Abstract
The changing patterns of goblet cell hyperplasia, intestinal epithelial cell turnover, and intestinal motility were studied in ICR and C57BL/6 mice infected with Gymnophalloides seoi (Digenea: Gymnophallidae). Whereas ICR mice retained G. seoi worms until day 7 post-infection (PI), C57BL/6 mice showed a rapid worm expulsion within day 3 PI. Immunosuppression with Depo-Medrol significantly delayed the worm expulsion in C57BL/6 mice. Goblet cell counts were increased in both strains of mice, peaking at day 1 PI in C57BL/6 mice and slowly increasing until day 7 PI in ICR mice. In C57BL/6 mice infected with G. seoi, newly proliferating intestinal epithelial cells were remarkably increased in the crypt, and the increase was the highest at day 1 PI. However, in ICR mice, newly proliferating intestinal epithelial cells increased slowly from day 1 to day 7 PI. Intestinal motility was increased in G. seoi-infected mice, and its chronological pattern was highly correlated with the worm load in both strains of mice. Meanwhile, immunosuppression of C57BL/6 mice abrogated the goblet cell proliferation, reduced the epithelial cell proliferation, and suppressed the intestinal motility. Goblet cell hyperplasia, increased intestinal epithelial cell turnover, and increased intestinal motility should be important mucosal defense mechanisms in G. seoi-infected C57BL/6 mice.
Keywords: Gymnophalloides seoi, worm expulsion, epithelial cell turnover, intestinal motility
INTRODUCTION
Gymnophalloides seoi Lee, Chai and Hong, 1993 (Digenea: Gymnophallidae) is a new human intestinal trematode originally reported from a patient with acute pancreatitis and gastrointestinal troubles in the Republic of Korea [1,2]. Subsequently, a highly endemic area with 49% egg positive rate and heavy worm loads was discovered on a southwestern coastal island (Aphaedo) of Shinan-gun, Jeollanam-do [3]. Thereafter, many small villages in the western and southern coastal areas were found to be endemic with G. seoi infection [4,5]. The infection is caused by eating raw oysters, Crassostrea gigas. The infected individuals complained of gastrointestinal troubles (abdominal pain and diarrhea), loss of appetite, loss of body weight, and fever [6]. Moreover, 2 diabetic patients were accompanied with G. seoi infection, and an association between these 2 diseases has been suspected [6]. However, host-parasite relationships including natural resistance and defense of animal hosts against G. seoi infection have been poorly understood.
Host-parasite relationships including expulsion mechanisms of helminths from the intestine of rodent hosts have been extensively studied in nematodes, including Trichinella spiralis, Nippostrongylus brasiliensis, Heligmosomoides polygyrus bakeri, and Trichuris muris [7,8]. In these models, intestinal mucosal mast cells (MMCs), goblet cells (GCs), secretory IgA, and cytokines were shown to be important effectors for worm expulsion. Intestinal epithelial cell turnover [9,10] and intestinal smooth muscle contraction [11,12,13] were also shown to be important in the expulsion mechanisms of nematodes from the intestine of rodent hosts. However, only a few studies have been performed on the worm expulsion mechanisms of trematodes, for example, in Echinostoma trivolvis [14,15], Echinostoma caproni [14], Metagonimus yokogawai [16,17], Echinostoma hortense [18], and Neodiplostomum seoulense [19,20]. Several immune effectors including intestinal intraepithelial T-lymphocytes, MMCs, and GCs are important in the defense against M. yokogawai [16,17], and MMCs function in the expulsion of E. hortense [18]. Important roles of GCs have been reported in E. trivolvis [14,15] and E. caproni [14]. However, MMCs and GCs are not critically important in the expulsion of N. seoulense from mice [19].
Once established in the intestine of mice, G. seoi is quickly expelled from day 3-21 post-infection (PI) in different strains of mice [21]. ICR and C3H/HeN mice were fairly susceptible to G. seoi infection, although the worm recovery at days 7-21 PI was not so high [21,22,23]. However, C57BL/6 mice were resistant to G. seoi infection, and worms were expelled by day 3 PI [23,24]. This resistance was due to their natural immunity and host defense mechanisms, and was suppressed by immunosuppression with prednisolone. GCs are an important effector in the expulsion of G. seoi worms from C57BL/6 mice, and their numbers are markedly reduced in immunosuppressed G. seoi-infected mice [23]. The GC hyperplasia and worm expulsion were suggested to be induced by signal transducer and activator of transcription (STAT6) signaling, and IL-13 may be involved as a triggering cytokine in this signaling [25]. CD4+ T-cells were important in regulating the GC responses in G. seoi-infected C57BL/6 mice [24]. Toll-like receptor 2 and MUC2 gene are highly expressed on intestinal epithelial cells stimulated with G. seoi antigen suggesting their roles in host defense mechanisms [26]. However, no studies have been performed on intestinal epithelial cell turnover and intestinal smooth muscle contraction in G. seoi-infected mice.
