Abstract
Most functional neuroimaging studies of panic disorder (PD) have focused on the resting state, and have explored PD in relation to healthy controls rather than in relation to other anxiety disorders. Here, PD patients, Posttraumatic stress disorder (PTSD) patients, and healthy control subjects were studied with functional magnetic resonance imaging utilizing an instructed fear conditioning paradigm incorporating both Threat and Safe conditions. Relative to PTSD and control subjects, PD patients demonstrated significantly less activation to the Threat condition and increased activity to the Safe condition in the subgenual cingulate, ventral striatum and extended amygdala, as well as in midbrain periaquaeductal grey, suggesting abnormal reactivity in this key region for fear expression. PTSD subjects failed to show the temporal pattern of activity decrease found in control subjects.
Keywords: anxiety disorders, panic disorder, posttraumatic stress disorder, subgenual cingulate cortex, ventral striatum, extended amygdala, brainstem, neuroimaging
1. Introduction
Panic disorder (PD) and posttraumatic stress disorder (PTSD) are anxiety disorders with evolving neurocircuitry models. Biological studies of anxiety disorders have focused on comparisons between patient groups and healthy controls, with only one neuroimaging study to date directly comparing PD and PTSD (Lucey et al., 1997). This resting state (single photon emission computed tomography, SPECT) study found significant cerebral blood flow (CBF) differences in obsessive compulsive disorder (OCD) and PTSD compared with PD and controls in bilateral superior frontal cortices and right caudate nuclei. However, to develop disorder-specific behavioral and pharmacological treatment approaches, knowledge of the differences in the underlying dysfunctional neurocircuitries in these disorders is required. Neurobehavioral and neurocircuitry models of PTSD suggest amygdalar hyperactivity and ventromedial prefrontal hypoactivity to external threat (Milad, Rauch, Pitman, & Quirk, 2006) whereas panic disorder appears to be marked by internally generated threat (Lissek et al., 2009) driven by dysfunctional ventromedial prefrontal (ACC), amygdalar and brainstem regions (Graeff & Del-Ben, 2008). Core components of panic disorder include autonomic signs like increased respiration, heart rate, and blood pressure which are modulated by key regions in the basal forebrain and the brainstem. The medial frontal cortical network (including Brodmann area 25) provides a major output to the hypothalamus and brain stem and contributes to this visceromotor system (Price, 1999). The ventral striatum, known for its central role in reward processing is implicated in coding emotional intensity and self-relatedness of a variety of stimuli, independent of their valence (Phan et al., 2004). The bed nucleus of the stria terminalis/extended amygdala may regulate fear perception and mediate anxiety (Davis & Shi, 1999).
Several studies have explored systemic pathophysiologic differences between PD and PTSD. PTSD and PD patients may have distinct profiles with respect to cortisol levels and HPA responsivity (Marshall et al., 2002); carbon dioxide sensitivity (Talesnik, Berzak, Ben-Zion, Kaplan, & Benjamin, 2007); polysomnography (Sheikh, Woodward, & Leskin, 2003); heart rate variability (Cohen et al., 2000), genetic contributions (Skre, Onstad, Torgersen, Lygren, & Kringlen, 1993); and acquisition of conditioned fear-potentiated startle to learned safety and danger cues (Lissek, et al., 2009). A study utilizing eyeblink electromyography, heart rate, and skin conductance responses (SCR) before and during treatment with alprazolam in PD and PTSD found a decrease in response probability and a decrease in the SCR in PD, but not in PTSD (Shalev, Bloch, Peri, & Bonne, 1998). Since both diseases share key symptoms (e.g. panic attacks) and both are thought to be elicited by abnormal fear conditioning/fear learning (Gorman, Kent, Sullivan, & Coplan, 2000; Phelps & LeDoux, 2005) direct experimental comparison can help to differentiate the neurobiological underpinnings of both diseases and give direction to specific therapeutic targets.
