Abstract
Research on intestinal bacteria began around the end of the 19th century. During the last 5 decades of the 20th century, research on the intestinal microbiota made rapid progress. At first, in my work, I first developed a method of comprehensive analysis of the intestinal microbiota, and then I established classification and identification methods for intestinal anaerobes. Using these methods I discovered a number of ecological rules governing the intestinal microbiota and the role of the intestinl microbiota in health and disease. Moreover, using germfree animals, it was proven that the intestinal microbiota has a role in carcinogenesis and aging in the host. Thus, a new interdisciplinary field, “intestinal bacteriology” was established.
Keywords: intestinal microbiota, intestinal bacteria, intestinal bacteriology, functional foods
INTRODUCTION
Research on intestinal bacteria began in 1719, when the first microscopic observations of fecal bacteria were made by Leeuwenhoeck. In 1886, Escherich isolated Bacillus coli (Escherichia coli) from infants. In 1899, Tissier of the Pasteur Institute isolated Bacillus bifidus (Bifidobacterium), a type of anaerobic lactic acid bacteria, from the feces of breast-fed infants, and this led to widespread research on infant nutrition and the intestinal microbiota in the pediatric field. In 1900, the Austrian pediatrician Moro discovered Bacillus acidophilus (Lactobacillus acidophilus). These two important types of enteric lactic acid bacteria appeared in rapid succession, and the debate on the classification of Lactobacillus, including the problem of differences from lactic acid bacteria and differentiation from yogurt-derived Lactobacillus, started in earnest.
Research on the intestinal microbiota began to determine if individual differences in enteric infections were caused by differences in intestinal microbiota, but the intestinal bacteria studied were mainly limited to aerobes such as E. coli and Enterococcus, which are easy to culture. In 1935, Eggerth and Gagnon reported that anaerobic bacteria of the genus Bacteroides outnumbered E. coli in human feces, but the conventional thinking that E. coli was the predominant enteric bacteria was firmly rooted and this new knowledge went unheeded for another 20 years.
In the 5th decade of the 20th century research on the intestinal microbiota began in Europe, Japan and the United States. Dwayne Savage summarized this matter in a review entitled “Microbial Biota of the Human Intestine: A Tribute to Some Pioneering Scientists” [1]. The present review describes the advances in intestinal microbiota research upon which “intestinal bacteriology” was established and functional foods were developed.
DEVELOPMENT OF METHODS FOR COMPREHENSIVE INVESTIGATION OF THE INTESTINAL MICROBIOTA
Development of methods of comprehensive analysis of the intestinal microbiota was carried out by Haenel et al. [2,3,4,5,6], Mitsuoka et al. [7,8,9,10,11] and Drasar [12].
“BL agar”
In 1953, I started graduate work on the intestinal microbiota. First, the fecal material of a human adult diluted with a diluent was prepared, Gram stained, and examined for the number and shape of bacteria under microscope. Then, gram-positive and gram-negative bacteria of different sizes and shapes were observed (Fig. 1). The number of bacteria in one gram was in the range of 3 to 5 ×1011. So, 0.01 ml of the diluted fecal material was smeared on a nutrient agar medium and incubated aerobically. Subsequently, only one or several kinds of coliform bacteria and enterococci colonies appeared on the agar. The number of bacteria per gram feces was usually between 105 and 108. This figure was far less than that estimated from direct smear observation under microscope.
Fig. 1.
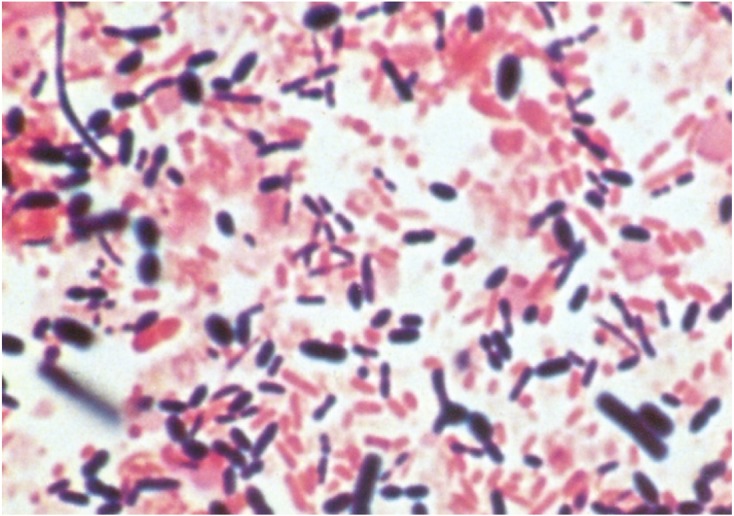
Photomicrograph of the Gram-stained fecal stool of an adult.
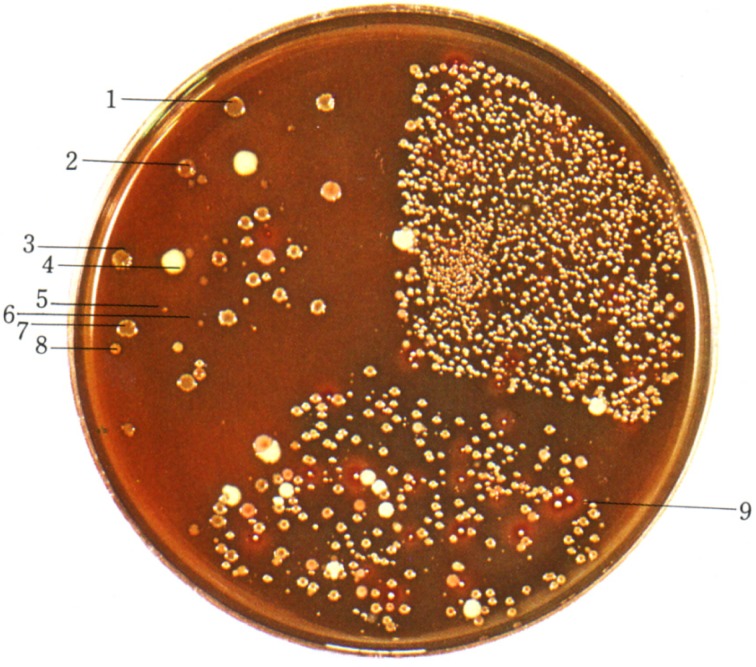
Therefore, I developed BL agar (glucose blood liver agar) for culture, differentiation, and isolation of various intestinal bacteria and cultivated the fecal material of human adults in an anaerobic jar. I discovered that obligately anaerobic bacteria such as Bifidobacterium and Bacteroides spp. constitute predominant microorganisms in human adult feces (Fig. 2). This discovery formed the basis for the establishment of “intestinal bacteriology” and the development of functional foods.
Fig. 2.
Discovery of BL agar and predominant flora of bifidobacteria in an adult fecal specimen.
1, 2, 3, 7: Bifidobacterium. 4: Megasphaera elsdenii. 5, 6: Bacteroides. 8: Eubacterium. 9: Veillonella
“Plate-in-bottle method”
In 1950, Hungate [13] developed the “anaerobic roll-tube method” for the isolation of cellulolytic anaerobic bacteria in the rumen. This culture technique was also applied to study the intestinal microbiota and made possible the growth of fastidious anaerobes from the intestinal materials of humans and animals. This method, however, is admittedly cumbersome for routine use. To overcome these difficulties, we developed the “plate-in-bottle method” (Fig. 3) [9], which made possible the growth of fastidious anaerobes as surface colonies on agar media without using special facilities.
Fig. 3.

Plate-in-bottle method.
This technical improvement enabled the cultivation of over 70% of the microscopic count of bacteria in animal feces, and often more than 90%.
Method for comprehensive investigation of intestinal microbiota
In 1969, we established a comprehensive method using the “plate-in-bottle method”, 4 nonselective media and 10 selective media [8] for analysis of the intestinal microbiota of humans and animals (Table 1). I described the details of this method in a book entitled “A Color Atlas of Anaerobic Bacteria” [11].
Table 1. The media and cultural method for comprehensive investigation of intestinal microbiota.
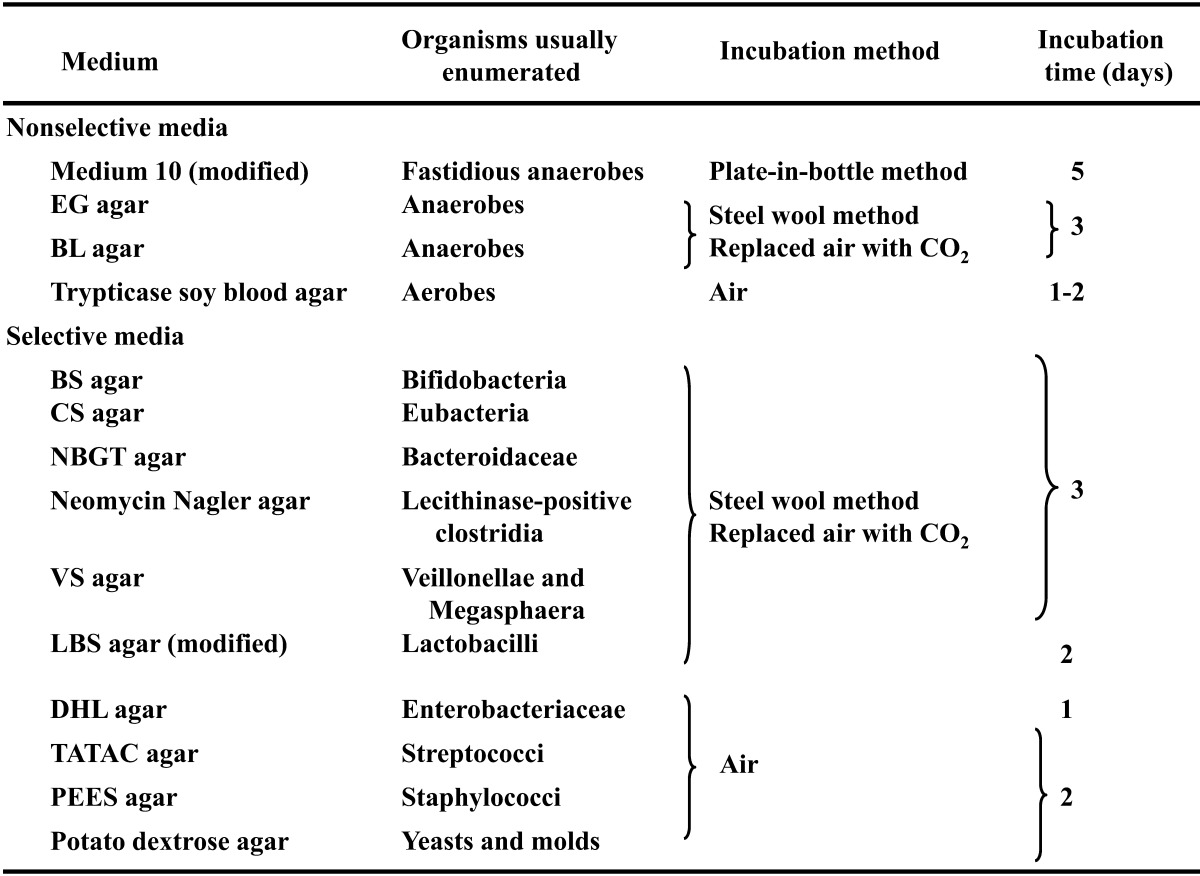
Despite explosive development in modern molecular techniques, it has to be emphasized that classical microbiological techniques are still not obsolete but are complementary to the molecular techniques.
CLASSIFICATION, DIFFERENTIATION AND HABITATS OF LACTIC ACID BACTERIA OF HUMANS AND ANIMALS
During the 1960s and 1980s, significant advances were made in the bacterial taxonomy of Lactobacillus, Bifidobacterium and other anaerobes. Moore and his coworkers published the “Anaerobe Laboratory Manual 4th Ed.” [14], and I published a color atlas for the isolation and identification of anaerobic bacteria [11].
Lactobacillus
Lactobacilli are isolated from the intestine of humans and animals. The predominant Lactobacillus flora are especially harbored in the intestines of animals such as pigs, chickens, dogs, mice, rats and hamsters.
Rogosa and Sharpe [15] provided a detailed description of L. acidophilus. Lerche and Reuter [16] grouped L. acidophilus strains into five biotypes, and I [17] expanded the number of biotypes to ten biotypes.
Johnson et al. [18] divided L. acidophilus species into six distinct homology groups, A-1, A-2, A-3, A-4, B-1 and B-2. Until recently, it was not possible to distinguish the genospecies recognized in the L. acidophilus group on the basis of phenotypic characteristics. Phenotypic criteria were developed that proved useful for the differentiation of genospecies of the L. acidophilus group as shown in Table 2, in which the differential characteristics of lactobacilli recovered from the gastrointestinal tract are also included. Homology groups A-4 and B-2 should be considered two new species, for which we proposed the names L. gallinarum and L. johnsonii, respectively [19]. The intestinal strains formerly identified as L. fermentum were classified as a new species, L. reuteri [20].
Table 2. Differential characteristics of Lactobacillus species recovered from the intestine of humans and animalsa.