The present study aimed to determine the role of intestinal epithelial cell turnover and intestinal motility in mucosal defense mechanisms and expulsion of G. seoi worms in C57BL/6 mice.
MATERIALS AND METHODS
Experimental animals
Two strains of specific pathogen-free mice (4-week-old males), i.e., ICR which is susceptible to G. seoi infection and C57BL/6 which is resistant to G. seoi infection [21], were purchased from the Samtaco Laboratory Animal Center (Osan-shi, Korea). Mice were kept in Animal Facility of Seoul National University College of Medicine, Seoul, Korea. The experiments were carried out in accordance with the guidelines of Institutional Animal Care and User Committee, Seoul National University College of Medicine.
Parasites
Naturally produced oysters, Crassostrea gigas, were collected from Aphaedo (Island), Shinan-gun, Jeollanam-do, an endemic area of G. seoi infection. To collect the metacercariae, the oyster shells were detached and the animal was gently stirred in 0.85% saline solution. The solution containing free metacercariae was serially filtered through meshes with progressively decreasing pore sizes of 600, 300, and 106 µm. Metacercariae on the last mesh were collected and counted using a stereomicroscope.
Immunosuppression
C57BL/6 mice were immunosuppressed by injecting intramuscularly with Depo-Medrol (15 mg/kg; Upjohn, Seoul, Korea) to the inner thighs every other day from 2 days prior to G. seoi infection until termination of infection (total 5 times; day -2, day 0, day 2, day 4, and day 6). Depo-Medrol is methylprednisolone acetate. Corticosteroids are known to suppress both humoral and cellular immunity when administered to humans and animals, and enhance the survival of G. seoi worms in the mouse intestine [21].
Worm recovery
ICR and C57BL/6 mice were infected orally with 200 metacercariae. At days 1, 3, 5, and 7 PI, 6 mice for each strain were sacrificed by hyperanesthesia with diethyl ether. Their small intestines were resected and opened longitudinally in Petri dishes containing physiological saline. The pieces of intestines were transferred to the top of the Baermman's apparatus. The flukes were collected from the bottom of the tube equipped in the apparatus. After incubation, intestinal segments were returned to Petri dishes to search for residual flukes under a stereomicroscope.
Staining of intestinal GCs
Mice were sacrificed, and intestinal segments about 2 cm in length were taken from the middle portion of the jejunum. The segments were washed twice with saline fixed in Carnoy's fixative for 2-3 days. The fixed tissues were embedded in paraffin and sectioned at about 5 µm thickness. Periodic acid Schiff (PAS) staining was done to visualize GCs. Briefly, the samples were oxidized with 1% periodic acid (Sigma-Aldrich, Poole, UK) for 5 min and reacted with Schiff's reagent to produce a colored end product. They were counterstained with hematoxylin (Sigma-Aldrich). GC numbers were counted per 10 villus-crypt unit (VCU).
Immunohistochemistry to measure intestinal epithelial cell turnover
C57BL/6 and ICR mice infected or uninfected with G. seoi were injected intraperitoneally with 10 mg 5-bromo-2'-deoxyuridine (BrdU; Sigma-Aldrich) in PBS. They were sacrificed 60 min later and the middle part of the jejunum was cut into 2-3 cm pieces. After 24 hr fixation in Carnoy's solution, 5-6 µm sections were obtained. Endogenous peroxidase activity was blocked by 10 min incubation using 3% hydrogen peroxide in PBS. The sections were then placed in 1 N HCl for 30 min at 37℃ for DNA denaturation and rinsed thoroughly. To decrease non-specific staining, the fixed sections were incubated in diluent consisting of 1% bovine serum albumin (BSA) and 0.6 v/v% Triton X-100 in PBS (pH 7.0) for 30 min, and washed 3 times for 5 min in PBS. Monoclonal anti-BrdU antibody (Sigma-Aldrich) was incubated for 2 hr at room temperature and samples were washed in PBS. Biotin-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA) was diluted 1:500 in BSA diluent for 1 hr. After washing in PBS, each sample was incubated in avidin-biotin-peroxidase complex (ABC Reagent; goat ABC Staining System; Santa Cruz Biotechnology) according to the manufacturer's instructions, prepared at least 30 min before use for 1 hr. Sections were washed in PBS and then transferred to TBS (0.1 M Tris-HCl, 0.15 M NaCl in distilled water, pH 8.0) and the peroxidase activity was demonstrated using 3,3'-diaminobenzidine (DAB) as substrate, followed by washes in TBS. After immunostaining, the sections were washed in distilled water, dehydrated, and mounted using Canada Balsam solution. Stained nuclei were counted in crypt layers of 10 VCU.