In this study, we used functional magnetic resonance imaging (fMRI) to compare neural responses in PD patients relative to PTSD patients and healthy comparison subjects during an instructed fear paradigm consisting of a Threat and a Safe condition (Butler et al., 2007; Phelps et al., 2001). In this task, the association of a previously neutral stimulus with a possible aversive event is learned by means of a verbal instruction given before the start of the scan. Symbolically acquired fear results in physiological fear responses and functional neuroimaging data comparable to the responses to a conditioned stimulus and its extinction in classical fear conditioning (Butler, et al., 2007; Phelps, et al., 2001). Based on Butler et al. we hypothesized that in healthy comparison subjects brain regions involved in fear processing, i.e. medial prefrontal, insula, ventral striatal, amygdalar and brainstem, would exhibit increased activity for Threat vs. Safe conditions and would habituate over time in a subset of those regions (Butler, et al., 2007). For PTSD subjects, we hypothesized that these same regions would not exhibit habituation from early to late runs of the experimental paradigm. For PD subjects, we hypothesized that these same regions would exhibit abnormal reactivity to the (external) Threat and Safe conditions.
2. Method
2.1. Participants
Participants were 8 subjects meeting DSM-IV criteria for PD (mean age=37 years, range=24–50); 8 subjects meeting DSM-IV criteria for PTSD (mean age=42 years, range=37–50); and 8 healthy comparison subjects (mean age=35 years, range=24–49). PTSD and comparison subjects were a matched subset of larger groups. Each group consisted of 4 female and 4 male right-handed subjects. Among the patients, there were current secondary diagnoses of generalized anxiety disorder, social phobia, specific phobia, major depressive disorder, dysthymia, and personality disorders (Table 1). Otherwise, all participants were free of other psychiatric diagnoses, substance abuse, and significant neurological or medical disorders. No subjects were on psychiatric medication, except one PD subject (sertraline, bupropion). Written informed consent was obtained from the participants in accordance with an IRB-approved protocol.
Table 1.
Subject Characteristics
| Primary | Age | Gender | Secondary Diagnosis |
|---|---|---|---|
| Diagnosis | |||
| None | 24 | Male | None |
| None | 28 | Male | None |
| None | 42 | Female | None |
| None | 33 | Male | None |
| None | 34 | Male | None |
| None | 31 | Female | None |
| None | 40 | Female | None |
| None | 49 | Female | None |
| Panic Disorder | 35 | Male | Generalized anxiety disorder (GAD), past Major depressive disorder (MDD) |
| Panic Disorder | 39 | Male | Social phobia, agoraphobia |
| Panic Disorder | 50 | Female | GAD, MDD |
| Panic Disorder | 24 | Male | None |
| Panic Disorder | 34 | Female | Past PTSD |
| Panic Disorder | 49 | Female | GAD, specific phobia, personality disorders (avoidant, obsessive compulsive, and paranoid) |
| Panic Disorder | 28 | Male | None |
| Panic Disorder | 36 | Female | None |
| PTSD | 45 | Male | Mild MDD, GAD (subthreshold) |
| PTSD | 37 | Female | Social phobia, specific phobia, OCD, dysthymia |
| PTSD | 36 | Female | None |
| PTSD | 38 | Male | None |
| PTSD | 41 | Male | Binge eating disorder |
| PTSD | 47 | Female | Past EtOH dependence, past substance induced mania |
| PTSD | 50 | Female | Past MDD, past EtOH and substance dependence |
| PTSD | 39 | Male | GAD, MDD |
2.2. Experimental Paradigm
Prior to scanning, subjects determined the level of electrodermal stimulation to be received during the scan via a standardized dial-up procedure to a level of intensity experienced as “uncomfortable but not painful” to standardize subjective stimulus aversiveness across subjects. The scanning session consisted of a “Threat” condition, about which participants were told “an electrodermal stimulation can occur at any time”, and a “Safe” condition during which participants were told they would receive no stimulations. Threat and Safe were signified by the presentation of easily-distinguishable colored squares via an MR-compatible screen. Presentation of stimuli was controlled by the Integrated Functional Imaging System (Invivo, Orlando, FL) using E-prime software (Psychology Software Tools, Pittsburgh, PA). Pairing of colors with conditions was counterbalanced across participants. Each color appeared for a period of 12s followed by a 18s rest period. There were five pseudo-randomly ordered blocks of each color per scanning run, and two scanning runs (first run = early run; second run = late run) per study session. Participants did not receive any electrodermal stimulation during scanning.
