where anoikis resistance is thought to lead to luminal filling,17 it was found that p-PERK was highly increased and heterogeneous when compared with the normal epithelial tissue.8,65 These data imply that ER stress pathways aid possibly in generating an anoikis resistance phenotype and also that PERK signaling may be important in promoting early carcinoma progression (Figure 2). Supporting evidence comes from later studies from Bobrovnikova-Marjon et al.67, where they observed that malignant mouse mammary tumor virus-Neu MG required PERK activity to aid tumor progression.
The role of p38SAPK signaling in mammary gland morphogenesis The p38SAPK (α, β, γ and δ isoforms) kinase pathway is part of a three-sequence phospho-relay system, activated by upstream MKKs (MKK3 and MKK6), which are in turn phosphorylated by MKKKs (TAK1, ASK1, TAO1-3).86 All p38 kinases can be categorized by a Thr-Gly-Tyr dual phosphorylation motif. Ultraviolet-light, heat, osmotic shock, inflammatory cytokines (tumor necrosis factor-α and interleukin-1) and growth factors (colony-stimulating factor-1) can all activate the p38 pathway.87,88 p38α/β participate in oocyte placental, lung and liver maturation, during early development and a breakthrough in the field was the advent of SB203580 compound, which expanded our understanding of the role of p38SAPK signaling in different systems.89 Only p38α and β are sensitive to SB inhibitors, and so p38γ and p38δ are much less well-characterized.90 As a bona fide tumor suppressor, p38α promotes growth arrest by inhibiting cyclin D1 expression91 and by activating the p53,-p21 and p16-Rb pathways.91–94 p38α also regulates the spindle assembly checkpoint27 and delays the G2 to M transition.95 Important for cancer is the amplification in ~18% of human breast tumors at chromosome 17q23 of the PPM1D gene encoding the WIP1 phosphatase,92,96 which can abrogate p38SAPK activity. Sporadic activating mutations in PPM1D have also been described recently, suggesting multiple mechanisms for activating this p38 (and other substrates97) phosphatase.
p38SAPK as a sensor of oxidative stress
As discussed previously, ROS has an important role in shaping and maintaining the normal structure of the mammary acinus by removing luminal cells.66,98 To accomplish this task, the PERK survival license must be issued only for a limited amount of time to ensure clearing of detached luminal cells and to avoid tumorigenic growth. Still, it appears that only after several hours of detachment, the anoikis program is fully executed. This suggests that (1) an additional ‘stress’ stimuli, rather than simple exhaustion of survival signaling, could have an essential role in MECs committing anoikis and (2) commitment to cell death may rely on transcriptional pathways that could significantly outweigh initial adaptation and compensatory mechanisms by PERK.
It could be proposed that p38SAPK integrates the ‘stress-activated transcription’ signals required to tilt the balance toward apoptosis in detached MECs. This may take place by integrating the signals propagated by high ROS levels. Dolado et al.,36 showed that p38α-deficient H-Ras-transformed fibroblasts were more tumorigenic and resistant to cell death compared with control cells. When p38α activity was reinduced, they found that tumor cells became sensitive to endogenous levels of ROS and could even inhibit H-Ras, N-Ras and Neu-induced tumorigenicity. More importantly, Dolado et al.,36 found that a commonality of breast cancer cell lines growing in soft agar despite high endogenous ROS levels was the overexpression of glutathione S-transferase (GSTm1 and GStm2) proteins, which specifically uncouple p38α apoptotic response from ROS accumulation. Together, these findings suggest that p38SAPK is an important player in ROS signaling and may be critical for detachment-induced cell death, which depends on an integration of ROS signals with apoptosis induction.