Charcoal meal gastrointestinal-transit test
Intestinal motility of G. seoi-infected C57BL/6 and ICR mice was measured using the charcoal meal gastrointestinal transit test with small modifications [27]. Animals were grouped into uninfected control (n=6) and G. seoi-infected (n=6) groups. Before experiment, mice were fasted for 18 hr and 0.2 ml charcoal meal (5% charcoal, 5% gum arabic in distilled water) was administered intragastrically. Mice were sacrificed 30 min after the charcoal meal administration, and the whole intestine was resected and separated from the omentum. The length of the intestine from the pyloric sphincter to the end of the large intestine and the distance traveled by the charcoal meal were both measured in control and infected mice at days 1, 3, 5, and 7 PI. The charcoal meal transit was measured by dividing the distance of travel by the length of the whole intestine and the rate was expressed as a percentage.
Statistical analysis
Experiments were repeated at least 3 times, and 1 representative set of data is presented. The statistical significance of the data was analyzed using the Student's t-test. P-value of <0.05 was considered significant.
RESULTS
Worm recovery in C57BL/6 and ICR mice
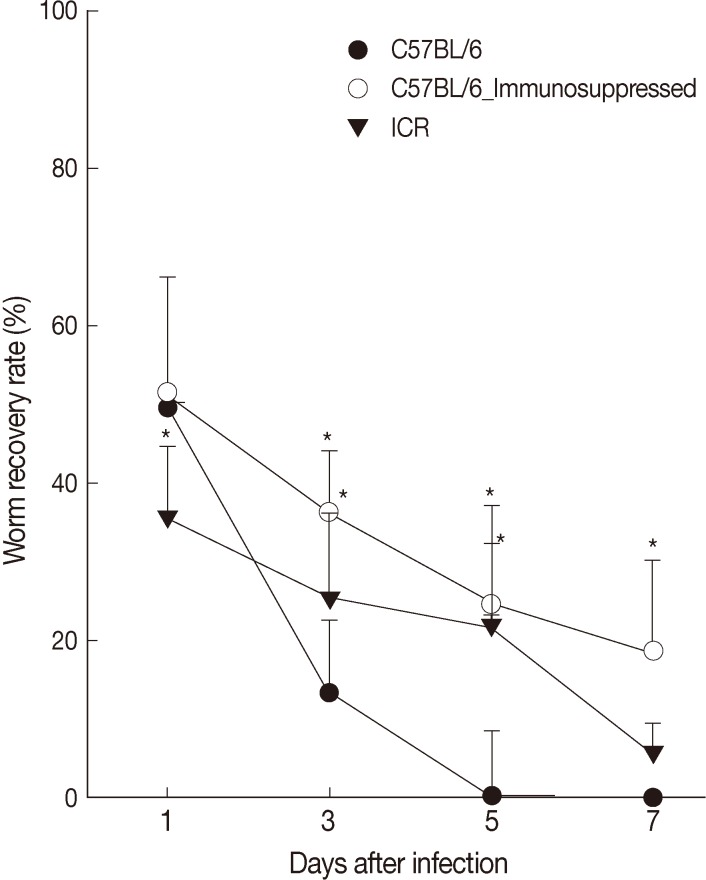
The worm recovery rate (WRR) of G. seoi was different between C57BL/6 and ICR mice through the 7 days of observation (Fig. 1). Rapid worm expulsion was observed in C57BL/6 mice (WRR at days 1, 3, 5, and 7 PI of 49.6%, 11.1%, 0.4%, and 0%, respectively), whereas slightly delayed worm expulsion was observed in ICR mice (WRR at days 1, 3, 5, and 7 PI of 35.5%, 25.5%, 22.0%, and 5.5%, respectively). Immunosuppression of C57BL/6 mice significantly delayed worm expulsion (WRR at days 1, 3, 5, and 7 PI of 51.5%, 36.2%, 24.5%, 18.5%, respectively). The values at days 3, 5, and 7 PI were significantly different between immunocompetent and immunosuppressed C57BL/6 mice (P<0.05).
Fig. 1.
Worm recovery according to mouse strains (C57BL/6 and ICR) after infection with 200 metacercariae of Gymnophalloides seoi from day 1 to day 7 PI. Statistically significant differences (P<0.05) compared with immunocompetent C57BL/6 mice are denoted by an asterisk (*).