2.3. Image Acquisition
Gradient echo echo-planar functional images (TR=1200; TE=30; flip angle=70°; FOV=240mm; fifteen 5mm slices; 1mm interslice gap; matrix=64×64) sensitive to blood oxygen level-dependent (BOLD) signal were obtained with a GE-Sigma 3T MRI scanner. Images were acquired using a modified z-shimming algorithm to minimize susceptibility artifact at the base of the brain (Gu et al., 2002). An identically sliced reference T1 weighted anatomical image was acquired to aid reorientation and co-registration. A high-resolution T1 weighted anatomical image was acquired using a spoiled gradient recalled acquisition sequence (TR/TE=30/8msec, flip angle=45, FOV=240mm, 100 1.5mm axial slices; matrix=256×256).
2.4. Image Processing and Data Analysis
Modified SPM software (Wellcome Department of Imaging Neuroscience) was used for processing the data, which included manual AC-PC re-orientation of all anatomical and EPI images; realignment of EPI images to correct for slight head movement between scans and for differential spin excitation history based on intracranial voxels; extraction of physiological fluctuations such as cardiac and respiratory cycles from the EPI image sequence (Frank, Buxton, & Wong, 2001); co-registration of functional EPI images to the corresponding high-resolution anatomical image based on the rigid body transformation parameters of the reference anatomical image to the latter for each individual subject; stereotactic normalization to a standardized coordinate space (Montreal MRI Atlas version of Talairach space) based on the high-resolution anatomical image; spatial smoothing with an isotropic Gaussian kernel (FWHM=7.5mm).
Using customized fmristat software (Worsley et al., 2002), a two-stage voxel-wise linear mixed-effects model was utilized to examine the key Group/Condition contrasts of interest. First, a whole-brain voxel-wise multiple linear regression model was employed at the individual subject level which comprised the regressor of interest, the covariates of no interest (the first-order temporal derivative of the regressor of interest, global and physiological fluctuations, realignment parameters, scanning period means, and baseline drift up to the third order polynomials) and an AR(1) model of the residual time series to accommodate temporal correlation in consecutive scans. Second, at the group level, a mixed-effects model was used, which accounts for intra- and inter-subject variability, and allows for population-based inferences to be drawn. Age and gender were used as covariates of no interest in an analysis of covariance setting.
A voxel-wise inference at the group level was then drawn according to Gaussian random field theory. Initial uncorrected threshold was p<0.001; comparisons were considered significant at p<0.05 in either whole brain correction or in small volume correction in a priori regions of interest (amygdala, basal ganglia and vmPFC) selected based on previous results (Butler, et al., 2007; Phelps, Delgado, Nearing, & LeDoux, 2004; Phelps, et al., 2001). BOLD activity t-maps are shown at voxel-wise p-values less than 0.01 for the purpose of presentation only.
3. Results
During debriefing all subjects indicated that they had expected to receive an electrodermal stimulation during the presentation of the threat stimulus and that this expectation was associated with the feeling of fear which decreased with repeated presentations over time.
In healthy control subjects significant activation was exhibited in the contrast of Threat versus Safety in bilateral anterior insula, bilateral basal ganglia and thalamus, bilateral dorsal anterior cingulate and bilateral dorsolateral prefrontal cortex (Butler, et al., 2007). In the contrast of safety versus Threat, increased activation was found in bilateral primary motor cortex, bilateral hippocampi/parahippocampi, bilateral posterior cingulate/precuneus and angular gyri as well as in bilateral medial and lateral orbitofrontal cortex from a larger sample (Butler et al 2007). Previous studies have shown an attenuation of amygdalar activity and relative decrease followed by an increase of vmPFC/sgACC activity over time (Butler, et al., 2007; Phelps, et al., 2004; Phelps, et al., 2001). Parametric modelling of trials over time revealed an initial decrease of amygdalar activation (Figure 1A; cp. (Butler, et al., 2007)) which was mainly driven by the Threat condition (Figure 1B) and accompanied by a co-variation in sgACC activity (Figure 1A).
