Abstract
Cardiac function analysis is the main focus of echocardiography. Left ventricular ejection fraction (LVEF) has been the clinical standard, however, LVEF is not enough to investigate myocardial function. For the last decade, speckle tracking echocardiography (STE) has been the novel clinical tool for regional and global myocardial function analysis. However, 2-dimensional imaging methods have limitations in assessing 3-dimensional (3D) cardiac motion. In contrast, 3D echocardiography also has been widely used, in particular, to measure LV volume measurements and assess valvular diseases. Joining the technology bandwagon, 3D-STE was introduced in 2008. Experimental studies and clinical investigations revealed the reliability and feasibility of 3D-STE-derived data. In addition, 3D-STE provides a novel deformation parameter, area change ratio, which have the potential for more accurate assessment of overall and regional myocardial function. In this review, we introduced the features of the methodology, validation, and clinical application of 3D-STE based on our experiences for 7 years.
Keywords: Three-dimensional echocardiography, Speckle tracking, Cardiac function
Introduction
For the last decade, speckle tracking echocardiography (STE) has been focused as a novel method in assessing cardiac chamber and regional myocardial functions. A numerical study reported the additional values of STE independent left ventricular ejection fraction (LVEF).1),2) Simultaneously, 3-dimensional (3D) echocardiography has been widely used in the clinical setting, and the trend of 3D imaging introduced commercially available 3D-STE systems.3),4),5),6),7) 3D-STE has not been a routine tool for cardiac function analysis, despite of various advantages and potentials compared to 2-dimensional (2D) imaging.
I often have been asked about the merits of 3D-STE in the clinical setting. Exactly, the advantage of 3D-STE should be clarified compared to 2D-STE and traditional Doppler echocardiographic assessments. Through this review, I try to summarize the current status of 3D-STE mainly based on our studies and clinical experiences.
Fundamental Preparation for 3D-STE Analysis
3D-speckle tracking
Of wall motion tracking methods of 3D-STE including the block-matching method, the elastic image registration method, and model-based approach,7) the block-matching method is solely used as the tracking method on the commercial available 3D-STE systems (Fig. 1).3),8),9) The motion vector between consecutive volume frames is detected by cubic matching technique.8) Comparing a template volume in the next volume searches the most similar point in the next volume. Finally, interpolation of the motion vectors is performed with a 3D interpolation algorithm. After these steps, arbitrary points of interest on the cardiac wall can be tracked by integrating the interpolated motions over all frames during one cardiac cycle.
Fig. 1.
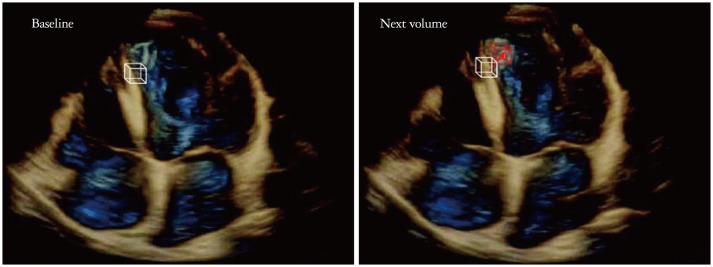
Speckle tracking with cubic template. The 3-dimensional speckle tracking method of the volume of interest (white line cubic template) from one volume (baseline volume) to the next volume (red line cubic template).
Data acquisitions
For 3D wall motion analysis, full-volume dataset is required. Multiple sectors are scanned with multiple beats and each dataset is automatically integrated into a wide-angle pyramidal data image covering the entire left ventricle.3) In the current available systems, the volume rate of each sector image has been increased at 30 Hz or more, which is enough to analysis LV function.10)
Automatic tracing
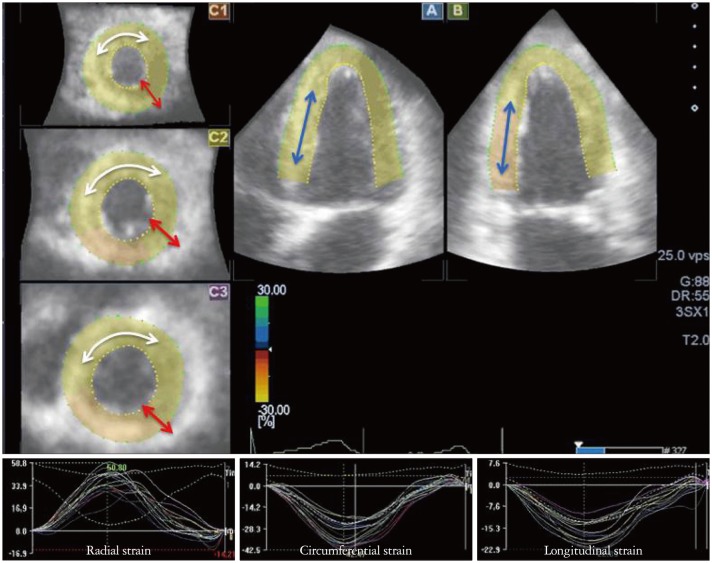
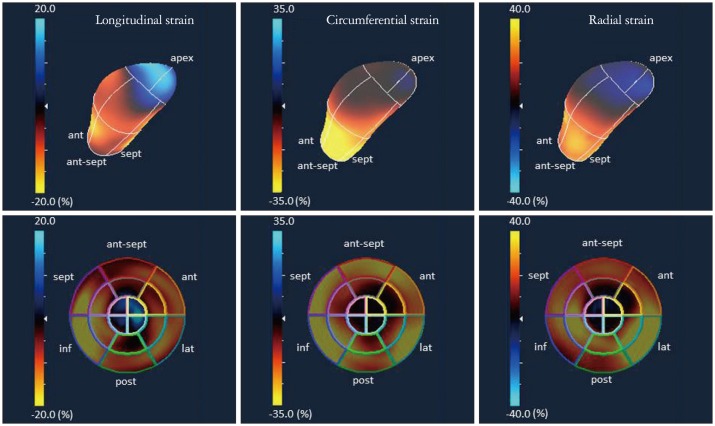
In general, data analysis is performed on the off-line system. The regions of interest are determined on the multiplanar reconstruction images (Fig. 2). Instead of manual tracing, automatic tracing of borders has been promising. The software automatically divides the left ventricle into 16 or 17 segments, and provides time-parameters curves for the assessment of magnitude of regional strain and timing of the maximum or minimum strain value.11)
Fig. 2.
