Abstract
Alzheimer’s disease (AD) is characterized by progressive impairments in cognitive and behavioral functions with deficits in learning, memory and executive reasoning. Growing evidence points toward brain insulin and insulin-like growth factor (IGF) resistance-mediated metabolic derangements as critical etiologic factors in AD. This suggests that indices of insulin/IGF resistance and their consequences, i.e. oxidative stress, neuro-inflammation, and reduced neuronal plasticity, should be included in biomarker panels for AD. Herein, we examine a range of metabolic, inflammatory, stress, and neuronal plasticity related proteins in early AD, late AD, and aged control postmortem brain, postmortem ventricular fluid (VF), and clinical cerebrospinal fluid (CSF) samples. In AD brain, VF, and CSF samples the trends with respect to alterations in metabolic, neurotrophin, and stress indices were similar, but for pro-inflammatory cytokines, the patterns were discordant. With the greater severities of dementia and neurodegeneration, the differences from control were more pronounced for late AD (VF and brain) than early or moderate AD (brain, VF and CSF). The findings suggest that the inclusion of metabolic, neurotrophin, stress biomarkers in AβPP-Aβ+pTau CSF-based panels could provide more information about the status and progression of neurodegeneration, as well as aid in predicting progression from early- to late-stage AD. Furthermore, standardized multi-targeted molecular assays of neurodegeneration could help streamline postmortem diagnoses, including assessments of AD severity and pathology.
Keywords: Biomarkers, Cerebrospinal fluid, Neurotrophins, Amyloid, Tau, Insulin resistance, Neurodegeneration, Glucose metabolism, Luminex
Introduction
Growing evidence supports the concept that in Alzheimer’s disease (AD), metabolic dysfunction, mediated by impairments in insulin and insulin-like growth factor (IGF) signaling [1–12], causes progressive deficits in brain glucose utilization, energy metabolism, cytoskeleton and myelin maintenance, and neuronal plasticity [13,14]. Further contributions from oxidative and endoplasmic reticulum stress, inflammation, and increased pro-death/anti-survival signaling, help drive neurodegeneration. Consequences include brain accumulations of amyloid beta (AβPP-Aβ) deposits and fibrils/oligomers, and phospho-tau-related neuronal cytoskeletal lesions [13–16]. In addition, insulin resistance down-regulates target genes needed for cholinergic function, further compromising neuronal plasticity, memory, and cognition [13,14]. The pivotal roles of insulin and IGF-1 resistance as mediators of cognitive impairment and neurodegeneration have been well documented in humans and experimental animals [7,11,12,17]. This concept is corroborated by the findings that cognitive impairment and neurodegeneration can be slowed, reduced in severity, or prevented in experimental animals and humans by treatment with insulin sensitizer agents, insulin, or long-acting glucagon-like peptide-1 (GLP-1)-related compounds [18–27].
Over the past two decades, robust efforts to develop non-invasive diagnostic assays for AD have led to protocols that measure cerebrospinal fluid (CSF) levels of Tau, hyperphosphorylated Tau (pTau) [28–31] and AβPP-Aβ [32–38], and positron emission tomography (PET) to image aberrant brain accumulations of AβPP-Aβ [39]. When combined with magnetic resonance imaging (MRI), functional MRI (fMRI), and PET studies of brain glucose utilization, CSF assays of Tau, pTau and AβPP-Aβ correlate well with intermediate and late stages of AD [33,40]. However, without the support of costly neuroimaging studies, the sensitivity, specificity, and reproducibility of highly restricted CSF-based assays are not sufficient to serve as stand-alone diagnostic aids or measures of treatment responses. On the other hand, PET studies of brain glucose metabolism and other means of assessing brain metabolic dysfunction, should be incorporated into AD diagnostic and assessment plans since the related abnormalities occur early and progress with severity of disease. Besides the proteins that directly pertain to insulin and IGF-1 resistance, i.e. the trophic factors/ligands themselves, associated molecules that further contribute to neurodegeneration, i.e. indices of oxidative stress, inflammation, and impaired neuronal plasticity, should be evaluated [41–43]. Here in, we examine the potential value of using CSF-based multi-pronged platforms for AD diagnosis and monitoring. The goal was to determine the degree to which indices of insulin/IGF resistance, neuroinflammation, oxidative stress, and neuronal plasticity in CSF correspond with those in postmortem ventricular fluid (VF) and brain tissue.
Methods
Human brain tissue, VF, and CSF
Fresh frozen samples of postmortem frontal lobe (Brodmann Area 8/9) from patients with no evidence of AD (Braak Stage 0–1), moderate AD (Braak States 3–4), or advanced AD (Braak Stage 6), were obtained from the Kathleen Price Bryan Brain Bank at Duke University Medical Center, the Massachusetts General Hospital Alzheimer’s Disease Research Center Brain Bank, and the Brown University Brain Bank [11,12,16,44]. With regard to #1 and #2, the subject in all groups had similar mean ages and specra of underlying diseases that contributed to death as described earlier [11,12,16,44]. The gender ratios were approximately even in control and moderate AD, but skewed with 75% female in the advanced AD (Braak 6) group as reported earlier [44]. Postmortem intervals were less than 14 hours and the tissues were stored at −80°C. Aqueous homogenates of brain tissue were used to obtain cytosolic and extracellular fluid proteins for comparison with VF and CSF results. To obtain these, brain tissue samples were homogenized in 5-volumes of phosphate-buffered saline (PBS; 10 mM phosphate, 0.9% sodium chloride, pH 7.35) containing 0.02% sodium azide and protease inhibitors [11,12]. Supernatants obtained by centrifuging the samples at 12000 × g for 10 minutes at 4°C were used in the studies. Protein concentrations were measured with the Nano-Orange Protein Quantification Kit (Pierce Chemical Company, Rockford, IL).
Postmortem VF was obtained from the Massachusetts General Hospital’s Alzheimer’s Disease Research Center Brain Bank. CSF samples were obtained for diagnostic or research purposes from patients fulfilling NINDS criteria for probable or definite AD (N=16) [45]. Control CSF samples were from patients undergoing evaluation for headache or back pain, and who were free of neoplastic, inflammatory and neurodegenerative diseases (N-16). CSF was obtained by lumbar puncture and stored at −80°C. Research CSF samples were obtained by written informed consent using guidelines set by the Human Studies Committees at the National Institutes of Health. All samples were de-identified and their use in this study was approved by the Lifespan Human Studies Committee and Investigational Review Board.]
Enzyme-linked immunosorbent assay (ELISA)
Aqueous anterior frontal lobe homogenates, VF, and CSF samples were serially diluted in PBS containing 1% bovine serum albumin (PBS-BSA). Immunoreactivity to insulin, nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), glial cell derived neurotrophic factor (GDNF), phospho-tau, AβPP, AβPP-Aβ, 8-hydroxydeoxyguanosine (8-OHdG), 4-hydroxynonenal (4-HNE), and advanced glycation end-products (AGE) was measured by direct binding ELISA using with horseradish peroxidase (HRP)-conjugated secondary antibody and Amplex Ultra Red soluble fluorophore [16,46]. Fluorescence intensity was measured (Ex 565 nm/Em 595 nm) in a Spectra Max M5 microplate reader (Molecular Devices, Sunnyvale, CA). All assays were performed in quadruplicate. Binding specificity was determined by omitting primary or secondary antibodies in parallel reactions. Immunoreactivity was normalized to protein content.
Multiplex ELISA
We used Luminex bead-based multiplex ELISA’s to measure immunoreactivity to pro-inflammatory cytokines, chemokines, growth factors, and insulin-related gut hormones (Millipore or Bio-Rad; Table 1). After re-centrifuging the brain, VF, and CSF samples to remove particulate debris (12000×g/10 minutes, 4°C), the supernatants were filtered (0.45 μm pores). For these assays, the samples were diluted in PBS (brain) or used undiluted (VF and CSF). Samples (200 μl) were incubated with the beads, and captured antigens were detected with biotinylated secondary antibody and phycoerythrin-conjugated Streptavidin according to the manufacturer’s protocol. Immunoreactivity was measured in a Bio-Plex 200 system (Bio-Rad, Hercules, CA). Data are expressed as fluorescence light units corrected for protein concentration.
Table 1.