Figure 1.
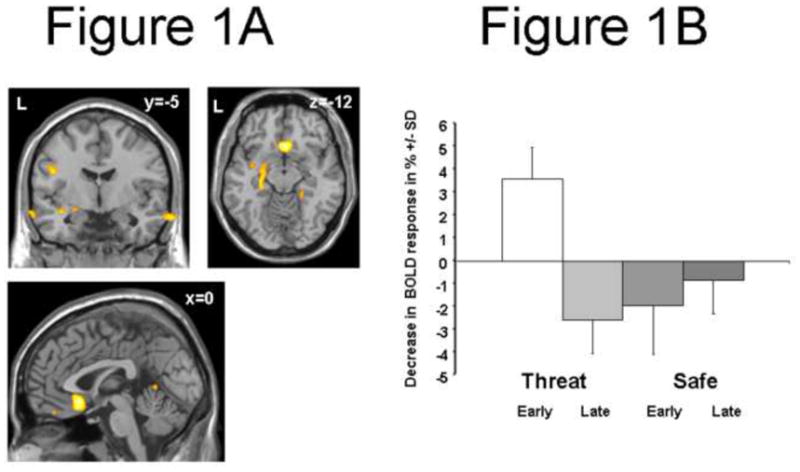
(A) Coronal (y = −5), axial (z=−12), and sagittal (x = 0) sections showing increased amygdala activity and subgenual cingulate (Brodmann area 25) activity in early runs (parametric modeling of the Threat vs. Safe by Early vs. Late interaction) in Normal Control subjects (p<0.01). (B) The bar plot shows the in BOLD response +/− SD (%) at the point showing maximum activity for the Threat vs. Safe by Early vs. Late interaction in the amygdala (MNI [−21, 0, −12]). BOLD response is shown for Normal Controls, conditions [Threat, Safe], and study session [broken into Early and Late run] relative to a resting baseline.
The main comparison of interest, PD versus PTSD patients, found less activation in the Threat versus Safe contrast in regions including the subgenual cingulate (Brodmann area 25), ventral striatum, and extended amygdala, with contrast maximum in the subgenual cingulate ([6, 12, −9], Z=−4.43, voxel-wise p<0.0001, p<0.05 [corrected]; Figure 2A, Table 2). These findings were due to an increase in activation to the Safe condition in PD patients, co-varying with an increased activity to the Threat condition in PTSD patients (Figure 2B). When the study sessions were broken into Early (first run) and Late (second run) components, healthy control subjects activated this region most strongly in the Early Threat condition while PTSD subjects activate this region equivalently in the Early and Late Threat conditions (Figure 2B).
Figure 2.
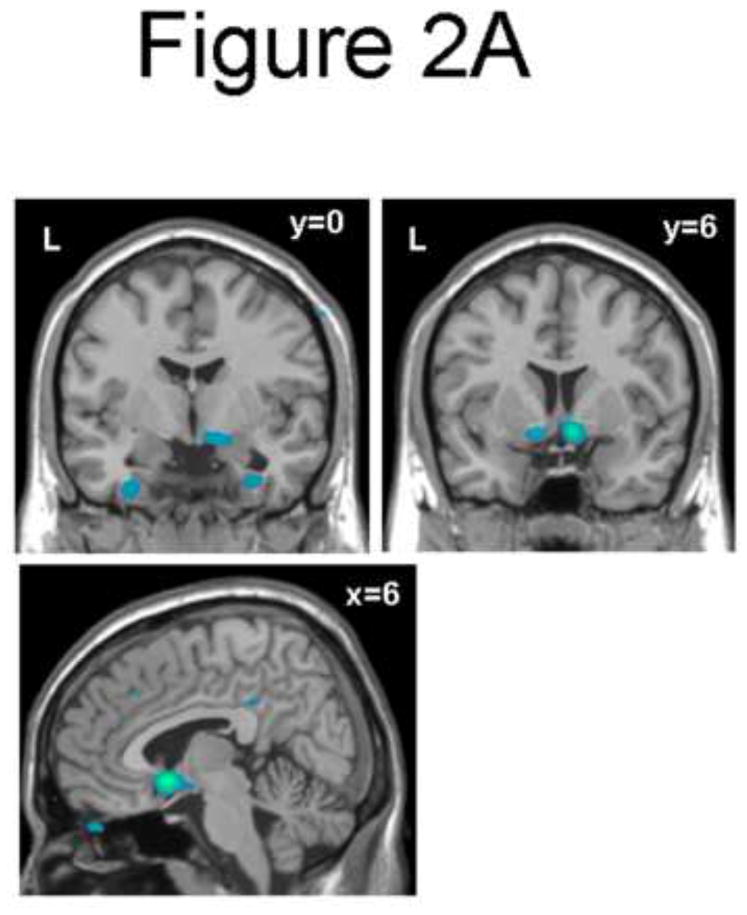
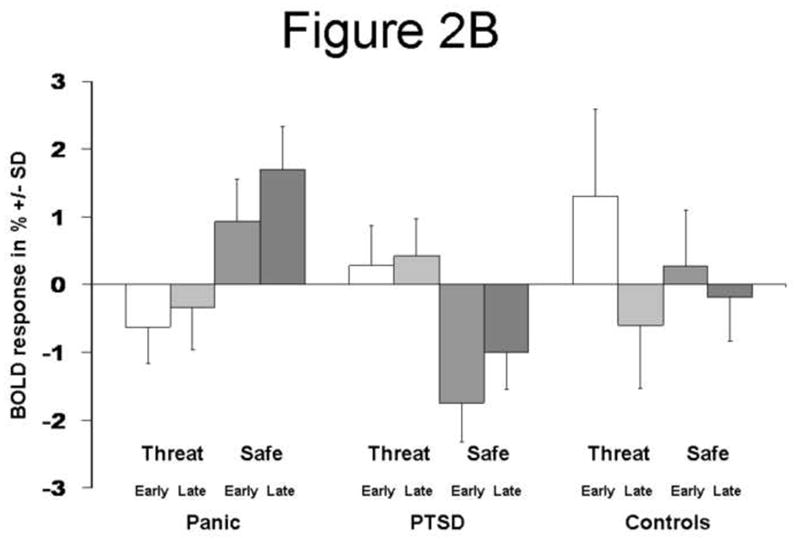