Parametric images of multiplanar reconstruction (MPR) and strain-time curves. On MPR images (upper panel), radial (red arrow), circumferential (white arrow), and longitudinal strain (blue arrow) are shown. Under panels show each strain-time curve.
3D-STE Derived Deformation Parameters
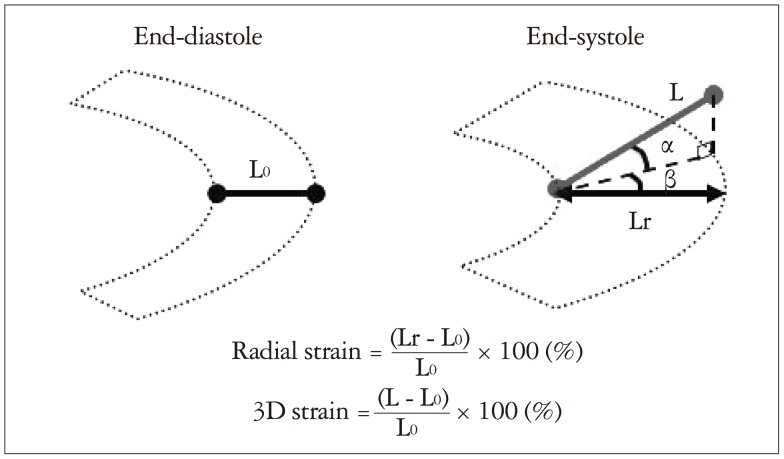
3D-STE can simultaneously provide the three orthogonal strain values, radial strain (RS), longitudinal strain (LS), and circumferential strain (CS) (Fig. 2).3),12) Since beat to beat variabilities of LV contraction may affect the strain measurements, it is important to obtain the multiple deformation indices in the same beat. Secondly, deformation parameters with 3D features are available; 3D-strain (Fig. 3) and the area change ratio (ACR) or area strain in a regional or the entire tracking area (Fig. 4).13) In this review, the area tracking-based deformation parameter is called as ACR since the strain means changes of a unit length, not an area. The ACR of the endocardium is attracting interest as a new measurement parameter along with the components of CS and LS. In addition, more accurate LV torsion values can be calculated through the simultaneous measures of rotations in each short-axis plane and the distance between the planes. Therefore, the novel parameter regional torsion is measureable, which represents the spatial distribution of torsion values.
Fig. 3.
3D-strain. The figures show deformation including shear strain. L0 means a baseline length between endo- and epicardium. Radial strain is calculated as (Lr - L0) / L0. If the shear (α degree) is caused between radial and longitudinal direction, the shear (β degree) is caused between radial and circumferential direction, and without shear between circumferential and longitudinal direction, the length between estimated endo- and epicardial points at end-systole is L. The novel parameter 3D-strain is calculated as (L - L0) / L0, then, 3D-strain is greater than 3D-radial strain because L is longer than Lr.
Fig. 4.
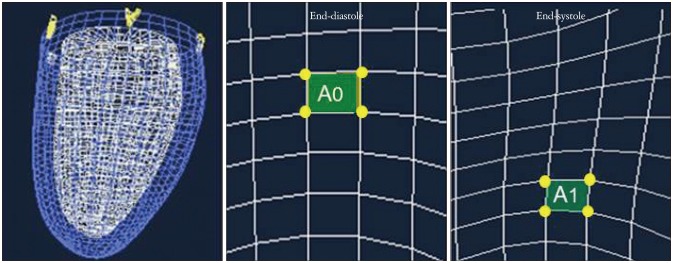
Area change ratio. The mid and right panels are the endocardial surface at end-diastole and systole, respectively. An area A0 is relocated and deformed to an area A1 by various wall motions including apical translation, regional rotation that causes shear strain, and longitudinal and circumferential contractions. The area change ratio is calculated as (A1 - A0) / A0.