GC counts
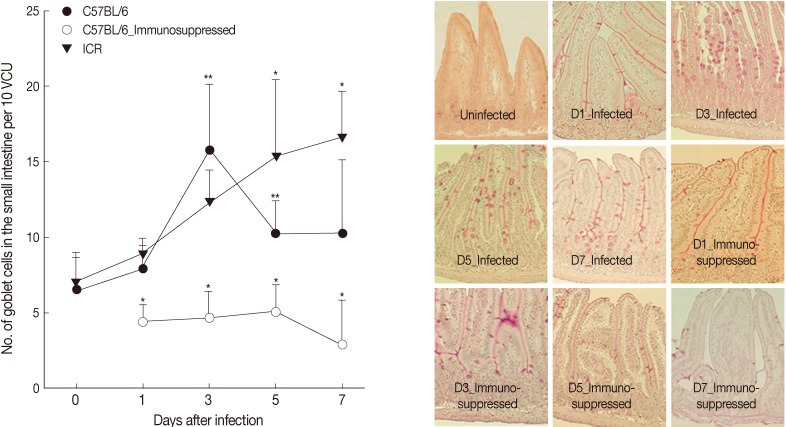
At day 0 PI (before infection), GC numbers per 10 VCU were 6.5±2.1 for both C57BL/6 and ICR mice. G. seoi infection increased the GC counts; however, the increasing patterns were different by mouse strain (Fig. 2). In C57BL/6 mice, the GC counts began to increase at day 1 PI (7.9±1.7 per 10 VCU) reaching a peak at day 3 PI (15.7±4.4; P<0.05) and then decreasing (10.2±2.2 and 10.3±4.8 at day 5 and 7, respectively; Fig. 2). In ICR mice, the GC counts increased (P<0.05) slowly from day 1 (8.8±1.1) to day 3 (12.3±2.1), day 5 (15.3±5.1), and day 7 PI (16.6±3.1). Immunosuppression inhibited the proliferation of goblet cells; the GC counts were lower than normal uninfected mice (Fig. 2).
Fig. 2.
Goblet cell (GC) counts of C57BL/6 and ICR mice. (A) GC counts in C57BL/6 (immunocompetent and immunosuppressed) and ICR mice infected with 200 G. seoi metacercariae. Statistically significant differences (P<0.05) compared with immunocompetent C57BL/6 mice were illustrated as an asterisk (*). In immunocompetent C57BL/6 mice, GC counts at day 3 (**) and day 5 (**) were significantly higher (P<0.05) than the figure of uninfected C57BL/6 mice (day 0). (B) Changes of GC counts in the small intestine of immunocompetent and immunosuppressed C57BL/6 mice infected with 200 G. seoi metacercariae. GCs were counted in the middle portion of the jejunum after PAS stain at days 0 (uninfected), 1, 3, 5, and 7 PI.
Intestinal epithelial cell turnover
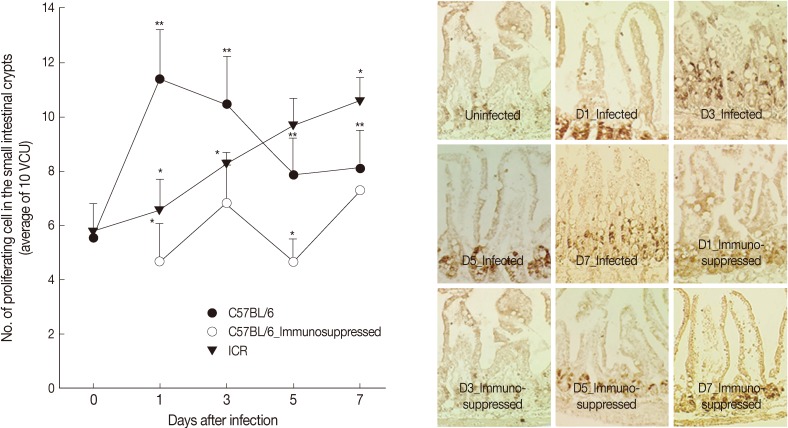
In C57BL/6 mice infected with G. seoi, remarkable increases (P<0.05) of newly proliferating epithelial cells were observed in the crypt layer at day 1 (11.5±2.6), day 3 (10.5±1.7), day 5 (7.9±1.4), and day 7 PI (8.1±1.4), compared with normal uninfected mice (5.6±0.3) (Fig. 3). Their increase was the highest at day 1 PI. However, in ICR mice, continuous increases of newly proliferating epithelial cells in the crypt were observed from day 1 (7.1±1.6) to 7 PI (10.6±0.9). Immunosuppression of C57BL/6 mice decreased the epithelial cell proliferation significantly (P<0.05) and the counts of newly proliferating epithelial cells were not much different from those of normal uninfected mice (Fig. 3).
