Abstract
Background and Objectives
Prior studies suggest a possible association between the use of neuraxial-general anesthesia and a decrease in prostate cancer recurrence after radical prostatectomy. We examine the correlation of a spinal anesthesia-only technique on prostate cancer recurrence.
Methods
Charts from consecutive radical prostatectomy patients of 3 experienced urologists from January 1999 to December 2005 were reviewed. In addition to the usual clinical and pathologic predictors of disease recurrence, patient records were queried for the type of anesthesia (general versus spinal) performed. A Cox proportional hazards model was used to determine the statistical significance of predictors of biochemical recurrence.
Results
A total of 1,964 patients—1,166 and 798 receiving spinal with sedation or general anesthesia, respectively—had complete preoperative and follow-up data. In univariate proportional hazards analysis, the use of general anesthesia was associated with a trend towards an increased risk of biochemical recurrence when compared with the use of spinal anesthesia (hazard ratio = 1.29, 95% confidence interval [CI] 0.99–1.66, P=0.053). In multi-variable analysis, the effect size (hazard ratio = 1.10, 95% CI 0.85–1.42, P=0.458) was diminished by clinical and pathologic variables.
Conclusions
This was a retrospective study of patients with prostate cancer who have undergone radical prostatectomy during a time period when the practice of anesthesia for prostatectomy at our institution was transitioned from spinal to general anesthesia. In our study, when controlling for other predictors of advanced prostate cancer, the type of anesthetic given during prostatectomy had no effect on the risk of biochemical recurrence.
INTRODUCTION
Increasing evidence has suggested that the use of regional anesthesia during oncologic surgery may modify the risk of cancer recurrence.1 As an adjunct to general endotracheal anesthesia, regional anesthesia can decrease volatile anesthestic requirements,(2, 3) decrease opioid requirements,(4, 5) block activation of the sympathetic nervous system,(6, 7) attenuate immunosuppression,(8, 9) improve tissue oxygenation,(3, 10) decrease post-operative pain(11), and promote innate anti-tumor factors through the effects of local anesthetic.(2)
For patients with prostate cancer, radical prostatectomy can be a curative treatment.(12) When the surgery is performed through an open incision, such as with radical retropubic prostatectomy (RRP), adequate anesthesia can be achieved through multiple modalities: general anesthesia, neuraxial (spinal or epidural) anesthesia, or a combination of general and neuraxial anesthesia. Prior studies evaluating the oncologic benefit of regional anesthesia have studied epidural anesthesia as an adjunct to general anesthesia versus general anesthesia alone. They found mixed results: either a significant reduction in risk of biochemical recurrence;(13) a significant reduction in risk of clinical recurrence, but not biochemical recurrence,(14, 15) or no significant difference.(16–18) Those authors who found a positive association between regional anesthesia and decreased cancer recurrence attributed the association to a combination of decreased volatile agent during general anesthesia and decreased opioid requirement intra- and post-operatively. None of these studies have examined the oncologic benefit of neuraxial anesthesia alone versus general anesthesia.
In the early 2000s, the preferred anesthetic modality for RRP at our institution was transitioned from spinal anesthesia with sedation to general anesthesia. This change created a dichotomous cohort of patients for whom a distinguishing factor was whether they received spinal anesthesia or general anesthesia, thus allowing us to examine the oncologic benefit of neuraxial (spinal) anesthesia without general anesthesia.
METHODS
This study was approved by the Johns Hopkins Institutional Review Board, which waived the requirement for written informed consent. Charts from consecutive radical prostatectomy patients of three experienced urologists (HBC, JLM, AWP) from January 1999 to December 2005 were reviewed. During this period, urologists at our institution changed their preferred mode of intraoperative anesthesia for RRP from spinal anesthesia with sedation to general endotracheal anesthesia in an attempt to decrease the total anesthesia time and the inter-provider variability in the efficacy of intrathecal anesthesia; no other major changes in delivery of anesthesia- or surgery-related care to RRP patients changed during this period. Twelve patients received epidural anesthesia during this study period; these patients were excluded from the analysis. All 3 urologists had extensive experience performing RRPs before the start of the study period, continued to operate at a consistent rate throughout the duration of the study, and had no significant changes to their surgical technique during this transition.
