Abstract
Hydatid worms, hosted by humans and animals, impose serious human health risk and cause significant livestock production loss. To better understand the disease infection status in Xinjiang, China, we investigated the disease epidemics in 4 livestock animals, i.e., cattle, sheep (both sheep and goat), camels, and horses, slaughtered at the abattoirs in Urumqi, Yining, Tacheng, and Altay areas. The results showed that the animals were infected at different rates, in the order of sheep (9.8%), cattle (8.4%), camels (6.8%), and horses (4.3%). The infection rates were found to be different between the abattoirs in various regions even for the same animals. For sheep, the rates increased significantly as the animals grew older. It was 1.9% before 1 year of age and increased to 8.2% in the age of 1-2 years, and further increased to 12.3% when the animals were 3-4 years old, and reached 17.2% when they were 5-6 year old. Sheep older than 6 years had an infection rate of 19.5%. This study demonstrates that the 4 livestock animals in the pastoral areas in Xinjiang were infected by the parasites to various extend. This study is the first systematic investigation of the hydatid worms in various livestock animals in Xinjiang, China, which provides epidemiological information about the infection of hydatid worms in livestock, and is valuable in developing strategies for prevention and control of the hydatid disease.
Keywords: hydatid cyst, prevalence, livestock animal, Xinjiang, China
Hydatid worms (Echinococcus granulosus) belong to the genus Echinococcus of the order Cyclophyllidea in the phylum Platyhelminthes. The adult worms are small and flat-shaped, parasitizing in the small intestines of canine carnivorous animals [1], while the larvae live in the liver, lung, and other organs of humans, cattle, sheep, pigs, and other animals [2,3,4,5,6,7], causing cystic hydatid disease, also known as cystic echinococcosis (CE). It is a serious zoonotic disease hazardous to humans [8,9] and livestocks [10,11], which is endemic in the ranges in China, particularly in Qinghai [12,13], Tibet, Gansu, Ningxia, Xinjiang, the western part of the Inner Mongolia Autonomous Region, Ganz and Aba, 2 Tibetan Autonomous Prefectures, Muli, the Tibetan Autonomous County in Sichuan [14], and northwestern Shaanxi [15]. It affects a human population of about 50 million, accounting for 5% of the national population. About 100 million livestock animals are subjected to disease infection, among them more than 70% are sheep [16].
Xinjiang is one of the China's 5 major pastoral areas. It is also the major endemic area of the diseases, where CE caused by hydatid worms is very common. This disease has therefore been listed as one of the major diseases to be prevented and controlled in Western China by the Chinese Ministry of Health. This study aimed to investigate the infection status of animals slaughtered in Urumqi, Yining, Tacheng, and Altay in Xinjiang, China with hydatid cysts.