(A) Coronal (y = 0 and y=6), and sagittal (x = 6) sections showing decreased subgenual cingulate (Brodmann area 25), ventral striatum, and extended amygdala activity for the Threat vs. Safe condition in Panic vs. PTSD subjects (p<0.01). (B) The bar plot shows BOLD response +/− SD (%) at the point showing maximum activity for the Threat vs. Safe condition in Panic vs. PTSD subjects [6, 12, −9]. This point is located in the subgenual anterior cingulate cortex. BOLD response is shown for groups [Panic, PTSD, Normal Controls], conditions [Threat, Safe], and study session [broken into Early and Late run] relative to a resting baseline.
Table 2.
Interaction contrast PD vs. PTSD x Threat vs. Safe
| Brain region | MNI coordinates x, y,z | Z-value | p-value uncorr | p-value SVC corr |
|---|---|---|---|---|
| Relative increased activity | ||||
| dorsal midbrain/mesial periaquaeductal grey | 6, −24, −18 | 4.49 | <0.0001 | 0.003 |
| right caudate | 9, 12, 3 | 3.72 | <0.0001 | 0.045 |
| Relative decreased activity | ||||
| subgenual cingulated, ventral striatum, and extended amygdala | 6, 12, −9 | −4.43 | <0.0001 | 0.05 |
The direct contrast of Early (first half) and Late (second half) components of the Threat versus Safe comparison revealed increased activity in PD versus PTSD patients, most prominently in the dorsal midbrain/mesial periaquaeductal grey (MNI [6, −24, −18], Z=4.49, voxel-wise p<0.0001, p<0.003 [corrected]; Figure 3A; Table 2) and right caudate (MNI [9, 12, 3], Z=3.72, voxel-wise p<0.0001, p<0.045 [corrected]; Figure 3A; Table 2). Inspection of the BOLD responses in the dorsal midbrain/mesial periaquaeductal grey (MNI [6, −24, −18]; Figure 3B) revealed a time-by-condition interaction in PD patients with a marked response to the late Safe condition.