Validation Studies for 3D-STE
3D-STE is a novel method using complex computations, therefore, validation studies are needed to identify the reliability and feasibility, and have been performed using experimental animal models.8),9),13),14),15)
Strain measurements
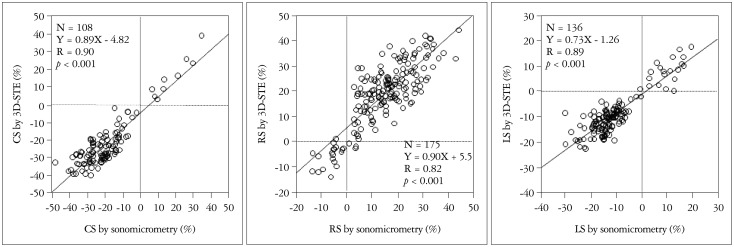
We performed the first validation study for the block matching method in the world, and found strong correlations between strain measurements by 3D-STE and sonomicrometry (LS: r = 0.89, RS: r = 0.84, CS: r = 0.90) (Fig. 5).8) However, CS and LS showed better accuracy for estimation of regional deformation than did RS. This discrepancy is due to each strain nature; LS and CS are calculated at the endocardial border, whereas RS is estimated by tracking of both the endocardial and epicardial borders. Since current 3D-STE systems increase the tracking errors in the epicardial regions, accuracy of RS may be lower than LS and CS. In addition, because the fundamental length for RS calculation is shorter than those of LS and CS, RS is more vulnerable to tracking errors.
Fig. 5.
The relation between strain by sonomicrometry and 3-dimensional speckle tracking echocardiography (3D-STE). CS: circumferential strain, RS: radial strain, LS: longitudinal strain.
Area change ratio
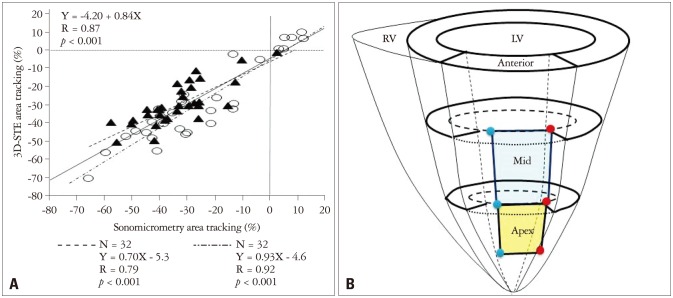
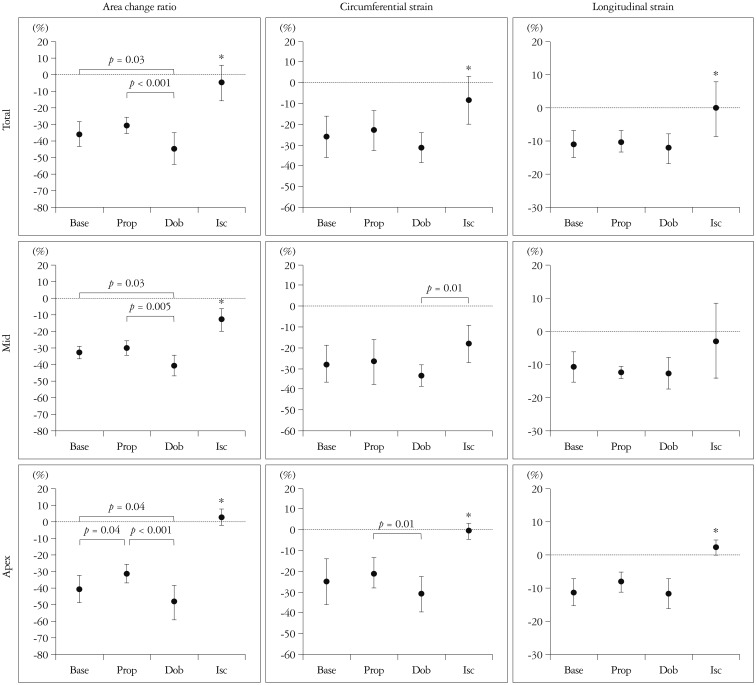
The second validation study was performed for ACR in a similar experimental study,13) and ACR measurements by 3D-STE were found to strongly correlate with those by sonomicrometry (r = 0.87) (Fig. 6). Interestingly, ACR was more sensitive to the changes of regional deformation compared to LS and CS (Fig. 7). The sensitiveness of ACR may be attributed by combined deformation data of LS and CS, since ACR can be calculated as a total sum of the product of LS and CS and the sum of them as follows; ACR = LS · CS + (LS + CS). As compared to 2D-STE, 3D-STE may cause tracking errors because of lower spatiotemporal resolutions. Therefore, combined data for 2 directional deformations can reduce the tracking error because of higher signal-noise ratio compared with that of LS and CS. Thus, ACR is more useful parameter to assess myocardial deformation.
Fig. 6.