Trophic factors and cytokines probed in alzheimer brain, ventricular fluid and cerebrospinal fluid samples.
| Trophic Factor | Abbreviation | Function |
|---|---|---|
| Basic Fibroblast Growth Factor | b–FGF | Present in basement membranes and sub-endothelial extracellular matrix. Regulates angiogenesis and cell survival, division, differentiation, and migration. Modulates nervous system development and wound healing. |
| Beta-Nerve Growth Factor | β–NGF | Neurotrophin family member that regulates survival and maintenance of sensory and sympathetic neurons. Implicated in neuronal growth, proliferation, differentiation and plasticity, as well as cognition. Functions through receptor tyrosine kinase. |
| Brain-Derived Neurotrophic Factor | BDNF | Neurotrophin family member that regulates synaptic transmission, activity-dependent plasticity and long-term potentiation in the hippocampus |
| Gastric Inhibitory Polypeptide | GIP-1 | Glucose-dependent insulinotropic peptide and secretin family member. Stimulates insulin secretion from pancreatic beta-cells following food ingestion. GIP receptors expressed in hippocampus, olfactory bulbs, and cerebellum. Promotes neural progenitor cell proliferation. |
| Ghrelin | GHRL | Stimulates hunger, craving, and growth hormone secretion from the pituitary. Functions in opposite ways compared to leptin. Essential for cognitive adaptations in changing environments. Receptor expressed in hypothalamus. |
| Glial Cell Line-Derived Neurotrophic Factor | GDNF | Isolated from glioma cells. Member of TGF-β superfamily. Neurotrophic factor that exerts neuroprotective and differentiation effects on dopaminergic and motor neurons |
| Glucagon-like Peptide 1 | GLP-1 | Incretin whose secretion is regulated by nutrients, e.g. carbohydrate, protein, and lipid. Promotes glucagon-dependent stimulation of insulin secretion, and survival and proliferation of pancreatic beta cells. Enhances insulin sensitivity and satiety. |
| Hepatocyte Growth Factor | HGF | Typically secreted by mesenchymal cells with actions on epithelial and endothelial cells. Mediates embryogenesis. Stimulates mitogenesis, cell motility, matrix invasion and angiogenesis via c-MET receptor tyrosine kinase. Regulates VEGF. Neuroprotective for cortical and hippocampal neurons during aging and ischemic injury. |
| Insulin | INS | Reduces blood glucose. Increases cellular permeability to monosaccharides, amino acids and fatty acids. Increases rates of glycolysis, pentose phosphate cycle, and glycogen synthesis in liver. |
| Leptin | LEP | Produced in adipocytes and regulates brain energy intake and expenditure, metabolism, and behavior. |
| Pancreatic Polypeptide (Human) | PP | Polypeptide secreted by PP endocrine cells in pancreas in response to hypoglycemia, fasting, or protein meal and decreased by glucose infusion or somatostatin. Closely related to neuropeptide Y and PP. |
| Peptide YY (tyrosine-tyrosine) | PYY | Secreted by intestinal L cells in response to feeding. Reduces appetite. Also produced in brainstem neurons, pancreatic islets. Improves nutrient absorption by slowing gastric motility and emptying. |
| Platelet-derived Growth Factor-AA | PDGF-AA | Regulates cell growth and angiogenesis, and mitogenic for glial and mesenchymal cells. Signals through PI3 Kinase to regulate cell growth and motility, tissue remodeling, differentiation, and migration. Maintains proliferation of oligodendrocyte progenitor cells. |
| Vascular Endothelial Growth Factor | VEGF | Stimulates angiogenesis and vasculogenesis and endothelial cell growth. Inhibits apoptosis and induces vascular permeability, revascularization of injured tissue, endothelial cell migration and proliferation. |
| Cytokine/Chemokine | Abbreviation | Function |
|---|---|---|
| Granulocyte Macrophage Colony-Stimulating Factor | GM-CSF | Stimulates the growth and differentiation of hematopoietic precursor cells from various lineages, including granulocytes, macrophages, eosinophils and erythrocytes. |
| Interferon-gamma | IFN-γ | Produced by innate NK cells, acquired antigen-specific cytotoxic CD4+ and effector CD8+ T cells. Activates macrophages and critical for innate and adaptive immune responses to intracellular pathogens, tumor control, and inhibition of viral replication. |
| Interferon-Gamma-Induced Protein | IP-10 | Produced by various cell types including monocytes, endothelial cells, fibroblasts, and keratinocytes. Induced by IFN-γ and TNF-α. Chemoattractant for activated T cells. |
| Interleukin-1, Beta | IL-1β | Produced by activated macrophages; mediates inflammatory responses, cell proliferation, and apoptosis. Induces Cox-2 in CNS, causing inflammatory pain |
| Interleukin-6 | IL-6 | Secreted by T cells and macrophages; triggers inflammation, acute phase response, fever. Anti-inflammatory effects include inhibiting TNF-α and IL-1, and activating IL-1Rα and IL-10. |
| Interleukin-8 | IL-8 | Made by macrophages and some epithelial and endothelial cells; Role in innate immune response. Major role in chemotaxis of neutrophils. Also mediates inflammatory response and angiogenesis. |
| Interleukin-10 | IL-10 | Produced by monocytes. Pleiotropic cytokine. As an anti-inflammatory cytokine, it inhibits macrophage and dendritic cell function, suppresses TNF-α. Acquires pro-inflammatory activity during immune response with IFN-α stimulation |
| Interleukin-16 | IL-16 | Secreted by lymphocytes. Pleiotropic cytokine. Functions as a chemo-attractant (CD4+ cells), modulates T cell activation, and inhibits HIV replication. |
| Interleukin-18 | IL-18 | Produced by macrophages and monocytes. Pro-inflammatory cytokine interacts with IL-12 to induce cell-mediated immune response with microbial infection and LPS, inducing severe inflammatory reactions. Stimulates NK and T cell release of IFN-γ, which activates macrophages. Inhibits IL4-dependent IgE, enhances B cell production. |
| Leukemia Inhibitory Factor | LIF | Induces myeloid cell differentiation, neuronal cell differentiation, and acute-phase protein synthesis. |
| Macrophage Inflammatory Protein 1 Beta | MIP-1β | Produced by macrophages. CCL4 chemokine that generates local inflammatory responses induces superoxide production by neutrophils. Chemotactic activity for lymphocytes, macrophages, NK cells, and monocytes with inflammation; down-regulates CCR5, inhibiting HIV-1 blocking. |
| Monocyte Chemotactic Protein-1 | MCP-1 | Expressed in monocytes, vascular endothelial cells, smooth muscle cells. CCL2 chemokine induces monocyte attraction, and degranulation of basophils with histamine release. Induced by IL-1, TNF-α, PDGF, TGF-β, and LIF |
| Stem Cell Factor | SCF | Regulates cell survival and proliferation, hematopoiesis, stem cell maintenance, gametogenesis, and mast cell development, migration and function. Promotes phosphorylation of PIK3R1 and activation of AKT1. Aids in activation of RAS, RAF1 and the MAP kinases MAPK1/ERK2 and/or MAPK3/ERK1 |
| Stromal Cell-Derived Factor-1α | SDF-1α | Expressed ubiquitously, except in blood cells. Small cytokine member of CXCL12 family of chemokines. Activates leukocytes due to strong chemotactic effects. Induced by pro-inflammatory stimuli, e.g. TNF-α and IL-1β. |
| TNF-Related Apoptosis Inducing Ligand | TRAIL | Secreted by macrophages, monocytes, neutrophils, T cells, NK cells after stimulation with LPS. CD4+ cells secrete TNF-α. Also made by astrocytes, microglial cells, smooth muscle cells, and fibroblasts. Mediates systemic inflammation, inhibits viral replication, and inhibits tumorigenesis. |
| Tumor Necrosis Factor-Alpha | TNF-α | Expressed broadly in tissues. Cytokine induces proapoptotic caspase activity by up-regulating pro-apoptotic Bcl proteins. Causes apoptosis in hepatocytes, neural cells, and thymocytes |
Statistical analysis
Inter-group comparisons of brain tissue results were made by analysis of variance (ANOVA) with linear trend and Fisher LSD post-hoc tests. VF and CSF AD versus control comparisons were made using Student t-tests. Box plots reflect group medians (horizontal bar), 95% confidence interval limits (upper and lower box limits) and range (whiskers). Data were analyzed using GraphPad Prism 6 software (GraphPad Software, Inc., San Diego, CA).
Results
AD and oxidative stress markers in postmortem brains
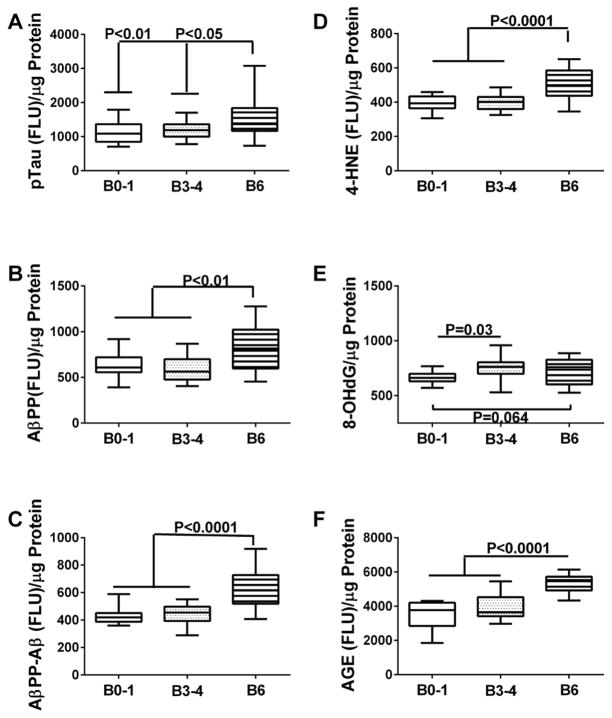
Direct-binding ELISA results for pTau, AβPP, AβPP-Aβ, 4-HNE, 8-OHdG, and AGE were analyzed with respect to Braak AD stage (Figure 1). One-way ANOVA tests demonstrated significant intergroup differences with respect to pTau (F=5.115; P=0.01), AβPP (F=7.47; P=0.0016), AβPP-Aβ (F=19.66; P<0.0001), 4-HNE (F=14.88; P<0.0001), and AGE (F=28.39; P<0.0001), and a significant trend for 8-OHdG (F=2.91; P=0.065). Post-hoc Fisher tests demonstrated higher levels of pTau (Figure 1A), AβPP (Figure 1B), AβPP-Aβ (Figure 1C), 4-HNE (Figure 1D), and AGE (Figure 1F) in AD Braak Stage 6 relative to the other groups. In contrast, 8-OHdG immunoreactivity was significantly elevated in Braak 3–4 AD brains, and only modestly increased at Braak 6 (Figure 1E) relative to control. This suggests that elevated levels of 8-OHdG may mark early to intermediate stages of neurodegeneration, and that a more sensitive assay may be required to assess DNA damage in late stages of disease. In addition, we observed a significant linear trend for increasing AGE levels and AD severity (F=28.39; P<0.0001), consistent with previous observations [47,48]. AGE marks oxidation related to insulin resistance.