Fig. 3.
Intestinal epithelial cell turnover of C57BL/6 and ICR mice. (A) Intestinal epithelial cell turnover was measured in C57BL/6 (immunocompetent and immunosuppressed) and ICR mice infected with 200 G. seoi metacercariae. The number of newly proliferating intestinal epithelial cells in the crypt was counted at 10 villus-crypt unit and averaged. Statistically significant differences (P<0.05) compared with C57BL/6 mice were expressed as an asterisk (*). In immunocompetent C57BL/6 mice, dark brown-stained epithelial cell counts at day 1 (**), day 3 (**), day 5 (**), and day 7 (**) were significantly higher (P<0.05) than the figure of uninfected C57BL/6 mice (day 0). (B) Changes of epithelial cell turnover in immunocompetent and immunosuppressed C57BL/6 mice in the middle part of the jejunum infected with 200 G. seoi metacercariae at days 0 (uninfected), 1, 3, 5, and 7 PI. The nuclei of proliferating epithelial cells are stained dark brown by bromo-2'-deoxyuridine (BrdU) immunohistochemical staining.
Intestinal motility
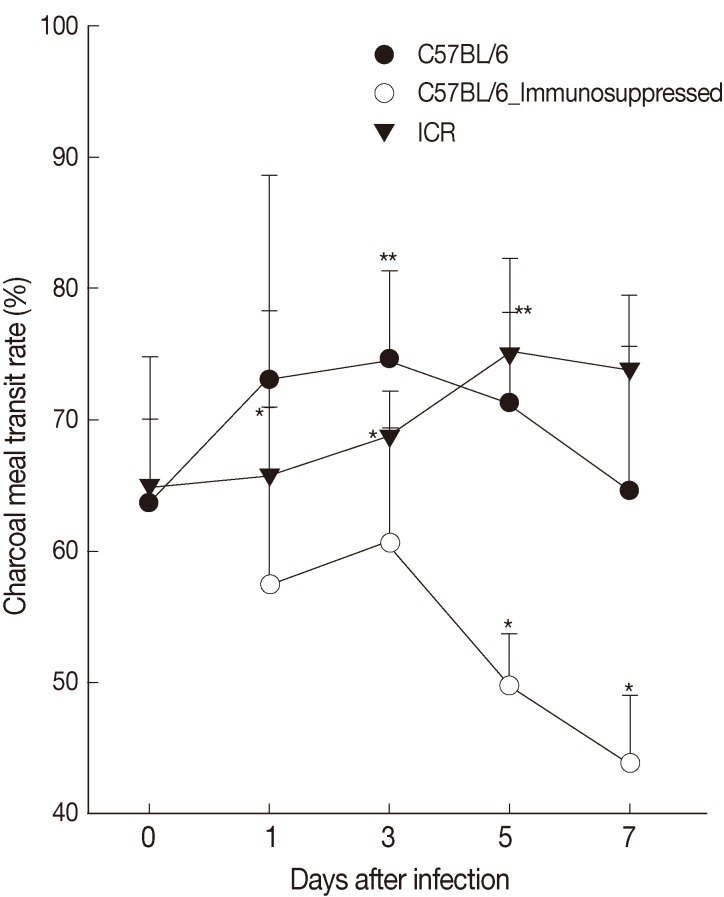
The intestinal motility or intestinal muscle contractility, the ratio (%) of charcoal meal travel per whole length of the small and large intestine, of C57BL/6 mice infected with G. seoi was 63.7±6.4, 73.1±15.5, 74.6±6.7 (P<0.05), 71.3±6.9 (P<0.05), 64.7±11.0 at days 0, 1, 3, 5, and 7 PI and that of ICR mice was 65.0±9.8, 65.7±12.6, 68.7±3.5, 75.0±7.3 (P<0.05), and 73.8±5.7 (P<0.05) at days 0, 1, 3, 5, and 7 PI, respectively (Fig. 4). Immunosuppression of C57BL/6 mice significantly (P<0.05) decreased the intestinal contractility rate (57.4±13.4, 61.0±8.7, 50.0±4.0, and 44.0±5.2 at days 1, 3, 5, and 7 PI) (Fig. 4).
Fig. 4.
Intestinal contractility of C57BL/6 (immunocompetent and immunosuppressed) and ICR mice as measured by charcoal meal gastrointestinal-transit test. Statistically significant differences (P<0.05) compared with uninfected mice are illustrated as an asterisk (*). In immunocompetent C57BL/6 mice, the ratio of charcoal meal transit at day 3 (**) and day 5 (**) were significantly higher (P<0.05) than the ratio of uninfected C57BL/6 mice (day 0).