The relation between area change ratio by sonomicrometry and 3-dimensional speckle tracking echocardiography. A: Scatter plot showing the relation between all measurements of area tracking by sonomicrometry and 3-dimensional speckle tracking echocardiography (3D-STE). The solid line shows a regression line of all measurements. The dashed dotted line shows a regression line at apex area (○). The dashed line shows a regression line at mid area (▴). B: Scheme of implanted positions of sonomicrometry crystals. Red and blue dots show each crystal position, and the blue area is a region area of mid anterior wall, and the yellow one is a region of apical one.
Fig. 7.
Comparisons of area change ratio, circumferential strain, and longitudinal strain during baseline (Base), propranolol infusion (Prop), dobutamine infusion (Dob), and acute ischemia (Isc). Upper panels are corresponding to total data (Total), mid ones to mid anterior wall (Mid), and bottom ones to apical anterior wall (Apex). Area change ratio clearly distinguished changes in myocardial function induced by pharmacological stress and acute ischemia in both mid and apical anterior wall. *p < 0.001 vs. others.
Advantages and Pit Fall of 3D-STE
LV volume and EF measurements
Since 3D-STE tracking is done on the LV endocardial boundaries, 3D-STE can directly measure LV volume through a cardiac cycle.16),17) We reported that as compared to volume measurements by cardiac magnetic resonance imaging (MRI), 3D-STE derived measurements correlated well with those by cardiac MRI (end-diastolic volume: r = 0.80, end-diastolic volume: r = 0.86), which slightly underestimated (LV end-diastolic volume: bias = -18 ± 37 mL; LV end-systolic volume: bias = -10 ± 34 mL).16)
LV global strain
Global LV function has been solely assessed by LVEF. However, numerical previous studies reported that 2D-STE derived LV global strain has been reported as a novel useful parameter of global LV function. The findings mean that global deformation parameters have a potential to assess intrinsic myocardial function compared to LVEF.
What are the differences between 3D-STE and 2D-STE derived strain values?
Previous studies reported that absolute CS values by 3D-STE are significantly higher than those by 2D-STE.18),19),20),21),22),23) The differences are affected by the out of plane phenomenon (Fig. 8). In our study, the absolute differences between 3D-STE and 2D-STE derived CS correlated significantly with the magnitude of longitudinal displacement (Fig. 9).24) In normal subjects, the differences were more significant in the free wall, where longitudinal displacements are larger than those in the septum. In contrast, the discrepancy was not apparent in patients with LV dysfunction with reduced longitudinal displacements. Previous studies reported LS values do not differ between 3D- and 2D-STE.19),20),21),22),23) However, Wu et al.22) reported LV twisting affects the LS values: larger twisting is a major determinant of differences between 2D and 3D LS values.
Fig. 8.
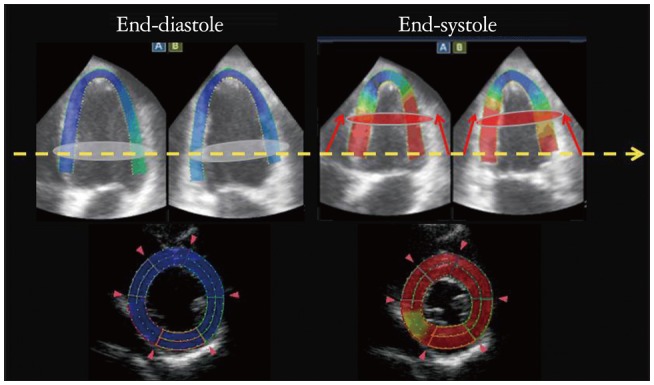
Out of plane phenomenon. Upper panels are 4 chamber and 2 chamber views at end-diastole and systole in a healthy subject. The color shows degree of longitudinal displacements during systole. White discs at end-diastole are moved to red discs level at end-systole, which distance is about 15 mm (red arrows). Lower panels show the 2-dimensional speckle tracking echocardiography (2D-STE) images at yellow dash arrow level. Since the white disc level is moved to apex at end-systole, the 2D-STE image at end-systole is changed to another plane of a basal level at end-diastole. Such changing of the plane of interest through a cardiac cycle is called as out of plane phenomenon.
Fig. 9.
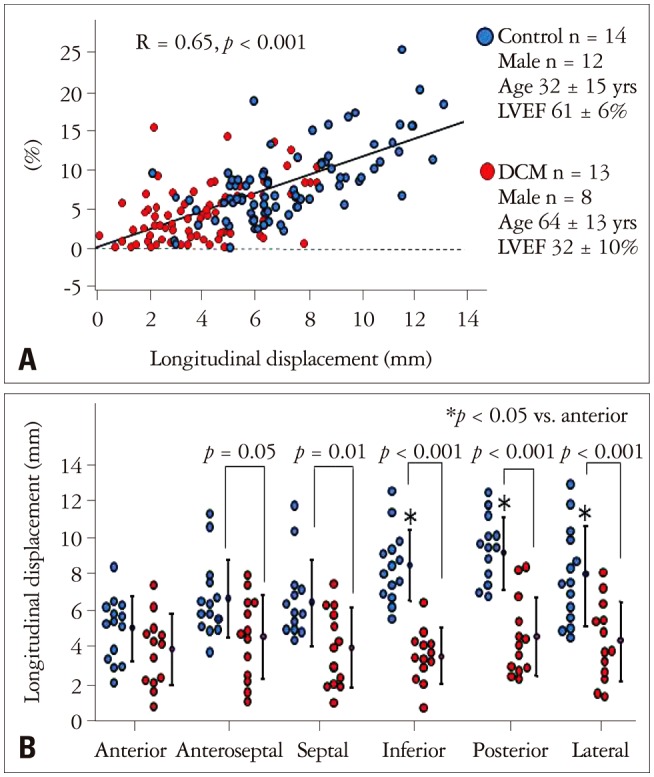
The affects of longitudinal displacements for the 2D- and 3D-circumferential strain (CS). Panel A shows the correlation between longitudinal displacement and the absolute differences between 2D- and 3D-CS. Panel B shows left ventricular segmental comparisons of longitudinal displacements between control (blue dots) and DCM (red dots). DCM: idiopathic dilated cardiomyopathy, LVEF: left ventricular ejection fraction.