Patient clinical characteristics that were extracted included age, weight, height, and American Society of Anesthesiologists (ASA) physical status. Commonly used pre- and postoperative pathologic predictors of prostate cancer progression—including prostate-specific antigen (PSA), clinical stage, Gleason sum, number of biopsy cores positive, maximum percentage core involvement, and pathologic stage—were extracted from the institutional radical prostatectomy database. The perioperative data—including total anesthesia time (anesthesia in-room time to anesthesia end time), total surgery time (surgery start to surgery end times), and primary mode of anesthesia—were extracted from the anesthesia record.
Biochemical recurrence was defined as a postoperative PSA ≥0.2 ng/mL.(19) Patients were considered to have the outcome of interest if they had biochemical recurrence, or if they had radiographic evidence of local recurrence or distant metastatic disease. Patients who did not experience the outcome of interest or died from other causes were censored at the time of their last follow-up or at the time of death, respectively.
Statistical Analysis
Preoperative characteristics were compared between men who had spinal and general anesthesia using appropriate comparative tests (t-test, rank-sum, and chi-squared). The cumulative incidence of disease progression was estimated using the Kaplan-Meier method and comparison was evaluated based on the log-rank test. Univariate Cox proportional hazards analysis was used to determine the statistical significance of predictors of time to biochemical recurrence. Because the allocation of patients to spinal or general anesthesia was not randomized, a propensity score was calculated for each patient using a logistic regression of pre-operative clinical characteristics to calculate the probability of receiving spinal versus general anesthesia. A multivariable model was created using predictors of clinical and statistical significance. Tests for nonproportional hazards using Schoenfeld residuals(20) and visual inspection resulted in non-significant findings in all analyses. Statistical significance was considered at P <0.05.
RESULTS
A total of 1,964 patients—1,166 and 798 receiving spinal with sedation and general anesthesia, respectively—had follow-up data available. Although spinal anesthesia was mostly given during the earlier portion of the study period and general anesthesia was mostly given during the latter portion of the study period, both modalities were used in cases throughout. Men who received general anesthesia tended to have more advanced disease, as evidenced by significantly more advanced clinical stage, higher biopsy and pathologic Gleason sum (Table 1). There was no significant change in the ASA status of patients, and while the anesthesia time decreased by a statistically significant amount, the surgery time also decreased (Table 1).
Table 1.
Demographic, clinical, and pathologic characteristics of patients undergoing radical prostatectomy
| Spinal (n=1,166) | General (n=798) | P-value† | |
|---|---|---|---|
|
| |||
| Median age (IQR) | 58 (54–62) | 58 (54–63) | 0.127 (rank-sum) |
|
| |||
| Median BMI, kg/m2 (IQR)‡ | 26.6 (24.8–28.9) | 26.9 (25.1–29.6) | 0.022 (rank-sum) |
|
| |||
| No. clinical stage (%)* | |||
|
| |||
| T1 | 918 (78.9) | 648 (81.6) | 0.067 |
| T2 | 242 (20.8) | 140 (17.6) | |
| T3 | 3 (0.3) | 6 (0.8) | |
|
| |||
| Median preop PSA, ng/ml (IQR) | 5.5 (4.2 – 7.6) | 5.4 (4.1 – 7.8) | 0.329 (t-test) |
|
| |||