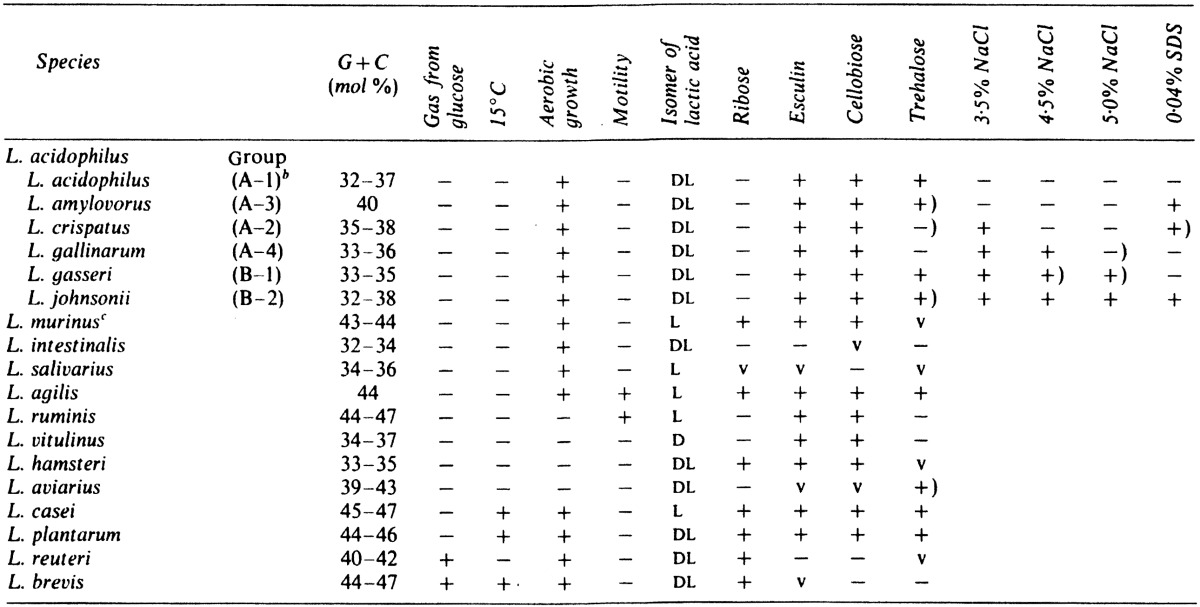
a Symbols: +, 90% or more strains positive; −, 90% or more strains negative; +), 80–90% strains positive; v, 21–79% strains positive.
b DNA homology group of Johnson et al. (1980).
c Synonymous with L. animalis.
Habitats of the genospecies of the L. acidophilus group and other Lactobacillus species are as shown in Table 3, L. acidophilus can probably be isolated from humans, mice and rats. L. amylovorus is mainly from pigs and occasionally isolated from cattle, and it can probably be isolated from chickens. L. crispatus is most common in the intestine of humans and chickens. L. gasseri is the most common isolate from humans, and it is occasionally isolated from cattle. L. johnsonii is more commonly isolated from chickens and is occasionally from humans and pigs. L. murinus is synonymous with L. animalis and is isolated from cattle, dogs, mice, and rats; it can also probably be isolated from pigs and chickens. L. intestinalis is common in mice and rats. L. salivarius has been commonly isolated from humans, pigs, and chickens. Finally, L. agilis is often isolated from pigs and chickens. Anaerobic lactobacilli have been isolated from the gastrointestinal tract of humans and animals, one strain being identified as L. ruminis [21] and others being identified as L. vitulinus [21], L. aviarius [22] and L. hamsteri [23]; L. ruminis is occasionally isolated from cattle and Bulgarian people; L. vitulinus is isolated from cattle and Papuan highlanders; L. hamsteri is the most common isolate from hamsters; and L. aviarius is a specific species from birds, including chickens and ducks.
Table 3. Habitats of Lactobacillus species in the intestine of humans and animalsa.
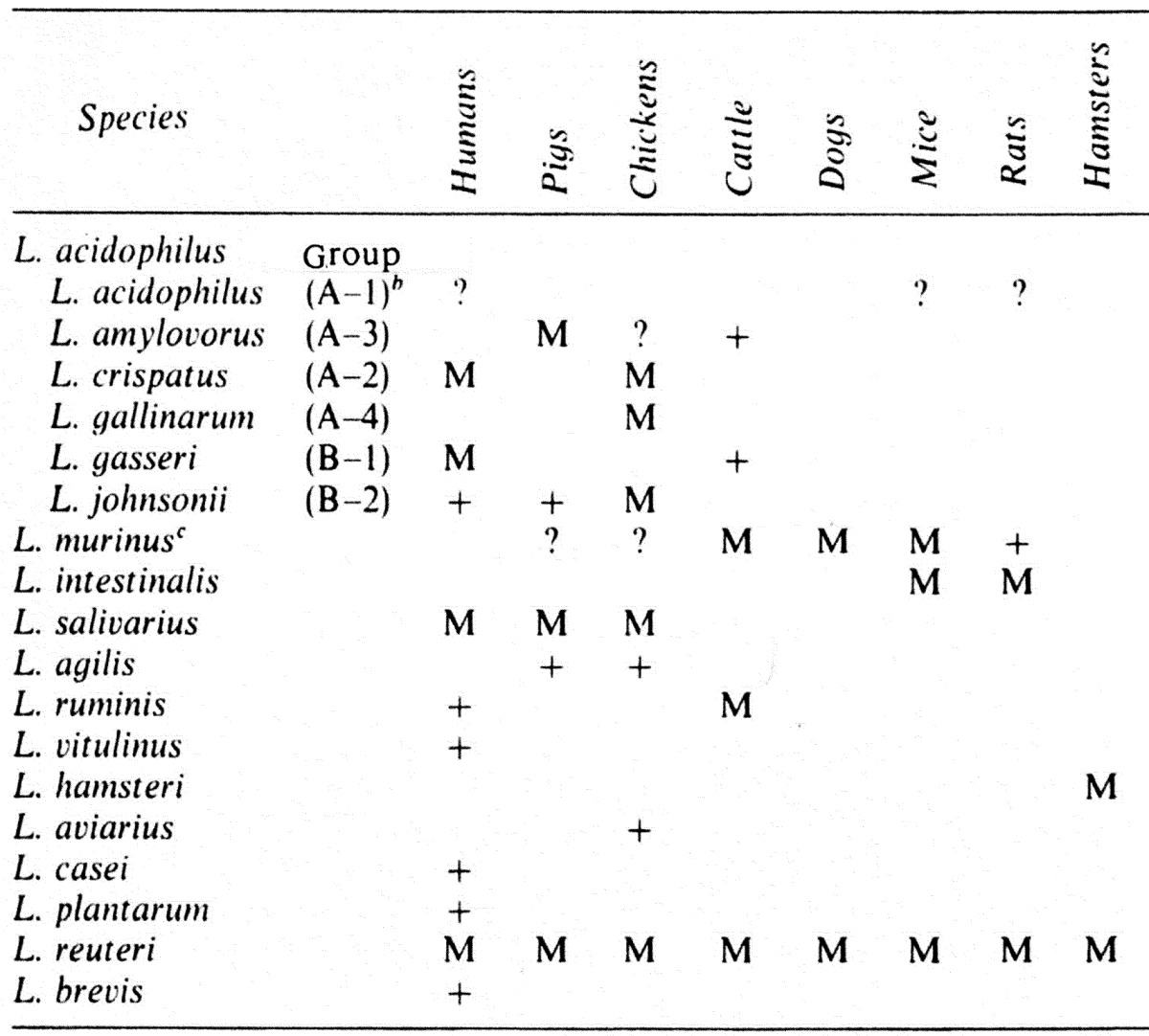
a Symbols: M, major component of Lactobacillus species; +, occasionally recovered; ?, questionable.
b DNA homology group of Johnson et al. (1980).
c Synonymous with L. animalis.
Bifidobacterium
For many years bifidobacteria were included in the genus Lactobacillus as Lactobacillus bifidus, but they are now classified in a separate genus Bifidobacterium as suggested by Orla-Jensen [24] on the basis of their characteristic morphology, biochemical characters, cell wall constituents and DNA base composition.
In the past, several workers recognized only one species in the genus Bifidobacterium, but Reuter has proposed eight species on the basis of carbohydrate fermentation and several biotypes for strains isolated from humans [25]. He recognized the following species and biotypes in the genus Bifidobacterium: Bifidobacterium bifidum a and b; B. infantis; B. parvulorum a and b; B. breve a and b; B. lactentis; B. liberorum; B. adolescentis a, b, c and d; and B. longum a and b.
In 1969, I [26] studied strains of bifidobacteria isolated from human feces, the rumen of cattle and sheep and the intestine of animals such as pigs, chickens, rats, mice, guinea pigs and rabbits and compared the results with those obtained by Reuter [25]. In his study, he clearly differentiated nonhuman strains from human strains by their carbohydrate fermentation patterns and ability to grow at 46.5 C and proposed the creation of new species, B. pseudolongum a, b, c, and d and B. thermophilum a, b, c, and d and a new variant, Bifidobacterium longum ss. animalis.
Subsequently, Scardovi and his coworkers [27,28,29,30,31] proposed, mainly based on DNA homology, the establishment of eleven additional species for organisms isolated from the intestine of honey bees, pigs, chickens and rabbits, the rumen of cattle and sheep and sewage. The eleven species were B. indicum, B. coryneforme, B. asteroides, B. ruminale, B. globosum, B. suis, B. pullorum, B. magnum, B. catenulatum, B. dentium, and B. angulatum.
Furthermore, these workers confirmed the separation of species B. bifidum and B. longum at the genetic level and proposed that B. lactentis and B. liberorum be merged into a single species, B. infantis. Likewise, they noted that B. parvulorum was considered a synonym for B. breve. They also proposed that B. ruminale and B. thermophilum be regrouped into a single species because of their similarities. They also found similarities between B. pseudolongum and B. globosum, but they did not consider that these organisms were enough alike to merge them into a single species. In 1974, Scardovi and Trovatelli [32] recognized the genetic dissimilarity between B. longum ss. longum and B. longum ss. animalis and proposed elevation of the subspecies animalis to the species level as B. animalis.
Actinomyces eriksonii was first described by Georg et al. [33] in 1965. This organism was isolated from human pleural fluid and a lung abscess. In 1972, Holdeman and Moore [14] reclassified A. eriksonii and placed it in the genus Bifidobacterium, as Bifidobacterium eriksonii. Subsequently, Mitsuoka et al. [34] suggested that A. eriksonii is synonymous with B. adolescentis type b.
The DNA hybridization technique, as used by Scardovi and coworkers, is a significant advance in determinative bacteriology and should help resolve much of the confusion previously encountered. However, on the basis of the fermentation patterns, B. adolescentis, B. angulatum, B. pseudocatenulatum, B. catenulatum and B. dentium; B. pseudolongum and B. globosum; B. animalis, B. suis, B. pullorum and B. gallinarum; B. thermophilum, B. boum and B. choerinum and B. coryneforme and B. asteroides cannot be differentiated from each other. At the present time, fermentation patterns are still the principle guidelines used for specific differentiation of bacteria. The differential scheme for a rapid routine identification of phenotypic species of bifidobacteria is shown in Table 4.
Table 4. Characteristics of Bifidobacterium species from humans and animalsa.