p38SAPK is required for normal mammary epithelial cell anoikis and breast tumor suppression
How does p38 limit ROS-induced damage and anoikis in epithelial cells? Others and we showed that loss of adhesion triggers p38SAPK activation in normal MECs.6,64 This stress signaling is functionally linked to a cell death program by the activation of ATF-2 and induction of c-Jun. These in turn induce BimEL, an anoikis activator that regulates apoptosis during MG development6 (Figure 1). These data are in agreement with studies showing that mice hypomorphic for ATF-2 developed lumen-occluded invasive ductal mammary carcinomas.99 Additionally, both p38α and ATF-2 knockdown show similar phenotypes in 3D culture assays and loss of these genes leads to anoikis resistance and lumen occlusion. Further ATF-2 phosphorylation by p38α leads to increased abundance of c-Jun in ECM-detached cells and this commits MECs to anoikis. These findings were validated in vivo where inhibition of p38 α/βSAPK signaling by both MKK3−/−/MKK6+/− knockout or by treatment of friend virus B-type (FVB) mice with SB203580 showed mammary ductal and lumen occlusion due to reduced anoikis6 (Figure 2). Although widely shown to negatively regulate cell proliferation, increased proliferation was not detected in p38-inhibited breast tissue from FVB mice treated with SB203580 and from MKK3/6 knockedout C57b mice.6 Nevertheless, significant increase in branching morphogenesis was detected upon p38 signaling inhibition, which is consistent with Dong et al.100 findings in Id4-null MGs with impairment of ductal expansion and branching morphogenesis due to increased p38 activity. On the basis of the role of p38 as integrator of ROS and apoptotic signals, we explored the possibility that p38-mediated anoikis of luminal cells during mammary acinar development could be a crucial point at which p38 might act to curtail breast cancer development. In mouse mammary tumor virus-Neu mammary tissue, SB203580 treatment accelerated epithelial tissue growth and the development of hyperplastic ducts with occluded lumens, supporting the notion that p38α/β signaling could limit the development of Her2/neu-induced hyperplasia.6 The massive expansion of the ductal tree in mouse mammary tumor virus-Neu mammary tissue upon p38α/β inhibition indicates that p38α/β acts to restrain HER2/neu signaling through ATF-2 activation99 and probably by p53 induction.101–104 Taken together, p38α is critical for the development of hollow ducts during MG development, a function that may explain its crucial role as an early breast cancer suppressor (Figures 1 and 2).
CONCLUSIONS AND OPEN QUESTIONS
In this review, we detailed the current understanding primarily of the role of PERK and p38 but also JNK stress signaling in MG morphogenesis and early breast cancer progression. In this section, we present important questions that may guide the field in coming years.
On how PERK and p38 crosstalk to regulate the survival to anoikis switch during MG development
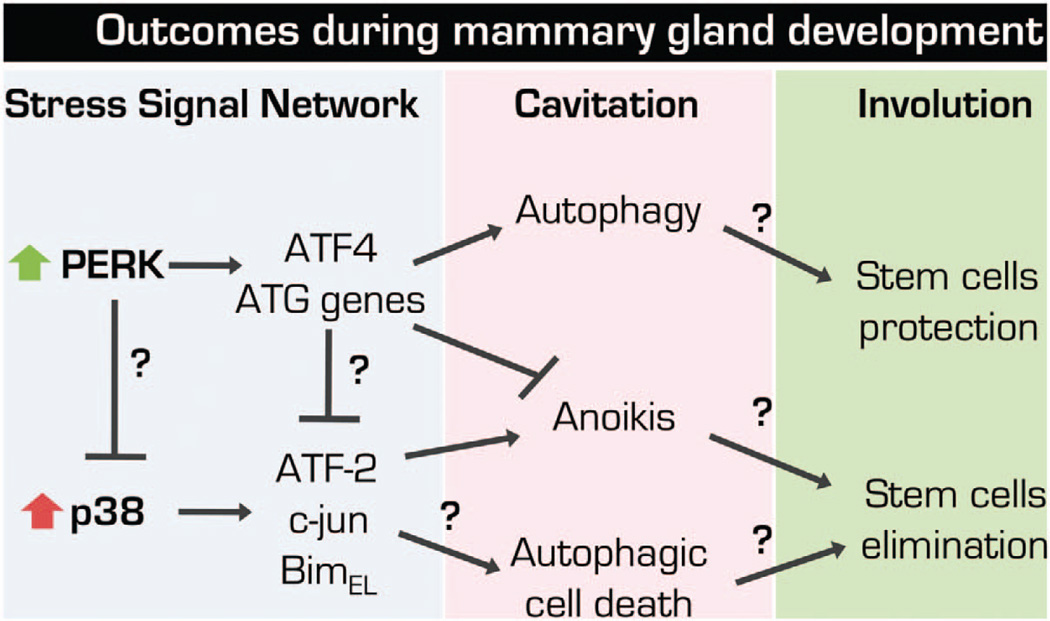
Whereas PERK signaling is located at the ER,105 the subcellular localization of activated p38 is debatable, with some evidence suggesting that it translocates from the cytoplasm to the nucleus, but other data show that it remains in the cytoplasm.88,106 Regardless of the physical location, it is unclear how both pathways interact and crosstalk following cell detachment and some key questions await elucidation. If PERK and p38SAPK pathways are simultaneously activated within minutes to hours following detachment, then (1) how is p38 pro-apoptotic effect inhibited during PERK-induced autophagy? (2) does p38 activation prime cells for autophagic cell death? (Figure 3) (3) what are the rate limiting mechanisms that antagonize p38 activation of ATF-2 and c-Jun to induce BimEL that mark the irreversible commitment to