| No. biopsy Gleason sum (%) | |||
|
| |||
| 5–6 | 934 (80.1) | 573 (71.8) | < 0.001 |
| 7 (3+4) | 154 (13.2) | 143 (17.9) | |
| 7 (4+3) | 57 (4.9) | 50 (6.3) | |
| 8–10 | 21 (1.8) | 32 (4.0) | |
|
| |||
| No. positive cores (%)¶ | |||
| 1 | 242 (41.1) | 195 (34.2) | 0.020 |
| 2 | 139 (23.6) | 132 (23.1) | |
| 3 or more | 208 (35.3) | 244 (42.7) | |
|
| |||
| No. max percent core involvement (%)§ | |||
| 1–5 | 33 (6.4) | 26 (5.1) | 0.004 |
| 6–50 | 309 (59.4) | 255 (50.4) | |
| 51–100 | 178 (34.2) | 225 (44.5) | |
|
| |||
| No. pathological Gleason sum (%) | |||
|
| |||
| 5–6 | 791 (67.8) | 492 (61.7) | 0.005 |
| 7 (3+4) | 248 (21.3) | 178 (22.3) | |
| 7 (4+3) | 73 (6.3) | 75 (9.4) | |
| 8–10 | 54 (4.6) | 53 (6.6) | |
|
| |||
| No. pathologic stage (%) | |||
|
| |||
| pT2 (organ confined) | 869 (74.5) | 572 (71.7) | 0.162 |
| pT3a/b (extraprostatic extension) | 254 (21.8) | 182 (22.8) | |
| pT3c (seminal vesicle invasion) | 33 (2.8) | 30 (3.8) | |
| Lymph node-positive | 10 (0.9) | 14 (1.7) | |
|
| |||
| No. with(out) biochemical recurrence (%) | |||
|
| |||
| No recurrence | 1041 (89.3) | 684 (85.7) | 0.018 |
| Recurrence | 125 (10.7) | 114 (14.3) | |
|
| |||
| Median ASA (IQR) | 2 (2 – 2) | 2 (2 – 2) | 0.147 (rank-sum) |
|
| |||
| Median anesthesia time, min (IQR) | 150 (136–165) | 144 (125–165) | < 0.001 (t-test) |
|
| |||
| Median surgery time, min (IQR) | 112 (99 – 125) | 107 (91 – 121) | 0.005 (t-test) |
|
| |||
| Median follow-up time, years (IQR) | 4 (2 – 9) | 5 (3 – 7) | 0.505 (t-test) |
Chi-square test except where noted.
In 1,143 and 503 men who received spinal and general anesthesia, respectively.
In 1,163 and 794 men who received spinal and general anesthesia, respectively.
In 589 and 571 men who received spinal and general anesthesia, respectively.
In 520 and 506 men who received spinal and general anesthesia, respectively.
Men who underwent surgery with general anesthesia also had a higher proportion that experienced biochemical recurrence. Men who had spinal anesthesia with sedation had an unadjusted 5- and 10-year biochemical recurrence-free survival (BFS) of 88.2% and 83.5%, compared with a 5- and 10-year BFS of 85.7% and 80.7% (log-rank test P = 0.049, Fig. 1) for men who underwent general anesthesia.
Figure 1.
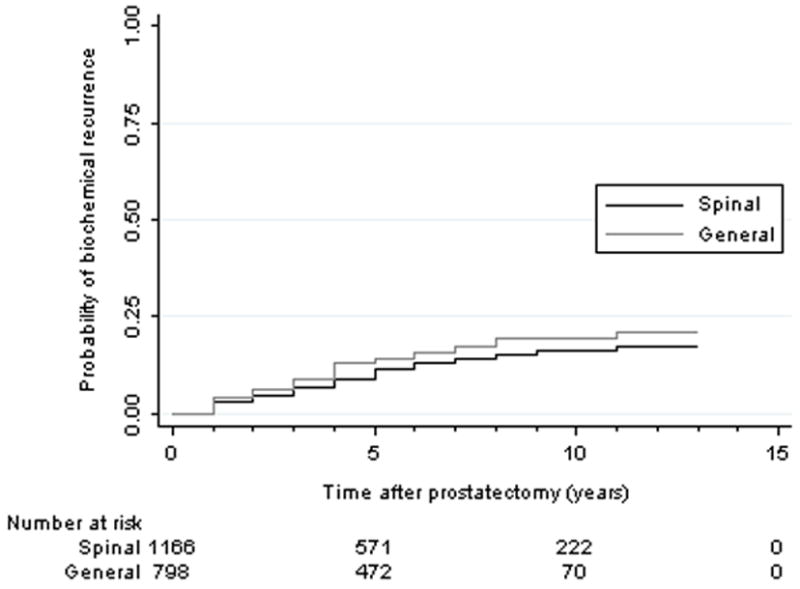
Kaplan-Meier unadjusted estimate of biochemical recurrence from prostate cancer after radical prostatectomy using spinal versus general anesthesia (p=0.049)
In univariate proportional hazards analysis, the use of general anesthesia was associated with a trend towards an increased risk of biochemical recurrence when compared with the use of spinal anesthesia with sedation (hazard ratio = 1.29, 95% confidence interval 0.99–1.66, p=0.053, table 2). However, when the type of anesthesia given was used in a multi-variable analysis, the effect size (hazard ratio = 1.10, 95% confidence interval 0.85–1.42, P=0.458) was diminished by clinical and pathologic variables. Both clinical (pre-operative PSA, clinical stage, Gleason sum) and pathologic (pathologic stage, Gleason sum) variables were significantly associated with increased risk of biochemical recurrence, although only pathologic variables were included in the multi-variable analysis due to the strong correlation between pre- and postoperative variables (Table 2). The addition of a variable representing the propensity score for spinal versus general anesthesia to the multivariable analysis did not change the significance of the other variables in the model.
