Abstract
The oocyst wall is severed by means of mechanical injury or chemical agents. This study reports the percentage of in vitro sporocyst release following mechanical shaking in the presence of varying sizes of glass beads. Glass beads measured 0.5, 1, and 3 mm in diameter and were shaken with the oocysts for different times ranging from 5 sec to 5 min. Approximately 80% of sporocysts were released with 5 min of shaking in the presence of 3 mm glass beads, as well as 30 sec with 0.5 mm beads and 1 mm glass beads. The release of sporocysts of E. tenella was most efficient using 1 mm glass beads and treatment times of 30 sec to 1 min. Therefore, the use of 1 mm glass beads with 30 sec to 1 min of agitation is recommended in order to maximize sporocyst release and recovery and to improve the yield of viable sporozoites for use in biochemical, tissue culture, and immunological applications of coccidia.
Keywords: Eimeria tenella, glass bead, oocyst, sporocyst
Avian Eimeria spp. are recognized as the parasitic protozoans with the greatest economic impact on poultry production worldwide [1]. Protozoan parasites of the genus Eimeria multiply in the intestinal tract and cause tissue damage and increase susceptibility to other diseases [2]. Sporulation of Eimeria spp. oocysts is affected by moisture, temperature, and oxygen levels [3]. Oocysts are enclosed in a thick outer shell and consist of a single cell that undergoes the process of sporulation to enter the infective stage after approximately 48 hr. Four sporocysts, each containing 2 sporozoites, are generally discernible within the oocysts. The oocyst wall has 2 distinguishable layers: an inner and outer layers [4]. In vivo, the oocyst wall may be opened through mechanical injury, such as being crushed in the gizzard of a bird. A high percentage of sporocysts are quickly released from crushed oocysts within the gizzard [5], and the sporozoites then enter the intestine. Sporozoites of Eimeria tenella invade chick cecal folds ~4 hr after infection, followed by sporozoite migration into the crypt epithelium. The first asexual replication occurs 48 hr later [6]. At least 2 generations of asexual reproduction occur before the sexual phase, where motile microgametes seek out and unite with macrogametes. The zygote matures into oocysts, which are then released from the intestinal mucosa and expelled in the feces. The life cycle of E. tenella is typical of all Eimeria.
To break the wall of Eimeria spp. oocysts for PCR analysis, glass beads, a mortar and a pestle, or chemical agents may be used [7]. Eimeria antigens are obtained from suspensions of oocysts in PBS by disruption with glass beads followed by repeated freezing and thawing [8]. The excystation of sporozoites from Eimeria oocysts is an important step for in vitro studies of these parasites. The aim of the present study was to assess the degree of released sporocysts of E. tenella oocysts using different sizes of glass beads and treatment durations.
Oocysts were field-isolated samples (Namwon Farm, Namwon, Korea). The correct species assignment and purity of samples were confirmed by singular ITS-PCR. Genes of E. tenella were collected using a Nucleic Acid Purification Kit (Exprep™ Plasmid DNA, GeneAll, Seoul, Korea). Genes were sequenced at the JeonJu Biomaterials Institute and submitted to GenBank (accession no. FJ447468). Since 2009, our laboratory has maintained generations of E. tenella on chickens. Feces were collected from 5 to 7 days post inoculation. Oocysts were suspended at ~3.0×105 per milliliter in 2% (w/v) potassium dichromate in a conical flask. Oocysts were sporulated under air supply within 5-8 days [9] and were checked for complete sporulation. A 200-µl sample of 3.0 ×105 oocysts per milliliter was added to 800 µl of saturated salt solution. The oocysts suspension was then mixed at a ratio of 1:1 with glass beads (0.5, 1, and 3 mm in diameter) [10] and vortexed for 5 sec, 30 sec, 1 min, or 5 min. For vortexing, Vortex-2 Genie (G 560, Scientific Industries, Springfield, Massachusetts, USA) was used. During vortexing, the conical tube was kept angular using a maximum speed (3,200 rpm). Same vortex speed was fixed in all groups. After oocysts were vortexed and added to 4 ml of saturated salt solution, the remaining oocysts were counted by the standard MacMaster method [11]. The data were subjected to ANOVA with SAS (Ver. 8x). Duncan's multiple range tests was used to determine significant differences among the various durations or bead sizes.