Based on the findings, 3D-STE can overcome LV translation and rotation effects for the quantification of ventricular contraction, and may provide reliable deformation data compared to 2D-STE.
Usefulness of ACR
ACR is a unique parameter with 3D imaging nature. Previous studies reported the usefulness of ACR to detect subclinical LV dysfunction in the clinical studies; ACR was reduced early stage heart failure or patients with early stage aortic valve diseases.25),26),27) Miyoshi et al.28) reported that reduced ACR was associated with the use of anthracycline even in patients with preserved LVEF. Thus, 3D-STE derived ACR may be a sensitive biomarker to detect early LV systolic dysfunction.
LV regional wall motion abnormality
An interesting study showed the disagreements (55%) between ACR and visual assessment in assessing hypokinesis, despite of high agreements in 91% of akinetic segments.29) Therefore, the quantifications of regional wall motion may contribute to accurate judgments of wall motion abnormalities. In addition, 3D imaging can confirm the extend of regions with wall motion abnormalities showing impressive images (Fig. 10).8),30) However, large scale studies have not been performed, and the superiorities of 3D-STE has not been established as compared to eyeball judgments or 2D-STE.
Fig. 10.
Regional wall motion abnormalities with 3-dimensional speckle tracking echocardiography. These images were obtained at experimental studies. Upper panels show plastic bag images and lower ones show polar map images at end-systole showing longitudinal strain (left panel), circumferential strain (central panel), and radial strain (right panel) during a coronary artery occlusion study. The blue area in the apex in each panel corresponds to dyskinetic motion induced by coronary artery occlusion.
Dyssynchrony
LV dyssynchrony have been studied in assessing cardiac resynchronization therapy responses during a last decade. Catheter based electrical 3D mapping system can clearly show the propagation of electrical activation. Recently, we have developed 3D-STE based activation imaging system, which is modeled after the electrical 3D mapping systems.31)
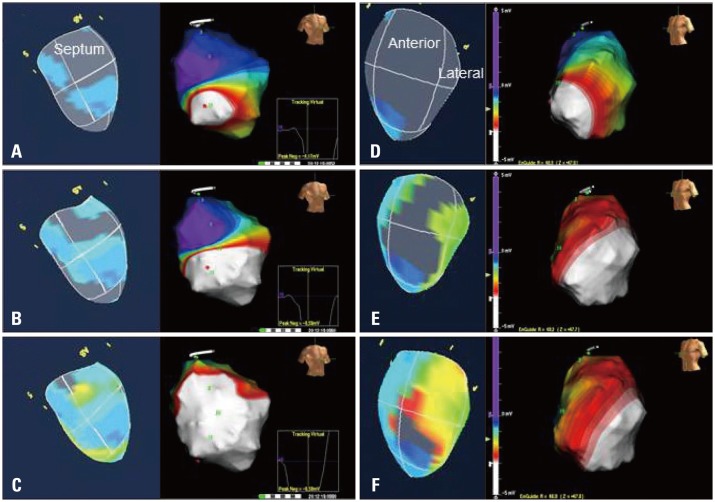
The activation imaging can visualize the intra-ventricular propagation pattern of regional contractions, which reliability was validated in comparison studies with electrical 3D mapping system (Fig. 11). For example, in the patients with left bundle branch block, the initial contraction is observed in the mid to apical septum. Subsequently, the contraction propagates to the basal lateral wall turning at the apex. Previous studies with an electrical 3D mapping system revealed the propagation pattern is the typical dyssynchrony pattern of left bundle branch block.32) Therefore, 3D-STE may provide a breakthrough in assessment studies for dyssynchrony.
Fig. 11.
Comparisons of activation propagation in left bundle branch block. Left figures show activation images by 3-dimensional speckle tracking echocardiography (3D-STE) and right ones show propagation images by Ensite voltage mapping system in each panel from A to F. Panels A to C show the images from septum, and blue areas in the 3D-STE are the earliest contraction sites. White areas in the Ensite images are the electrical activation area. Panels D to F show the images from free wall, and yellow or orange areas are the latest contraction sites. Note the U shape propagation with functional block area in anterior wall.