anoikis? and (4) does ATF4 repress anoikis genes or does PERK-induced translational repression attenuate the expression of genes required for the execution of the p38 anoikis program? (Figure 3) Within this context, it is important to consider that during life the MG undergoes cycles of expansion and involution and during the former tissue-resident progenitors and stem cells have critical roles.107 In this context (1) does autophagy protect stem cells during MG involution? (2) is p38 activity differentially regulated in stem cells an/or progenitors during MG cycles of proliferation, differentiation, cell death, involution and remodeling? Or alternatively, does p38 signaling proceed to anoikis in stem and progenitor cells as well to maintain a balanced number of these cells during lifetime of the tissue (Figure 3).
Figure 3.
Hypothetical implications and open questions on the crosstalk between p38SAPK and PERK pathways during MG morphogenesis. p38SAPK and PERK are activated simultaneously after ECM-detachment; however, how can such opposing activities (PERK pro-survival and p38SAPK pro-apoptotic) be reconciled in the comprehensive setup of the mammary gland epithelium is largely unknown. It is possible that PERK signaling directly inhibit p38SAPK activity via post-translational modifications, through the translational repression of p38SAPK-dependent anoikis executors (that is, BimEL) or via upregulation of ATF4. However, these signals are integrated during the survival and anoikis stages of cell fate may markedly influence during hollow lumen morphogenesis. Finally, this network may have a critical role during MG development cycle. We hypothesize that PERK-dependent arm could protect the stem cell compartment during MG involution, whereas p38 signaling could compensate to maintain a homeostatic number of these cells and progenitors that if in excess could result in an exacerbated proliferation in the expansion phase. Thus, both pathways may be critical to maintain the homeostasis of the stem and progenitor cell compartments and proper MG morphogenesis. ATG genes, autophagy genes.
On how ERK1/2 activity and phosphatases regulate p38 activity
ERK survival signaling is inhibited in detached MECs, and this decrease in activity has been shown to be required for pro-apoptotic Bim protein induction to trigger anoikis.64 Activation of the MKK6-p38α pathway is essential for the increase in BimEL and in part responsible for the inhibition of the ERK1/2 pathway in detached cells.6 However, the nature of this p38-dependent pathway inhibition of ERK1/2, which occurs within minutes, is unclear. Phosphatases regulate ERK and p38 activity but there is little insight into how phosphatases such as PP2A, MKPs-inhibitors of ERK pathway activity, and PPM1D-inhibitor of p38 activity may regulate p38-induced anoikis in normal and tumorigenic settings, and research is much needed.
On the identification of specific and effective MKK6/p38 activators?
Although p38 activation may be subverted to promote some tumor cell malignancy processes, many of these data were derived from cell lines.28,108,109 In contrast, the vast majority of in vivo data from mouse models argue that loss of p38 signaling is detrimental by favoring tumorigenesis and progression.6,93,100,110,111 Thus, identifying strategies to restore p38 signaling may be an effective manner to suppress tumor progression in combination with drugs such as Raf and Mek inhibitors or RTK inhibitors. To this end, it has been shown that the level of p38 signaling activation required to achieve tumor growth inhibition is significantly lower than that required to suppress cell growth in vitro.112 Specific activators of p38 have not been discovered. However, these would offer an opportunity because compounds that activates the MKK6/p38 pathway not even maximally, could serve as anticancer agents.