DISCUSSION
The 3 major mechanisms for worm expulsion of intestinal nematodes, for example, T. muris, are mucin production by intestinal GCs, intestinal epithelial cell turnover, and intestinal muscle hypercontractility [10]. Among these mechanisms, the involvement of GCs and their mucins has been most extensively studied. In rodents infected with nematodes or trematodes, such as T. spiralis, N. brasiliensis, and Echinostoma sp., intestinal GC hyperplasia occurred around the time of worm expulsion [15,28]. A goblet cell-derived factor, RELMb, which is regulated by IL-13, affects the chemosensory apparatus of the intestine-dwelling nematodes, impairing their survival [29]. GC mucins are also an important component of innate defense against T. muris infection, because mucin gene deficient mice have impaired host resistance against parasite infection [30]. In human intestinal epithelial cells cultured in vitro, MUC2 gene expression is upregulated upon stimulation with G. seoi antigen [26]. In the present study, GC hyperplasia was closely associated with expulsion of G. seoi worms regardless of the mouse strain, which was consistent with our previous reports [23,24]. In C57BL/6 mice, the number of GCs peaked at day 3 PI, and on the same day the WRR began to subtantially decrease. In contrast, in ICR mice the number of GCs increased slowly from day 1 to day 7 PI, and the WRR became the lowest on day 7 PI. In immunosuppressed C57BL/6 mice, the WRR remained fairly high until day 7 PI and GC counts were significantly depressed.
The importance of intestinal epithelial cell turnover in expulsion of intestinal nematodes has been highlighted recently [9,10]. Enterocytes migrate from the crypt to the villus, undergoing proliferation, differentiation, and maturation before programmed cell death and extrusion into the intestinal lumen (which was referred to as epithelial cell turnover) [31]. An increase in epithelial cell turnover in the intestine, which is controlled by IL-13, acts like an epithelial escalator to expel Trichuris worms from the epithelial layer toward the lumen [9]. In contrast, the chemokine CXCL10 (IFN-γ-induced protein 10) reduces the rate of epithelial cell turnover and negatively affects worm expulsion [32]. On the other hand, in susceptible animals, the parasite promotes its own survival by inducing IFN-γ production, which inhibits epithelial cell proliferation [9,33]. However, the importance of intestinal epithelial cell turnover had never been firmly defined in intestinal trematode models. In the present study, the intestinal epithelial cell proliferation was most active at the time of worm expulsion, at days 1-3 PI for C57BL/6 and days 5-7 PI for ICR mice infected with G. seoi. This strongly suggests that the intestinal epithelial cell turnover is an important mechanism of G. seoi expulsion from the host. Studies on this aspect are needed in other species of trematodes, such as Echinostoma spp., M. yokogawai, and N. seoulense.
Intestinal smooth muscle contractility or intestinal motility is the third important factor for nematode expulsion from the gut [10]. In mice infected with N. brasiliensis or H. polygyrus bakeri, increased intestinal muscle contractility was suggested to be an important expulsion mechanism of worms [12]. This reaction is STAT6-dependent and controlled by IL-9 [34,35]. However, in trematodes, intestinal muscle contractility and motility affecting worm expulsion has not been well documented. In the present study, the intestinal motility of immunocompetent C57BL/6 mice infected with G. seoi was significantly higher than that of immunosuppressed C57BL/6 mice, which had higher WRR than immunocompetent counterparts.
IL-4 and IL-13 are Th2-type cytokines that share a common peptide chain in their receptors and display closely related and overlapping biological activities [36]. These 2 cytokines have been implicated as key players in the expulsion of parasitic nematodes through the induction of intestinal muscle hypercontractility and GC hyperplasia [10,34,35,37]. Moreover, in nematode infections, induction of intestinal muscle hypercontractility and GC hyperplasia by IL-4 and IL-13 is STAT6 dependent [11,38]. This STAT6 signaling has seldom been studied in trematode models. The only paper available is on STAT6 expression and IL-13 production in association with GC hyperplasia and worm expulsion in G. seoi infection in mice [25]. In E. caproni infection, IL-13 is the main immune component responsible for the host protection, and it seems that IL-13 binds IL-4R and activates STAT6 signaling [39].
Conclusively, the present study demonstrates the importance of increased GC numbers, increased intestinal epithelial cell turnover, and enhanced intestinal motility in mucosal defense mechanisms and worm expulsion of G. seoi in C57BL/6 mice having innate resistance against this trematode infection.
ACKNOWLEDGMENT
This study was supported by a grant from Seoul National University Hospital (2014).
Footnotes
We have no conflict of interest related to this work.