Figure 1. Biomarker indices of AD and oxidative stress in postmortem brains.
Frontal lobe aqueous homogenates from subjects with Braak Stage (B) 0–1 (controls), B3–4 (moderate AD), or B6 (late AD) pathology (N=8/group) were used to measure (A) pTau, (B) amyloid precursor protein (AβPP), (C) amyloid precursor protein-Abeta (AβPP-Aβ), (D) 4-hydroxynonenol (HNE), (E) 8-hydroxydeoxyguanosine (8-OHdG), and (F) advanced glycation end-product (AGE) by direct binding ELISA. Immunoreactivity was detected with HRP-conjugated secondary antibody and Amplex Red soluble fluorophor. Fluorescence light units (FLU) were measured (Ex 579 nm/Em 595 nm) in a Spectromax M5, and results were normalized to sample protein content. Results were analyzed by one-way repeated measures ANOVA with post hoc Fisher tests.
Insulin resistance and related proteins
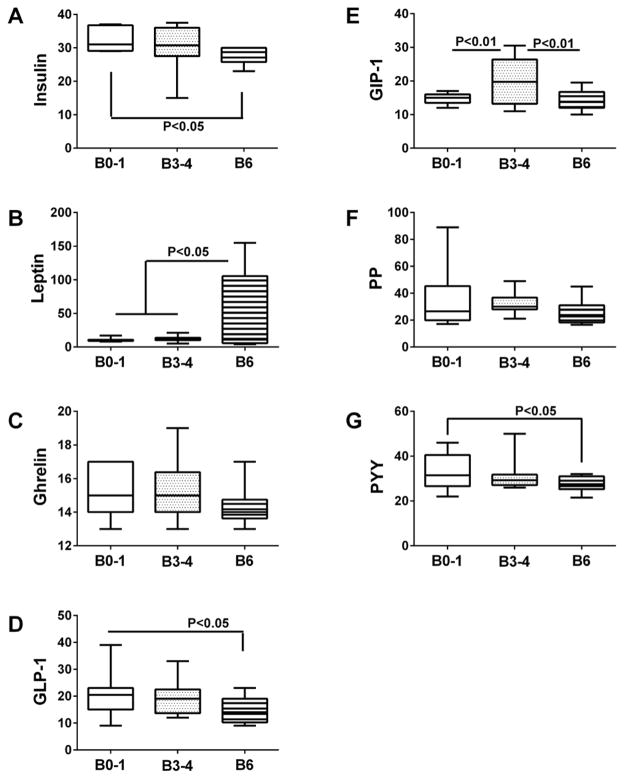
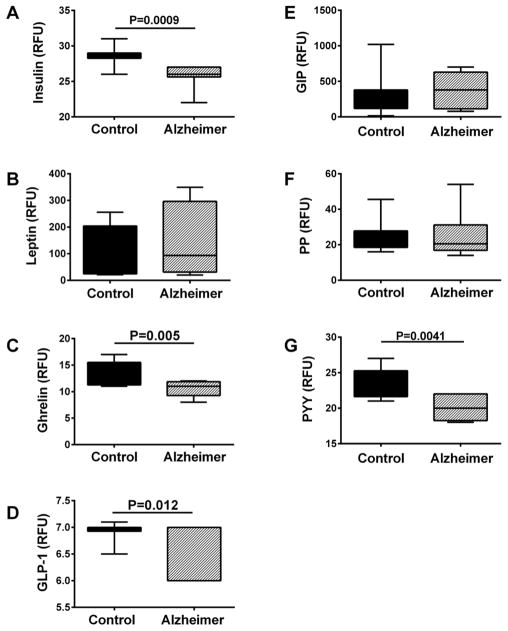
A Gut Hormone Multiplex ELISA panel was used to measure insulin, leptin, ghrelin, glucagon-like peptide-1 (GLP-1), gastric inhibitory polypeptide (GIP-1), pancreatic polypeptide (PP), and Peptide tyrosine tyrosine (PYY) in brain tissue (Figure 2) and CSF (Figure 3). For postmortem brain tissue, ANOVA tests demonstrated significant inter-group differences with respect to insulin (F=3.305; P=0.046), GLP-1(F=3.47; P=0.041), GIP-1 (F=7.45; P=0.0016), leptin (F=5.38; P=0.008), and PYY (F=3.04; P<0.05). Post-hoc Fisher tests revealed significantly reduced levels of insulin (Figure 2A), GLP-1 (Figure 2D), and PYY (Figure 2G), and increased levels of leptin (Figure 2B) at Braak Stage 6 relative to control. In addition, GIP-1 immunoreactivity was significantly elevated at Braak Stage 3–4 relative to control and Braak 6 brains (Figure 2E). Furthermore, trend line post-hoc tests demonstrated significant progressive declines in brain insulin (P=0.017), GLP-1 (P=0.016), and PYY (P=0.02) with increasing AD stage, AD stage-associated trend reductions in ghrelin (P=0.089) and PP (P=0.087), and significant increases in leptin (P=0.005) with AD severity. Analysis of CSF samples revealed significantly lower levels of insulin (Figure 3A), ghrelin (Figure 3C), and GLP-1 (Figure 3D) in subjects with probable AD relative to normal aging, but no significant inter-group differences with respect to leptin (Figure 3B), GIP-1 (Figure 3E), PP (Figure 3F), or PYY (Figure 3G). Therefore, concordant results between postmortem brain and clinical CSF assays were obtained with respect to insulin, GLP-1, and to some extent, ghrelin.
Figure 2. Insulin resistance biomarkers in brain.
Frontal lobe aqueous homogenates from subjects with Braak Stage (B) 0–1 (controls), B3–4 (moderate AD), or B6 (late AD) pathology (N=8/group) were used to measure (A) insulin, (B) leptin, (C) ghrelin (D) GLP-1, (E) GIP-1, (F) PP, and (G) PYY by multiplex bead-based ELISA. Immunoreactivity is expressed in fluorescence light units (FLU) normalized to protein content. Data were analyzed by one-way repeated measures ANOVA with post hoc Fisher significance tests.
Figure 3. CSF indices of insulin resistance.
Lumbar puncture CSF from controls (N=12) and patients with early probable AD (N=16) were used to measure (A) insulin, (B) leptin, (C) ghrelin (D) GLP-1, (E) GIP-1, (F) PP, and (G) PYY by multiplex bead-based ELISA. Immunoreactivity is expressed in fluorescence light units (FLU) normalized to protein content. Data were analyzed with Student T tests.
Trophic factors
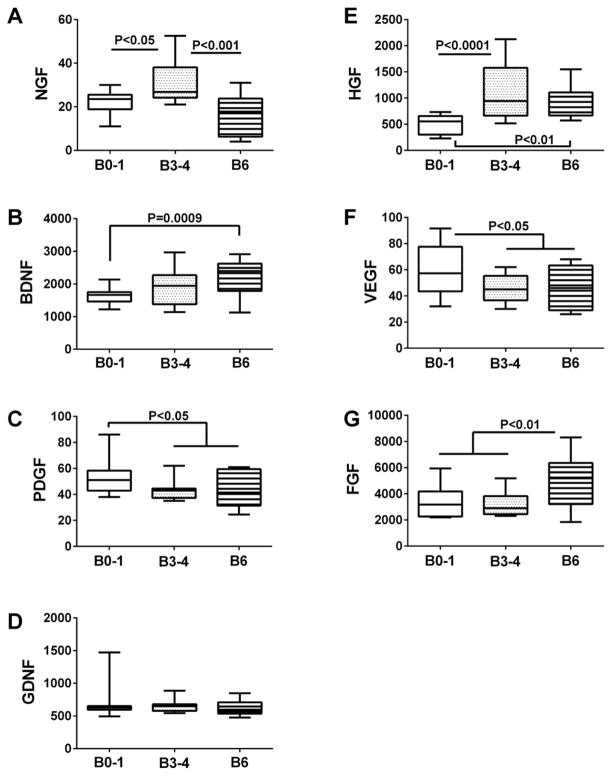
Immunoreactivity to nerve growth factor (NGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (β-FGF) were measured in brain (Figure 4), VF (Table 2), and CSF (Table 3) by multiplex ELISA. In addition, brain derived neurotrophic factor (BDNF) and glial derived neurotrophic factor (GDNF) were measured in brain homogenates by direct binding ELISAs (Figure 4). ANOVA tests demonstrated significant inter-group differences in postmortem brain levels of NGF (F=12.85; P<0.0001), BDNF (F=6.26; P=0.004), PDGF (F=3.624; P=0.035), HGF (F=10.08; P=0.0002), VEGF (F=3.54; P=0.037), and β-FGF (F=6.95; P=0.0023) (Table 2). Post-hoc trend line analysis demonstrated significant AD Braak stage dependent increases in BDNF (P=0.0009), HGF (P=0.0044), and β-FGF (P=0.0028), and decreases in PDGF (P=0.026) and VEGF (P=0.031). Fisher post-hoc inter-group comparisons demonstrated significantly higher NGF levels in Braak 3–4 compared with control and Braak 6 brains (Figure 4A), higher BDNF levels in Braak 6 relative to control (Figure 4B), HGF levels in Braak 3–4 and Braak 6 relative to control (Figure 4E), and β-FGF in Braak 6 relative to both control and Braak 3–4 brains (Figure 4G). In contrast, brain PDGF (Figure 4C) and VEGF (Figure 4F) levels were significantly lower at Braak 3–4 and Braak 6 relative to control. GDNF protein levels did not change in relation to AD Braak stage (Figure 4D).
