Abstract
Pulmonary arterial hypertension (PAH) is a progressive disorder in which endothelial dysfunction and vascular remodeling obstruct small pulmonary arteries, resulting in increased pulmonary vascular resistance and pulmonary pressures. This leads to reduced cardiac output, right heart failure, and ultimately death. In this review, we attempt to answer some important questions commonly asked by patients diagnosed with PAH pertaining to the disease, and aim to provide an explanation in terms of classification, diagnosis, pathophysiology, genetic etiologies, demographics, and prognostic factors. Furthermore, important molecular pathways that are central to the pathogenesis of PAH are reviewed, including nitric oxide, prostacyclin, endothelin-1, reactive oxygen species, and endothelial and smooth muscle proliferation.
Keywords: pulmonary arterial hypertension, clinical assessment, molecular pathogenesis, hemodynamics, nitric oxide
Pulmonary arterial hypertension (PAH) is a progressive disease of the lung vascular system, primarily affecting the small pulmonary arterioles. A combination of endothelial dysfunction and increased contractility of small pulmonary arteries, proliferation and remodeling of endothelial and smooth muscle cells, and in situ thrombosis leads to progressive narrowing of the blood vessels. This results in a progressive resistance to blood flow and an increase in pulmonary artery pressures.
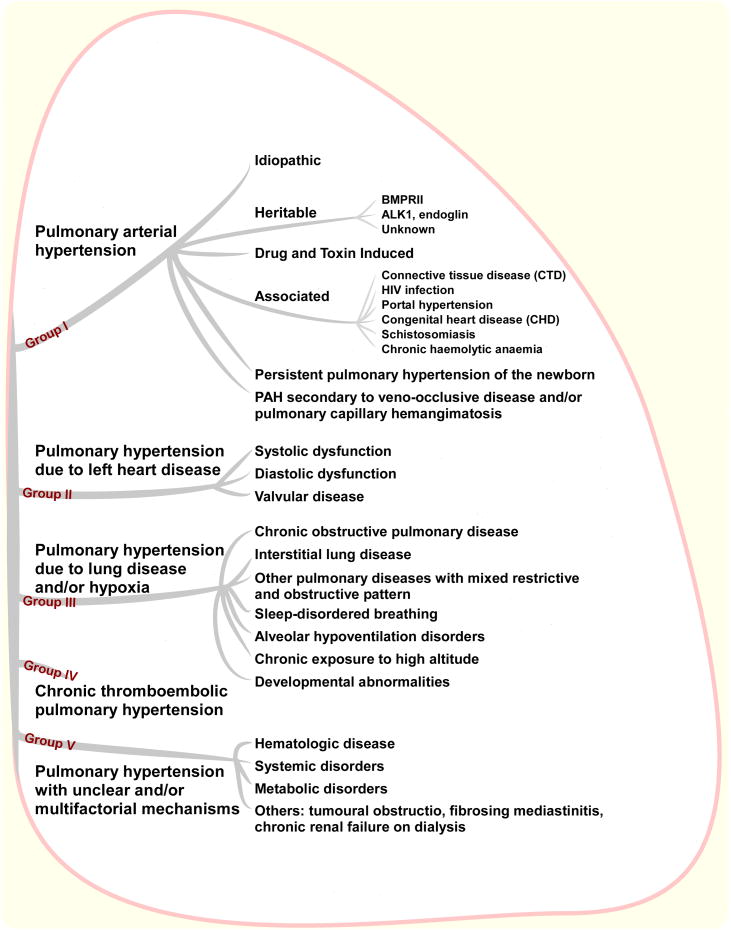
According to the clinical classification of pulmonary hypertension (PH, see Figure 1) from the 4th WHO symposium (Dana Point, 2008), PAH can be idiopathic (IPAH, caused by reasons that are unknown), heritable/familial or associated with other medical conditions such as connective tissue disease, HIV infection, portal hypertension (liver disease), sickle cell disease and congenital heart disease. PAH has also been associated with drug and toxin exposures, especially to the use of anorexigens, such as fenfluramine and dexfenfluramine as well as toxic rapeseed oil.1 Rarely is PAH caused by pulmonary veno-occlusive disease or is related to persistent pulmonary hypertension of the newborn.
Figure 1. Clinical classification of PH from Dana Point.