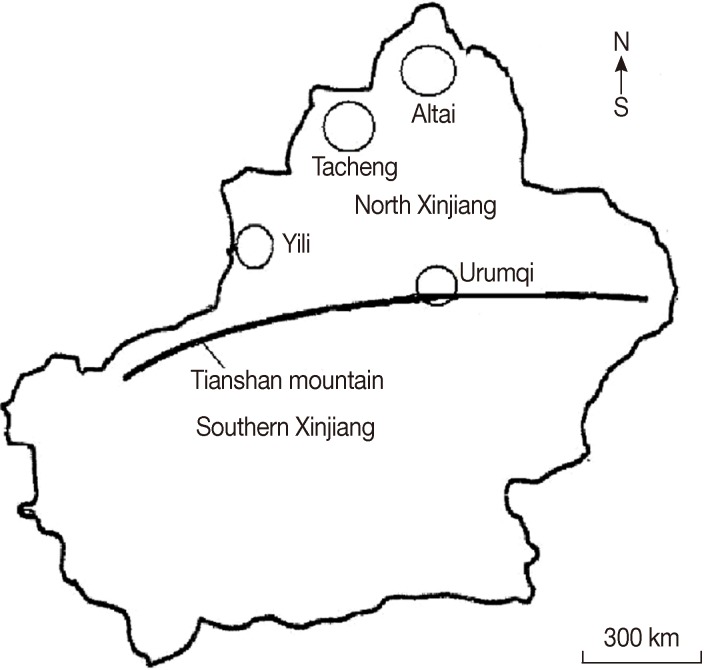
This investigation was conducted between January 2011 and December 2012. Animals examined were all subjects during the study period at those abattoirs at the 4 governmental slaughterhouses in Xinjiang, each for 1 of the 4 regions. The areas, called Northern Xingjiang Ranges (NXR), are temperate continental with arid and semi-arid climate [17], which are located between latitudes 44° to 48° N and altitudes 83° to 88° E (Fig. 1). In total, 12,539 sheep, 1,407 cattle, 192 camels, and 352 horses were examined for the presence of hydatid cysts in their internal organs. The animals were local varieties in the pastoral regions and had been raised outdoor. More than 70% (9,565/14,490) of the animal slaughtered were males, and about 60% of sheep (both sheep and goats) were aged less than 1 year and about 10% were over 6 years old.
Fig. 1.
Geographic locations of the 4 survey areas in north Xinjiang, China.
Surveys were made on the slaughtered animals at the slaughterhouses. After opening of the belly, the presence of the cysts was checked, mainly in the liver, lungs, spleen, heart, kidneys, and other organs. The organs were examined by visual inspection and palpation. When cysts were found, their quantity, morphology, and fertility were determined by visual and microscopic examinations. The cyst numbers were recorded for each affected animals and organs. Data were analyzed with the Chi square (χ2) tests using the SPSS for windows software version 13.0. P-values<0.05 were considered statistically significant.
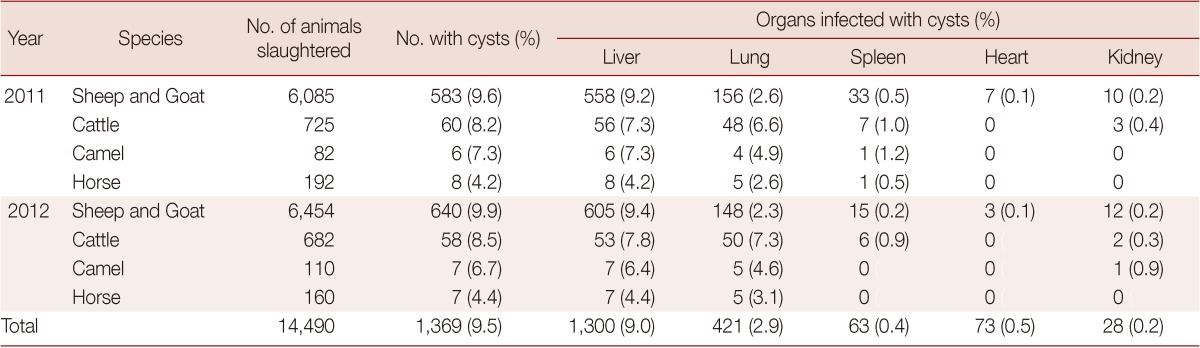
A total of 12,539 sheep, 1,407 cattle, 192 camels, and 352 horses were examined in 2 years at the 4 slaughterhouses. Among them, the animals with hydatis cysts were 1,223, 118, 13, and 1, respectively. The infection rates and their distributions among the different organs (liver, lungs, spleen, heart, and kidneys) are shown in Table 1. There were different infection rates in the animals, with the order of sheep (9.8%), cattle (8.4%), camels (6.8%), and horses (4.3%). However, the organ infection rates were different between the 4 animals. For sheep, the highest rate was seen in the liver (9.3%), which is significantly higher than that in lungs (2.4%), spleen (0.4%), heart (0.1%) and kidneys (0.2%) (P<0.01). For cattle, the rates were 7.5% for the liver, not significantly different from that of the lungs (7.0%), but these 2 rates were significantly higher than that for the spleen (0.9%), heart (0%), and kidneys (0.4%) (P<0.01). Camels had a liver infection rate of 6.8%, significantly higher than that of the lungs (4.7%) (P<0.05). In horses, 4.3% of the livers were infected, a figure significantly higher than that of the lungs (2.8%) (P<0.05). However, they had very low infections in other organs such as the spleen (0.3%), heart (0%), and kidneys (0%).