Figure 4. Trophic factor measurements in postmortem brains.
Frontal lobe aqueous homogenates from subjects with Braak Stage (B) 0–1 (controls), B3–4 (moderate AD), or B6 (late AD) pathology (N=8/group) were used to measure (A) NGF, (B) BDNF, (C) PDGF-AA, (D) glial cell derived neurotrophic factor (GDNF), (E) hepatocyte growth factor (HGF), and (F) basic fibroblast growth factor (FGF) by direct binding ELISA. Immunoreactivity was detected with HRP-conjugated secondary antibody and Amplex Red soluble fluorophor. Fluorescence was measured (Ex 579 nm/Em 595 nm) in a Spectromax M5, and results were normalized to sample protein content. Data were analyzed by one-way repeated measures ANOVA with post hoc Fisher significance tests.
Table 2.
Trophic Factor and Cytokine Levels in Post-mortem Ventricular Fluid.
| Protein | Control | Alzheimer | P- Value |
|---|---|---|---|
| Trophic Factors | |||
| β-NGF | 41.76 ± 4.26 | 48.60 ± 8.14 | |
| PDGF-AA | 111.52 ± 9.94 | 59.73 ± 4.69 | 0.0002 |
| HGF | 1305.97 ± 299.12 | 3887.81 ± 691.31 | 0.0001 |
| VEGF | 195.74 ± 12.52 | 120.72 ± 9.74 | 0.0003 |
| β-FGF | 948.78 ± 81.77 | 546.07 ± 46.79 | 0.0005 |
| Cytokines | |||
| IL-1β | 123.40 ± 44.97 | 34.23 ± 7.75 | 0.028 |
| IL-6 | 316.64 ± 108.93 | 126.60 ± 91.79 | 0.009 |
| IL-8 | 806.30 ± 321.41 | 929.34 ± 185.10 | |
| IL-10 | 18.20 ± 1.19 | 13.95 ± 0.97 | 0.008 |
| Il-16 | 2769.95 ± 244.39 | 3058.46 ± 219.60 | |
| IL-18 | 365.60 ± 48.50 | 672.60 ± 218.45 | |
| TNF-α | 12.95 ± 1.03 | 15.93 ± 2.72 | |
| TRAIL | 61.14 ± 13.65 | 25.67 ± 2.84 | 0.01 |
| LIF-1 | 58.19 ± 3.02 | 51.27 ± 3.06 | |
| SCF | 30.14 ± 3.14 | 28.14 ± 1.29 | |
| MCP-1 (CCL2) | 1793.48 ± 559.87 | 1123.61 ± 496.75 | 0.001 |
| SDF (CXCL12) | 79.25 ± 11.76 | 29.75 ± 2.76 | <0.0001 |
| GM-CSF | 39.05 ± 1.29 | 33.54 ± 2.83 | |
| MIP-1 | 2279.33 ± 791.99 | 1177.78 ± 142.71 | |
| IFN-γ | 6.66 ± 0.37 | 6.93 ± 0.55 | |
| IP-10 | 734.19 ± 479.61 | 398.13 ± 96.21 |
Postmortem ventricular fluid samples from aged controls or patients with documented late-stage AD (N=10/group) were used to measure immunoreactivity to trophic factors by direct binding ELISAs, and cytokines by multiplex bead-based ELISAs. Immunoreactivity was normalized to protein concentration and data are expressed as mean ± SEM of fluorescence light units (arbitrary). Inter-group comparisons were made with the Student T-test. Significant P values are listed in the right column.
Table 3.
CSF Trophic Factor and Cytokine Levels in Probable AD.
| Protein | Control | Alzheimer | P- Value |
|---|---|---|---|
| Trophic Factors | |||
| β-NGF | 5.69 ± 0.77 | 4.68 ± 0.17 | P=0.10 |
| PDGF | 19.88 ±1.27 | 19.19 ± 0.36 | |
| HGF | 98.00 ± 7.09 | 111.5 ± 10.44 | |
| VEGF | 89.00 ± 2.15 | 103.9 ± 2.45 | P<0.0001 |
| β-FGF | 89.81 ± 2.50 | 81.81 ± 2.29 | P=0.025 |
| Cytokines | |||
| IL-1β | 6.25 ± 0.62 | 4.69 ± 0.17 | P=0.018 |
| IL-6 | 21.00 ± 3.05 | 31.19 ± 4.42 | P=0.067 |
| IL-8 | 68.25 ± 8.50 | 77.00 ± 7.70 | |
| IL-10 | 10.81 ± 0.51 | 11.50 ± 0.43 | |
| Il-16 | 14.75 ± 0.71 | 17.44 ± 0.59 | P=0.0035 |
| IL-18 | 11.81 ± 1.98 | 8.94 ± 0.83 | P=0.095 |
| TNF-α | 7.31 ± 0.66 | 6.38 ± 0.26 | 0.098 |
| TRAIL | 14.94 ± 1.05 | 14.13 ± 0.39 | |
| LIF | 36.56 ± 1.82 | 41.56 ± 1.34 | P=0.017 |
| SCF | 57.63 ± 6.13 | 64.0 ± 5.08 | |
| MCP-1 (CCL2) | 2971 ± 80.11 | 3165 ± 118.3 | P=0.093 |
| SDF (CXCL12) | 21.88 ± 2.11 | 22.06 ± 1.51 | |
| GM-CSF | 28.38 ± 1.27 | 29.00 ± 0.71 | |
| MIP-1β | 85.94 ± 10.94 | 98.13 ± 6.90 | |
| IFN-γ | 3.75 ± 0.62 | 3.00 ± 0.05 | |
| IP-10 | 1578 ± 155.9 | 1739 ± 121.0 |
CSF samples from controls (N=12) or patients with clinically diagnosed probable AD (N=16; confirmed by postmortem exam) were used to measure immunoreactivity to trophic factors by direct binding ELISAs, and cytokines by multiplex bead-based ELISAs. Immunoreactivity was normalized to protein concentration and data are expressed as mean ± SEM of fluorescence light units (arbitrary). Inter-group comparisons were made with the Student T-test. Significant P values are listed in the right column.
In postmortem VF samples, we detected significantly lower levels of PDGF (P=0.0002), VEGF (P=0.0003), and β-FGF (P=0.0005), and higher levels of HGF (P=0.0001) in AD (Braak Stage 5–6) relative to control (Table 2). In CSF from patients diagnosed with probable AD (confirmed by postmortem examination), VEGF (P<0.0001) levels were increased while β-FGF (P=0.025) levels were reduced relative to normal aged controls (Table 3). In addition, a trend for reduced NGF (P=0.10) in AD CSF was observed. The slightly higher CSF levels of HGF in the AD group were not statistically significant. Note that the VF and CSF results were concordant with respect to HGF and β-FGF, but discordant with respect to β-NGF, PDGF, and VEGF, indicating that the trends in trophic factor expression/secretion may shift with AD progression.