References
- 1.Lee SH, Chai JY, Hong ST. Gymnophalloides seoi n. sp. (Digenea: Gymnophallidae), the first report of human infection by a gymnophallid. J Parasitol. 1993;79:677–680. [PubMed] [Google Scholar]
- 2.Chai JY, Choi MH, Yu JR, Lee SH. Gymnophalloides seoi: a new human intestinal trematode. Trends Parasitol. 2003;19:109–112. doi: 10.1016/s1471-4922(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, Chai JY, Lee HJ, Hong ST, Yu JR, Sohn WM, Kho WG, Choi MH, Lim YJ. High prevalence of Gymnophalloides seoi infection in a village on a southwestern island of the Republic of Korea. Am J Trop Med Hyg. 1994;51:281–285. doi: 10.4269/ajtmh.1994.51.281. [DOI] [PubMed] [Google Scholar]
- 4.Chai JY, Park JH, Han ET, Shin EH, Kim JL, Hong KS, Rim HJ, Lee SH. A nationwide survey of the prevalence of human Gymnophalloides seoi infection on western and southern coastal islands in the Republic of Korea. Korean J Parasitol. 2001;39:23–30. doi: 10.3347/kjp.2001.39.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guk SM, Park JH, Shin EH, Kim JL, Lin A, Chai JY. Prevalence of Gymnophalloides seoi infection in coastal villages of Haenam-gun and Yeongam-gun, Republic of Korea. Korean J Parasitol. 2006;44:1–5. doi: 10.3347/kjp.2006.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SH, Chai JY. A review of Gymnophalloides seoi (Digenea: Gymnophallidae) and human infections in the Republic of Korea. Korean J Parasitol. 2001;39:85–118. doi: 10.3347/kjp.2001.39.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman RA, deSchoolmeester ML, Hurst RJM, Wright SH, Pemberton AD, Else KJ. The goblet cell is the cellular source of the anti-microbial angiotensin 4 in the large intestine post Trichuris muris infection. PLoS One. 2012;7:e42248. doi: 10.1371/journal.pone.0042248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasnain SZ, Gallagher AL, Grencis RK, Thornton DJ. A new role for mucins in immunity: insights from gastrointestinal nematodes. Int J Biochem Cell Biol. 2013;45:364–374. doi: 10.1016/j.biocel.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 10.Klementowicz JE, Travis MA, Grencis RK. Trichuris muris: a model of gastrointestinal parasitic infection. Semin Immunopathol. 2012;34:815–828. doi: 10.1007/s00281-012-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226–G232. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- 12.Zhao A, Mcdermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 13.Finkelman FD, Shea-donohue T, Morris SC, Gilea L, Strait R, Madden KB, Schope L, Urban JF., JR Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujino T, Fried B, Ichikawa H, Tada I. Rapid expulsion of the intestinal trematodes Echinostoma trivolvis and E. caproni from C3H mice by trapping with increased goblet cell mucins. Int J Parasitol. 1996;26:319–324. doi: 10.1016/0020-7519(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 15.Fujino T, Ichikawa H, Fukuda K, Fried B. The expulsion of Echinostoma trivolvis caused by goblet cell hyperplasia in severe combined immunodeficient (SCID) mice. Parasite. 1998;5:219–222. doi: 10.1051/parasite/1998053219. [DOI] [PubMed] [Google Scholar]
- 16.Chai JY, Kim TH, Kho WG, Chung SW, Hung SW, Hong ST, Lee SH. Mucosal mast cell responses to experimental Metagonimus yokogawai infection in rats. Korean J Parasitol. 1993;31:129–134. doi: 10.3347/kjp.1993.31.2.129. [DOI] [PubMed] [Google Scholar]
- 17.Chai JY, Lee SH. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol Int. 2002;51:129–154. doi: 10.1016/s1383-5769(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim I, Im JA, Lee KJ, Ryang YS. Mucosal mast cell responses in the small intestine of rats infected with Echinostoma hortense. Korean J Parasitol. 2000;38:139–143. doi: 10.3347/kjp.2000.38.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai JY, Kim TK, Cho WH, Seo M, Kook J, Guk SM, LEE SH. Intestinal mastocytosis and goblet cell hyperplasia in BALB/c and C3H mice infected with Neodiplostomum seoulense. Korean J Parasitol. 1998;36:109–119. doi: 10.3347/kjp.1998.36.2.109. [DOI] [PubMed] [Google Scholar]