Right ventricular function analysis
Right ventricular (RV) function has a key role in patients with left heart failure.33),34),35) Because of the complex RV geometry, 3D imaging may be more useful to analysis global and regional function in right ventricle than left ventricle. Our colleagues reported a preliminary study of RV 3D-STE, which used a modified system for the left ventricle (Fig. 12).36) In particular, we found intraventricular differences in the systolic ACR values and dyssynchrony in normal control subjects. The 3D-STE system could reveal lower ACR in patients with RV dysfunction than normal control. In future, a RV specific 3D-STE system will be introduced.
Fig. 12.
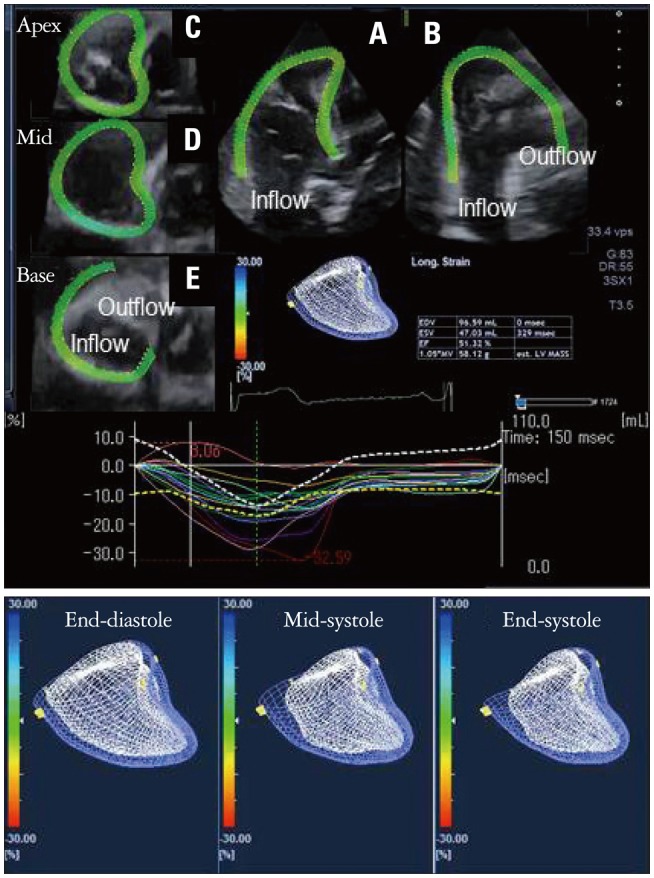
Right ventricular 3-dimensional speckle tracking echocardi-ography (3D-STE) images. Panels A to E show right ventricular (RV) multiplanar reconstruction images in a healthy subject; short axis view of apex (C), mid (D), base (E), apical 4 chamber view (A) and its orthogonal view (B). 3D-STE images using mesh imaging, and time area change ratio curves also are shown. The lower left panel shows the RV-STE at end-diastole, the center panel at mid-systole, and the right panel at end-systole.
Current Issues of 3D-STE Systems
Tracking depends on image quality
B-mode image quality strongly affects accuracy of STE measurements, which is the common issue of STE. In general, 3D data sets are comprised of multiple sectors, and stitching noise may be caused around the border between sectors. In addition, the multi beats system is not available in patients with atrial fibrillation. Recent advanced systems with a single beat might overcome the limitations. However, the spatiotemporal resolution might be deteriorated in such a system, therefore, reliable validation data should be reported to use the advanced system in the clinical setting.
Vendor dependency
A comparison study between vendors reported the vendor-dependent variability of deformation data.37) Due to the high vendor dependence of 3D-STE data, inter-vendor discordance of data must be taken into account when interpreting 3D deformation data. However, further studies are needed to confirm whether vendor differences have clinical impacts in assessing cardiac function and to discriminate the risk level.
Conclusions
3D-STE has great promise as a clinical feasible tool for evaluating myocardial function, and could provide novel pathophysiological insights based on 3D cardiac mechanics.
The visibility of 3D-STE is steadily increasing in the study field, however, the clinical uses are not enough. Therefore, information about the current status of 3D-STE should be spread, and we thank for the opportunity of this review.