Table 1.
Percent of animals and their organs infected with E. granulosus between January 2011 and December 2012
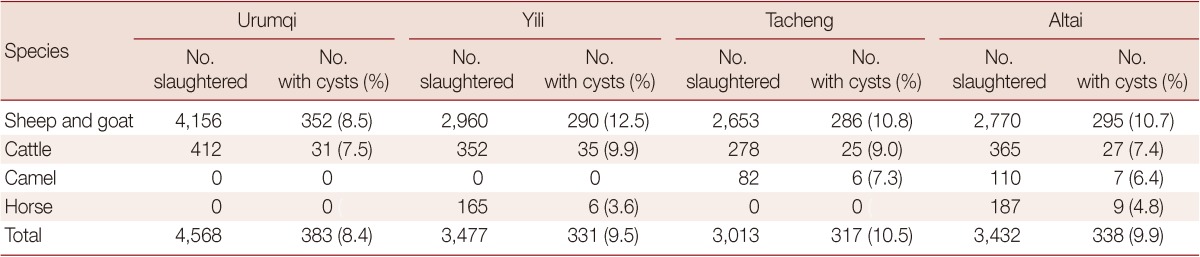
The types and quantities of the animals slaughtered at the 4 abattoirs are shown in Table 2. There were different infection rates for the same animals in different locations. For sheep, the highest infection rate was seen at Yining slaughterhouse (12.5%), and the lowest at Urumqi slaughterhouse, which differed significantly (P<0.05) each other. The camel's infection rates were found to be similar at 7.3% and 6.4% at Tacheng slaughterhouse and Altay slaughterhouse. The horse's infection rates were lower at Yining slaughterhouse (3.6%) than Altay slaughterhouse (4.8%).
Table 2.
The types and quantities of slaughtered animals infected with E. granulosus in different locations
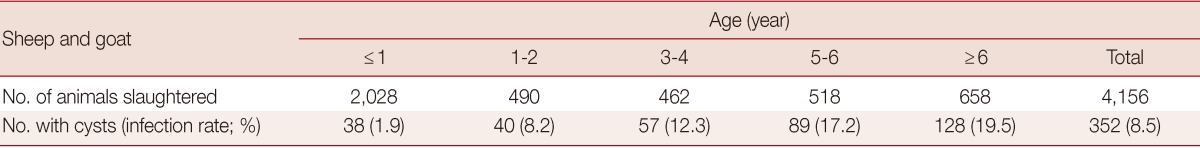
To reveal the relationship between animal ages and the infection rates, we reviewed the historic data at Urumqi slaughterhouse. As shown in Table 3, the infection rates for sheep were 1.9% before they were 1 year old, increased to 8.2% between 1 and 2 years old, which climbed to 12.3% at 3 and 4 years old, and reached 17.2% at 5 to 6 years old. The animals older than 6 years were infected at 19.5%, a clear indication of positive increase of the infection with age.
Table 3.
E. granulosus infection rates in sheep at different ages
E. granulosus occurs worldwide while Echinococcus multilocularis is mostly in the northern hemisphere [5]. E. granulosus has adapted to a wide range of intermediate hosts [5,8,11,18], of which were livestock animals in the NXR, such as cattle, sheep, camels, and horses [3,4,10], as well as humans. In this survey, we investigated the disease infections in 4 livestock animals (sheep, cattle, camels, and horses) in NXR at 4 slaughterhouses. Statistical analysis indicates that sheep had the highest infection rate. This is because sheep is the most suitable intermediate host for the disease, and may also be associated with the herding dog, which are used to guard the sheep. In addition, the herdsman often feed the dogs with inedible sheep internal organs, which often contain the parasite larvae.