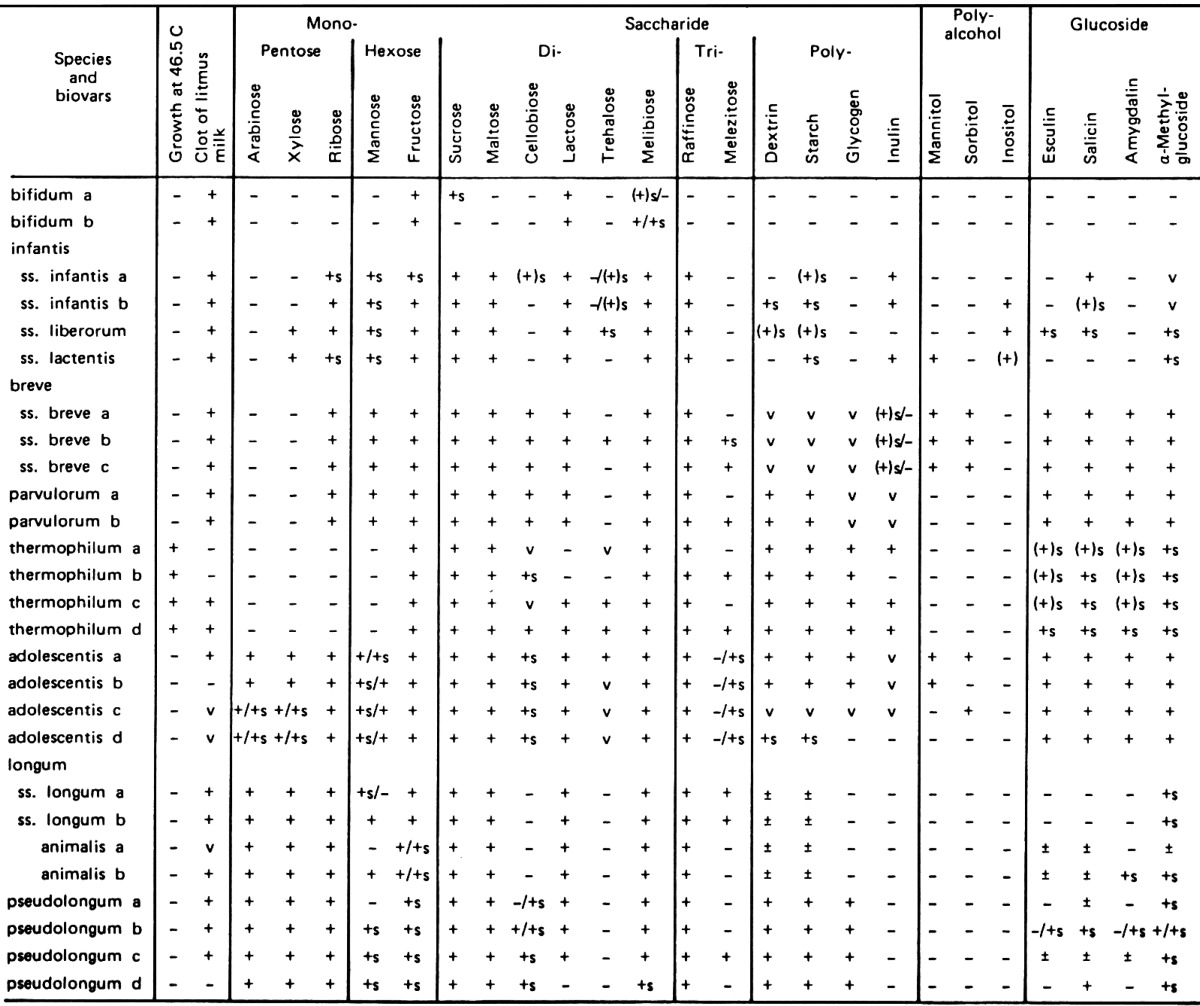
aSymbols: +, 90% or more of strains are positive; −, 90% or more of strains are negative; v, 11–89% of strains are positive.
In view of the relationship between Bifidobacterium species or biotypes and the ecological distribution in humans and animals, it must be stressed that, in general, each animal species harbors certain species or biotypes of bifidobacteria (Table 5). The great majority of the animal strains, with the exception of the strains isolated from monkeys and dogs, were clearly differentiated from human strains by their growth temperature and carbohydrate fermentation patterns and classified as new species, B. thermophilum, B. pseudolongum and B. animalis [26]. The isolates from chickens and pigs were identified as B. thermophilum and B. pseudolongum, while the strains from rodents (mice, rats and guinea pigs) and ruminants (cattle and sheep) belonged to B. pseudolongum and/or B. animalis. On the other hand, isolates from dogs were identified as B. adolescentis, B. longum and B. pseudolongum, and the strains from monkeys were identified as B. adolescentis [35].
Table 5. Habitats of Bifidobacterium species in the intestine of humans and animalsa.
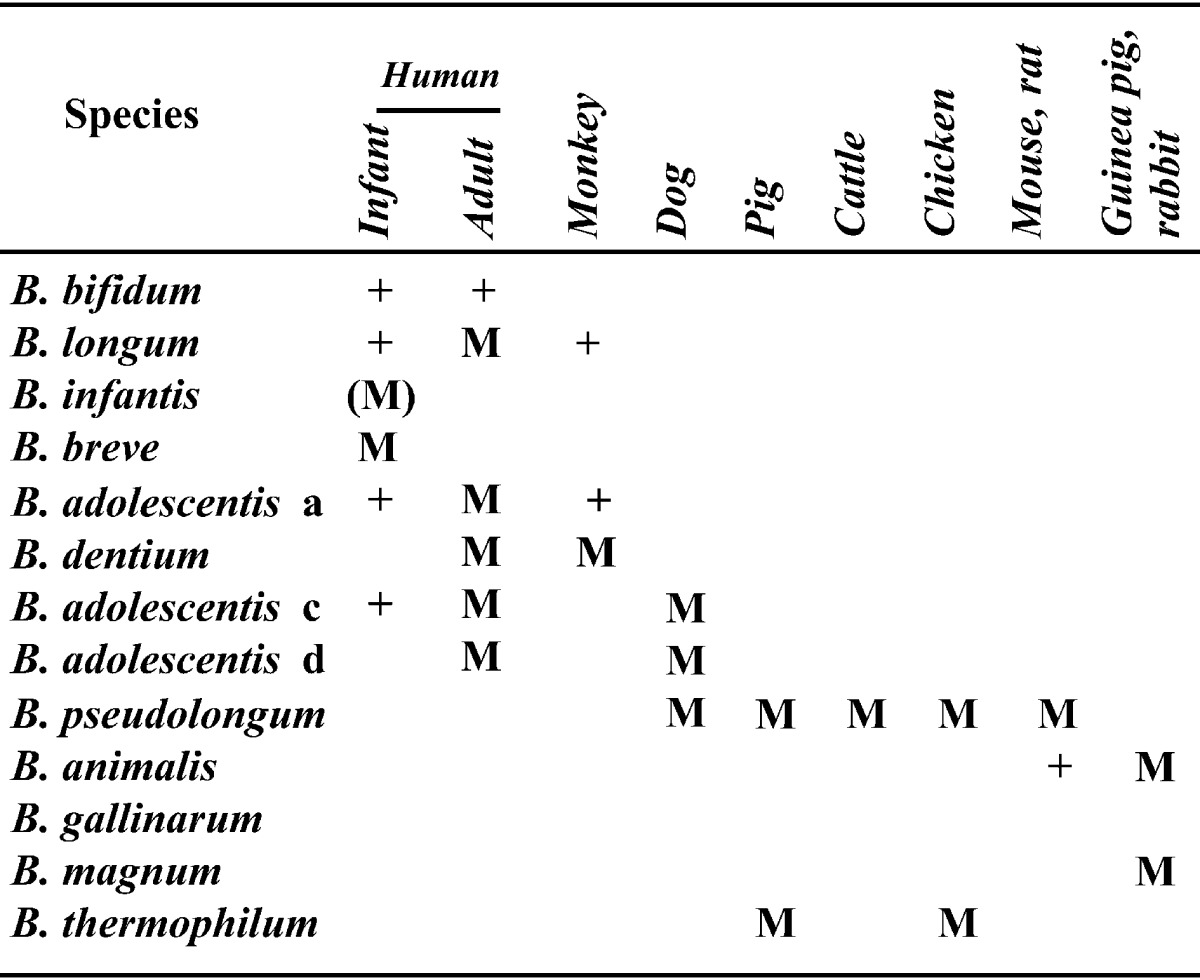
aSymbols: M, major component; (M), formerly major component; +, occasionally isolated.
ECOLOGICAL RULES GOVERNING THE INTESTINAL MICROBIOTA
Major bacterial groups composing the intestinal microbiota
The major bacteria detected in the intestinal microbiota are broadly categorized as follows: lactic acid bacteria, including Bifidobacterium, Lactobacillus and Enterococcus; putrefactive bacteria, including Clostridium including C. perfringens, Bacteroidaceae, Peptococcaceae, Veillonella, Escherichia coli, Staphylococcus, Pseudomonas aeruginosa; and other bacteria, including Eubacterium, Ruminococcus, Megasphaera, Mitsuokella, C. butyricum, and Candida. These bacteria are also divided into anaerobes and aerobes on the basis of the oxygen requirement or into non-pathogens and pathogens on the basis of the pathogenicity.
Composition of the intestinal microbiota of human adults
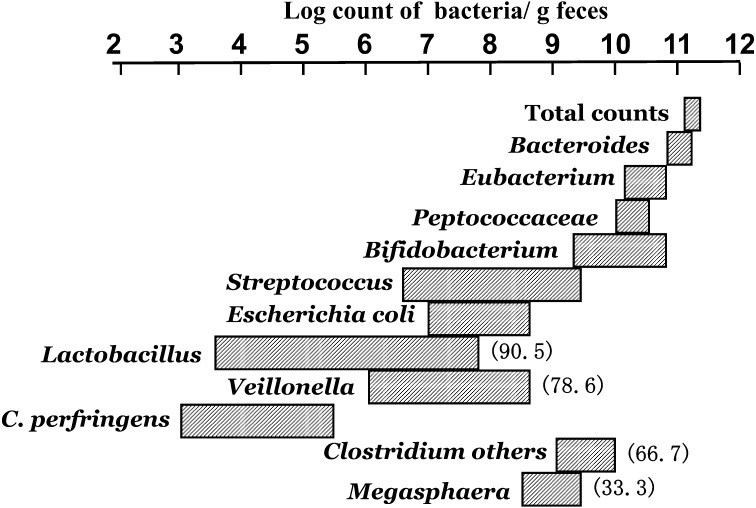
With the use of strict anaerobic culturing techniques, it is now accepted that the most prevalent microorganisms in human adult feces are obligately anaerobes, while easily culturable aerobes are expected to account for less than 10−3 of anaerobe numbers [14] (Fig. 4). The most prevalent anaerobe is Bacteroidaceae, and the average count of this organism is 1010.9 per gram of wet feces. The second most prevalent microorganisms are those of the genus Eubacterium, with average counts of 1010.4 per gram. The third most prevalent microorganisms are those of the Peptococcaceae family, including Ruminococcus, Coprococcus, Peptostreptococcus and Peptococcus. The average count for these bacteria is 1010.2 per gram. The fourth most prevalent microorganisms are those of the genus Bifidobacterium with an average count of 1010.0 per gram. Other anaerobes often found in human feces include Clostridium, Megasphaera and Veillonella. The aerobic microbiota is predominantly represented by E. coli, Streptococcus including Enterococcus, and Lactobacillus, but their counts are less than 108 per gram feces.
Fig. 4.
Composition of fecal microbiota of adults.
Figures in parentheses indicate the frequency (%) of detection. Bacterial groups without parentheses: the frequency of detection is 100%.
Microbiota in each region of the gastrointestinal tract of human adults
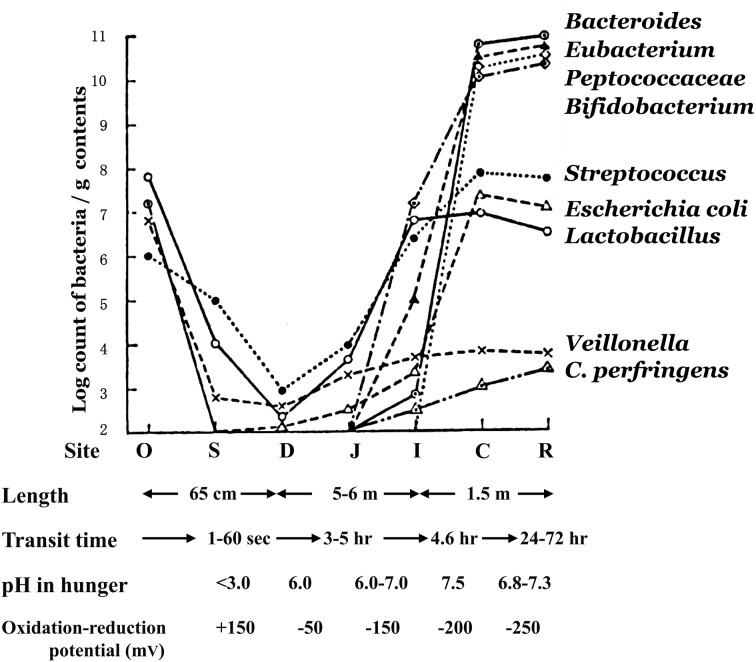
With respect to total bacterial counts in the oral cavity of the adult, there are already 107 bacteria per gram of saliva (Fig. 5). However, the number of bacteria transiently decreases in the stomach as a result of gastric acid; only about 102 to 103 bacteria are detectable in each gram of gastric juice. Although the number of organisms gradually increases beginning in the small intestine, the bacterial count in the upper small intestine is still (103 to 104 /g), with Lactobacillus, Streptococcus and Veillonella being the primary bacteria detected in this region.
Fig. 5.
Microbiota of various regions of the GI tract of human adults.
O, oral cavity; S, stomach; D, duodenum; J, jejunum, I, ileum; C, cecum; R, rectum.
The lower part of the ileum in the small intestine shows increasing total bacterial counts and exhibits some of the flora seen on transition into the large intestine. A mixed flora is detected here consisting of bacteria that make up the flora of the upper small intestine as well as bacteria seen in the microbiota of the large intestine.
A marked change in the flora then occurs as from the ileocecal valve into the large intestine. The total bacterial count rises abruptly to 1011 or greater per gram. Anaerobic bacteria including Bacteroides, Eubacterium, Peptostreptococcus, Clostridium and Bifidobacterium predominate here. Enterobacteriaceae, Enterococcus, Lactobacillus, Veillonella and Staphylococcus are only detected at levels of about 105 to 107/g.