Table 2.
Hazard ratio (HR) estimates for risk of biochemical recurrence after radical prostatectomy in 1,964 men with prostate cancer (of whom 239 had evidence of biochemical recurrence)
| Univariate HR | P-value | Multivariate HR | P-value | |
|---|---|---|---|---|
|
| ||||
| Age | 1.00 (0.98–1.02) | 0.818 | 0.99 (0.97–1.01) | 0.380 |
|
| ||||
| BMI* | 1.05 (1.01–1.10) | 0.016 | ||
|
| ||||
| Clinical stage† | ||||
| T1 (ref) | ||||
| T2 | 2.03 (1.54–2.68) | < 0.001 | ||
| T3 | 4.83 (1.79–13.0) | 0.002 | ||
|
| ||||
| PSA | ||||
| < 10 ng/ml (ref) | ||||
| 10 ng/ml or greater | 3.51 (2.65–4.64) | < 0.001 | 1.73 (1.28–2.33) | < 0.001 |
|
| ||||
| Biopsy Gleason sum | ||||
| 5–6 (ref) | ||||
| 7 (3+4) | 2.92 (2.15–3.97) | < 0.001 | ||
| 7 (4+3) | 5.81 (4.03–8.36) | < 0.001 | ||
| 8–10 | 6.59 (4.21–10.3) | < 0.001 | ||
|
| ||||
| Positive cores‡ | ||||
| 1 (ref) | ||||
| 2 | 2.18 (1.37–3.45) | 0.001 | ||
| 3 or more | 2.33 (1.54–3.53) | < 0.001 | ||
|
| ||||
| Max percent core involvement§ | ||||
| 1–5 (ref) | ||||
| 6–50 | 1.06 (0.46–2.44) | 0.896 | ||
| 51–100 | 1.64 (0.71–3.77) | 0.247 | ||
|
| ||||
| Pathological Gleason sum | ||||
| 5–6 (ref) | ||||
| 7 (3+4) | 3.93 (2.75–5.62) | < 0.001 | 2.52 (1.73–3.67) | < 0.001 |
| 7 (4+3) | 11.7 (8.09–16.9) | < 0.001 | 5.75 (3.84–8.62) | < 0.001 |
| 8–10 | 15.3 (10.5–22.3) | < 0.001 | 7.64 (5.05–11.6) | < 0.001 |
|
| ||||
| Pathologic stage | ||||
| pT2 (organ confined, ref) | ||||
| pT3a/b (extraprostatic extension) | 5.25 (3.93–7.02) | < 0.001 | 3.06 (2.24–4.17) | < 0.001 |
| pT3c (seminal vesicle invasion) | 14.7 (9.80–21.9) | < 0.001 | 5.20 (3.35–8.08) | < 0.001 |
| Lymph node-positive | 17.1 (9.82–29.9) | < 0.001 | 4.81 (2.66–8.70) | < 0.001 |
|
| ||||
| ASA | 1.18 (0.87–1.59) | 0.280 | ||
|
| ||||
| Anesthesia time, min | 1.00 (1.00–1.01) | 0.029 | 1.00 (0.99–1.01) | 0.596 |
|
| ||||
| Surgery time, min | 1.00 (1.00–1.01) | 0.034 | ||
|
| ||||
| Anesthesia type | ||||
| Spinal (ref) | ||||
| General | 1.29 (0.99–1.66) | 0.053 | 1.10 (0.85–1.42) | 0.458 |
In 1,646 men, of whom 200 had biochemical recurrence.
In 1,957 men, of whom 238 had biochemical recurrence.
In 1,160 men, of whom 147 had biochemical recurrence.
In 1,026 men, of whom 141 had biochemical recurrence.