ACKNOWLEDGEMENTS
This study was supported by the grant from Samuel Waxman Cancer Research Foundation Tumor Dormancy Program, NIH/National Cancer Institute (CA109182, CA163131), NIEHS (ES017146) and NYSTEM to JAA-G.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuperwasser C, Hurlbut GD, Kittrell FS, Dickinson ES, Laucirica R, Medina D, et al. Development of spontaneous mammary tumors in BALB/c p53 heterozygous mice. A model for Li-Fraumeni syndrome. Am J Pathol. 2000;157:2151–2159. doi: 10.1016/S0002-9440(10)64853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandriota SJ, Buser R, Lesne L, Stouder C, Favaudon V, Maechler P, et al. Ataxia telangiectasia mutated (ATM) inhibition transforms human mammary gland epithelial cells. J Biol Chem. 2010;285:13092–13106. doi: 10.1074/jbc.M109.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivaraman L, Conneely OM, Medina D, O’Malley BW. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proc Natl Acad Sci USA. 2001;98:12379–12384. doi: 10.1073/pnas.221459098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sequeira SJ, Ranganathan AC, Adam AP, Iglesias BV, Farias EF, Aguirre-Ghiso JA. Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation. PLoS One. 2007;2:e615. doi: 10.1371/journal.pone.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen HC, Avivar-Valderas A, Sosa MS, Girnius N, Farias EF, Davis RJ, et al. p38alpha signaling induces anoikis and lumen formation during mammary morphogenesis. Sci Signal. 2011;4:ra34. doi: 10.1126/scisignal.2001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M, Cavener DR, Thompson CB, et al. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci USA. 2008;105:16314–16319. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall HG, Farson DA, Bissell MJ. Lumen formation by epithelial cell lines in response to collagen overlay: a morphogenetic model in culture. Proc Natl Acad Sci USA. 1982;79:4672–4676. doi: 10.1073/pnas.79.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol. 2010;3:a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateman JF, Boot-Handford RP, Lamande SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat Rev Genet. 2009;10:173–183. doi: 10.1038/nrg2520. [DOI] [PubMed] [Google Scholar]
- 12.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ossowski L, Biegel D, Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979;16:929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- 14.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, et al. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development. 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- 17.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 18.Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Belmonte F, Yu W, Rodriguez-Fraticelli AE, Ewald AJ, Werb Z, Alonso MA, et al. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jechlinger M, Podsypanina K, Varmus H. Regulation of transgenes in three-dimensional cultures of primary mouse mammary cells demonstrates oncogene dependence and identifies cells that survive deinduction. Genes Dev. 2009;23:1677–1688. doi: 10.1101/gad.1801809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lockshin RA, Zakeri Z. Cell death in health and disease. J Cell Mol Med. 2007;11:1214–1224. doi: 10.1111/j.1582-4934.2007.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kultz D. Evolution of the cellular stress proteome: from monophyletic origin to ubiquitous function. J Exp Biol. 2003;206:3119–3124. doi: 10.1242/jeb.00549. [DOI] [PubMed] [Google Scholar]
- 23.Smith ML, Fornace AJ., Jr Mammalian DNA damage-inducible genes associated with growth arrest and apoptosis. Mutat Res. 1996;340:109–124. doi: 10.1016/s0165-1110(96)90043-3. [DOI] [PubMed] [Google Scholar]
- 24.Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol. 2001;13:738–747. doi: 10.1016/s0955-0674(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 25.Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, Yaffe MB. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell. 2005;17:37–48. doi: 10.1016/j.molcel.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Seong YS, Kamijo K, Lee JS, Fernandez E, Kuriyama R, Miki T, et al. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J Biol Chem. 2002;277:32282–32293. doi: 10.1074/jbc.M202602200. [DOI] [PubMed] [Google Scholar]