The composition of the microbiota in the lower large intestine is the same as that of the fecal flora.
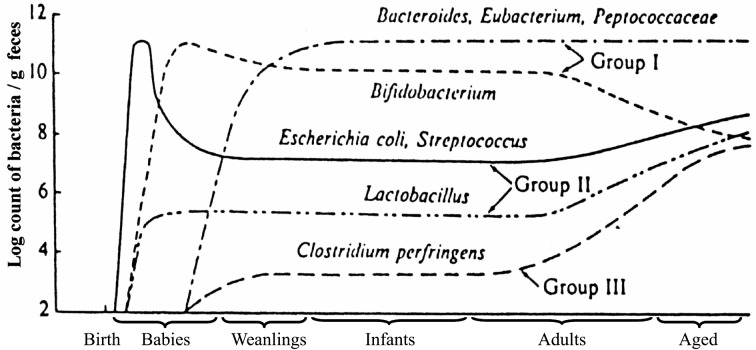
Succession of human intestinal microbiota associated with age
The fetus lives in a completely germfree environment in utero in the mother.
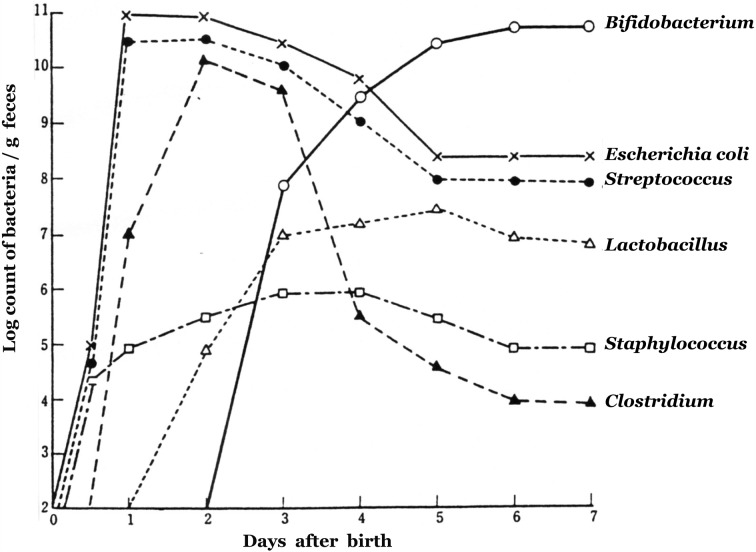
After birth, it rapidly becomes colonized by bacteria. On the first day after birth, E. coli, Enterococcus, Lactobacillus, Clostridium and Staphylococcus are found in the stools of almost all newborns, with total bacterial counts reaching 1011 per gram.
In breast-fed infants, Bifidobacterium generally begins to appear about 3 days after birth, and the previously appearing bacterial groups begin to decrease. On the fourth to seventh day, Bifidobacterium becomes predominant, with counts of 1010 to 1011 per gram. In contrast, the bacterial counts of E. coli, Streptococcus, Staphylococcus, Bacteroides and Clostridium are reduced to about 1 percent of those of Bifidobacterium, with Bifidobacterium accounting for close to 100 percent of the overall microbiota. By about day 7, the “balance” of the intestinal microbiota is nearly stable [36] (Fig. 6).
Fig. 6.
Changes in the fecal microbiota during the first 7 days after birth.
As the weaning period approaches, the intestinal microbiota begins to resemble that of the predominant Gram-negative rod flora seen in adults. The median total bacterial counts per gram of stool are about 1011/g. There are increased counts of Bacteroides, Eubacterium, anaerobic Streptococcus and often Clostridium. Bifidobacterium then decreases to about 10 percent of the total flora. In addition, the pattern of Bifidobacterium (species and biovars) changes from an infantile pattern, consisting mainly of B. infantis and B. breve, to an adult pattern, consisting of B. longum and B. adolescentis [37].
The intestinal flora then begins to again show changes during the transition from middle age to old age. Along with slight reductions in total bacterial counts, there are reductions of Bifidobacterium. There are some individuals in whom Bifidobacterium becomes completely undetectable. However, the detection rate and number of Clostridium perfringens markedly increases. Lactobacillus, Enterobacteriaceae, and Enterococcus also increase. This phenomenon is thought to result from the effect that aging of physiologic function in the host has on the intestinal bacterial microbiota. This itself may also further accelerate the aging process (Table 6, Fig. 7).
Table 6. Fecal microbiota of different age groups of humans.
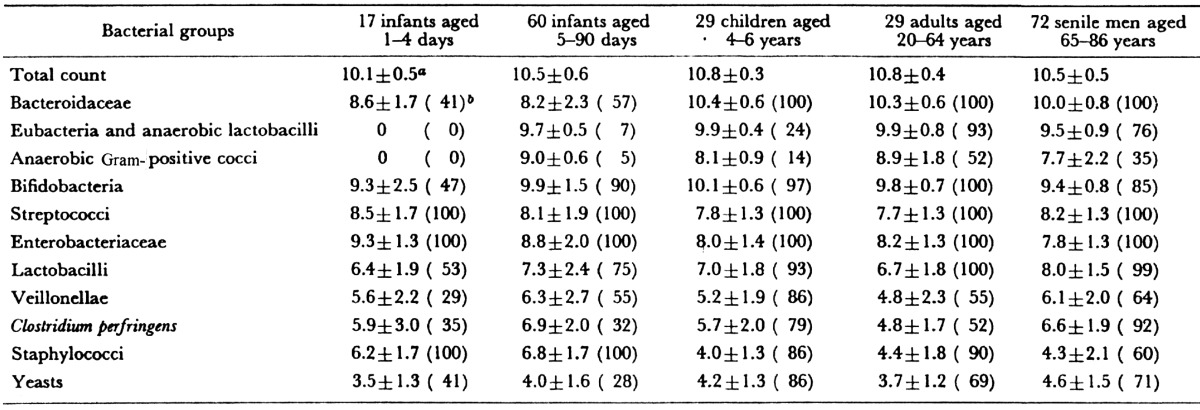
a Mean ± SD of log bacterial counts (when present). b Frequency of occurrence (%).
Fig. 7.
Changes in the fecal microbiota with increasing age.
Composition of the intestinal microbiota of various animal species
The compositions of the fecal microbiota of healthy adult animals of 12 different species have been analyzed [38] (Table 7). In general, animals of the same species had a common pattern of fecal microbiota, but patterns different from those of other species. In almost all animal species, the most predominant fecal microbiota were anaerobes, including Bacteroidaceae, Bifidobacterium, Eubacterium, Lactobacillus, Peptococcaceae and anaerobic curved rods. The numbers of lactobacilli and bifidobacteria varied with the species of animal. In the feces of humans, monkeys and guinea pigs, bifidobacteria outnumbered lactobacilli. Regarding the feces of chickens, pigs, dogs, mice, rats and hamsters, bifidobacteria were present in almost all individuals but in smaller quantities than lactobacilli. In the feces of rabbits and horses, bifidobacteria were occasionally demonstrated as being present but not consistently. They were never demonstrated as being present in the feces of cats and minks.
Table 7. Fecal microbiota of twelve adult animals of different species.
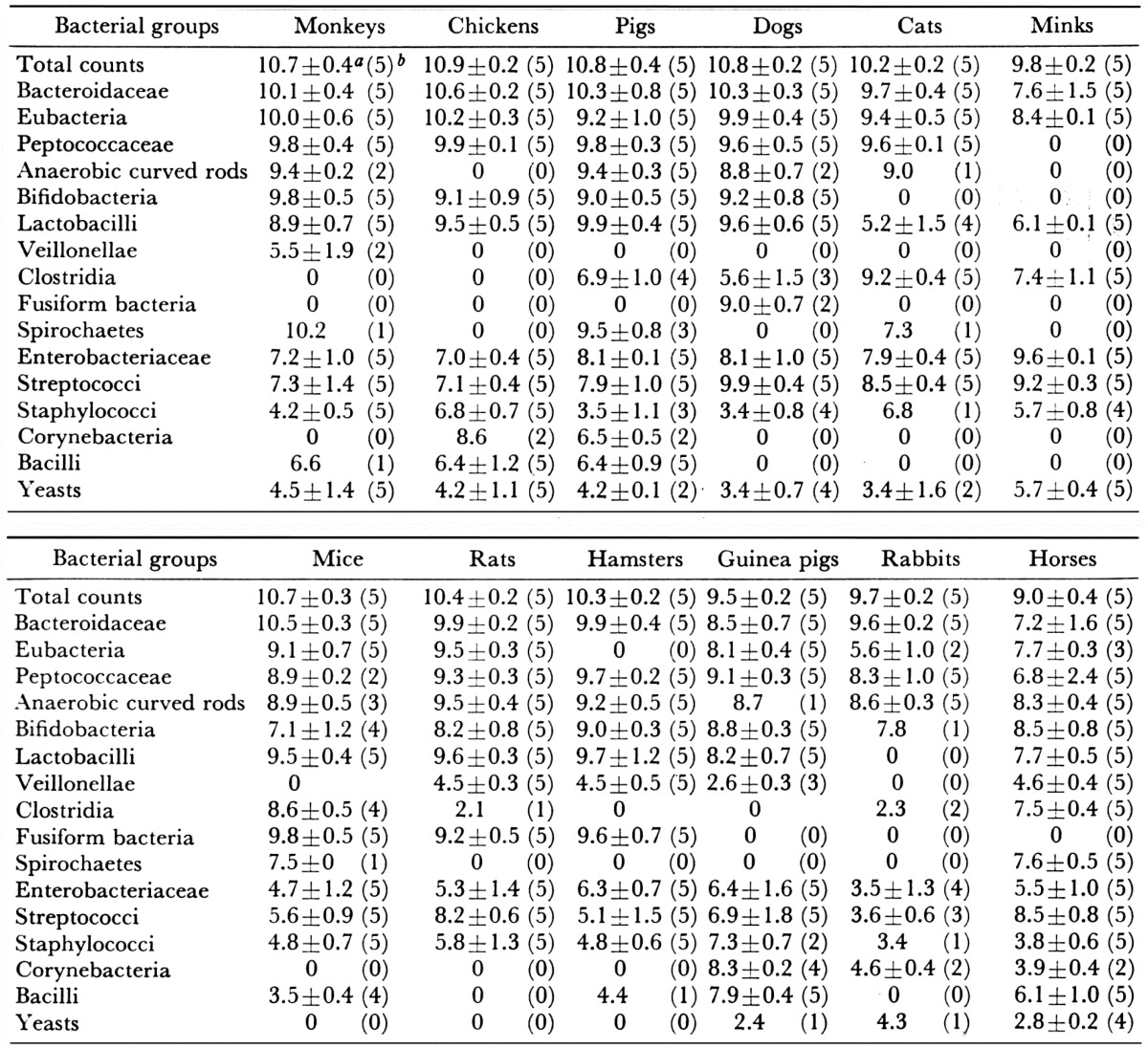
a Mean ± SD of log bacterial counts (when present). b Figures in parentheses refer to the number of subjects that harbor the organism.
FACTORS AFFECTING THE COMPOSITION OF THE HUMAN INTESTINAL MICROBIOTA
Individual and day-to-day differences
Individual differences in the intestinal microbiota have also been observed in adults. The intestinal microbiota of six adults were investigated in seven samples collected from each individual during a two-month period Table 8 [39]. As a result, it was revealed that the balances of the intestinal microbiota were nearly stable; day-to-day variations in the predominating bacterial populations were markedly small, but in the minor bacterial populations, they were unstable. The possible causes of the difference in balance of the intestinal flora are physical conditions of the digestive tract, such as peristalsis and the excretion of gastric juice or bile acids, and dietary habits.
Table 8. Individual and daily differences in the fecal microbiota of 6 healthy adults.