DISCUSSION
To our knowledge, this is the first published study of spinal anesthesia as the primary anesthesia modality (versus general anesthesia) to examine the correlation of intraoperative type of anesthesia and cancer recurrence for RRP patients. In our retrospective study of prostate cancer patients undergoing radical prostatectomy during a time period when the practice of anesthesia for prostatectomy at our institution was transitioned from spinal anesthesia with sedation to general endotracheal anesthesia, the type of anesthetic given during prostatectomy had no effect on the risk of biochemical recurrence, after controlling for other predictors of advanced prostate cancer. Prior clinical studies have suggested a possible link between anesthetic type during oncologic surgery and cancer recurrence, particularly for breast,(21) colorectal,(22–24) and gynecologic(25) tumors. The evidence for an association between decreased prostate cancer recurrence and neuraxial anesthesia, however, has been mixed.
Prior studies examining this issue have compared a combined neuraxial (epidural)-general intraoperative anesthesia to general anesthesia. Early studies evaluating the oncologic benefit of regional anesthesia for prostatectomy found that epidural anesthesia as an adjunct to general anesthesia conferred a protective effect compared with general anesthesia alone. The first study published on the subject was able to show a statistically significant decrease in the risk of biochemical recurrence among patients who received an epidural in addition to their general anesthetic, when compared to those who did not have an epidural.(13) The authors proposed the difference could be attributed to a reduction in volatile anesthetic use or post-operative opioid use, although neither of these was quantified in those studies. Subsequent positive studies have found a significant reduction in clinical recurrence—which was defined as radiographic evidence of recurrent disease—for those who received epidural-general anesthesia.(14, 15) However, no other study found an increased risk of biochemical recurrence.
Other studies, however, have not found a significant difference in clinical or biochemical recurrence.(16–18) These negative studies include the only trial with patients randomized to either general anesthesia or combined general-epidural for radical prostatectomy.(16) In that trial, the primary outcome had been pain control and blood loss, and a secondary analysis was performed to evaluate disease recurrence.
In each of these previous studies, all of the study patients received general anesthesia. The addition of epidural analgesia was thought to reduce patient exposure to opioids during and after surgery through improved pain control. Patients may also have had decreased exposure to volatile anesthetics, as well, if the epidural was used intra-operatively. Our study is unique because patients who received spinal anesthesia at our institution did not receive any volatile anesthetic intraoperatively, although they did receive sedation, which typically consisted of a propofol infusion.
The strengths of our study include the large sample size, which comes from being at a referral center where 2 of the 3 urologists studied perform prostatectomy exclusively, as well as follow-up data that includes a median of 4 to 5 years for both groups. However, there are several potential limitations to our work. This was a retrospective study where the choice of anesthestic type was not randomized, but based primarily on the time period in which the patient had their surgery. Although no obvious changes in surgical technique, anesthesia monitoring, or other perioperative care took place during this time period, we cannot exclude every potential temporal confounder. We were also not able to capture and account for other factors (eg, temperature, blood administration) that may contribute to perioperative cancer recurrence. One possible reason for the absence of a positive finding in our study is the relatively lower rate of biochemical recurrence in our cohort, when compared with prior studies. Also, other studies have suggested that part of the decrease in cancer recurrence from the use of epidurals may be modulated by a decrease in intraoperative and postoperative opioid use, which was not captured in our dataset. It is possible that intraoperative neuraxial anesthesia may have a favorable oncologic benefit if the physiologic benefits of neuraxial anesthesia are extended well into the postoperative period; however, none of our patients received postoperative epidural analgesia.
In summary, we examined the oncologic effects of spinal anesthesia as the primary anesthesia modality (versus general anesthesia) in a large cohort of RRP patients. Although this is one of the largest cohorts studied to date, we found no difference in prostate cancer recurrence between patients who received intraoperative spinal anesthesia versus general anesthesia. The absence of a positive association between prostate cancer recurrence and spinal anesthesia in our study suggests that the relationship between neuraxial anesthesia and prostate cancer may have more facets than previously estimated, including the contribution of postoperative analgesia or other intraoperative factors. The results of ongoing randomized clinical trials of neuraxial anesthesia in surgery for breast (NCT 00418457), lung (NCT 01179308, NCT 00684229), and colon (NCT 00684229) cancer may help elucidate some of those relationships.
Acknowledgments
Funding: This work was received support from NIH SPORE Grant P50CA58236 and from the Departments of Anesthesiology and Critical Care Medicine and Urology, The Johns Hopkins University School of Medicine
Footnotes
Institution: Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine
Prior Presentation:
This report was previously presented, in part, at the 38th Annual Regional Anesthesiology and Acute Pain Medicine Meeting, May 3, 2013, Boston, MA; and the New York Academy of Medicine Anesthesiology Residents’ Night, October 25, 2013, New York, NY.