Our investigation at the 4 slaughter sites also showed that there was a close relationship between the infection rates and the age of the animals. The animals surveyed at Urumqi were mostly less than 1 year old (60%). The hydatid infection rate was very low. However, the rates increased as sheep aged, indicating that older sheep have higher chance to contact the pathogen.
The survey also found that the liver and lungs are more susceptible to the parasite. This may be because these organs have special blood circulation characteristics. In the liver, blood is abundantly supplied from both portal veins and hepatic arteries; 25% of the blood flow into the liver comes from the hepatic arteries, and used as the major supply of oxygen required for the liver. The remaining 75% is from the portal veins (jointly from the veins of the stomach, intestine, spleen, and pancreas), transporting various nutrients and hazardous substances from digestive tracts to the liver to process for recycling into the body's circulation systems. The lungs have intensive capillary networks, and they are the frequent designation of the 6-hooked larvae cycled in with the blood from the intestine to continue to develop.
During the survey, we also found that the high infection rate can be partially attributed to the lack of understanding of the herdsmen to the life cycle of the worms. Most of herdsmen do not know that dogs are the major sources of the infective stage (=eggs) of the parasite. It is therefore highly recommended that scientific educations and effective measures be undertaken in the regions to improve the awareness of disease prevention and to prevent the spread of the pathogen.
In conclusion, we investigated on the hydatid cyst infection in livestock animals in Xinjiang, China, which provided epidemiological information about hydatid infection in livestock, and is valuable in developing strategies for prevention and control of hydatid disease.
ACKNOWLEDGMENTS
We thank the field staff from Urumqi, Yining, Tacheng, and Altay, Xinjiang Province, China who provided the samples for this study. This work was supported by the "State Special Fund for Agro-Scientific Research in the Public Interest" (grant no. 201303037), National Natural Science Foundation of China (31260601, 31360596), International Science and Technology Cooperation Program of Xinjiang Production and Construction Corps (2012BC006), China.
Footnotes
The authors declare that they do not have any conflict of interest.
References
- 1.Staebler S, Grimm F, Glaus T, Kapel CM, Haller M, Hasler A, Hanosset R, Deplazes P. Serological diagnosis of canine alveolar echinococcosis. Vet Parasitol. 2006;141:243–250. doi: 10.1016/j.vetpar.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Haridy FM, Ibrahim BB, Elshazly AM, Awad SE, Sultan DM, El-Sherbini GT, Morsy TA. Hydatidosis granulosus in Egyptian slaughtered animals in the years 2000-2005. J Egypt Soc Parasitol. 2006;36:1087–1100. [PubMed] [Google Scholar]
- 3.Ibrahim MM. Study of cystic echinococcosis in slaughtered animals in Al Baha region, Saudi Arabia: interaction between some biotic and abiotic factors. Acta Trop. 2010;113:26–33. doi: 10.1016/j.actatropica.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Kassem HH, Gdoura NK. Hydatidosis in camels (Camelus dromedarius) slaughtered at Sirt Abattoir, Libya. J Egypt Soc Parasitol. 2006;36:1–10. [PubMed] [Google Scholar]