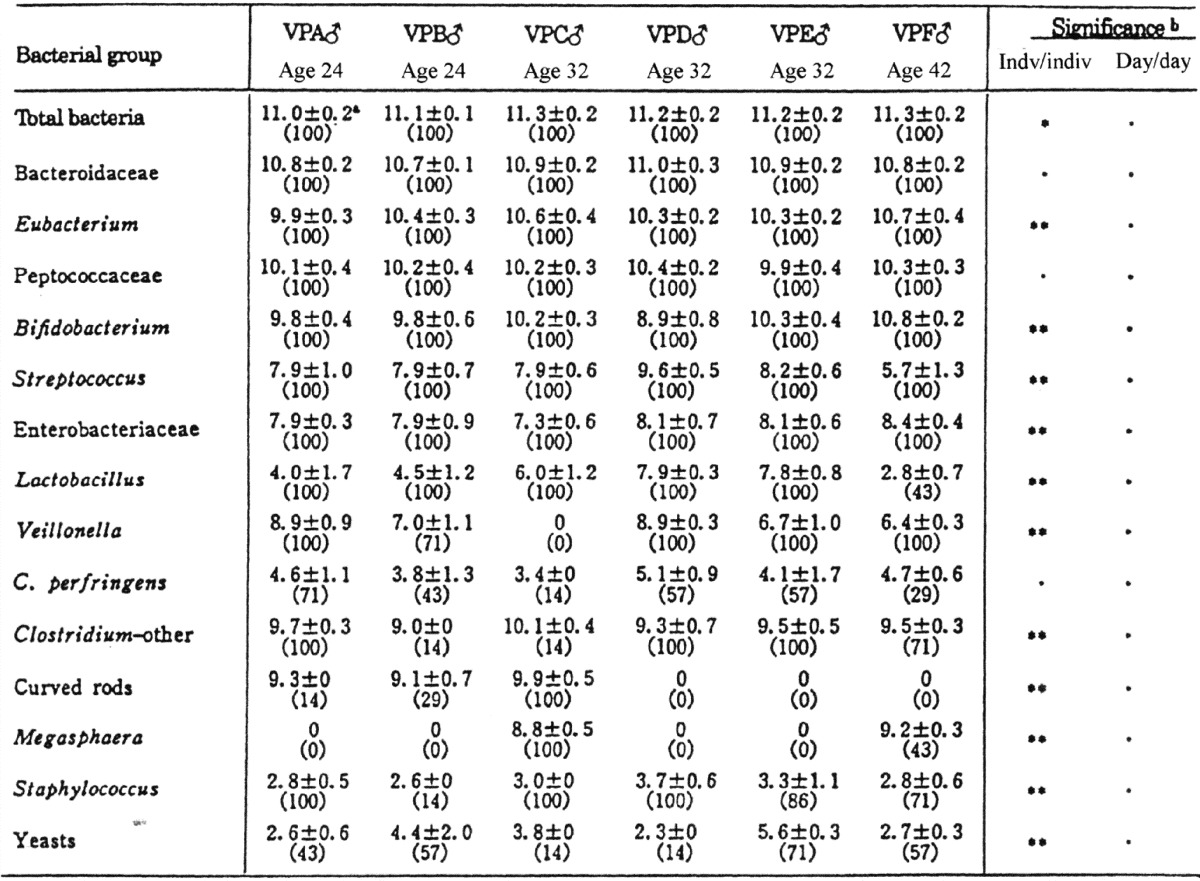
a Mean ± SD of log bacterial counts (%, the rate of occurrence).
b Significant difference: *p<0.05; **p<0.01; ***p<0.001.
Age-related differences in Bifidobacterium species or biotypes
Differences in the predominant Bifidobacterium species or biotypes in different age groups of humans were observed [37]. The results are shown in Table 9. The bifidobacteria isolated from infants belonged to the species B. bifidum type b, B. infantis ss. infantis, B. breve ss. breve, B. breve ss. parvulorum, and B. longum ss. longum type b. These species and biotypes, except B. longum biotype b, occurred almost exclusively in infants. In contrast, B. adolescentis types a-b and B. longum type a were found in high numbers in the intestine of children, adults and senile men, although in the senile men, the occurrence of B. adolescentis type b was significantly higher than in the other age groups. These species and biotypes were only occasionally isolated from infants.
Table 9. Frequency of occurrence of species and biotypes of bifidobacteria in feces of various age groups of humansa.
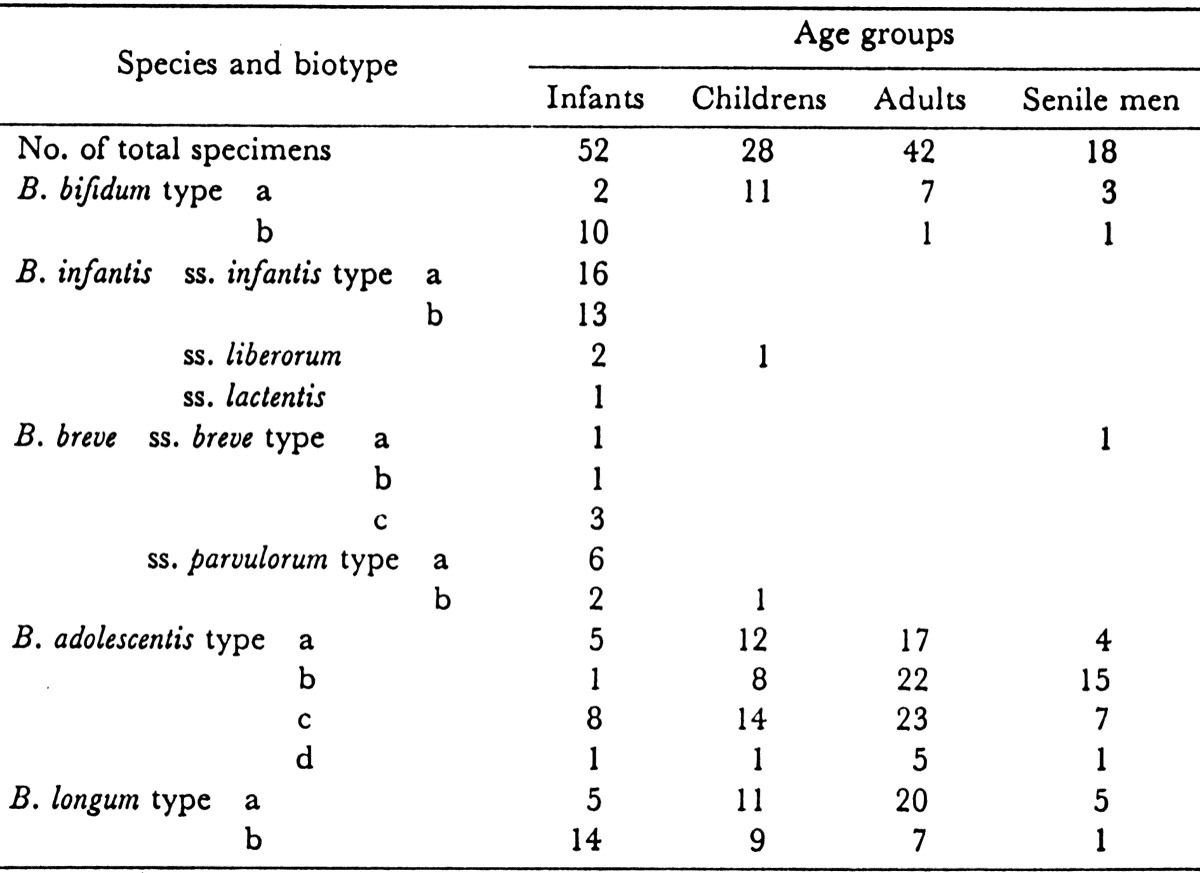
a Figures indicate the numbers of specimens.
Age-related differences in Lactobacillus species
Lactobacilli isolated from infants belonged mainly to the L. acidophilus group, L. salivarius and L. fermentum (L. reuteri). After weanings, L. plantarum, L. casei, L. brevis, and L. buchneri, which are thought to be of diet origin, were also detected (Table 10).
Table 10. Differences in Lactobacillus species in different age groups.
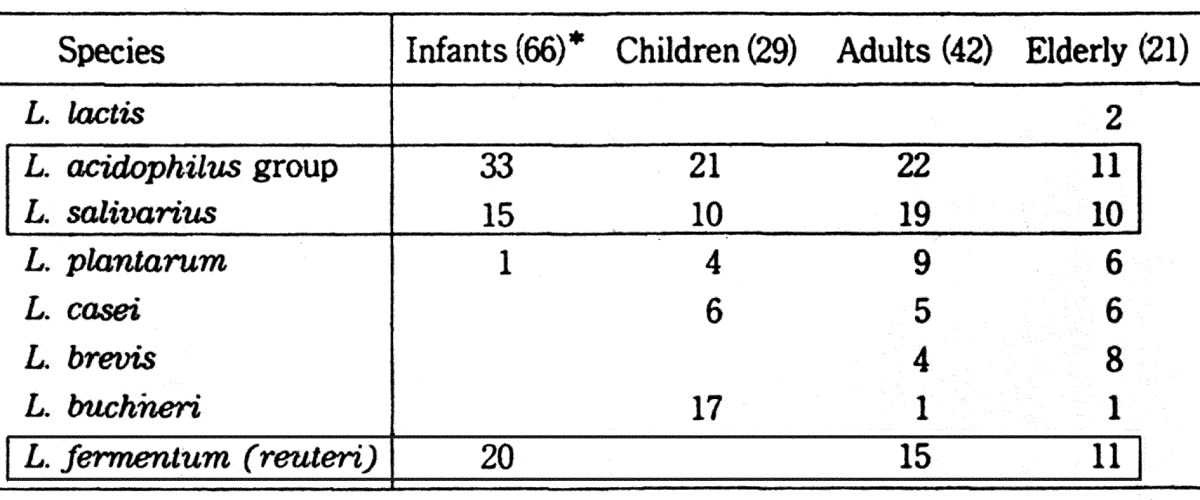
*Number of subjects.
Differences of the intestinal microbiota in breast-fed and bottle-fed infants
Since the work of Tissier, it has been believed that bifidobacteria are found exclusively in feces of breast-fed infants, whereas in bottle-fed infants, Lactobacillus acidophilus is the most commonly found organism. According to our study [40], however, the differences in the occurrences and numbers of bifidobacteria between breast-fed and bottle-fed infants were not significant. Bifidobacteria were present in all 30 breast-fed infants and in 29 bottle-fed infants, with mean counts (when present) of 1010.7/g and 1010.0/g, respectively (Table 11). The principal difference between breast-fed and bottle-fed infants was that in bottle-fed infants, the numbers of enterobacteriaceae, streptococci and anaerobes other than bifidobacteria were significantly greater than in breast-fed infants.
Table 11. Comparison of fecal microbiota between bottle-fed infants and breast-fed infants.
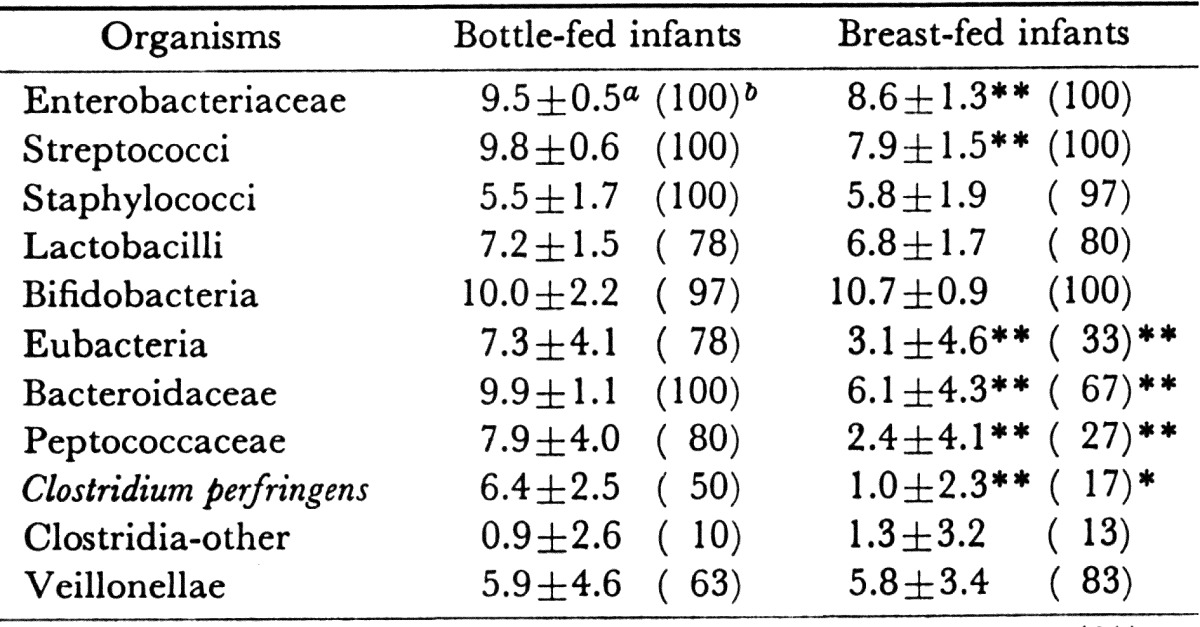
a Mean ± SD of log bacterial counts.
b Frequency of occurrence (%).
Significant difference: **p<0.01; *p<0.05.
Clinic-dependent Bifidobacterium species or biotypes in the infant intestine
Variations were observed in the occurrences of Bifidobacterium species or types in infants in different clinics [37]. In general, all infants in the same clinic harbored a similar predominating Bifidobacterium strain(s). B. longum type b was a relatively common species isolated from the infants of Clinic A but was only occasionally isolated from the infants of Clinics B and C. B. infantis ss. infantis type b was a predominant species among infants of Clinic B and was less frequently isolated from infants of Clinic A and C. B. infantis ss. infantis type a was the most common bifidobacteria isolated from infants in Clinic C but was not isolated in other clinics. This finding suggested that one Bifidobacterium strain may be transmitted from infant to infant through the hands of nurses.