- 27.Takenaka K, Moriguchi T, Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- 28.del Barco Barrantes I, Nebreda AR. Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans. 2012;40:79–84. doi: 10.1042/BST20110676. [DOI] [PubMed] [Google Scholar]
- 29.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 30.Ellis RJ, Hartl FU. Principles of protein folding in the cellular environment. Curr Opin Struct Biol. 1999;9:102–110. doi: 10.1016/s0959-440x(99)80013-x. [DOI] [PubMed] [Google Scholar]
- 31.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 32.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 33.Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35:7475–7484. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horak P, Crawford AR, Vadysirisack DD, Nash ZM, DeYoung MP, Sgroi D, et al. Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci USA. 2010;107:4675–4680. doi: 10.1073/pnas.0907705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolado I, Swat A, Ajenjo N, De Vita G, Cuadrado A, Nebreda AR. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Puri PL, Wu Z, Zhang P, Wood LD, Bhakta KS, Han J, et al. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000;14:574–584. [PMC free article] [PubMed] [Google Scholar]
- 38.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 39.Das M, Garlick DS, Greiner DL, Davis RJ. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25:634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waetzig V, Herdegen T. A single c-Jun N-terminal kinase isoform (JNK3-p54) is an effector in both neuronal differentiation and cell death. J Biol Chem. 2003;278:567–572. doi: 10.1074/jbc.M207391200. [DOI] [PubMed] [Google Scholar]
- 41.Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Qiu J, Wang J, Zhong Y, Zhu J, Chen Y. Corticosterone-induced rapid phosphorylation of p38 and JNK mitogen-activated protein kinases in PC12 cells. FEBS Lett. 2001;492:210–214. doi: 10.1016/s0014-5793(01)02254-2. [DOI] [PubMed] [Google Scholar]
- 43.Miller AL, Garza AS, Johnson BH, Thompson EB. Pathway interactions between MAPKs, mTOR, PKA, and the glucocorticoid receptor in lymphoid cells. Cancer Cell Int. 2007;7:3. doi: 10.1186/1475-2867-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, et al. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–1583. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- 45.Qi AQ, Qiu J, Xiao L, Chen YZ. Rapid activation of JNK and p38 by glucocorticoids in primary cultured hippocampal cells. J Neurosci Res. 2005;80:510–517. doi: 10.1002/jnr.20491. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Rosen JM. Glucocorticoid regulation of rat whey acidic protein gene expression involves hormone-induced alterations of chromatin structure in the distal promoter region. Mol Endocrinol. 1994;8:1328–1335. doi: 10.1210/mend.8.10.7854350. [DOI] [PubMed] [Google Scholar]
- 47.Rosen JM, Zahnow C, Kazansky A, Raught B. Composite response elements mediate hormonal and developmental regulation of milk protein gene expression. Biochem Soc Symp. 1998;63:101–113. [PubMed] [Google Scholar]
- 48.Zettl KS, Sjaastad MD, Riskin PM, Parry G, Machen TE, Firestone GL. Glucocorticoid-induced formation of tight junctions in mouse mammary epithelial cells in vitro. Proc Natl Acad Sci USA. 1992;89:9069–9073. doi: 10.1073/pnas.89.19.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murtagh J, McArdle E, Gilligan E, Thornton L, Furlong F, Martin F. Organization of mammary epithelial cells into 3D acinar structures requires glucocorticoid and JNK signaling. J Cell Biol. 2004;166:133–143. doi: 10.1083/jcb.200403020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cellurale C, Girnius N, Jiang F, Cavanagh-Kyros J, Lu S, Garlick DS, et al. Role of JNK in mammary gland development and breast cancer. Cancer Res. 2011;72:472–481. doi: 10.1158/0008-5472.CAN-11-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cellurale C, Weston CR, Reilly J, Garlick DS, Jerry DJ, Sluss HK, et al. Role of JNK in a Trp53-dependent mouse model of breast cancer. PLoS One. 2010;5:e12469. doi: 10.1371/journal.pone.0012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almholt K, Green KA, Juncker-Jensen A, Nielsen BS, Lund LR, Romer J. Extra-cellular proteolysis in transgenic mouse models of breast cancer. J Mammary Gland Biol Neoplasia. 2007;12:83–97. doi: 10.1007/s10911-007-9040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 54.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 55.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 56.Liang G, Yang J, Wang Z, Li Q, Tang Y, Chen XZ. Polycystin-2 down-regulates cell proliferation via promoting PERK-dependent phosphorylation of eIF2alpha. Hum Mol Genet. 2008;17:3254–3262. doi: 10.1093/hmg/ddn221. [DOI] [PubMed] [Google Scholar]