Neuro-inflammation
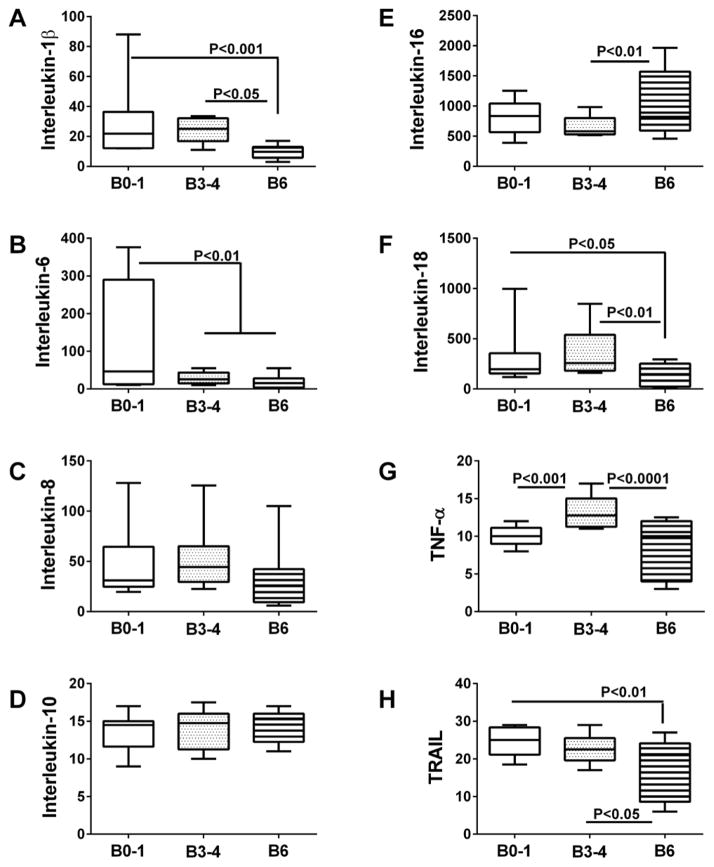
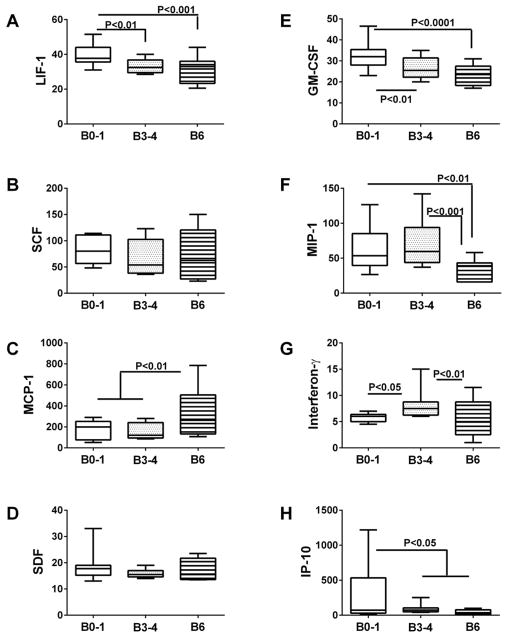
Multiplex bead-based ELISAs were used to measure pro-inflammatory cytokines and chemokines in postmortem brain tissue (Figures 5 and 6), VF (Table 2), and CSF (Table 3). In brain, we observed four distinct trends pertaining to inflammatory mediators such that the levels of immunoreactivity: 1) declined progressively from Braak 0–1 to Braak 6 AD (LIF-1 (F=7.774; P=0.0013), GM-CSF (F=11.05; P=0.0001) and TRAIL (F=6.214, P=0.0041)); 2) were significantly but similarly reduced at Braak Stages 3–4 and 6 relative to control(IL-6 (F=6.17; P=0.0043) and IP10 (F=3.97, P=0.026)), 3) were similar for Braak 0–1 and 3–4, but significantly increased (MCP-1 (F=6.47, P=0.0034) and IL-16 (F=4.132, P=00225)), or decreased (IL-1β (F=6.85; P=0.0025), IL-18 (F=4.56; P=0.0158), and MIP-1(F=7.91. P=0.001)) at Braak Stage 6; and 4) higher at Braak Stage 3–4 compared with Braak Stage 0–1 and/or Braak Stage 6 (TNF-α(F=13.54, P<0.0001) and Interferon-γ (F=4.93, P=0.012)). In contrast, no significant differences were observed with respect to IL-8, IL-10, SCF, or SDF (Figures 5 and 6). Fisher post-hoc tests confirmed that inflammatory mediator levels were significantly suppressed in the late stages (IL-1β, IL-18, TRAIL, MIP-1) or both the intermediate and late stages (IL-6, LIF-1, GM-CSF, IP-10) of AD (Figures 5 and 6). However, selected inflammatory markers were increased either at Braak stage 3–4 (Interferon-γ and TNF-α), or Braak Stage 6 (IL-16 and MCP-1).
Figure 5. Inflammatory mediators in postmortem brains-1.
Frontal lobe aqueous homogenates from subjects with Braak Stage (B) 0–1 (controls), B3–4 (moderate AD), or B6 (late AD) pathology (N=8/group) were used to measure (A) Interleukin-1β, (B) Interleukin-6, (C) Interleukin-8, (D) Interleukin-10, (E) Interleukin-16, (F) Interleukin-18, (G) Tumor necrosis factor-α (TNF-α), and (H) TRAIL by multiplex bead-based ELISA. Immunoreactivity is expressed in fluorescence light units (FLU) normalized to protein content. Data were analyzed by one-way repeated measures ANOVA with post hoc Fisher significance tests.
Figure 6. Inflammatory mediators in postmortem brains-2.
Frontal lobe aqueous homogenates from subjects with Braak Stage (B) 0–1 (controls), B3–4 (moderate AD), or B6 (late AD) pathology (N=8/group) were used to measure (A) LIF-1, (B) scatter factor (SCF), (C) (MCP), (D) SDF, (E) GM-CSF, (F) MIP-1, (G) Interferon-γ, and (H) (IP-10) by multiplex bead-based ELISA. Immunoreactivity is expressed in fluorescence light units (FLU) normalized to protein content. Data were analyzed by one-way repeated measures ANOVA with post hoc Fisher significance tests.
Analysis of postmortem VF samples demonstrated significantly reduced levels of IL-1β, IL-6, IL-10, TRAIL, MCP-1, and SDF in AD relative to control (Table 2). MIP-1, IP-10, GM-CSF, and LIF levels were also reduced in AD, but the differences did not reach statistical significance. Although other cytokines and chemokines were somewhat increased in AD (IL-8, IL-16, IL-18, TNF-α, and Interferon-γ), the differences did not reach statistical significance. Together, these findings suggest that AD is mainly associated with broad-based suppression of inflammatory cytokine responses, and modest increases in selected cytokines. The findings in postmortem VF were largely concordant with results obtained for brain tissue, with notable exceptions including IL-10, IL-18, MCP-1, and SDF, in which the results were contradictory.
In contrast to the findings in brain and VF, cytokine and chemokine levels were mainly increased in AD relative to control CSF (Table 3). Significant differences or trends corresponding to increased inflammation in AD were observed for IL-6, IL-16, LIF, and MCP-1. In addition, CSF levels of IL-8, SCF, MIP-1b, and IP-10 were also increased in AD, although the differences from control did not reach statistical significance. However, as observed in brain and VF, evidence for AD-associated CNS suppression of inflammatory mediators was marked by the significant reductions or trends in reduced levels of IL-1β, IL- 18, and TNF-α. The findings with respect to neuro-inflammation in CSF at the early and intermediate stages of AD were largely discordant with postmortem brain and VF results. On the other hand, the reduced levels of IL-1β and IL-18, and increased levels of IL-16 and MCP-1 in AD CSF did correspond with the postmortem findings in Braak 6 AD brains (Figures 5 and 6), and to some extent (IL-1β and IL-16) postmortem VF. Overall, the most informative and consistent correlate of AD was IL-1β suppression in brain, VF, and CSF. In contrast, neuro-inflammatory indices in CSF (early or moderate AD) seem not to inform about the levels of neuroinflammation in brains with Braak Stage 3–4 AD.
Discussion
In AD, significant impairments in brain insulin signaling begin early in the clinical course and progress with disease severity [7,11,14]. A probable role for brain metabolic dysfunction in the pathogenesis of AD is further supported by the: 1) findings of cognitive impairment and neurodegeneration in experimental models of brain insulin/IGF resistance [6,49–53]; 2) halting or reversal of cognitive deficits by insulin, GLP-1 analog, or insulin sensitizer treatments in humans and experimental animals [18–27]; and 3) effectiveness of lifestyle changes for reducing insulin resistance and preserving cognition [54–57]. Despite these conceptual gains, AD diagnostic panels have not been revised to accommodate the metabolic/insulin resistance hypothesis, and instead remain largely focused on detecting altered levels of AβPP-Aβ and pTau in CSF. Data from neuroimaging and human brain studies strongly suggest that CNS metabolic indices, particularly those related to brain insulin signaling, could help with early detection of AD, monitoring the clinical course, and evaluating responses to treatment. This concept is reinforced by evidence of brain mitochondrial dysfunction which reflects significant perturbations in brain energy metabolism and begins early in the course of AD [43,44,58]. Finally, independent evidence suggests that consequences of, or co-factors in brain metabolic dysfunction, e.g. neuro-inflammation and oxidative stress, should be considered as they likely exacerbate or perpetuate the cascade of neurodegeneration [15,16,43]. The present study attempts to address these concepts by identifying clusters of additional potential biomarker indices that might be incorporated into AD diagnostic panels. The long-range objectives are to enhance sensitivity and specificity of AD detection, particularly in the early and most treatable phases of disease.