Succession of the species and biovars of bifidobacteria found in infants
In 1900, Tissier discovered B. bfidum in the feces of infants. In 1950, Norris et al. [41] isolated Lactobacillus parabifidus strain Timberlain (ATCC 17930) from infant feces. This strain is considered synonymous to B. infantis ss. liberorum György [42] originally designated the organism L. bifidus var. penn (subsequently changed to L. bifidus var. pennsylvanicus) isolated from infants. This strain belongs to B. bifidum. Dehnert [43] reported his group IV (B. infantis ss. lactentis) as the predominant bifidobacterial group in the stools of breast-fed infants. Reuter [44] and I [17] reported that B. infantis ss. infantis predominated in the feces of breast-fed infants. Seeliger and Werner [45] reported that Dehnert’s group III (B. infantis) was predominant in Bonn (West Germany), although group IV (B. infantis ss. lactentis) was also present. Petuely and Lindner [46] noted the predominance of Dehnert’s group IV (B. infantis ss. lactentis) in the stools of breast-fed infants. Werner [47] also reported the prevalence of Dehnert’s group IV in breast-fed infants and stated that group IV had been found only in the stools of breast-fed infants or of infants fed maternal milk together with cow’s milk. In the year after 1980, all of the subspecies of B. infantis became very difficult to isolate from infant feces, and B. breve ss. breve predominates in the feces of both breast-fed and bottle-fed infants. The factors affecting such succession of the predominant bifidobacterial species and biovars are still unknown (Table 12).
Table 12. Succession of species and biovars of bifidobacteria occurring in the feces of infants.
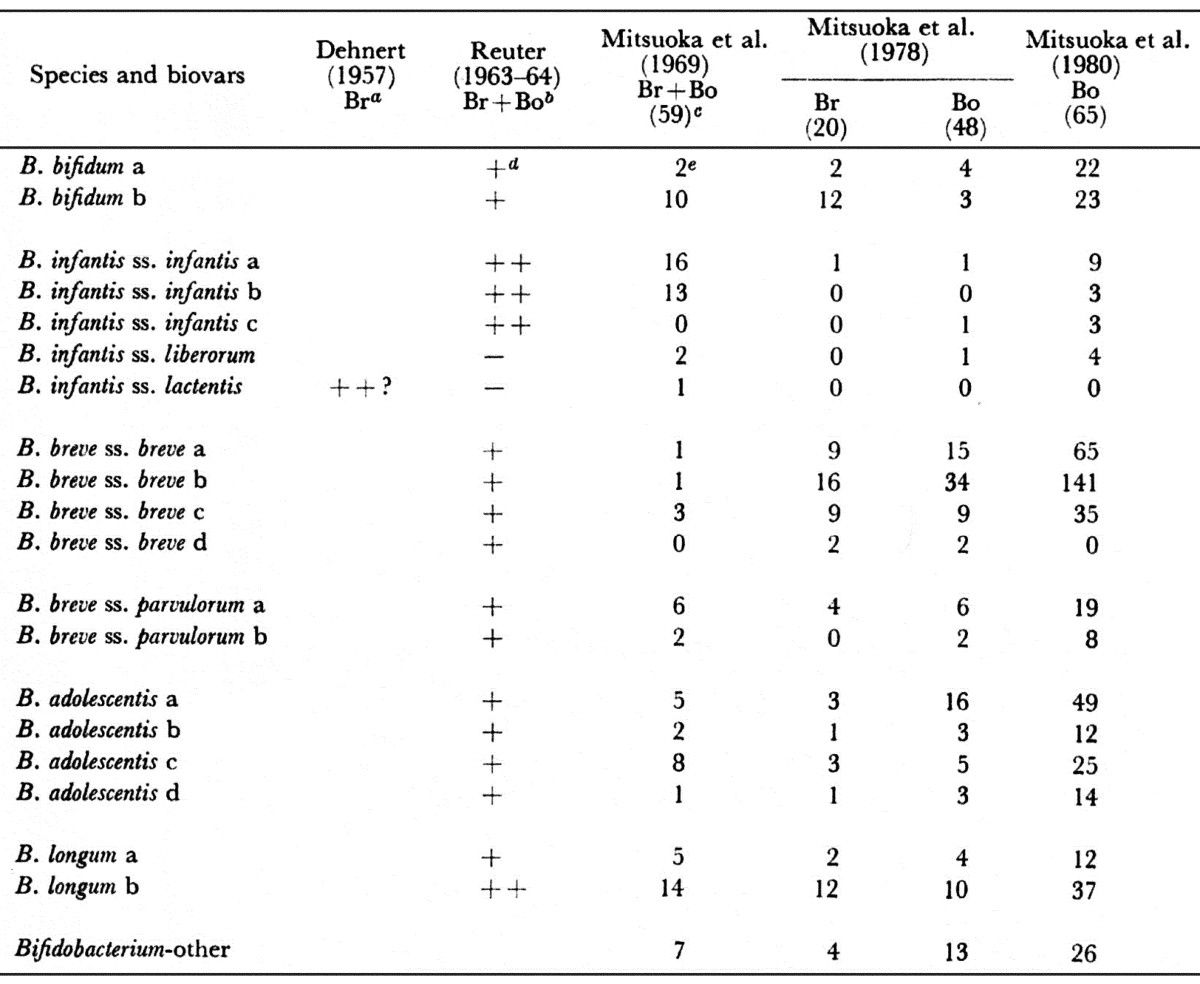
a Br: Breast-fed infant. b Bo: bottle-fed infant. c Number of speciments. d Frequency of isolation. e Number of specimens harbored.
Influence of diet
It is often reported that the compositions of intestinal microbiota are influenced by diet. The fecal microbiota of 15 healthy elderly persons with a median age of 84 years in a rural area whose inhabitants tend to be long-lived (Yuzurihara, Uenohara, Yamanashi Prefecture) was compared with the microflora of individuals with a median age of 68 years in an urban area (Tokyo). The diet of the elderly persons in the Yuzurihara area is characterized by a high intake of dietary fiber. Total numbers of anaerobic bacteria were significantly smaller in the elderly persons in the Yuzurihara area than those of the individuals in the Tokyo area. A significantly large number of bifidobacteria, but not of lecithinase-negative clostridia, was observed in the elderly persons in the Yuzurihara area. Large numbers and high incidences of bacilli and lecithinase-positive clostridia (mainly C. perfringens) were found in the elderly persons in the Tokyo area (Table 13) [48]. Differences in the fecal microbiota between elderly persons in the Yuzurihara area and those in the Tokyo area might be due to a difference in the intake of dietary fiber.
Table 13. Comparison of the fecal microbiota of aged persons in Yuzurihara and Tokyo.
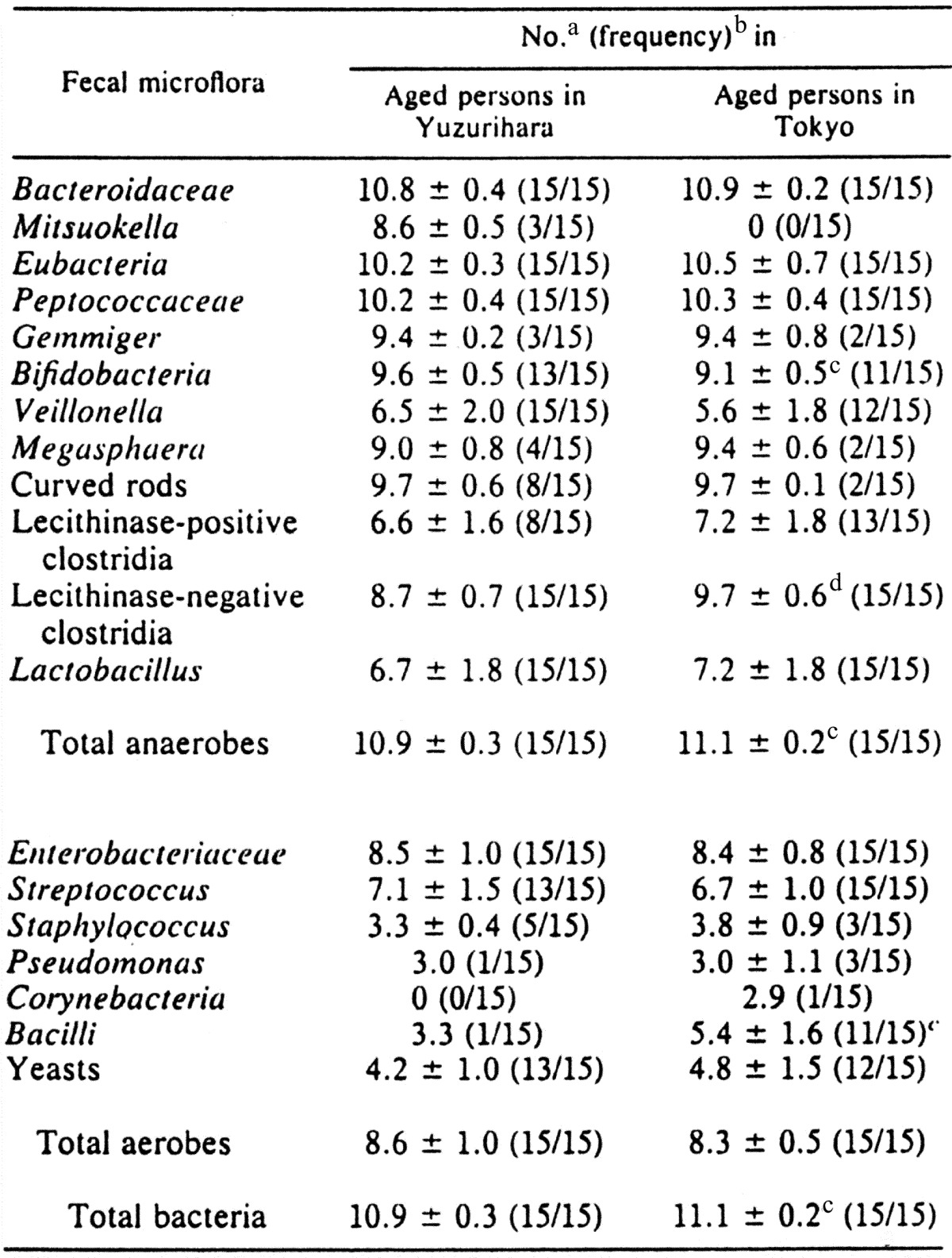
a Bacterial counts are expressed as the mean ± standard deviation of log10 per gram (wet weight) of feces.
b Frequency occurrence is expressed as the number of subjects with the organism detected/number of subjects examined.
c Statistically significant at the p<0.05 level when compared with elderly persons in the rural area (Student’s t test for the bacterial counts and chi-square test for the frequency of occurrence).
d Statistically significant at the p<0.001 level when compared with elderly persons in the rural area.
Influence of diseases
a) Colorectal cancer and stomach cancer
The fecal microbiota of 54 healthy adults was compared with that of 44 patients with colorectal cancer and that of 39 patients with stomach cancer. In the patients with colorectal cancer, the Eubacterium and Bacteroides melaninogenicus counts were significantly higher, and in the patients with stomach cancer, the Bifidobacterium and Bacteroidaceae counts were significantly lower and those of C. perfringens and Streptococcus were significantly higher. In addition, the detection rates of Micrococcaceae and Pseudomonas were significantly higher [49].
b) Ulcerative colitis and Crohn’s disease
Comparing the fecal microbiota of patients with ulcerative colitis and Crohn’s disease with those of healthy adults, it was found that total bacterial counts, especially those of Bacteroidaceae, Eubacterium, Peptococcaceae and Pseudomans, were significantly decreased in both groups of patients. In addition, the Lactobacillus, Streptococcus and Pseudomanas counts were significantly decreased and that for Enterobacteriacceae was significantly increased in patients with ulcerative colitis [49].
c) Dementia senilis
Examination of the fecal microbiota of 7 patients (aged 61 to 90 years old) with dementia senilis showed that the total bacterial counts were moderately lower and the counts of patients with dementia senilis were significantly higher, as compared with our earlier study examined healthy 72 senile persons (aged 65–86 years old). This result suggested that the fecal microbiota is related to dementia senilis [49].