Figure 3.
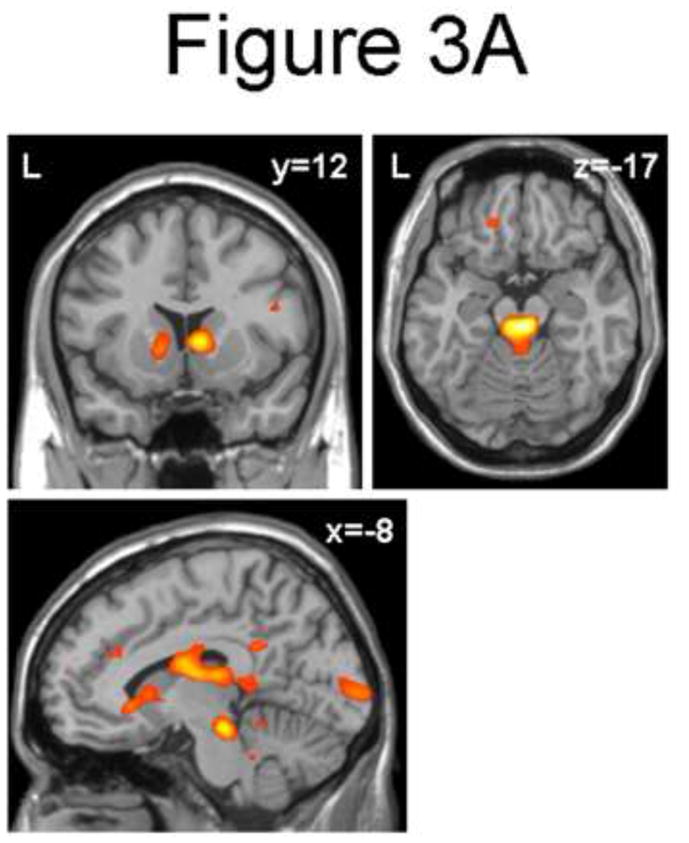
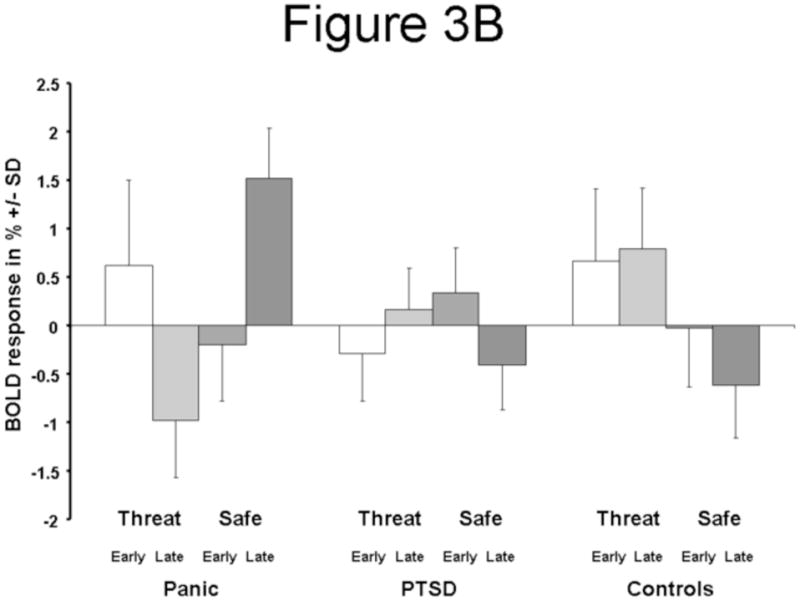
(A) Coronal (y = 12), axial (z=−17), and sagittal (x = −8) sections showing increased dorsal midbrain/mesial periaquaeductal grey and (right) caudate for the Threat vs. Safe by Early vs. Late interaction in Panic vs. PTSD
4. Discussion
Using cognitively instructed fear, this study demonstrates significantly less activation to threat cues and increased activity to safety cues in the subgenual cingulate, ventral striatum, extended amygdala and midbrain periaquaeductal grey in PD patients, suggesting abnormal reactivity in these regions for fear expression. PTSD subjects, in comparison, failed to show the temporal pattern of activity decrease found in control subjects.