References
- 1.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234–243. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Kawagishi T. Speckle tracking for assessment of cardiac motion and dyssynchrony. Echocardiography. 2008;25:1167–1171. doi: 10.1111/j.1540-8175.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- 4.Elen A, Choi HF, Loeckx D, Gao H, Claus P, Suetens P, Maes F, D'hooge J. Three-dimensional cardiac strain estimation using spatio-temporal elastic registration of ultrasound images: a feasibility study. IEEE Trans Med Imaging. 2008;27:1580–1591. doi: 10.1109/TMI.2008.2004420. [DOI] [PubMed] [Google Scholar]
- 5.Crosby J, Amundsen BH, Hergum T, Remme EW, Langeland S, Torp H. 3-D speckle tracking for assessment of regional left ventricular function. Ultrasound Med Biol. 2009;35:458–471. doi: 10.1016/j.ultrasmedbio.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Takeguchi T, Nishiura M, Abe Y, Ohuchi H, Kawagishi T. Practical considerations for a method of rapid cardiac function analysis based on three-dimensional speckle tracking in a three-dimensional diagnostic ultrasound system. J Med Ultrason. 2010;37:41–49. doi: 10.1007/s10396-009-0249-8. [DOI] [PubMed] [Google Scholar]
- 7.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. quiz 453-5. [DOI] [PubMed] [Google Scholar]
- 8.Seo Y, Ishizu T, Enomoto Y, Sugimori H, Yamamoto M, Machino T, Kawamura R, Aonuma K. Validation of 3-dimensional speckle tracking imaging to quantify regional myocardial deformation. Circ Cardiovasc Imaging. 2009;2:451–459. doi: 10.1161/CIRCIMAGING.109.858480. [DOI] [PubMed] [Google Scholar]
- 9.Duan Q, Parker KM, Lorsakul A, Angelini ED, Hyodo E, Homma S, Holmes JW, Laine AF. Quantitative validation of optical flow based myocardial strain measures using sonomicrometry. Proc IEEE Int Symp Biomed Imaging. 2009;2009:454–457. doi: 10.1109/ISBI.2009.5193082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yodwut C, Weinert L, Klas B, Lang RM, Mor-Avi V. Effects of frame rate on three-dimensional speckle-tracking-based measurements of myocardial deformation. J Am Soc Echocardiogr. 2012;25:978–985. doi: 10.1016/j.echo.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Jasaityte R, Heyde B, D'hooge J. Current state of three-dimensional myocardial strain estimation using echocardiography. J Am Soc Echocardiogr. 2013;26:15–28. doi: 10.1016/j.echo.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Seo Y, Ishizu T, Enomoto Y, Sugimori H, Aonuma K. Endocardial surface area tracking for assessment of regional LV wall deformation with 3D speckle tracking imaging. JACC Cardiovasc Imaging. 2011;4:358–365. doi: 10.1016/j.jcmg.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Heyde B, Bouchez S, Thieren S, Vandenheuvel M, Jasaityte R, Barbosa D, Claus P, Maes F, Wouters P, D'Hooge J. Elastic image registration to quantify 3-D regional myocardial deformation from volumetric ultrasound: experimental validation in an animal model. Ultrasound Med Biol. 2013;39:1688–1697. doi: 10.1016/j.ultrasmedbio.2013.02.463. [DOI] [PubMed] [Google Scholar]
- 15.Ashraf M, Zhou Z, Nguyen T, Ashraf S, Sahn DJ. Apex to base left ventricular twist mechanics computed from high frame rate two-dimensional and three-dimensional echocardiography: a comparison study. J Am Soc Echocardiogr. 2012;25:121–128. doi: 10.1016/j.echo.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamura R, Seo Y, Ishizu T, Atsumi A, Yamamoto M, Machino-Ohtsuka T, Nakajima H, Sakai S, Tanaka YO, Minami M, Aonuma K. Feasibility of left ventricular volume measurements by three-dimensional speckle tracking echocardiography depends on image quality and degree of left ventricular enlargement: validation study with cardiac magnetic resonance imaging. J Cardiol. 2014;63:230–238. doi: 10.1016/j.jjcc.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Nesser HJ, Mor-Avi V, Gorissen W, Weinert L, Steringer-Mascherbauer R, Niel J, Sugeng L, Lang RM. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: comparison with MRI. Eur Heart J. 2009;30:1565–1573. doi: 10.1093/eurheartj/ehp187. [DOI] [PubMed] [Google Scholar]
- 18.Saito K, Okura H, Watanabe N, Hayashida A, Obase K, Imai K, Maehama T, Kawamoto T, Neishi Y, Yoshida K. Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. J Am Soc Echocardiogr. 2009;22:1025–1030. doi: 10.1016/j.echo.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Maffessanti F, Nesser HJ, Weinert L, Steringer-Mascherbauer R, Niel J, Gorissen W, Sugeng L, Lang RM, Mor-Avi V. Quantitative evaluation of regional left ventricular function using three-dimensional speckle tracking echocardiography in patients with and without heart disease. Am J Cardiol. 2009;104:1755–1762. doi: 10.1016/j.amjcard.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 20.Hayat D, Kloeckner M, Nahum J, Ecochard-Dugelay E, Dubois-Randé JL, Jean-François D, Guéret P, Lim P. Comparison of real-time three-dimensional speckle tracking to magnetic resonance imaging in patients with coronary heart disease. Am J Cardiol. 2012;109:180–186. doi: 10.1016/j.amjcard.