Abstract
Fatty acids can function as signaling molecules, acting through receptors in the cytosol or on the cell surface. G-Protein Receptor (GPR)120 is a membrane-bound receptor mediating anti-inflammatory and insulin-sensitizing effects of the omega-3 fatty acid docohexaenoic acid (DHA). GPR120 dysfunction is associated with obesity in humans. Cellular localization of GPR120 and the influence of maternal obesity on GPR120 protein expression in the placenta are unknown. Herein we demonstrate that GPR120 is predominantly expressed in the microvillous membrane (MVM) of human placenta and that the expression level of this receptor in MVM is not altered by maternal body mass index (BMI).
Keywords: Pregnancy, BMI, DHA receptor, FFAR4, O3FAR1
Introduction
Fatty acids are an important source of nutrients and energy, but also act as signaling molecules regulating cell function. In primary human trophoblast cells (PHTs) fatty acids influence inflammatory responses, lipid accumulation, and transport functions [1–5]. Fatty acids can exert cellular effects via several different mechanisms, including receptors on the cell surface. In 2005, the membrane-bound protein GPR120 was identified as a receptor for unsaturated long-chain fatty acids [6]. Subsequently GPR120 has been shown to mediate the anti-inflammatory effects of DHA [7]. In obese individuals adipose tissue GPR120 expression is increased [8] and dysfunction of this receptor is implicated in the pathophysiology of obesity [7–9].
Obesity in pregnancy is associated with increased placental inflammation [10–12], which may be modulated by altered GPR120 signaling. GPR120 is expressed at the mRNA level in the human placenta and placental GPR120 mRNA expression correlates inversely with maternal BMI in male fetuses [13]. However, the cellular localization and influence of fetal or maternal adiposity on placental GPR120 protein expression is currently unknown.
Methods
Placenta collection
Placental tissue was collected with informed written consent (Institutional Review Board approved protocol: HSC20100262H). De-identified placental tissue and relevant medical information were added to a tissue repository. Thirty women with uncomplicated, term pregnancies (>37 weeks of gestation) were selected for this study. All deliveries were by Cesarean-sections performed before onset of labor. Placentas were collected immediately after delivery, decidua basalis and chorionic plate removed, and villous tissue rinsed in ice-cold physiological saline.
Immunohistochemistry
Villous tissue was fixed in formalin, embedded in paraffin, and cut into 5 µm sections. Immunohistochemistry was performed as described previously [14]. The anti-GPR120 antibody was purchased from Abcam (Cambridge, UK; ab97272), diluted in blocking serum (final concentration 10 µg/ml; negative control without primary antibody) and incubated overnight (4°C).
MVM-vesicle isolation
All procedures were performed on ice. Villous tissue was homogenized in ice-cold buffer (250 mM sucrose, 10 mM Hepes, pH 7.4) containing protease and phosphatase inhibitors; isolation of syncytiotrophoblast MVM-vesicles from placental homogenates was accomplished by Mg2+ precipitation [15]. Alkaline phosphatase enrichment was at least tenfold higher in MVM-vesicles compared to homogenates and did not significantly differ between the groups (Table 1).
Table 1.
Clinical Characteristics
| Normal BMI (BMI<25 kg/m2) |
Overweight (BMI 25–30 kg/m2) |
Obese (BMI>30 kg/m2) |
P-value (ANOVA) |
|
|---|---|---|---|---|
| Mother | ||||
| N | 10 | 10 | 10 | - |
| Age | 27.7 ± 1.8 | 27.4 ± 1.4 | 27.0 ± 1.9 | 0.96 |
| BMI | 21.6 ± 0.7 | 26.8 ± 0.4** | 36.3 ± 1.3*** | <0.0001 |
| Ethnicity (% Hispanic) | 70% | 70% | 80% | - |
| Newborn | ||||
| GA at delivery | 39.3 ± 0.3 | 39.1 ± 0.1 | 39.5 ± 0.3 | 0.64 |
| Fetal sex (female/male) | 5/5 | 5/5 | 5/5 | - |
| Birth weight (g) | 3326 ± 79 | 3477 ± 175 | 3701 ± 120 | 0.14 |
| Birth length (cm) | 51.0 ± 0.5 | 50.4 ± 0.7 | 51.2 ± 0.6 | 0.68 |
| Ponderal Index (100 × g/cm) | 2.5 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 0.10 |
| Placenta | ||||
| Weight (g) | 718 ± 61 | 766 ± 46 | 804 ± 49 | 0.52 |
| Alk. Phos. † activity | 14.9 ± 1.7 | 16.4 ± 1.8 | 14.6 ± 0.6 | 0.66 |
Data are presented as mean ± SEM. Maternal BMI based on pre-pregnancy or first trimester weight.
P<0.01 vs. Normal BMI;
P<0.001 vs. Normal BMI and Overweight evaluated by one-way ANOVA followed by Tukey’s post hoc test. GA, gestational age;
Alk. Phos., alkaline phosphatase activity enrichment in isolated MVM-vesicles compared to placental homogenate.