SIGNIFICANCE OF THE INTESTINAL MICROBIOTA IN HEALTH AND DISEASE
Relationships between the intestinal microbiota and the host
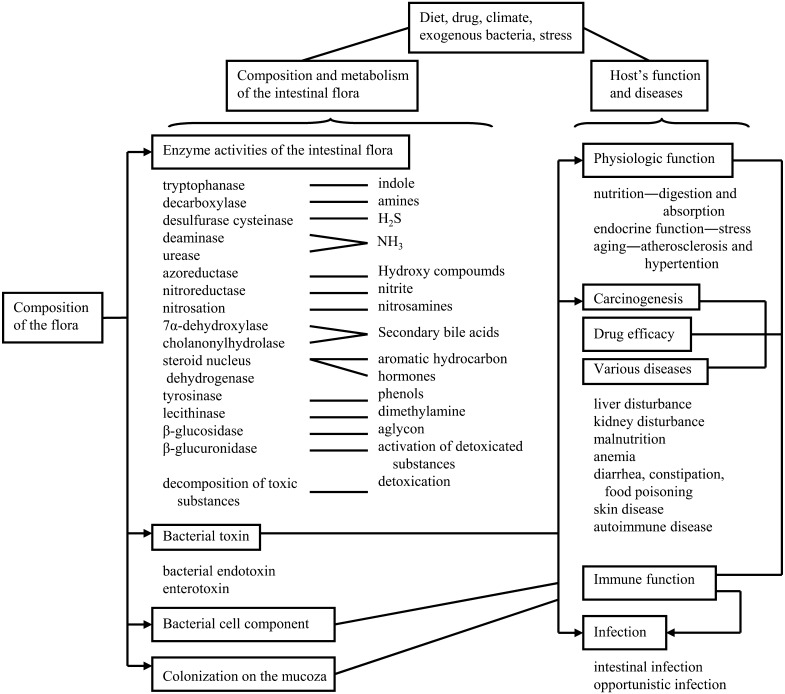
The intestinal flora possess diverse enzymes that perform extremely varied types of metabolism in the intestine, converting substances into compounds that can be beneficial or detrimental to the host (Fig. 8) [50]. In general, metabolism within the tissues of an organism, particularly hepatic metabolism, involves oxidation and biosynthesis with glucuronic acid and sulfuric acid conjugation. This leads to production of polar, water-soluble substances. In contrast, metabolism by the intestinal flora primarily involves reduction and hydrolysis. The harmful bacteria may form substances that are noxious to the host. Among these are certain putrefactive substances (ammonia, hydrogen sulfide, amines, phenols, indoles, etc.) and secondary bile acids. These substances may injure the intestine directly and are also partially absorbed, potentially contributing throughout the host’s life to aging and geriatric diseases such as cancer, arteriosclerosis, hypertension, liver disorders, autoimmune diseases and immunosuppression. These substances may affect nutrition, physiologic function, drug efficacy, carcinogenesis and aging, as well as the host’s resistance to infection.
Fig. 8.
The metabolic activities of intestinal bacteria.
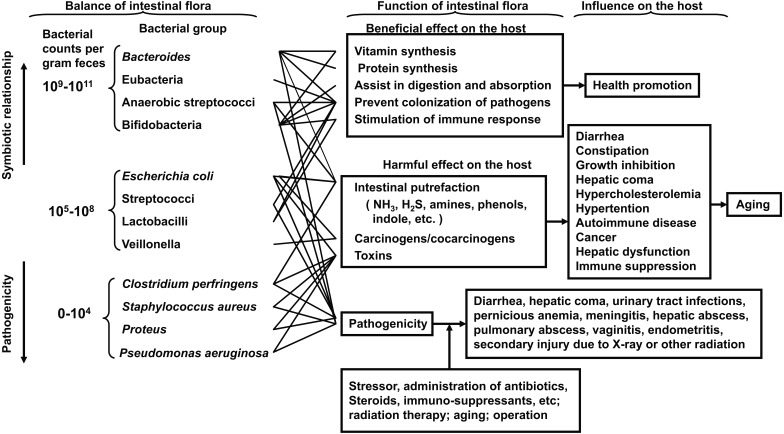
Intestinal bacteria influence both the health and disease of the host. I have postulated three groups of intestinal bacteria: the first group consists of organisms symbiotic to the host and constitutes the predominant flora, the second group consists of ubiquitous organisms such as E. coli or Streptococcus group but with counts in the normal host not being predominant, and the third group of bacteria consists of true pathogenic bacteria, sometimes producing infection, with counts that are normally low (Fig. 9) [50].
Fig. 9.
Relationship between intestinal bacteria and the host.
The bacterial cell components are known to stimulate immunity. The intestinal microbiota is known to be important in the development of many lymphoid cells in the gut-associated lymphoid tissue (GALT) and the induction of a mucosal immune response. Since lymphocytes stimulated in GALT subsequently migrate to other lymphoid tissues, the intestinal microbiota may also influence the proliferative response of spleen and lymph node cells.
Lactic acid bacteria possess various immunological functions, including mitogenic activity, adjuvant activity, macrophage activation, antibody production enhancement, interferon production and antitumor effects. Furthermore, both the cell wall and cytoplasm of lactic acid bacteria were found to have induced mitogenic responses of spleen cells.
On the other hand, some intestinal bacteria have potential pathogenicity. Aging; the administration of antibiotics, immunosuppressive agents, anticancer agents or adrenocortical (steroid) hormones; stresses; or radiation therapy can lead to decreased resistance of the host, and some intestinal bacteria can then invade the viscera through the gastrointestinal tract and exhibit pathogenicity. These organisms can cause a so-called opportunistic infection: sepsis; endocarditis; brain, hepatic or pulmonary abscesses; cystitis; or vaginitis.
The composition of the intestinal flora reflects intestinal metabolism. This also has a variety of effects on the organism. The ingredients of the food ingested each day and substances secreted and excreted by the intestine are converted into various substances. This therefore has a profound influence on factors such as nutrition, drug effects, physiologic function, aging, carcinogenicity, immunity and infection within the host.
Microbial populations in the gastrointestinal tract are known to form a barrier against the proliferation of exogenous pathogens. Thus, host susceptibility to specific enteric infections is influenced by the nature of the intestinal microbiota. One of the mechanisms may be related to the colonization of the indigenous flora, which prevents colonization of the invader by competing effectively for essential nutrients or attachment sites on the epithelium. Another mechanism to prevent colonization of exogenous pathogens is production of bacteriocidal or bacteriostatic agents.
Usefulness of gnotobiotes and human flora animals
Gnotobiotic animals associated with human intestinal bacteria and ex-germfree animals associated with human fecal flora provide useful models for studying the role of the intestinal flora in carcinogenesis and ageing.
a) Liver tomor experiment
The effect of intestinal bacteria on liver tumorigenesis in gnotobiotic C3H/He male mice monoassociated, diassociated or polyassociated with various intestinal bacteria has been investigated [51]. As shown in Table 14, the incidence of liver tumors was higher in most of the gnotobiotes and conventional mice than in the germfree mice. Liver tumors were observed in 100% of mice associated with a bacterial combination of E. coli, S. faecalis and C. paraputrificum, while they were only observed in 30% of germfree mice and 75% of conventional mice. However, this tumor-promoting effect of intestinal bacteria was suppressed by 46% by addition of B. longum to the promoting combination and by 65% by addition of L. acidophilus. These results suggest that intestinal bacteria are related to both promotion and prevention of cancer. The mechanism of the suppressive effect of bifidobacteria on liver tumors might be related to their ability to stimulate immunity of the host or to detoxify carcinogens.
Table 14. Incidence of liver tumors in gnotobiotic C3H/He male mice associated with human intestinal bacteria.
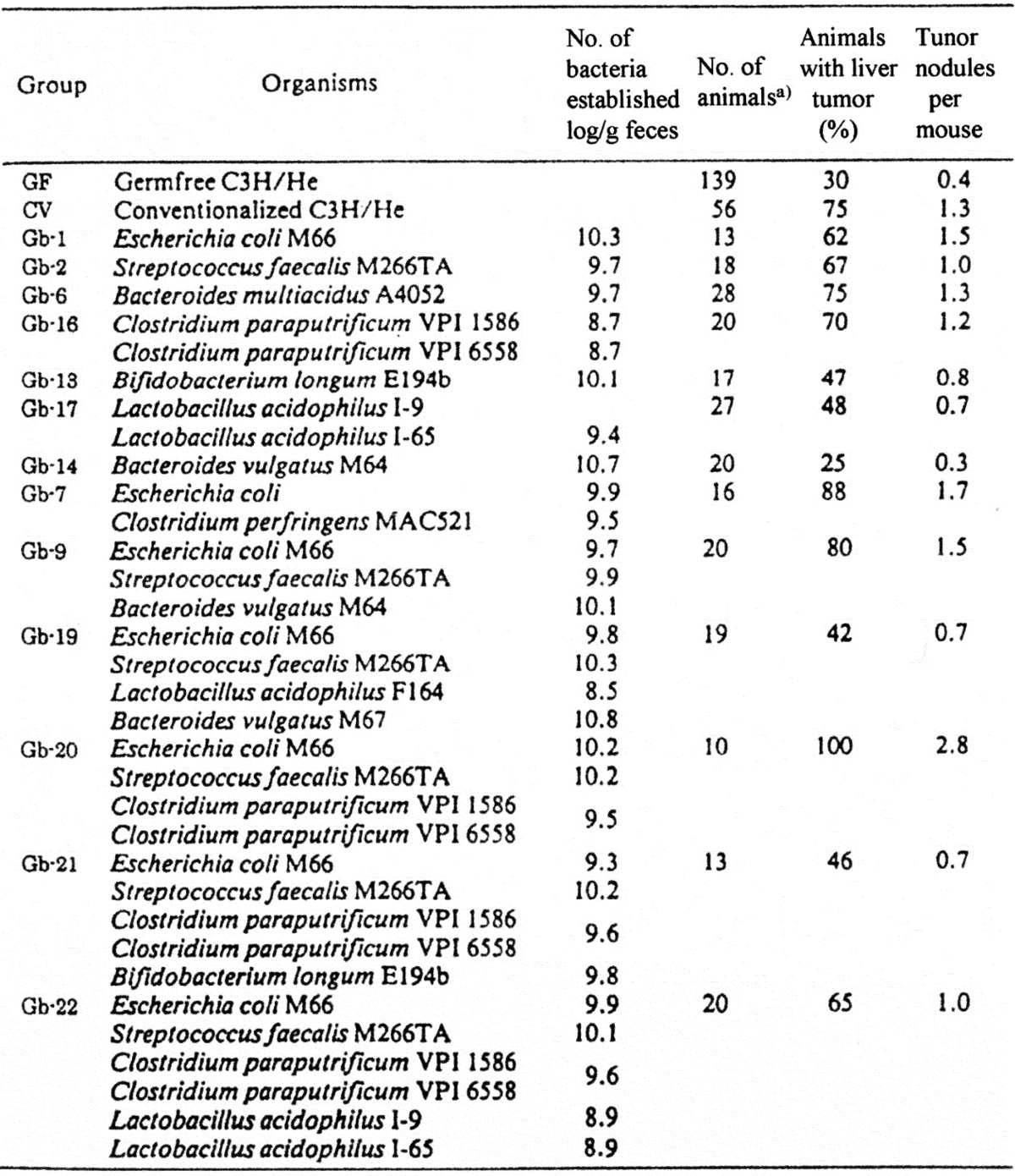
a) Mice were euthanized under ether anesthesia when they were 12 months old.
b) Aging experiment
The effect of intestinal flora on life span has also been studied. Germfree, conventional and gnotobiotic (GB) CF#1 female mice were produced. GB-1 were associated with E. coli, Enterococcus faecalis, Bacteroides vulgatus, Eubacterium aerofaciens, Bifidobacterium longum and C. perfringens, and those associated with the same combination of intestinal bacteria without B. longum (GB-2) or C. perfringens (GB-3) were produced and maintained until their natural deaths [49] (Table 15). The verage life spans were longest in germfree mice (96.3 weeks) and shortest in conventional (78.2 weeks) mice. Of the gnotobiotes, the average life spans were shorter in GB-2 (80.7 weeks) than in GB-1 and GB-3 (87.1 weeks). These findings suggest that the presence of B. longum may be related to longevity in gnotobiotic animals.
Table 15. Bacterial strains associated and bacterial number of bacteria (log/g feces) in gnotobiotic (GB) DF#1 mice and differences in the longevity among germfree, gnotobiotic and conventional CF#1 female mice.
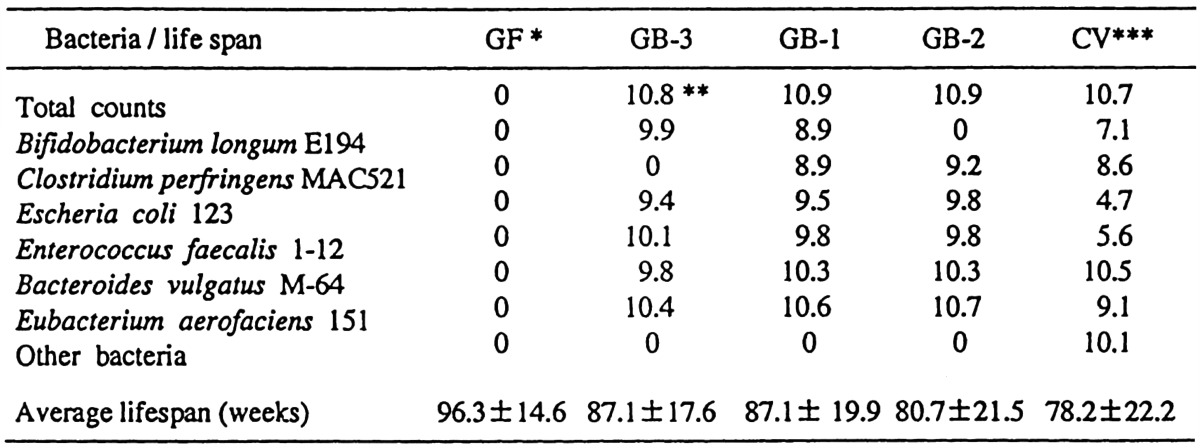
*GF: germfree mice. **Log. Number/gram feces. *** Bacterial strains and number of CVs (conventional mice) are indicated as bacterial groups and their numbers in conventional DF#1 mice.