Consistent with the well-characterized neuropathology of AD and studies utilizing pTau and AβPP-Aβ CSF-based biomarker assays to diagnose or monitor AD [59–61], we detected increased levels of pTau and AβPP-Aβ in postmortem brains that had intermediate (Braak 3–4) or advanced (Braak 6) stages of AD. In addition, we observed increased levels of lipid peroxidation (4-HNE), advanced glycation end-products, and a trend for increased DNA damage (8-OHdG) with severity of AD. In sporadic AD, which was present in all AD cases in this study, Tau pathology is caused by aberrant activation of kinases that cause hyper-phosphorylation of the protein, leading to the formation of insoluble aggregates that undergo ubiquitination. Fibrillar aggregates of pTau promote oxidative injury and stress, which activate or exacerbate AβPP-Aβ pathology, neuro-inflammation, and cell death cascades [62]. Hyper-phosphorylated tau-associated lesions in AD are recognized as neurofibrillary tangles, dystrophic neurites, and neuropil threads, and their accumulations correlate with clinical severity of dementia. AβPP-Aβ pathology results from aberrant cleavage of AβPP, resulting in AβPP-Aβ fibril accumulation. Fibrillar aggregates of AβPP-Aβ undergo ubiquitination and promote oxidative stress, which can trigger or worsen Tau pathology, as well as promote cellular stress-related injury and inflammation [63]. In addition, soluble, diffusible and toxic AβPP-Aβ oligomers, which accumulate late in the course of AD, may have a role in AD progression due to neurotoxic injury [64] and inhibitory effects on insulin signaling [13].
Activation of glycogen synthase kinase 3β (GSK-3β) which has a pivotal role in promoting Tau hyper-phosphorylation [65], is a major consequence of impaired insulin signaling and insulin resistance [66–70]. Increased GSK-3β activation promotes oxidative stress and DNA damage [71], and oxidative stress is sufficient to increase AβPP-Aβ accumulation and Tau phosphorylation [72]. Likewise, insulin resistance promotes brain accumulations of pTau and AβPP-Aβ [49,52]; AβPP-Aβ toxic fibrils impair insulin signaling by down-regulating insulin receptors [13]. Together, these responses promote oxidative stress, neuro-inflammation, neurotoxicity, and synaptic dysfunction through a positive feedback loop that exacerbates insulin/IGF resistance [13,15]. Given this scenario, it is likely that multi-pronged biomarkers that detect different components of the neurodegeneration cascade will provide a more informative and sensitive diagnostic aid for detecting and monitoring AD at different stages of disease. Moreover, this strategy holds promise for early detection of AD, when the disease is most likely to respond to treatment. Lastly, simple, cost-effective biochemical and molecular tools are needed to objectively monitor therapeutic responses, as well as help to streamline postmortem diagnoses of AD.
Herein, we examined three clusters of potential biomarker for detecting AD neurodegeneration: insulin resistance, trophic factors, and inflammatory indices. The goals were to assess: 1) trends in AD-associated abnormalities in insulin resistance-related proteins; 2) AD-associated abnormalities in trophic factors that support different functions in the brain, including neuronal plasticity; and 3) patterns of neuro-inflammation in brain versus VF and CSF.
The results obtained with the multiplex gut hormone panel were reassuring with regard to the roles of insulin resistance and metabolic dysfunction in AD because they reported AD Braak stage declines in insulin and GLP-1, and increases in leptin. The significant reductions in GLP-1 correspond with the reduced insulin levels in brain and CSF. The trends with regard to GIP-1 and PYY were novel and suggest further studies should be done to characterize the full spectrum of AD-associated abnormalities in gut-pancreatic type polypeptides that occur over the course of disease. It is particularly noteworthy that the reductions in GLP-1 and GIP-1 could exacerbate the deficiencies in brain insulin levels and worsen impairments in brain insulin signaling, since both GLP-1 and GIP-1 are incretins with insulinotropic functions, and they are important regulators of glucose metabolism [73] that could be used therapeutically to treat cognitive impairment and neurodegeneration in AD [18,74]. Reduced levels of these polypeptides in AD correlate with decreased levels of insulin, thereby supporting their use in diagnostic panels as well as targeted therapy for AD. In addition, since many of the insulin resistance-related abnormalites in AD also occur in metabolic syndrome which contributes to cognitive decline [75], both CNS and systemic factors mediating brain metabolic dysfunction and insulin resistance could serve as therapeutic targets in AD [75–77].
Increased levels of leptin [78] and reduced levels of PYY [79] are features of obesity with peripheral insulin resistance [80]. The presence of similar abnormalities in AD brains suggests that leptin and PYY levels could also serve as indices of brain insulin resistance. Finally, ghrelin, a ligand for growth hormone secretagogue receptor, is downregulated in aging [81] and morbid obesity, which are insulin resistance states [82]. Therefore, reduced levels of ghrelin in AD correspond with brain aging and insulin resistance. The constellation of insulin, GLP-1, and GIP-1 deficiencies, together with alterations in other polypeptides that report brain insulin resistance is consistent with the hypothesis that AD represents Type 3 diabetes with combined features of insulin deficiency and resistance in the brain [12,14]. The consequences of these metabolic derangements were reflected by the increased levels of AGE, HNE, and 8-OHdG in postmortem AD brains.
The findings with respect to trophic factors were interesting because most of the trends showed increased expression levels in relation to AD severity. Exceptions were PDGF and VEGF, which declined, and GDNF, which was unchanged. Increased levels of NGF and BDNF in AD could reflect effects of receptor resistance, particularly given the impairments in neuronal plasticity and the roles these neurotrophins play in synaptic remodeling [83,84]. Despite its name, HGF is expressed in the brain, particularly the hippocampus and may have neurotrophic properties [85]. Its prominent localization in the CA3–CA4 regions of the hippocampus [85] where neurogenesis occurs [86], further suggests that HGF plays a key role in maintaining neuronal populations as well as mediating synaptic plasticity. The increased levels of HGF in AD brains corresponds with previous observations [87], and the somewhat higher levels in AD VF and CSF could also reflect HGF receptor resistance given the progressive impairments in neuronal plasticity that occur with AD progression. The findings with regard to β-FGF are entirely consistent with earlier observations in human postmortem brains [88]. Previous studies correlated increased β-FGF expression in AD with increased gliosis [88], which characteristically marks several aspects of neurodegeneration, including loss of neurons and fibers.
VEGF is expressed in microglia and endothelial cells. Alterations in VEGF expression occur in cerebral microvascular disease and in AD. In addition to its role in angiogenesis, VEGF has neuroprotective actions that may have relevance for treatment of AD and other neurodegenerative diseases [89]. In this regard, low VEGF levels have been shown to mediate neurodegeneration, which could be due to hypoperfusion or reduced neuronal protection from oxidative stress [89]. Therefore, reduced levels of VEGF in AD brains and VF could mark the presence and/or severity of neurodegeneration mediated by brain hypo-perfusion and neuronal death. This concept opens the door to additional treatment modalities for AD, as well as investigating whether the VEGF responses in AD are primary or secondary. For example, insulin and IGF-1 regulate expression of VEGF [90], and impairments in brain insulin and IGF-1 levels begin early in the course of AD [11].
Platelet-derived growth factor (PDGF) mediates β-γ secretase mediated cleavage of AβPP [91]. In addition, PDGF-BB, which is only expressed in neurons, is abundant in neurofibrillary tangles and associated with synaptic loss and dystrophic sprouting, whereas PDGF-AA is vascular associated [92] and mediates oligodendrocyte development. PDGF-AA, which was measured in the gut hormone panel, has an important role in myelin maintenance [93]. PDGF-A receptor is regulated by β-FGF [93], and PDGF regulates oligodendrocyte progenitor cells functions, including myelination [94,95]. Therefore, the reduced levels of PDGF in AD correspond with the previously demonstrated early loss of white matter and hypomyelination in this disease [96,97]. The fact that PDGF expression was reduced in AD brain and VF samples but not in the clinical CSF samples suggests that these abnormalities may be detectable in CSF only in the later stages of disease.
Neuro-inflammation remains a focus of research in AD because it occurs early in the course of disease [98], and already has been addressed in several clinical trials [99,100]. The failure to obtain conclusive evidence that anti-inflammatory measures are neuroprotective and can halt neurodegeneration most likely reflects the complexity and non-static nature of the problem. For example, inflammation may mediate disease at selected stages rather than throughout its clinical course. Multiplex ELISAs are an efficient way to simultaneously assess arrays of pro-inflammatory mediators in human subject material. A major unexpected finding was the broad-based suppression rather than activation of pro-inflammatory mediators in AD brains and postmortem ventricular fluid. In brain tissue, only IL-16, TNF-α, MCP-1, and Interferon-γ were elevated at either Braak Stage 3–4 or 6. For the other 12 cytokines/chemokines measured, 8 were expressed at significantly lower levels in brains with Braak Stage 6 or both Braak 3–4 and 6 AD relative to control. Similarly, in VF samples, only 5 of the 16 cytokines measured were elevated in AD but none of those differences were statistically significant. In contrast, in CSF, 4 cytokines were significantly elevated in AD, and 11 were moderately although not significantly elevated relative to control. However, since TNF-α and Interferon-γ expression were elevated in brains with Braak Stage 3–4 but not Braak 6 AD, and higher percentages of the inflammatory mediators were up-regulated in CSF as compared with brain or VF, conceivably the activation of neuro-inflammation occurs early in the course of AD, but as disease progresses, neuroinflammation subsides or is suppressed. The mechanisms and consequences of these responses are not known. However, the findings suggest that as a tool for evaluating AD diagnosis, severity, and responses to treatment, pro-inflammatory cytokines do not represent viable targets. On the other hand, the higher levels and profiles of inflammatory mediators in the clinical CSF samples from patients with probable AD suggest that anti-inflammatory therapeutic approaches may have value in the early stages of disease.