- 57.Wei J, Sheng X, Feng D, McGrath B, Cavener DR. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J Cell Physiol. 2008;217:693–707. doi: 10.1002/jcp.21543. [DOI] [PubMed] [Google Scholar]
- 58.Merksamer PI, Papa FR. The UPR and cell fate at a glance. J Cell Sci. 2010;123:1003–1006. doi: 10.1242/jcs.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta S, Read DE, Deepti A, Cawley K, Gupta A, Oommen D, et al. Perk-dependent repression of miR-106b-25 cluster is required for ER stress-induced apoptosis. Cell Death Dis. 2012;3:e333. doi: 10.1038/cddis.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu CL, Li X, Hu GL, Li RJ, He YY, Zhong W, et al. Salubrinal protects against tunicamycin and hypoxia induced cardiomyocyte apoptosis via the PERK-eIF2alpha signaling pathway. J Geriatr Cardiol. 2012;9:258–268. doi: 10.3724/SP.J.1263.2012.02292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talukder AH, Wang RA, Kumar R. Expression and transactivating functions of the bZIP transcription factor GADD153 in mammary epithelial cells. Oncogene. 2002;21:4289–4300. doi: 10.1038/sj.onc.1205529. [DOI] [PubMed] [Google Scholar]
- 64.Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Espina V, Mariani BD, Gallagher RI, Tran K, Banks S, Wiedemann J, et al. Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS One. 2010;5:e10240. doi: 10.1371/journal.pone.0010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sequeira SJ, Wen HC, Avivar-Valderas A, Farias EF, Aguirre-Ghiso JA. Inhibition of eIF2alpha dephosphorylation inhibits ErbB2-induced deregulation of mammary acinar morphogenesis. BMC Cell Biol. 2009;10:64. doi: 10.1186/1471-2121-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Avivar-Valderas A, Bobrovnikova-Marjon E, Alan Diehl J, Bardeesy N, Debnath J, Aguirre-Ghiso JA. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene. 2013;32:4932–4940. doi: 10.1038/onc.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci USA. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brewer JW, Hendershot LM, Sherr CJ, Diehl JA. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci USA. 1999;96:8505–8510. doi: 10.1073/pnas.96.15.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farmer SR, Ben-Ze’av A, Benecke BJ, Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978;15:627–637. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- 74.Benecke BJ, Ben-Ze’ev A, Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978;14:931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- 75.Gorrini C, Loreni F, Gandin V, Sala LA, Sonenberg N, Marchisio PC, et al. Fibronectin controls cap-dependent translation through beta1 integrin and eukaryotic initiation factors 4 and 2 coordinated pathways. Proc Natl Acad Sci USA. 2005;102:9200–9205. doi: 10.1073/pnas.0409513102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19:797–806. doi: 10.1091/mbc.E07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 80.Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- 81.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, et al. ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest. 2012;122:4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milani M, Rzymski T, Mellor HR, Pike L, Bottini A, Generali D, et al. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009;69:4415–4423. doi: 10.1158/0008-5472.CAN-08-2839. [DOI] [PubMed] [Google Scholar]
- 84.Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, et al. PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy. 2008;4:513–515. doi: 10.4161/auto.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Debnath J. Detachment-induced autophagy during anoikis and lumen formation in epithelial acini. Autophagy. 2008;4:351–353. doi: 10.4161/auto.5523. [DOI] [PubMed] [Google Scholar]