Western blot
Western blots were performed on pre-cast gels (BioRad, Hercules, CA) and proteins transferred to PVDF membranes. Membranes were stained for total protein with Amido Black stain (Sigma-Aldrich, St. Louis, MO) [16], blocked in 5% non-fat milk, and probed with anti- GPR120 antibody (ab97272, Abcam; final concentration 1µg/ml) overnight (4°C). Immunolabeling was visualized with peroxidase-labeled secondary antibody and SuperSignal Dura West detection solution (Thermo Scientific, Rockford, IL) in a G:Box (Syngene, Cambridge, UK). GPR120 expression was adjusted for total protein loaded.
Statistics
Statistical differences were evaluated by t-test, one-way ANOVA (Tukey’s post-hoc test) or Pearson’s correlation using GraphPad Prism 5 (La Jolla, CA). P<0.05 was considered significant.
Results and Discussion
Maternal, newborn, and placental characteristics did not differ between the three groups, except for maternal BMI which by design was significantly different (P<0.001; Table 1). Newborn ponderal index (r=0.437, P<0.05) correlated positively with maternal BMI.
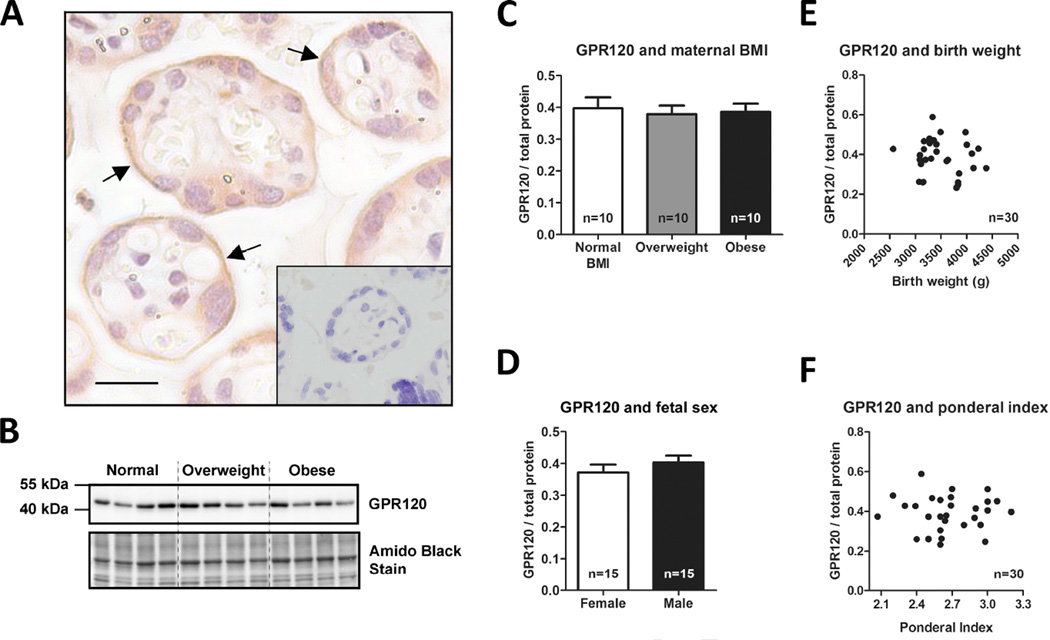
Immunohistochemical staining of GPR120 was predominantly observed in the MVM (Figure 1A). This suggests that fatty acids in the maternal circulation have direct access to GPR120 in the syncytiotrophoblast and could affect trophoblast signaling through this receptor.
Figure 1. GPR120 is expressed in the MVM of human placenta.
Immunohistochemistry of GPR120 in human term placenta (A); MVM (black arrow); scale bar 20 µm; negative control shown as insert. Nuclei stained with hematoxylin. Representative Western blots of isolated MVM vesicles (B). GPR120 expression in isolated MVM vesicles adjusted for total protein stain: data grouped according to maternal pre-/early-pregnancy BMI (C) or fetal sex (D). Data are presented as mean + SEM. Correlations between GPR120 expression in isolated MVM vesicles and birth weight (E) or newborn ponderal index (F).