In conclusion, this study demonstrates the utility of evaluating indices of insulin resistance, neuronal plasticity, glial function, and oxidative stress in conjunction with pTau and AβPP-Aβ in CSF-based multiplex assays. This multi-pronged approach to assess different aspects of the neurodegeneration cascade will likely be more informative with respect to using streamlined biochemical and molecular assays for clinical as well as postmortem diagnoses, monitoring the clinical course of AD, and evaluating responses to treatment. This study suggests that the use of neuro-inflammatory markers will likely not be beneficial due to the transient nature of their activation in relation to disease severity. Future studies should assess the time course of shifts in biomarker indices in relation to cognitive decline and structural and functional neuroimaging abnormalities.
Acknowledgments
Supported by AA-11431, AA-12908, and AA-16126 from the National Institutes of Health.
References
- 1.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, et al. Insulin Resistance and Alzheimer-like Reductions in Regional Cerebral Glucose Metabolism for Cognitively Normal Adults With Prediabetes or Early Type 2 Diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 3.Krikorian R, Eliassen JC, Boespflug EL, Nash TA, Shidler MD. Improved cognitive-cerebral function in older adults with chromium supplementation. Nutr Neurosci. 2010;13:116–122. doi: 10.1179/147683010X12611460764084. [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20:723–736. doi: 10.3233/JAD-2010-091687. [DOI] [PubMed] [Google Scholar]
- 5.Neumann KF, Rojo L, Navarrete LP, Farías G, Reyes P, et al. Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Curr Alzheimer Res. 2008;5:438–447. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- 6.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson GS, Craft S. Insulin resistance, inflammation, and cognition in Alzheimer’s Disease: lessons for multiple sclerosis. J Neurol Sci. 2006;245:21–33. doi: 10.1016/j.jns.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer S. The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: an update. J Neural Transm. 2002;109:341–360. doi: 10.1007/s007020200028. [DOI] [PubMed] [Google Scholar]
- 10.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 12.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 13.de la Monte SM. Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease. Drugs. 2012;72:49–66. doi: 10.2165/11597760-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10:1049–1060. [PMC free article] [PubMed] [Google Scholar]
- 15.de la Monte SM. Triangulated mal-signaling in Alzheimer’s disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis. 2012;30(Suppl 2):S231–249. doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Monte SM, Re E, Longato L, Tong M. Dysfunctional pro-ceramide, ER stress, and insulin/IGF signaling networks with progression of Alzheimer’s disease. J Alzheimers Dis. 2012;30(Suppl 2):S217–229. doi: 10.3233/JAD-2012-111728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3:1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- 18.Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- 19.Biswas SC, Buteau J, Greene LA. Glucagon-like peptide-1 (GLP-1) diminishes neuronal degeneration and death caused by NGF deprivation by suppressing Bim induction. Neurochem Res. 2008;33:1845–1851. doi: 10.1007/s11064-008-9646-4. [DOI] [PubMed] [Google Scholar]
- 20.Harkavyi A, Whitton PS. Glucagon-like peptide 1 receptor stimulation as a means of neuroprotection. Br J Pharmacol. 2010;159:495–501. doi: 10.1111/j.1476-5381.2009.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClean PL, Gault VA, Harriott P, Hölscher C. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: a link between diabetes and Alzheimer’s disease. Eur J Pharmacol. 2010;630:158–162. doi: 10.1016/j.ejphar.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Reger MA, Watson GS, Frey WH, 2nd, Baker LD, Cholerton B, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007;86:136–142. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- 24.Djupesland PG. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;71:864. doi: 10.1212/01.wnl.0000327291.47162.ed. [DOI] [PubMed] [Google Scholar]
- 25.Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, et al. Intranasal Insulin Therapy for Alzheimer Disease and Amnestic Mild Cognitive Impairment: A Pilot Clinical Trial. Arch Neurol. 2011;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landreth G. Therapeutic use of agonists of the nuclear receptor PPARgamma in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:159–164. doi: 10.2174/156720507780362092. [DOI] [PubMed] [Google Scholar]
- 28.Kahle PJ, Jakowec M, Teipel SJ, Hampel H, Petzinger GM, et al. Combined assessment of tau and neuronal thread protein in Alzheimer’s disease CSF. Neurology. 2000;54:1498–1504. doi: 10.1212/wnl.54.7.1498. [DOI] [PubMed] [Google Scholar]
- 29.De La Monte SM, Wands JR. The AD7c-NTP neuronal thread protein biomarker for detecting Alzheimer’s disease. J Alzheimers Dis. 2001;3:345–353. doi: 10.3233/jad-2001-3310. [DOI] [PubMed] [Google Scholar]
- 30.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haense C, Buerger K, Kalbe E, Drzezga A, Teipel SJ, et al. CSF total and phosphorylated tau protein, regional glucose metabolism and dementia severity in Alzheimer’s disease. Eur J Neurol. 2008;15:1155–1162. doi: 10.1111/j.1468-1331.2008.02274.x. [DOI] [PubMed] [Google Scholar]
- 32.Pauwels EK, Volterrani D, Mariani G. Biomarkers for Alzheimer’s disease. Drug News Perspect. 2009;22:151–160. doi: 10.1358/dnp.2009.22.3.1354128. [DOI] [PubMed] [Google Scholar]
- 33.Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, et al. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–637. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, He J, Hong T. Biomarkers of Alzheimer’s disease in body fluids. Sci China Life Sci. 2010;53:490–496. doi: 10.1007/s11427-010-0081-9. [DOI] [PubMed] [Google Scholar]
- 35.Bloudek LM, Spackman DE, Blankenburg M, Sullivan SD. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis. 2011;26:627–645. doi: 10.3233/JAD-2011-110458. [DOI] [PubMed] [Google Scholar]
- 36.Cummings JL. Biomarkers in Alzheimer’s disease drug development. Alzheimers Dement. 2011;7:e13–44. doi: 10.1016/j.jalz.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Rosenmann H. CSF biomarkers for amyloid and tau pathology in Alzheimer’s disease. J Mol Neurosci. 2012;47:1–14. doi: 10.1007/s12031-011-9665-5. [DOI] [PubMed] [Google Scholar]
- 38.Zetterberg H, Blennow K. Cerebrospinal fluid biomarkers for Alzheimer’s disease: more to come? J Alzheimers Dis. 2013;33(Suppl 1):S361–369. doi: 10.3233/JAD-2012-129035. [DOI] [PubMed] [Google Scholar]
- 39.Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 40.Jack CR., Jr Alliance for aging research AD biomarkers work group: structural MRI. Neurobiol Aging. 2011;32(Suppl 1):S48–57. doi: 10.1016/j.neurobiolaging.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Skoumalová A, Hort J. Blood markers of oxidative stress in Alzheimer’s disease. J Cell Mol Med. 2012;16:2291–2300. doi: 10.1111/j.1582-4934.2012.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattsson N, Blennow K, Zetterberg H. CSF biomarkers: pinpointing Alzheimer pathogenesis. Ann N Y Acad Sci. 2009;1180:28–35. doi: 10.1111/j.1749-6632.2009.04944.x. [DOI] [PubMed] [Google Scholar]
- 44.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimers Dis. 2006;9:167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 45.Cummings JL. Definitions and diagnostic criteria. Informa UK Limited; London: 2007. [Google Scholar]
- 46.Tong M, Dong M, de la Monte SM. Brain insulin-like growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. J Alzheimers Dis. 2009;16:585–599. doi: 10.3233/JAD-2009-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, et al. A multimodal RAGE-specific inhibitor reduces amyloid Î2-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahmadi A, Steiner N, Münch G. Advanced glycation endproducts as gerontotoxins and biomarkers for carbonyl-based degenerative processes in Alzheimer’s disease. Clin Chem Lab Med. 2011;49:385–391. doi: 10.1515/CCLM.2011.079. [DOI] [PubMed] [Google Scholar]
- 49.Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebro ventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- 50.Hoyer S, Müller D, Plaschke K. Desensitization of brain insulin receptor. Effect on glucose/energy and related metabolism. J Neural Transm Suppl. 1994;44:259–268. doi: 10.1007/978-3-7091-9350-1_20. [DOI] [PubMed] [Google Scholar]
- 51.Labak M, Foniok T, Kirk D, Rushforth D, Tomanek B, et al. Metabolic changes in rat brain following intracerebro ventricular injections of streptozotocin: a model of sporadic Alzheimer’s disease. Acta Neurochir Suppl. 2010;106:177–181. doi: 10.1007/978-3-211-98811-4_32. [DOI] [PubMed] [Google Scholar]
- 52.Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, et al. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 53.de la Monte SM, Tong M, Bowling N, Moskal P. si-RNA inhibition of brain insulin or insulin-like growth factor receptors causes developmental cerebellar abnormalities: relevance to fetal alcohol spectrum disorder. Mol Brain. 2011;4:13. doi: 10.1186/1756-6606-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kidd PM. Alzheimer’s disease, amnestic mild cognitive impairment, and age-associated memory impairment: current understanding and progress toward integrative prevention. Altern Med Rev. 2008;13:85–115. [PubMed] [Google Scholar]