- 20.Shin EH, Kim TH, Hong SJ, Park JH, Guk SM, Chai JY. Effects of anti-allergic drugs on intestinal mastocytosis and worm expulsion of rats infected with Neodiplostomum seoulense. Korean J Parasitol. 2003;41:81–87. doi: 10.3347/kjp.2003.41.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Park SK, Seo M, Guk SM, Choi MH, Chai JY. Susceptibility of various species of animals and strains of mice to Gymnophalloides seoi infection and the effects of immunosuppression in C3H/HeN mice. J Parasitol. 1997;83:883–886. [PubMed] [Google Scholar]
- 22.Chai JY, Chung WJ, Hung WJ, Kook J, Seo M, Park YK, Guk SM, Choi MH, Lee SH. Growth and development of Gymnophalloides seoi in immunocompetent and immunosuppressed C3H/HeN mice. Korean J Parasitol. 1999;37:21–26. doi: 10.3347/kjp.1999.37.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo M, Guk SM, Han ET, Chai JY. Role of intestinal goblet cells in the expulsion of Gymnophalloides seoi from mice. J Parasitol. 2003;89:1080–1082. doi: 10.1645/GE-3182RN. [DOI] [PubMed] [Google Scholar]
- 24.Guk SM, Lee JH, Kim HJ, Kim WH, Shin EH, Chai JY. CD4+ T-cell-dependent goblet cell proliferation and expulsion of Gymnophalloides seoi from the intestine of C57BL/6 mice. J Parasitol. 2009;95:581–590. doi: 10.1645/GE-1807.1. [DOI] [PubMed] [Google Scholar]
- 25.Lee JJ, Kim D, Pyo KH, Kim MK, Kim HJ, Chai JY, Shin EH. STAT6 expression and IL-13 production in association with goblet cell hyperplasia and worm expulsion of Gymnophalloides seoi from C57BL/6 mice. Korean J Parasitol. 2013;51:589–594. doi: 10.3347/kjp.2013.51.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KD, Guk SM, Chai JY. Toll-like receptor 2 and MUC2 expression on human intestinal epithelial cells by Gymnophalloides seoi adult antigen. J Parasitol. 2010;96:58–66. doi: 10.1645/GE-2195.1. [DOI] [PubMed] [Google Scholar]
- 27.Kook J, Nawa Y, Lee SH, Chai JY. Pathogenicity and lethality of a minute intestinal fluke, Neodiplostomum seoulense, to various strains of mice. J Parasitol. 1998;84:1178–1183. [PubMed] [Google Scholar]
- 28.Brunet LR, Joseph S, Dunne DW, Fried B. Immune responses during the acute stages of infection with the intestinal trematode Echinostoma caproni. Parasitology. 2000;120:565–571. doi: 10.1017/s0031182099006009. [DOI] [PubMed] [Google Scholar]
- 29.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, Grencis RK, Thornton DJ, Khan WI. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 2010;138:1763–1771. doi: 10.1053/j.gastro.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki S, Yoneyama H, Suzuki K, Suriki H, Aiba T, Watanabe S, Kaqauchi Y, Kawachi H, Shimizu F, Matsushima K, Asakura H, Narumi S. Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. Eur J Immunol. 2002;32:3197–3205. doi: 10.1002/1521-4141(200211)32:11<3197::AID-IMMU3197>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Artis D, Potten CS, Else KJ, Finkelman FD, Grencis RK. Trichuris muris: host intestinal epithelial cell hyperproliferation during chronic infection is regulated by interferon-gamma. Exp Parasitol. 1999;92:144–153. doi: 10.1006/expr.1999.4407. [DOI] [PubMed] [Google Scholar]
- 34.Khan WI, Blennerhasset P, Ma C, Matthaei KI, Collins SM. Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol. 2001;23:39–42. doi: 10.1046/j.1365-3024.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- 35.Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, Snick JV, Collins SM. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun. 2003;71:2430–2438. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mckenzie AN. Regulation of T helper type 2 cell immunity by interleukin-4 and interleukin-13. Pharmacol Ther. 2000;88:143–151. doi: 10.1016/s0163-7258(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 37.Khan WI, Vallance BA, Blennerhassett PA, Deng Y, Verdu EF, Matthaei KI, Collins SM. Critical role for signal transducer and activator of transcription factor 6 in mediating intestinal muscle hypercontractility and worm expulsion in Trichinella spiralis-infected mice. Infect Immun. 2001;69:838–844. doi: 10.1128/IAI.69.2.838-844.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trelis M, Sotillo J, Monteagudo C, Fried B, Marcilla A, Estaban JG, Toledo R. Echinostoma caproni (Trematoda): differential in vivo cytokine responses in high and low compatible hosts. Exp Parasitol. 2011;127:387–397. doi: 10.1016/j.exppara.2010.09.004. [DOI] [PubMed] [Google Scholar]