In the two gnotobiotic studies reported here, almost all of the bacteria inoculated were established in high numbers in the intestine of gnotobiotic mice, and the bacterial numbers differed significantly from the numbers of the same bacteria in the human intestine, but it was not possible to monocontaminate animals with L. acidophilus. Thus, it is not easy to extrapolate from an experiment in laboratory animals to humans.
CONCLUSION
In 1953, our group began research on the intestinal microbiota. At first, we made it possible to cultivate and isolate intestinal bacteria for comprehensive investigation. Then, we developed a classification and identification system of intestinal anaerobes and clarified the characteristic composition and habitat of the intestinal microbiota and lactic acid bacteria of humans and animals. Based on these results, we have carried out ecological research on the intestinal microbiota of humans and animals, and I have put forward a hypothesis concerning the “relationships between the intestinal microbiota and the host”. Furthermore, using gnotobiotic mice, the effects of intestinal bacteria on longevity and tumorigenesis in mice have been studied, and it was clarified that food excerts a profound effect on the intestinal microbiota and health. At this point of time, I judged that a new interdisciplinary science “intestinal bacteriology”, had been established.
REFERENCES
- 1.Savage DC. 2001. Microbial biota of the human intestine: a tribute to some pioneering scientists. Curr Issues Intest Microbiol 2: 1–15 [PubMed] [Google Scholar]
- 2.Haenel H. 1960. Aspekte der mikroökologischen Beziehungen des Makroorganisms. Zbl Bakt Hyg I Ref 176: 305–425 [Google Scholar]
- 3.Haenel H. 1963. Über die Mikrobkologie alter Menschen. Zbl Bakt Hyg I Orig 188: 219–230 [Google Scholar]
- 4.Haenel H. 1965. Gesetzmäßigkeiten in der Zusammensetzung der fäkalen Mikroflora: Eubiose und Dysbiose der menschlichen Darmbesiedlung. Ernährungsforsch 10: 289–301 [Google Scholar]
- 5.Haenel H, Müller-Beuthow W. 1963. Untersuchungen an deutschen und bulgarischen jungen Männern über die intestinale Eubiose. Zbl Bakt Hyg I Orig 188: 70–80 [Google Scholar]
- 6.Haenel H, Müller-Beuthow W, Grütte FK. 1970. Zur fäkalen Mikroökologie des Säuglings in Abhängigkeit von der Ernährung: Zusammensetzung der Mikroflora sowie Vorkommen der Laktobacillus bifidus-Typen. Zbl Bakt Hyg I Orig 215: 333–347 [PubMed] [Google Scholar]
- 7.Mitsuoka T, Ohno K, Benno Y, Namba K, Suzuki K. 1976. Die Fackalfiora bei Menschen. IV. Mitteilung. Vergleich des neu entwickelten Verfahrens mit dem bisherigen jüblichen Verfahren zur Darmfloraanalyse. Zbl Bakt Hyg I Orig 234: 219–233 [PubMed] [Google Scholar]
- 8.Mitsuoka T, Sega T, Yamamoto S. 1965. Eine verbesserte Methodik der qualitativen und quantitaven Analyse der Darmflora von Menschen und Tieren. Zbl Bakt Hyg I Orig 195: 445–469 [PubMed] [Google Scholar]
- 9.Mitsuoka T, Terada A, Morishita Y, Yamamoto S. 1969. A simple method (‘Plate-in-bottle’) for the cultivation of fastidious anaerobes. Jpn J Microbiol 13: 383–385 [DOI] [PubMed] [Google Scholar]
- 10.Mitsuoka T. 1970. An improved method for comprehensive investigation of intestinal flora. Proc. VIII. International Congress on Nutrition, pp. 422–424.
- 11.Mitsuoka T. 1984. A Color Atlas of Anaerobic Bacteria. Sobunsha, Tokyo. [Google Scholar]
- 12.Drasar BS. 1967. Cultivation of anaerobic intestinal bacteria. J Pathol Bacteriol 94: 417–427 [DOI] [PubMed] [Google Scholar]
- 13.Hungate RE. 1950. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev 14: 1–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holdeman LV, Cato EP, Moore WEC. 1977. Anaerobe Laboratory Manual, 4th ed. Virginia Polytechnic Institute and State University, Blacksburg, Virginia. [Google Scholar]
- 15.Rogosa M, Sharpe ME. 1959. An approach to the classification of the lactobacilli. J Appl Bacteriol 22: 329–340 [Google Scholar]
- 16.Reuter G. 1965. Das Vorkommen von Laktobazillen in Lebensmitteln and ihr Verhalten im menschlichen Intestinaltrakt. Zbl Bakt Hyg I Orig 197: 468–487 [Google Scholar]
- 17.Mitsuoka T. 1969. Vergleichende Untersuchungen über die Laktobazillen aus den Faeces von Menschen, Schweinen, und Hünern. Zbl Bakt Hyg I Orig 210: 32–51 [PubMed] [Google Scholar]
- 18.Johnson JL, Phelps CF, Cummings CS, London L, Gasser F. 1980. Taxonomy of the Lactobacillus acidophilus group. Int J Syst Evol Microbiol 30: 53–68 [Google Scholar]
- 19.Mitsuoka T. 1992. The human gastrointestinal tract. In The Lactic Acid Bacteria in Health and Disease, Wood BJB (ed), Elsevier, London, pp. 69–114. [Google Scholar]
- 20.Kandler O, Stetter KO, Kohl LR. 1980. Lactobacillus reuteri sp. nov., a new species of heterofermentative lactobacilli. Zbl Bakt Hyg I Orig C1: 264–269 [Google Scholar]
- 21.Sharpe ME, Latham MJ, Garvie E, Zirngibl J, Kandler O. 1973. Two species of Lactobacillus isolated from the rumen, Lactobacillus ruminis sp. nov. and Lactobacillus vitulinus sp. nov. J Gen Microbiol 77: 37–49 [DOI] [PubMed] [Google Scholar]
- 22.Fujisawa T, Shirasaka S, Watabe J, Mitsuoka T. 1984. Lactobacillus aviarius sp. nov.: a new species isolated from the intestine of chickens. Syst Appl Microbiol 5: 414–420 [Google Scholar]
- 23.Mitsuoka T, Fujisawa T. 1987. Lactobacillus hamsteri, a new species from the intestine of hamsters. Proc Jpn Acad B 63: 269–272 [Google Scholar]
- 24.Orla-Jensen S. 1924. La classification des bacteries lactiques. Lait 4: 468–474 [Google Scholar]
- 25.Reuter G. 1963. Vergleichende Untersuchungen über die Bifidus-Flora im Säuglings- und Erwacksenenstuhl. Zbl Bakt Hyg I Orig 191: 486–507 [PubMed] [Google Scholar]
- 26.Mitsuoka T. 1969. Vergleichende Untersuchungen über die Bifidobakterien aus dem Verdauungstrakt von Menschen and Tieren (zugleich die Beschreibung von B. thermophilum nov. spec.). Zbl Bakt Hyg I Orig 210: 52–64 [PubMed] [Google Scholar]
- 27.Scardovi V, Crochiani F. 1974. Bifidobacterium catenulatum, B. dentium, and B. angulatum. Three new species and their deoxyribonucleic acid homology relationships. Int J Syst Bacteriol 24: 6–20 [Google Scholar]
- 28.Scardovi V, Trovatelli LD, Crociani F, Sgarbati B. 1969. Bifidobacterium: B. globosum n. sp. and B. ruminale n. sp. Arch Mikrobiol 68: 278–294 [PubMed] [Google Scholar]
- 29.Scardovi V, Zani G. 1974. Bifidobacterium magnum sp. nov., a large acidophilic bifidobacterium isolated from rabbit feces. Int J Syst Bacteriol 24: 29–34 [Google Scholar]
- 30.Trovatelli LD, Crociani F, Pedinotti M, Scardovi V. 1974. Bifidobacterium pullorum sp. nov.: a new species isolated from chicken feces and a related group of bifidobacteria isolated from rabbit feces. Arch Microbiol 98: 187–198 [DOI] [PubMed] [Google Scholar]
- 31.Matteuzzi D, Crochiani F, Zani G, Trovatteri LD. 1971. Bifidobacterium suis n. sp.: a new species of the genus Bifidobacterium isolated from pig feces. Z Allg Mikrobiol 11: 387–395 [DOI] [PubMed] [Google Scholar]
- 32.Scardovi V, Trovatelli LD. 1974. Bifidobacterium animalis (Mitsuoka) comb. nov. and the “minimum” and “subtile” groups of new bifidobacteria found in sewage. Int J Syst Bacteriol 24: 21–28 [Google Scholar]
- 33.Georg LK, Robertstad S, Brinkman A, Hicklin MD. 1965. A new pathogenic anaerobic Actinomyces species. J Infect Dis 115: 88–99 [DOI] [PubMed] [Google Scholar]
- 34.Mitsuoka T, Morishita Y, Terada A, Watanabe K. 1974. Actinomyces eriksonii Georg, Robertstad, Brinkman and Hicklin 1965 identisch mit Bifidobacterium adolescentis Reuter 1963. Zbl Bakt Hyg I Orig 226: 257–263 [PubMed] [Google Scholar]
- 35.Mitsuoka T, Kimura N, Kobayashi A. 1976. Untersuchungen über die Zusammensetzung der Faekalflora gesunder Hunde unter besonderer Berücksichtigung der Laktobazillen- und Bifidobakterienflora. Zbl Bakt Hyg I Orig 225: 485–493 [PubMed] [Google Scholar]
- 36.Mitsuoka T, Hayakawa K. 1973. Die Faekal Flora bei Menschen I. Die Zusammensetzung der Faekalflora der verschiedenen Altersgruppen. Zbl Bakt Hyg I Orig 233: 333–342 [PubMed] [Google Scholar]
- 37.Mitsuoka T, Hayakawa K, Kimura N. 1974. Die Faekal Flora bei Menschen II. Die Zusammensetzung der Bifidobacterienflora der verschiedenen Altersgruppen. Zbl Bakt Hyg I Orig 226: 469–478 [PubMed] [Google Scholar]
- 38.Mitsuoka T. 1982. Recent trends in research on intestinal flora. Bifidobact Microflora 1: 3–24 [Google Scholar]
- 39.Mitsuoka T, Ohno K. 1977. Die Faekalflora bei Menschen. V. Mitteilung: Die Schwankungen in der Zusammensetzung der Faekalflora gesunder Erwachsener. Zbl Bakt Hyg I Orig 238: 228–236 [PubMed] [Google Scholar]
- 40.Yuhara T, Isojima S, Tsuchiya F, Mitsuoka T. 1983. On the intestinal flora of bottle-fed infant. Bifidobact Microflora 2: 33–39 [Google Scholar]
- 41.Norris RF, Flanders T, Tomarelli RM, György P. 1950. The isolation and cultivation of Lactobacillus bifidus. A comparison of branched and unbranched strains. J Bacteriol 60: 681–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.György P. 1953. A hitherto unrecognized bio-chemical difference between human milk and cow’s milk. Pediatrics 11: 98–108 [PubMed] [Google Scholar]
- 43.Dehnert J. 1957. Untersuchung über die gram-positive Stuhlflora des Brust- milchkindes. Zbl Bakt Hyg I Orig 169: 66–79 [PubMed] [Google Scholar]
- 44.Reuter G. 1963–64. Vergleichende Untersuchungen über die Bifidus-Flora im Säuglings- und Erwachsenenstuhl. Zbl Bakt Hyg I Orig 169: 66–79 [PubMed] [Google Scholar]
- 45.Seeliger HPR, Werner H. 1963. Recherches qualitatives et quantitatives sur la flora intestinale de l’homme. Ann Inst Pasteur (Paris) 105: 911–936 [PubMed] [Google Scholar]
- 46.Petuely F, Linder G. 1955. Kritische Untersuchung über die Darmflora. III. Mit- teilung: Bedeutung quantitativer Züchtungs-methoden. Die Darmflora des Brustkindes. Zbl Bakt Hyg I Orig 195: 347–384 [PubMed] [Google Scholar]
- 47.Werner H. 1966. The gram positive nonsporing anaerobic bacteria of the human intestine with particular reference to the Corynebacteria and Bifidobacteria. J Appl Bacteriol 29: 138–146 [Google Scholar]
- 48.Benno Y, Endo K, Mizutani T, Namba Y, Komori T, Mitsuoka T. 1989. Comparison of fecal microflora of elderly persons in rural and urban areas of Japan. Appl Environ Microbiol 55: 1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsuoka T. 2002. Research in intestinal flora and functional foods. Journal of Intestinal Microbiology 15: 57–89 [Google Scholar]
- 50.Mitsuoka T. 1992. Intestinal flora and aging. Nutr Rev 50: 438–446 [DOI] [PubMed] [Google Scholar]
- 51.Mizutani T, Mitsuoka T. 1980. Inhibitory effect of some intestinal bacteria on liver tumorigenesis in gnotobiotic C3H/He male mice. Cancer Lett 11: 89–95 [DOI] [PubMed] [Google Scholar]