There are other pathologies in which PH presents as a secondary disease and it is always important to rule out secondary disease during initial comprehensive evaluation of a patient with PAH. Some examples are shown in Figure 1 and include left heart disease (Group II), chronic lung diseases and/or hypoxia (Group III), chronic thromboembolic pulmonary hypertension (CTEPH, Group IV), or pulmonary hypertension related to other unclear/multifactorial mechanisms (Group V, e.g., lymphangiomatosis, histiocytosis X, and sarcoidosis). It is important to note that the diagnosis of PAH, particularly IPAH, is a diagnosis of exclusion.
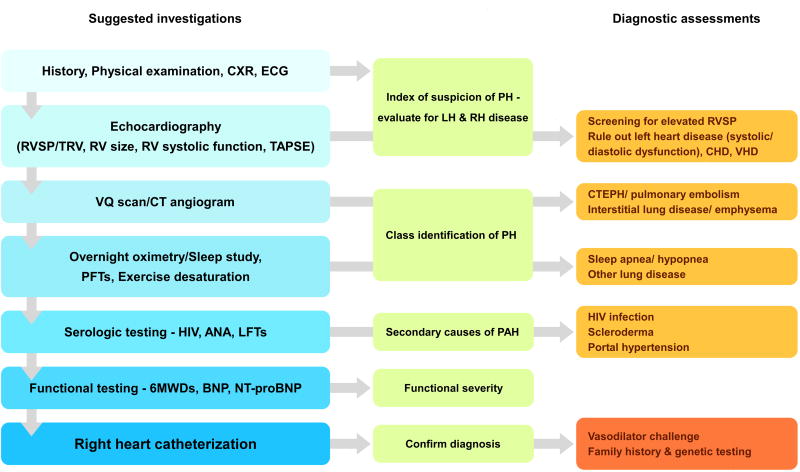
The evaluation of a patient with suspected PAH requires a combination of tests, including history, physical exam, echocardiography, imaging, laboratory screenings, functional assessment/exercise tolerance, and right heart catheterization. For a standard approach to the diagnosis of PAH at our institution, see Figure 2. Classic symptoms of PAH, such as fatigue, lethargy, dyspnea, and exertional presyncope/syncope, are often the first clue to PH. Physical examination findings will depend on the severity of the disorder in the pulmonary vasculature and the degree of right ventricular dysfunction.2 In all subtypes of PH, a loud P2 (second pulmonic closure sound) is frequently heard in association with tricuspid regurgitation murmur. A right-sided third or fourth heart sound may be present depending on the severity of right heart decompensation. Chest x-ray (CXR) may show right heart enlargement and abnormal lung vessels. Electrocardiogram (ECG) frequently shows alterations in heart rhythm and changes compatible with right ventricular hypertrophy. Echocardiography is also used to estimate pulmonary artery pressure based on tricuspid regurgitant jet velocity; however, the accuracy of this estimation is low. Thus, despite echocardiography being a good screening tool to suggest PAH, it should never be used to establish a diagnosis of PAH without proceeding with a right heart catheterization. Additionally, laboratory tests should be judiciously applied as indicated by history and physical examination. Chest computed tomography (CT) may be warranted to evaluate for interstitial lung disease or emphysema if symptoms or previous radiographs are suggestive. Ventilation-perfusion (VQ) scan and, if needed, pulmonary angiogram should be performed in those patients with a suggestive history for pulmonary embolism and CTEPH. Overnight oximetry or polysomnography can also be used to exclude obstructive sleep apnea/hypopnea. In excluding alternative diagnoses to PH, serologic studies should be ordered for patients with a suspicion of collagen vascular disease, HIV, or hepatitis.
Figure 2. Algorithm for diagnosis and severity rating of PH.