- 86.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 88.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 89.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 90.Kumar S, McDonnell PC, Gum RJ, Hand AT, Lee JC, Young PR. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem Biophys Res Commun. 1997;235:533–538. doi: 10.1006/bbrc.1997.6849. [DOI] [PubMed] [Google Scholar]
- 91.Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R, et al. PRAK is essential for ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 92.Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31:210–215. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 93.Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, et al. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet. 2004;36:343–350. doi: 10.1038/ng1317. [DOI] [PubMed] [Google Scholar]
- 94.Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mikhailov A, Patel D, McCance DJ, Rieder CL. The G2 p38-mediated stress-activated checkpoint pathway becomes attenuated in transformed cells. Curr Biol. 2007;17:2162–2168. doi: 10.1016/j.cub.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lambros MB, Natrajan R, Geyer FC, Lopez-Garcia MA, Dedes KJ, Savage K, et al. PPM1D gene amplification and overexpression in breast cancer: a qRT-PCR and chromogenic in situ hybridization study. Mod Pathol. 2010;23:1334–1345. doi: 10.1038/modpathol.2010.121. [DOI] [PubMed] [Google Scholar]
- 97.Ruark E, Snape K, Humburg P, Loveday C, Bajrami I, Brough R, et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2012;493:406–410. doi: 10.1038/nature11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 99.Maekawa T, Shinagawa T, Sano Y, Sakuma T, Nomura S, Nagasaki K, et al. Reduced levels of ATF-2 predispose mice to mammary tumors. Mol Cell Biol. 2007;27:1730–1744. doi: 10.1128/MCB.01579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dong J, Huang S, Caikovski M, Ji S, McGrath A, Custorio MG, et al. ID4 regulates mammary gland development by suppressing p38MAPK activity. Development. 2011;138:5247–5256. doi: 10.1242/dev.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akaogi K, Ono W, Hayashi Y, Kishimoto H, Yanagisawa J. MYBBP1A suppresses breast cancer tumorigenesis by enhancing the p53 dependent anoikis. BMC Cancer. 2013;13:65. doi: 10.1186/1471-2407-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Danes CG, Wyszomierski SL, Lu J, Neal CL, Yang W, Yu D. 14-3-3 zeta down-regulates p53 in mammary epithelial cells and confers luminal filling. Cancer Res. 2008;68:1760–1767. doi: 10.1158/0008-5472.CAN-07-3177. [DOI] [PubMed] [Google Scholar]
- 103.Ravid D, Maor S, Werner H, Liscovitch M. Caveolin-1 inhibits cell detachment-induced p53 activation and anoikis by upregulation of insulin-like growth factor-I receptors and signaling. Oncogene. 2005;24:1338–1347. doi: 10.1038/sj.onc.1208337. [DOI] [PubMed] [Google Scholar]
- 104.Yazinski SA, Westcott PM, Ong K, Pinkas J, Peters RM, Weiss RS. Dual inactivation of Hus1 and p53 in the mouse mammary gland results in accumulation of damaged cells and impaired tissue regeneration. Proc Natl Acad Sci USA. 2009;106:21282–21287. doi: 10.1073/pnas.0904965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 106.Ben-Levy R, Leighton IA, Doza YN, Attwood P, Morrice N, Marshall CJ, et al. Identification of novel phosphorylation sites required for activation of MAPKAP kinase-2. EMBO J. 1995;14:5920–5930. doi: 10.1002/j.1460-2075.1995.tb00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruno RD, Smith GH. Reprogramming non-mammary and cancer cells in the developing mouse mammary gland. Semin Cell Dev Biol. 2012;23:591–598. doi: 10.1016/j.semcdb.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim MS, Lee EJ, Kim HR, Moon A. p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res. 2003;63:5454–5461. [PubMed] [Google Scholar]
- 109.Mao L, Yuan L, Slakey LM, Jones FE, Burow ME, Hill SM. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010;12:R107. doi: 10.1186/bcr2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O’Sullivan AW, Wang JH, Redmond HP. p38 MAP kinase inhibition promotes primary tumour growth via VEGF independent mechanism. World J Surg Oncol. 2009;7:89. doi: 10.1186/1477-7819-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Timofeev O, Lee TY, Bulavin DV. A subtle change in p38 MAPK activity is sufficient to suppress in vivo tumorigenesis. Cell Cycle. 2005;4:118–120. doi: 10.4161/cc.4.1.1342. [DOI] [PubMed] [Google Scholar]