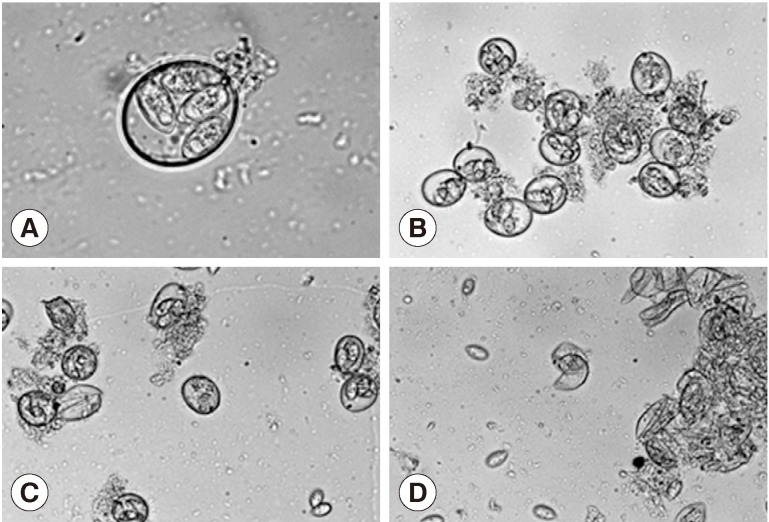
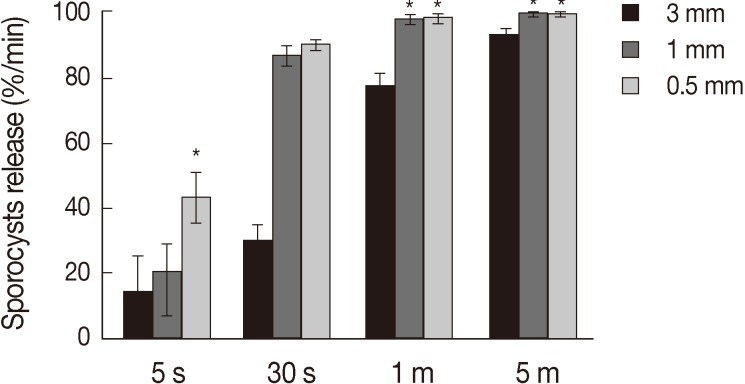
Treatment of the oocytes with 1 mm glass bead (Fig. 1A, B) for 5 sec caused approximately 20% sporocysts release (Fig. 1C), while treatment for 1 min caused the release of more than 90% of sporocysts (Fig. 1D). Release rates of sporocysts using 0.5 mm glass beads (56.8±7.3%) were higher than those with other bead sizes after 5 sec of treatment (P<0.01). Release rates of sporocysts with 0.5 mm and 1 mm glass beads were not significantly different after 30 sec of treatment, but release rates using 0.5 mm and 1 mm glass beads were higher than 3 mm after 1 min and 5 min of treatment (P<0.01). Change of treatment times was highest at 5 min (P<0.01). For all bead sizes, the release rates of sporocysts increased as the agitation time increased (Fig. 2).
Fig. 1.
Effect of glass beading on the excystation of Eimeria tenella oocysts. (A) Sporulated oocysts. ×1,000. (B) No treatment of oocysts. ×400. (C) Oocysts were vortexed with 1 mm glass bead for 5 sec. ×400. (D) Oocysts were vortexed with 1 mm glass bead for 1 min. ×400.
Fig. 2.
Sporocyst release rate of Eimeria tenella oocysts at different glass beads and treatment times. Oocysts were vortexed with 0.5, 1, and 3 mm glass beads for 5 sec, 30 sec, 1 min, and 5 min. Oocysts release of sporocysts is depicted as the mean release of 4 times±SD. *Significance (P<0.01).
During in vitro experiments, oocysts may be made to release 80% of viable sporocysts [16]. There are several methods that can be used to rupture the cell wall of Eimeria species; the most common includes sonication, microwave, heating, and pestle and mortar. The greatest advantage of using glass beads for wall rupture is that they are an efficient way to inexpensively process large quantities of oocysts. The concept of mechanical crushing with glass beads came from the in vivo mechanism by which oocysts are crushed in the gizzard [12]. Our study for oocyst disruption of Eimeria did not require enzymes. Landers [13] demonstrated that excystation of E. nieschulzi could not be achieved using pepsin or trypsin. It was also found that, for release of E. separta sporocysts, hydrochloric acid, pepsin, and trypsin were required [14]. Pre-treatment of E. nieschulzi oocysts with pepsin destabilizes the oocyst wall structure, ensuring that mechanical disruption by vortexing with glass beads is more efficient [15]. Chai et al. [16] described that the wall structure of E. tenella oocysts was ruptured using a teflon-coated tissue homogenizer. The high mechanical resistance of Eimeria spp. oocysts required a greater number of glass beads during vortexing. Dulski and Turner [17] described a protocol similar to ours for disrupting E. tenella oocysts using 3 mm glass beads. They found that roughly 60% of oocysts were broken by orbital shaking at 200 rpm. Sporocysts have also been released from oocysts by agitating oocyst suspensions with 4 mm glass beads [18]. E. tenella oocysts placed in an equal volume of 0.5 mm glass beads and exposed to the maximum speed of agitation resulted in invisible sporocysts [19]. If too many glass beads are used, sporocysts may be damaged; in order to ensure a high yield of undamaged sporocysts, the agitation procedure must be stopped after a very specific duration.