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Tanaka H, Kaneko A, Ryo K, Fukuda Y, Tatsumi K, Kawai H, Hirata K. Contractile reserve assessed by three-dimensional global circumferential strain as a predictor of cardiovascular events in patients with idiopathic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25:1299–1308. doi: 10.1016/j.echo.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Wu VC, Takeuchi M, Otani K, Haruki N, Yoshitani H, Tamura M, Abe H, Lin FC, Otsuji Y. Effect of through-plane and twisting motion on left ventricular strain calculation: direct comparison between two-dimensional and three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2013;26:1274–1281.e4. doi: 10.1016/j.echo.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Luo XX, Fang F, Lee AP, Sun JP, Li S, Zhang ZH, Sanderson JE, Kwong JS, Zhang Q, Wang J, Yu CM. What can three-dimensional speckle-tracking echocardiography contribute to evaluate global left ventricular systolic performance in patients with heart failure? Int J Cardiol. 2014;172:132–137. doi: 10.1016/j.ijcard.2013.12.314. [DOI] [PubMed] [Google Scholar]
- 24.Seo Y, Ishizu T, Atsumi A, Kawamura R, Aonuma K. Three-dimensional speckle tracking echocardiography. Circ J. 2014;78:1290–1301. doi: 10.1253/circj.cj-14-0360. [DOI] [PubMed] [Google Scholar]
- 25.Ishizu T, Seo Y, Kameda Y, Kawamura R, Kimura T, Shimojo N, Xu D, Murakoshi N, Aonuma K. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension. 2014;63:500–506. doi: 10.1161/HYPERTENSIONAHA.113.02149. [DOI] [PubMed] [Google Scholar]
- 26.Galderisi M, Esposito R, Schiano-Lomoriello V, Santoro A, Ippolito R, Schiattarella P, Strazzullo P, de Simone G. Correlates of global area strain in native hypertensive patients: a three-dimensional speckle-tracking echocardiography study. Eur Heart J Cardiovasc Imaging. 2012;13:730–738. doi: 10.1093/ehjci/jes026. [DOI] [PubMed] [Google Scholar]
- 27.Li CM, Li C, Bai WJ, Zhang XL, Tang H, Qing Z, Li R. Value of three-dimensional speckle-tracking in detecting left ventricular dysfunction in patients with aortic valvular diseases. J Am Soc Echocardiogr. 2013;26:1245–1252. doi: 10.1016/j.echo.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi T, Tanaka H, Kaneko A, Tatsumi K, Matsumoto K, Minami H, Kawai H, Hirata KI. Left Ventricular Endocardial Dysfunction in Patients with Preserved Ejection Fraction after Receiving Anthracycline. Echocardiography. 2013 doi: 10.1111/echo.12473. {Epub ahead of print} [DOI] [PubMed] [Google Scholar]
- 29.Kleijn SA, Aly MF, Terwee CB, van Rossum AC, Kamp O. Three-dimensional speckle tracking echocardiography for automatic assessment of global and regional left ventricular function based on area strain. J Am Soc Echocardiogr. 2011;24:314–321. doi: 10.1016/j.echo.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Baccouche H, Maunz M, Beck T, Fogarassy P, Beyer M. Echocardiographic assessment and monitoring of the clinical course in a patient with Tako-Tsubo cardiomyopathy by a novel 3D-speckle-tracking-strain analysis. Eur J Echocardiogr. 2009;10:729–731. doi: 10.1093/ejechocard/jep064. [DOI] [PubMed] [Google Scholar]
- 31.Seo Y, Yamasaki H, Kawamura R, Ishizu T, Igarashi M, Sekiguchi Y, Tada H, Aonuma K. Left ventricular activation imaging by 3-dimensional speckle-tracking echocardiography. Comparison with electrical activation mapping. Circ J. 2013;77:2481–2489. doi: 10.1253/circj.cj-13-0380. [DOI] [PubMed] [Google Scholar]
- 32.Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, Kloss M, Klein H. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109:1133–1139. doi: 10.1161/01.CIR.0000118502.91105.F6. [DOI] [PubMed] [Google Scholar]
- 33.Kjaergaard J, Akkan D, Iversen KK, Køber L, Torp-Pedersen C, Hassager C. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail. 2007;9:610–616. doi: 10.1016/j.ejheart.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Chrysohoou C, Antoniou CK, Kotrogiannis I, Metallinos G, Aggelis A, Andreou I, Brili S, Pitsavos C, Stefanadis C. Role of right ventricular systolic function on long-term outcome in patients with newly diagnosed systolic heart failure. Circ J. 2011;75:2176–2181. doi: 10.1253/circj.cj-11-0296. [DOI] [PubMed] [Google Scholar]
- 35.Guendouz S, Rappeneau S, Nahum J, Dubois-Randé JL, Gueret P, Monin JL, Lim P, Adnot S, Hittinger L, Damy T. Prognostic significance and normal values of 2D strain to assess right ventricular systolic function in chronic heart failure. Circ J. 2012;76:127–136. doi: 10.1253/circj.cj-11-0778. [DOI] [PubMed] [Google Scholar]
- 36.Atsumi A, Ishizu T, Kameda Y, Yamamoto M, Harimura Y, Machino-Ohtsuka T, Kawamura R, Enomoto M, Seo Y, Aonuma K. Application of 3-dimensional speckle tracking imaging to the assessment of right ventricular regional deformation. Circ J. 2013;77:1760–1768. doi: 10.1253/circj.cj-12-1445. [DOI] [PubMed] [Google Scholar]
- 37.Gayat E, Ahmad H, Weinert L, Lang RM, Mor-Avi V. Reproducibility and inter-vendor variability of left ventricular deformation measurements by three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2011;24:878–885. doi: 10.1016/j.echo.2011.04.016. [DOI] [PubMed] [Google Scholar]