- 55.Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, et al. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743–752. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGough EL, Kelly VE, Logsdon RG, McCurry SM, Cochrane BB, et al. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “up & go” test. Phys Ther. 2011;91:1198–1207. doi: 10.2522/ptj.20100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isobe C, Abe T, Terayama Y. Increase in the oxidized/total coenzyme Q-10 ratio in the cerebrospinal fluid of Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2009;28:449–454. doi: 10.1159/000256209. [DOI] [PubMed] [Google Scholar]
- 59.Kang JH, Vanderstichele H, Trojanowski JQ, Shaw LM. Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer’s disease. Methods. 2012;56:484–493. doi: 10.1016/j.ymeth.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Blennow K, Zetterberg H, Fagan AM. Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006221. doi: 10.1101/cshperspect.a006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drago V, Babiloni C, Bartrés-Faz D, Caroli A, Bosch B, et al. Disease tracking markers for Alzheimer’s disease at the prodromal (MCI) stage. J Alzheimers Dis. 2011;26(Suppl 3):159–199. doi: 10.3233/JAD-2011-0043. [DOI] [PubMed] [Google Scholar]
- 62.Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Di Carlo M. Beta amyloid peptide: from different aggregation forms to the activation of different biochemical pathways. Eur Biophys J. 2010;39:877–888. doi: 10.1007/s00249-009-0439-8. [DOI] [PubMed] [Google Scholar]
- 64.Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, et al. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Bhat R, Xue Y, Berg S, Hellberg S, Ormö M, et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem. 2003;278:45937–45945. doi: 10.1074/jbc.M306268200. [DOI] [PubMed] [Google Scholar]
- 66.De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer’s disease. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 67.Fraser PE, Yu G, Lévesque L, Nishimura M, Yang DS, et al. Presenilin function: connections to Alzheimer’s disease and signal transduction. Biochem Soc Symp. 2001:89–100. doi: 10.1042/bss0670089. [DOI] [PubMed] [Google Scholar]
- 68.Grilli M, Ferrari Toninelli G, Uberti D, Spano P, Memo M. Alzheimer’s disease linking neurodegeneration with neurodevelopment. Funct Neurol. 2003;18:145–148. [PubMed] [Google Scholar]
- 69.Mudher A, Chapman S, Richardson J, Asuni A, Gibb G, et al. Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase. J Neurosci. 2001;21:4987–4995. doi: 10.1523/JNEUROSCI.21-14-04987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimura M, Yu G, Levesque G, Zhang DM, Ruel L, et al. Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of beta-catenin, a component of the presenilin protein complex. Nat Med. 1999;5:164–169. doi: 10.1038/5526. [DOI] [PubMed] [Google Scholar]
- 71.de la Monte SM. Therapeutic targets of brain insulin resistance in sporadic Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:1582–1605. doi: 10.2741/482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen GJ, Xu J, Lahousse SA, Caggiano NL, de la Monte SM. Transient hypoxia causes Alzheimer-type molecular and biochemical abnormalities in cortical neurons: potential strategies for neuroprotection. J Alzheimers Dis. 2003;5:209–228. doi: 10.3233/jad-2003-5305. [DOI] [PubMed] [Google Scholar]
- 73.Larsen PJ, Holst JJ. Glucagon-related peptide 1 (GLP-1): hormone and neurotransmitter. Regul Pept. 2005;128:97–107. doi: 10.1016/j.regpep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 74.Holscher C. Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer’s disease. Recent Pat CNS Drug Discov. 2010;5:109–117. doi: 10.2174/157488910791213130. [DOI] [PubMed] [Google Scholar]
- 75.Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, et al. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev. 2010;9:399–417. doi: 10.1016/j.arr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Cai H, Cong WN, Ji S, Rothman S, Maudsley S, et al. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr Alzheimer Res. 2012;9:5–17. doi: 10.2174/156720512799015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease--the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull. 2012;89:144–149. doi: 10.1016/j.brainresbull.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 79.Small CJ, Bloom SR. Gut hormones and the control of appetite. Trends Endocrinol Metab. 2004;15:259–263. doi: 10.1016/j.tem.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Chessler SD, Fujimoto WY, Shofer JB, Boyko EJ, Weigle DS. Increased plasma leptin levels are associated with fat accumulation in Japanese Americans. Diabetes. 1998;47:239–243. doi: 10.2337/diab.47.2.239. [DOI] [PubMed] [Google Scholar]
- 81.DiStefano PS, Curtis R, Geddes BJ. Insulin resistance, glycemic control and adiposity: key determinants of healthy lifespan. Curr Alzheimer Res. 2007;4:153–157. doi: 10.2174/156720507780362038. [DOI] [PubMed] [Google Scholar]
- 82.Rigamonti AE, Pincelli AI, Corrà B, Viarengo R, Bonomo SM, et al. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002;175:R1–5. doi: 10.1677/joe.0.175r001. [DOI] [PubMed] [Google Scholar]
- 83.Cuello AC, Bruno MA, Bell KF. NGF-cholinergic dependency in brain aging, MCI and Alzheimer’s disease. Curr Alzheimer Res. 2007;4:351–358. doi: 10.2174/156720507781788774. [DOI] [PubMed] [Google Scholar]
- 84.Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Achim CL, Katyal S, Wiley CA, Shiratori M, Wang G, et al. Expression of HGF and cMet in the developing and adult brain. Brain Res Dev Brain Res. 1997;102:299–303. doi: 10.1016/s0165-3806(97)00108-9. [DOI] [PubMed] [Google Scholar]
- 86.Sawada M, Sawamoto K. Mechanisms of neurogenesis in the normal and injured adult brain. Keio J Med. 2013;62:13–28. doi: 10.2302/kjm.2012-0005-re. [DOI] [PubMed] [Google Scholar]
- 87.Tsuboi Y, Kakimoto K, Nakajima M, Akatsu H, Yamamoto T, et al. Increased hepatocyte growth factor level in cerebrospinal fluid in Alzheimer’s disease. Acta Neurol Scand. 2003;107:81–86. doi: 10.1034/j.1600-0404.2003.02089.x. [DOI] [PubMed] [Google Scholar]
- 88.Stopa EG, Gonzalez AM, Chorsky R, Corona RJ, Alvarez J, et al. Basic fibroblast growth factor in Alzheimer’s disease. Biochem Biophys Res Commun. 1990;171:690–696. doi: 10.1016/0006-291x(90)91201-3. [DOI] [PubMed] [Google Scholar]
- 89.Storkebaum E, Carmeliet P. VEGF: a critical player in neurodegeneration. J Clin Invest. 2004;113:14–18. doi: 10.1172/JCI200420682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu M, Amano S, Miyamoto K, Garland R, Keough K, et al. Insulin-induced vascular endothelial growth factor expression in retina. Invest Ophthalmol Vis Sci. 1999;40:3281–3286. [PubMed] [Google Scholar]
- 91.Gianni D, Zambrano N, Bimonte M, Minopoli G, Mercken L, et al. Platelet-derived growth factor induces the beta-gamma-secretase-mediated cleavage of Alzheimer’s amyloid precursor protein through a Src-Rac-dependent pathway. J Biol Chem. 2003;278:9290–9297. doi: 10.1074/jbc.m211899200. [DOI] [PubMed] [Google Scholar]
- 92.Masliah E, Mallory M, Alford M, Deteresa R, Saitoh T. PDGF is associated with neuronal and glial alterations of Alzheimer’s disease. Neurobiol Aging. 1995;16:549–556. doi: 10.1016/0197-4580(95)00050-o. [DOI] [PubMed] [Google Scholar]
- 93.Watzlawik JO, Warrington AE, Rodriguez M. PDGF is required for remyelination-promoting IgM stimulation of oligodendrocyte progenitor cell proliferation. PLoS One. 2013;8:e55149. doi: 10.1371/journal.pone.0055149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Butt AM, Hornby MF, Kirvell S, Berry M. Platelet-derived growth factor delays oligodendrocyte differentiation and axonal myelination in vivo in the anterior medullary velum of the developing rat. J Neurosci Res. 1997;48:588–596. [PubMed] [Google Scholar]
- 95.Butt AM, Hornby MF, Ibrahim M, Kirvell S, Graham A, et al. PDGF-alpha receptor and myelin basic protein mRNAs are not coexpressed by oligodendrocytes in vivo: a double in situ hybridization study in the anterior medullary velum of the neonatal rat. Mol Cell Neurosci. 1997;8:311–322. doi: 10.1006/mcne.1996.0590. [DOI] [PubMed] [Google Scholar]
- 96.de la Monte SM. Quantitation of cerebral atrophy in preclinical and end-stage Alzheimer’s disease. Ann Neurol. 1989;25:450–459. doi: 10.1002/ana.410250506. [DOI] [PubMed] [Google Scholar]
- 97.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 98.Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, et al. Neuroinflammation - an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener Dis. 2010;7:38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 99.Blasko I, Jungwirth S, Jellinger K, Kemmler G, Krampla W, et al. Effects of medications on plasma amyloid beta (Abeta) 42: longitudinal data from the VITA cohort. J Psychiatr Res. 2008;42:946–955. doi: 10.1016/j.jpsychires.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: the epidemiological evidence. CNS Neurol Disord Drug Targets. 2010;9:132–139. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]