Considering the role of these regions in the regulation of visceromotor, autonomic, and emotional circuitry (Price, 1999), decreased activation of the subgenual cingulate, extended amygdala, and ventral striatum in the Threat versus Safe contrast in PD versus PTSD patients is notable. This same network appears to be activated in healthy control subjects under the most threatening condition (Early Threat), and in PTSD subjects fails to habituate over time under threat conditions, supporting models of failure of PTSD patients to habituate to threatening stimuli (Protopopescu et al., 2005).
Studies in rats and humans have shown that the medial prefrontal cortex plays a critical role in the retention and expression of extinction memory (Milad, et al., 2006; Morgan, Romanski, & LeDoux, 1993), with subgenual cingulate cortex specifically mediating successful extinction learning and retention (Phelps, et al., 2004). Deficits in fear extinction have been hypothesized to play a central role in PTSD (Milad, et al., 2006), and such deficits have recently been demonstrated in psychophysiologic studies of PTSD (Blechert, Michael, Vriends, Margraf, & Wilhelm, 2007) as well as PD (Michael, Blechert, Vriends, Margraf, & Wilhelm, 2007). Our results complement these findings that PTSD and PD might, in part, be related to deficits in extinction learning subserved by the same neuroanatomical region (subgenual cingulate) but by different functional/neuronal mechanisms (cp. Fig. 2B). While normal control subjects show strong ventromedial prefrontal cortex activity in the early threat condition alone, PTSD subjects show weaker ventromedial prefrontal cortex activity which persists across the early and late threat conditions.
The increased activation of the medial frontal cortical network and, in the late phase, the brainstem to the Safe condition in PD patients (a reversal of the activation pattern seen in PTSD patients and healthy controls) is particularly interesting and is intriguingly in-line with recent behavioral evidence for an impairment of discrimination learning in PD (Lissek, et al., 2009) which might reflect elevated fear responding to learned safety cues. One possible explanation for this finding is that unlike PTSD, in which dysfunction is related to external threat, PD is largely concerned with internal viscero-somatic threat, possibly generated in the brainstem (Gorman, Kent, Sullivan, & Coplan, 2000; Protopopescu et al., 2006).
The ventromedial prefrontal cortex and brainstem findings in this study are especially interesting in light of a recent fMRI study demonstrating that threat imminence elicits a prefrontal-periaqueductal gray shift in humans (Mobbs et al., 2007). This study used electrodermal “shock” stimuli in concert with a virtual predator maze task, and showed that activity in the (mesial) periaquaeductal gray correlated with increased subjective sense of dread and decreased confidence of escape (Mobbs, et al., 2007). Conversely, in the same study, decreased dread and increased confidence of escape was associated with increased activity in the ventromedial prefrontal cortex. In the current study, PD subjects in the early threat (strongest external threat) condition activated the brainstem but not the ventromedial prefrontal cortex (in line with the Mobbs findings on “imminent threat”). This contrasts with the healthy control subjects who activated ventromedial prefrontal cortex in addition to brainstem in the early threat condition. PD subjects in the late safe condition (perhaps the strongest internal visero-somatic threat condition as it is the farthest from task defined external threat) demonstrated their strongest brainstem and ventromedial prefrontal cortex activations, in contrast to healthy control and PTSD subjects, who had their lowest activations in these regions in this late “Safe” condition.