After the oocysts wall is crushed, the sporocysts and glass beads are collected separately. The use of smaller glass beads may result in greater difficulty of recovering sporocysts. Our results suggest that sporocyst release from oocysts was efficient after vortexing with 0.5 mm glass beads for 5 to 30 sec, with 1 mm glass beads for 30 sec to 1 min and with 3 mm glass beads for 1 to 5 min. However, the use of 0.5 mm glass beads presented greater difficulties in recovering the sporocysts compared to larger glass beads. Therefore, the present study recommended the use of 1 mm glass beads for 30 sec to 1 min in order to maximize sporocyst release and recovery. Oocyst walls of Eimeria spp. have various characteristics. Some Eimeria species are more susceptible to chemical agents while others are more susceptible to mechanical injury. Because each Eimeria species is unique, with each being lysed upon application of different degrees of either mechanical or chemical stresses, different methods should be tested to improve the yield of viable sporozoites for use in biochemical, tissue culture, and immunological applications of coccidia.
ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (No. KRF-2008-313-E00610).
Footnotes
The author has no conflict of interest related to this work.
References
- 1.Ruff MD. Important parasites in poultry production systems. Vet Parasitol. 1999;84:337–347. doi: 10.1016/s0304-4017(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 2.Morris GM, Gasser RB. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotechnol Adv. 2006;24:590–603. doi: 10.1016/j.biotechadv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002;15:58–65. doi: 10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson DJ, Belli SI, Smith NC, Wallach MG. The development of the macrogamete and oocyst wall in Eimeria maxima: immuno-light and electron microscopy. Int J Parasitol. 2003;33:1329–1340. doi: 10.1016/s0020-7519(03)00185-1. [DOI] [PubMed] [Google Scholar]
- 5.Doran DJ, Farr MM. Excystation of the poultry coccidium, Eimeria acervulina. J Protozool. 1962;9:154–161. doi: 10.1111/j.1550-7408.1962.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 6.Daszak P. Zoite migration during infection: parasite adaptation to host defences. Parasitol Today. 1999;15:67–72. doi: 10.1016/s0169-4758(98)01379-9. [DOI] [PubMed] [Google Scholar]
- 7.Kaya G, Dale C, Maudlin I, Morgan K. A novel procedure for total nucleic acid extraction from small numbers of Eimeria species oocysts. Turkiye Parazitol Derg. 2007;31:180–183. [PubMed] [Google Scholar]
- 8.Rose ME, Hesketh P, Rennie M. Coccidiosis: rapid depletion of circulating lymphocytes after challenge of immune chickens with parasite antigens. Infect Immun. 1984;45:166–171. doi: 10.1128/iai.45.1.166-171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldenstedt L, Elwinger K, Lunden A, Thebo P, Uggla A. Sporulation of Eimeria maxima oocysts in litter with different moisture contents. Poult Sci. 2001;80:1412–1415. doi: 10.1093/ps/80.10.1412. [DOI] [PubMed] [Google Scholar]
- 10.Kurth M, Entzeroth R. Improved excystation protocol for Eimeria nieschulzi (Apicomplexa, Coccidia) Parasitol Res. 2008;102:819–822. doi: 10.1007/s00436-007-0868-1. [DOI] [PubMed] [Google Scholar]
- 11.Dunn A, Keymer A. Factors affecting the reliability of the McMaster technique. J Helminthol. 1986;60:260–262. doi: 10.1017/s0022149x00008464. [DOI] [PubMed] [Google Scholar]
- 12.Jordan FTW, Pattison M. Poultry Diseases. 6th ed. London, UK: W.B. Saunders Company Ltd; 1996. p. 975. [Google Scholar]
- 13.Landers EJ. Studies on excystation of coccidial oocysts. J Parasitol. 1960;46:195–200. [PubMed] [Google Scholar]
- 14.Kowalik S, Zahner H. Eimeria separata: method for the excystation of sporozoites. Parasitol Res. 1999;85:496–499. doi: 10.1007/s004360050584. [DOI] [PubMed] [Google Scholar]
- 15.Krucken J, Hosse RJ, Mouafo AN, Entzeroth R, Bierbaum S, Marinovski P, Hain K, Greif G, Wunderlich F. Excystation of Eimeria tenella sporozoites impaired by antibody recognizing gametocyte/oocyst antigens GAM22 and GAM56. Eukaryotic Cell. 2008;7:202–211. doi: 10.1128/EC.00292-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chai JY, Lee SH, Kim UH, Yun CK. Development of Eimeria tenella in MDCK cell culture with a note on enhancing effect of preincubation with chicken spleen cells. Korean J Parasitol. 1989;27:87–100. doi: 10.3347/kjp.1989.27.2.87. [DOI] [PubMed] [Google Scholar]
- 17.Dulski P, Turner M. The purification of sporocysts and sporozoites from Eimeria tenella oocysts using percoll density gradients. Avian Dis. 1988;32:235–239. [PubMed] [Google Scholar]
- 18.Wagenbach GE. Purification of Eimeria tenella sporozoites with glass bead columns. J Parasitol. 1969;55:833–838. [PubMed] [Google Scholar]
- 19.Tomley F. Techniques for isolation and characterization of apical organelles from Eimeria tenella sporozoites. Methods. 1997;13:171–176. doi: 10.1006/meth.1997.0509. [DOI] [PubMed] [Google Scholar]