GPR120 was detected as a band at ~42–43 kDa (Figure 1B). Because obesity is associated with altered GPR120 expression in human adipose tissue [8], we investigated the effect of maternal BMI on GPR120 MVM-expression. However, maternal adiposity does not influence GPR120 MVM-expression, as expression levels were similar between placentas from normal weight, overweight, and obese women (Figure 1C). This finding is inconsistent with a previous report showing that placental GPR120 mRNA expression in male fetuses correlate inversely with maternal BMI [13]; suggesting that whole tissue mRNA expression does not reflect membrane-bound protein levels of this receptor. Even if MVM GPR120 protein expression is unaffected by maternal obesity, there are numerous mechanisms by which GPR120 signaling could modulate the placental inflammation reported in pregnancies complicated by obesity [10–12]. For example, maternal obesity is likely to be associated with changes in circulating levels of endogenous ligands, the GPR120 receptor may be dysfunctional as reported in adipose tissue of obese individuals [8], or the intracellular signaling pathway may be impaired. In contrast to the reported differential effects of fetal sex on placental GPR120 mRNA expression [13], GPR120 MVM-expression was unaffected by fetal sex (Figure 1D). Furthermore, GPR120 MVM-expression levels were not associated with fetal adiposity (estimated by ponderal index) or birth weight (Figure E–F).
In conclusion, GPR120 is expressed primarily in the MVM of human, term placenta. This observation suggests that fatty acids in the maternal circulation could affect trophoblast cellular signaling mediated by GPR120 receptor activation. The functional importance and downstream effects of activating this receptor in the placenta remains to be determined.
We show that the fatty acid receptor GPR120 is expressed in human placenta
GPR120 is predominantly localized to the MVM of the syncytiotrophoblast
Expression of placental GPR120 is not affected by maternal or fetal characteristics
Acknowledgements
We are grateful to E. Miller, patients, and staff at Labor & Delivery, University Hospital for placenta collection, E. Dudley (technical assistance), and W.G.D. Frederick II (valuable comments on manuscript). Grant support: NIDDK R01DK089989 (T.L.P.), Swedish Research Council (S.L.), and Swedish Society of Endocrinology (S.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magnusson-Olsson AL, Lager S, Jacobsson B, Jansson T, Powell TL. Effect of maternal triglycerides and free fatty acids on placental LPL in cultured primary trophoblast cells and in a case of maternal LPL deficiency. Am J Physiol Endocrinol Metab. 2007;293(1):E24–E30. doi: 10.1152/ajpendo.00571.2006. [DOI] [PubMed] [Google Scholar]
- 2.Scifres CM, Chen B, Nelson DM, Sadovsky Y. Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts. J Clin Endocrinol Metab. 2011;96(7):E1083–E1091. doi: 10.1210/jc.2010-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathmaperuma AN, Mana P, Cheung SN, Kugathas K, Josiah A, Koina ME, Broomfield A, Delghingaro-Augusto V, Ellwood DA, Dahlstrom JE, Nolan CJ. Fatty acids alter glycerolipid metabolism and induce lipid droplet formation, syncytialisation and cytokine production in human trophoblasts with minimal glucose effect or interaction. Placenta. 2010;31(3):230–239. doi: 10.1016/j.placenta.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Elchalal U, Schaiff WT, Smith SD, Rimon E, Bildirici I, Nelson DM, Sadovsky Y. Insulin and fatty acids regulate the expression of the fat droplet-associated protein adipophilin in primary human trophoblasts. Am J Obstet Gynecol. 2005;193(5):1716–1723. doi: 10.1016/j.ajog.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Lager S, Gaccioli F, Ramirez VI, Jones HN, Jansson T, Powell TL. Oleic Acid Stimulates System A Amino Acid Transport in Primary Human Trophoblast Cells Mediated by Toll-Like Receptor 4. J Lipid Res. 2013;54(3):725–733. doi: 10.1194/jlr.M033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 7.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin MR, Korner A, Kiess W, Pigeyre M, Caiazzo R, Van Hul W, Van Gaal L, Horber F, Balkau B, Levy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu TA, Wolowczuk I, Tsujimoto G, Froguel P. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483(7389):350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 9.Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol Sci. 2011;32(9):543–550. doi: 10.1016/j.tips.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, Hor K, Jabbour HN, Norman JE, Denison FC. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32(3):247–254. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Aye ILMH, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, Powell TL. Activation of distinct placental inflammatory signaling pathways in maternal obesity. Biol Reprod. 2014 doi: 10.1095/biolreprod.113.116186. Appected for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westhoff G, Grayson B, Barker D, Morris C, Thornburg K, O'Tierney P. The expression of the omega-3 fatty acid receptor, GPR120, is more sensitive to inflammatory cytokines and maternal obesity in male infants than in females. American Journal of Obstetrics and Gynecology. 2012;206(1):S360-S. Abstract. [Google Scholar]
- 14.Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod. 2005;20(2):521–530. doi: 10.1093/humrep/deh596. [DOI] [PubMed] [Google Scholar]
- 15.Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta. 1990;1029(2):218–226. doi: 10.1016/0005-2736(90)90157-j. [DOI] [PubMed] [Google Scholar]
- 16.Lanoix D, St-Pierre J, Lacasse AA, Viau M, Lafond J, Vaillancourt C. Stability of reference proteins in human placenta: general protein stains are the benchmark. Placenta. 2012;33(3):151–156. doi: 10.1016/j.placenta.2011.12.008. [DOI] [PubMed] [Google Scholar]