- 5.Latif AA, Tanveer A, Maqbool A, Siddiqi N, Kyaw-Tanner M, Traub RJ. Morphological and molecular characterization of Echinococcus granulosus in livestock and humans in Punjab, Pakistan. Vet Parasitol. 2010;170:44–49. doi: 10.1016/j.vetpar.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Lidetul D, Hutchinson GW. The prevalence, organ distribution and fertility of cystic echinoccosis in feral pigs in tropical North Queensland, Australia. Onderstepoort J Vet Res. 2007;74:73–79. doi: 10.4102/ojvr.v74i1.140. [DOI] [PubMed] [Google Scholar]
- 7.Pour AA, Hosseini SH, Shayan P. The prevalence and fertility of hydatid cysts in buffaloes from Iran. J Helminthol. 2012;86:373–377. doi: 10.1017/S0022149X11000514. [DOI] [PubMed] [Google Scholar]
- 8.Bardonnet K, Piarroux R, Dia L, Schneegans F, Beurdeley A, Godot V, Vuitton DA. Combined eco-epidemiological and molecular biology approaches to assess Echinococcus granulosus transmission to humans in Mauritania: occurrence of the 'camel' strain and human cystic echinococcosis. Trans R Soc Trop Med Hyg. 2002;96:383–386. doi: 10.1016/s0035-9203(02)90369-x. [DOI] [PubMed] [Google Scholar]
- 9.Cetinkaya U, Hamamcı B, Kaya M, Gücüyetmez S, Kuk S, Yazar S, Sahin I. Investigation of anti-Echinococcus granulosus antibodies in patients with suspected cystic echinococcosis. Turkiye Parazitol Derg. 2012;36:57–60. doi: 10.5152/tpd.2012.15. [DOI] [PubMed] [Google Scholar]
- 10.Sissay MM, Uggla A, Waller PJ. Prevalence and seasonal incidence of larval and adult cestode infections of sheep and goats in eastern Ethiopia. Trop Anim Health Prod. 2008;40:387–394. doi: 10.1007/s11250-007-9096-z. [DOI] [PubMed] [Google Scholar]
- 11.Tolosa T, Tigre W, Teka G, Dorny P. Prevalence of bovine cysticercosis and hydatidosis in Jimma municipal abattoir, South West Ethiopia. Onderstepoort J Vet Res. 2009;76:323–326. doi: 10.4102/ojvr.v76i3.37. [DOI] [PubMed] [Google Scholar]
- 12.Han XM, Wang H, Cai HX, Ma X, Liu YF, Wei BH, Ito A, Craig PS. Epidemiological survey on echinococcosis in Darlag County of Qinghai Province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27:22–26. (in Chinese) [PubMed] [Google Scholar]
- 13.Yu SH, Wang H, Wu XH, Ma X, Liu PY, Liu YF, Zhao YM, Morishima Y, Kawanaka M. Cystic and alveolar echinococcosis: an epidemiological survey in a Tibetan population in southeast Qinghai, China. Jpn J Infect Dis. 2008;61:242–246. [PubMed] [Google Scholar]
- 14.Zhao YM, Tong SX, Jing T, Chong SG, Cai XP, Jing ZZ, Han J. Investigation on echinococcosis in animals in Gannan Tibetan Autonomous Prefecture. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27:27–30. (in Chinese) [PubMed] [Google Scholar]
- 15.Zheng ZX, Liu L, Li DB, Yang XZ. Epidemiological investigation and analysis on hydatid disease in Dingbian County of Shaanxi from January to August, 2011. J Med Pest Control. 2012;28:907–908. (in Chinese) [Google Scholar]
- 16.Craig PS, Liu D, Ding Z. Hydatid disease in China. Parasitol Today. 1991;7:46–50. doi: 10.1016/0169-4758(91)90188-t. [DOI] [PubMed] [Google Scholar]
- 17.Wu ZT, Zhang HJ, Crystal MK, Neil SC. Climate change and human activities: a case study in Xinjiang, China. Climatic Change. 2010;99:457–472. [Google Scholar]
- 18.MacPherson CN, Karstad L, Stevenson P, Arundel JH. Hydatid disease in the Turkana District of Kenya. III. The significance of wild animals in the transmission of Echinococcus granulosus, with particular reference to Turkana and Masailand in Kenya. Ann Trop Med Parasitol. 1983;77:61–73. [PubMed] [Google Scholar]