Conflict of Interest:
The authors declare no conflict of interest.
References
- 1.Sessler DI. Does regional analgesia reduce the risk of cancer recurrence? A hypothesis. Eur J Cancer Prev. 2008;17:269–272. doi: 10.1097/CEJ.0b013e3282f0c005. [DOI] [PubMed] [Google Scholar]
- 2.Santamaria LB, Schifilliti D, La Torre D, Fodale V. Drugs of anaesthesia and cancer. Surg Oncol. 2010;19:63–81. doi: 10.1016/j.suronc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–115. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 4.Afsharimani B, Cabot P, Parat MO. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011;30:225–238. doi: 10.1007/s10555-011-9285-0. [DOI] [PubMed] [Google Scholar]
- 5.Mathew B, Lennon FE, Siegler J, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112:558–567. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald PJ. Is norepinephrine an etiological factor in some types of cancer? Int J Cancer. 2009;124:257–263. doi: 10.1002/ijc.24063. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Diz PG, Gandara Rey JM, Garcia-Garcia A. Beta-adrenergic receptors in cancer: therapeutic implications. Oncol Res. 2010;19:45–54. doi: 10.3727/096504010x12828372551867. [DOI] [PubMed] [Google Scholar]
- 8.Ahlers O, Nachtigall I, Lenze J, et al. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth. 2008;101:781–787. doi: 10.1093/bja/aen287. [DOI] [PubMed] [Google Scholar]
- 9.Bar-Yosef S, Melamed R, Page GG, Shakhar G, Shakhar K, Ben-Eliyahu S. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94:1066–1073. doi: 10.1097/00000542-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–75. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page GG. Surgery-induced immunosuppression and postoperative pain management. AACN Clin Issues. 2005;16:302–309. doi: 10.1097/00044067-200507000-00004. quiz 416–418. [DOI] [PubMed] [Google Scholar]
- 12.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 14.Wuethrich PY, Hsu Schmitz SF, Kessler TM, Thalmann GN, Studer UE, Stueber F, et al. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: a retrospective study. Anesthesiology. 2010;113:570–576. doi: 10.1097/ALN.0b013e3181e4f6ec. [DOI] [PubMed] [Google Scholar]
- 15.Scavonetto F, Yeoh TY, Umbreit EC, et al. Association between neuraxial analgesia, cancer progression, and mortality after radical prostatectomy: a large, retrospective matched cohort study. Br J Anaesth. 2013 doi: 10.1093/bja/aet467. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsui BC, Rashiq S, Schopflocher D, et al. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can J Anaesth. 2010;57:107–112. doi: 10.1007/s12630-009-9214-7. [DOI] [PubMed] [Google Scholar]
- 17.Forget P, Tombal B, Scholtes JL, et al. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? Eur J Anaesthesiol. 2011;28:830–835. doi: 10.1097/EJA.0b013e32834b7d9a. [DOI] [PubMed] [Google Scholar]
- 18.Wuethrich PY, Thalmann GN, Studer UE, Burkhard FC. Epidural Analgesia during Open Radical Prostatectomy Does Not Improve Long-Term Cancer-Related Outcome: A Retrospective Study in Patients with Advanced Prostate Cancer. PLoS One. 8:e72873. doi: 10.1371/journal.pone.0072873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld D. Residuals for the proportional hazards regresssion model. Biometrika. 1982;69:239–241. [Google Scholar]
- 21.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–332. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Bjornsson A, Fredriksson M, Hallbook O, Eintrei C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. Br J Anaesth. 2011;107:164–170. doi: 10.1093/bja/aer100. [DOI] [PubMed] [Google Scholar]
- 24.Gottschalk A, Ford JG, Regelin CC, You J, Mascha EJ, Sessler DI, et al. Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology. 2010;113:27–34. doi: 10.1097/ALN.0b013e3181de6d0d. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira GS, Jr, Ahmad S, Schink JC, Singh DK, Fitzgerald PC, McCarthy RJ. Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in ovarian cancer patients after primary cytoreductive surgery. Reg Anesth Pain Med. 2011;36:271–277. doi: 10.1097/AAP.0b013e318217aada. [DOI] [PubMed] [Google Scholar]