One limitation of this study is that a subset of PD and PTSD subjects had a range of psychiatric comorbidities, typical in most PD and PTSD diagnosed individuals, and reflecting an overlap of clinical and likely biological features. However, no systematic disparity in comorbid diagnoses was present between groups, making it unlikely that comorbid diagnoses explain any systematic variance or the central findings of the present study. Furthermore, condition- and group-specific activity in the hypothesized regions was exhibited despite those comorbidities in a mixed-effects model that is considered statistically more stringent and capable of addressing inter- and intrasubject variability and generalizable to the larger population. The same line of arguments holds true for another issue to consider, namely the current medication of one PD subject. Yet another limitation is the small number of participants in each group mainly limited by the number of recruitable PD subjects. To mitigate this limitation, subjects were matched across groups as closely as possible and, as above, a mixed-effects model was used to address inter- and intrasubject variability and to improve generalizability to the general population. Nevertheless, it will be important in the future to conduct studies with additional patients and larger sample sizes, to extend and test the replicability of these findings, and to further address medication and comorbidity issues.
5. Conclusion
These findings contribute to the growing literature examining the potentially unique neurocircuitry subserving distinct anxiety disorders. Key findings in the present study may suggest a heightened sensitivity to internally generated, viscerosomatic threat in PD versus heightened sensitivity to external threat in PTSD, as well as to impaired discrimination learning in PD versus impaired extinction learning in PTSD on the behavioral level. Neuroimaging studies contributing to the characterization of more specific pathophysiological mechanisms underlying PD and PTSD may identify diagnostically and therapeutically relevant biomarkers.
Acknowledgments
Funding for this study was provided by the NIMH Grant P50 MH58911-S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45(9):2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Butler T, Pan H, Tuescher O, Engelien A, Goldstein M, Epstein J. Human fear-related motor neurocircuitry. Neuroscience. 2007;150(1):1–7. doi: 10.1016/j.neuroscience.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000;96(1):1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The Extended Amygdala: Are the Central Nucleus of the Amygdala and the Bed Nucleus of the Stria Terminalis Differentially Involved in Fear versus Anxiety? In: McGinty JF, editor. Advancing from the Ventral Striatum to teh Extended Amygdala. New York, N.Y: New York Academy of Sciences; 1999. pp. 281–291. [DOI] [PubMed] [Google Scholar]
- Frank LR, Buxton RB, Wong EC. Estimation of respiration-induced noise fluctuations from undersampled multislice fMRI data. Magn Reson Med. 2001;45(4):635–644. doi: 10.1002/mrm.1086. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157(4):493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Del-Ben CM. Neurobiology of panic disorder: from animal models to brain neuroimaging. Neurosci Biobehav Rev. 2008;32(7):1326–1335. doi: 10.1016/j.neubiorev.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Gu H, Feng H, Zhan W, Xu S, Silbersweig DA, Stern E. Single-shot interleaved z-shim EPI with optimized compensation for signal losses due to susceptibility-induced field inhomogeneity at 3 T. Neuroimage. 2002;17(3):1358–1364. doi: 10.1006/nimg.2002.1274. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behav Res Ther. 2009;47(2):111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey JV, Costa DC, Adshead G, Deahl M, Busatto G, Gacinovic S. Brain blood flow in anxiety disorders. OCD, panic disorder with agoraphobia, and post-traumatic stress disorder on 99mTcHMPAO single photon emission tomography (SPET) Br J Psychiatry. 1997;171:346–350. doi: 10.1192/bjp.171.4.346. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Blanco C, Printz D, Liebowitz MR, Klein DF, Coplan J. A pilot study of noradrenergic and HPA axis functioning in PTSD vs. panic disorder. Psychiatry Res. 2002;110(3):219–230. doi: 10.1016/s0165-1781(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Michael T, Blechert J, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in panic disorder: Enhanced resistance to extinction. J Abnorm Psychol. 2007;116(3):612–617. doi: 10.1037/0021-843X.116.3.612. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317(5841):1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163(1):109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Price JL. Prefrontal Cortical Networks Related to Visceral Function and Mood. In: McGinty JF, editor. Advancing from the Ventral Striatum to teh Extended Amygdala. New York, N.Y: New York Academy of Sciences; 1999. pp. 383–396. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien A. Increased brainstem volume in panic disorder: a voxel-based morphometric study. Neuroreport. 2006;17(4):361–363. doi: 10.1097/01.wnr.0000203354.80438.1. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57(5):464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]