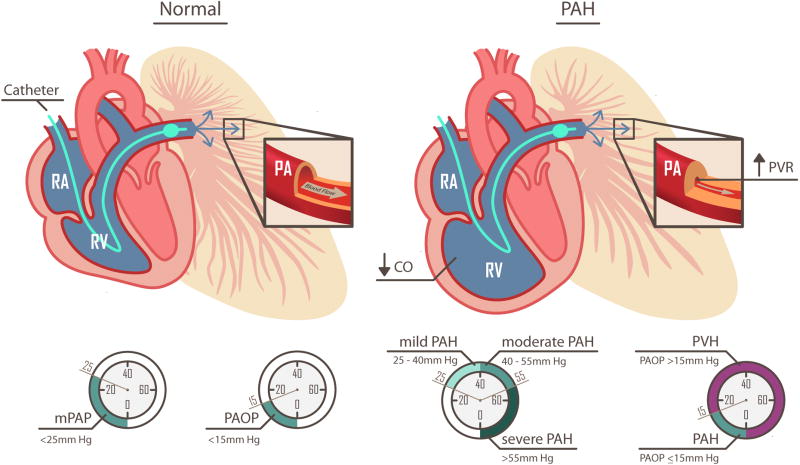
As we mentioned above, right heart catheterization is an absolute requirement for proper evaluation of suspected PAH patients. Diagnosis of PAH by right heart catheterization is confirmed when the mean pulmonary artery pressure (mPAP) exceeds 25 mm Hg and that pulmonary artery occlusion pressure (PAOP) or pulmonary capillary wedge pressure (PCWP) is less than 15 mm Hg. The latter can be measured by passing a balloon tipped pulmonary artery catheter from the jugular, femoral, or brachial vein (jugular and femoral approaches are typically used in our institution) through the right atrium (RA) and the right ventricle (RV) and into the pulmonary artery (PA). The catheter is advanced until the balloon tip occludes or “wedges” into a small PA. The standard PA catheter will measure the pressure at the very tip of the catheter, beyond the balloon, which now blocks the pressure coming from the RV and main PA. In the wedge position, the catheter can measure the reflected pressure coming backwards from the end-diastolic left ventricular pressure, the left atrium, and through the pulmonary veins and capillaries to the pulmonary artery. It is important to note that PAOP or PCWP at the end of expiration is measured in all patients. Inferior vena cava (IVC) occlusion is used in complex cases; however, fluid challenge or exercise helps to diagnose left ventricular dysfunction. If there is further concern for coronary disease or discrepancy from previous right heart catheterization, left ventricular end-diastolic pressure (LVEDP) can be measured by left heart catheterization.
When the mPAP is elevated above 25 mm Hg at rest or 30 mm Hg with exercise and the PAOP or PCWP is less than 15 mm Hg, the vascular resistance is at the pulmonary arterioles and capillaries (e.g., pre-capillary) and this defines patients with PAH. Some definitions have also included pulmonary vascular resistance (PVR) > 3.0 Wood units.
If, on the other hand, the PAOP is elevated above 15 mm Hg, the pressure elevation comes from the left ventricle (LV) or pulmonary veins and is referred to as pulmonary venous hypertension (PVH or WHO group II; see Figure 1). PVH can occur secondary to systolic or diastolic dysfunction of the LV. The latter is more commonly referred to as heart failure with preserved ejection fraction (HFpEF) or non-systolic heart failure. With chronic elevations in left ventricular pressure, the pulmonary vasculature can vasoconstrict and undergo pathological remodeling, leading to an elevated PVR and high transpulmonary pressure gradient. These patients often exhibit PH out of proportion to the degree of left ventricular dysfunction or have persistent PAH after therapies that lower their PAOP, such as diuretics and systemic blood pressure control. These patients exhibit a form of PH, which resembles WHO Group I PAH, however the prognosis or response to current PAH therapeutics is not known.3
The physiologic basis of symptoms in PAH
The symptoms of PAH, such as shortness of breath with exercise or exertion, fluid retention and lower extremity edema, and presyncope/syncope, are almost entirely related to a progressive decline in right heart function, also called right heart failure. This is caused by a progressive increase in afterload or PVR to a point where the right heart starts to dilate and fail as it is unable to meet the increased demand of the afterload. The RV is a very thin walled crescent shaped structure that is designed to pump blood through a low pressure and high flow pulmonary vascular system. The rapid exchange of carbon dioxide and oxygen between the circulating red blood cells and the air in the alveoli within a brief one-second transit time requires a very thin “sheet” of blood exposed to a massive surface area of air. This is accomplished by the alveolar gas exchange unit, the alveolus, which is composed of a one cell thin alveolar epithelial cell on the air-environment side and one thin endothelial cell on the lung-blood capillary side. The entire cardiac output (CO) of 4-6 liters of blood flowing per minute must spread out over a surface area the size of a tennis court for such efficient gas exchange; this requires a very high flow at a very low pressure in order to limit alveolar injury and pulmonary edema (fluid leak into the lung). For this reason, the RV is designed to accept a large blood volume (pre-load) and pump blood against a low resistance (low afterload).
The hemodynamic system can be compared to an electrical circuit subject to Ohm's law (V=IR), which states that voltage (V), or the potential difference across a resistor, is proportional to the electrical current (I) times the resistance (R). The pulmonary circulation hemodynamics is governed by the same relationship: mPAP-PAOP = CO × PVR; where mPAP-PAOP is the pressure gradient (analogous to voltage or potential across a resistor) and quantifies the gradient across the pulmonary resistance arterioles, CO is analogous to current (I), and PVR is analogous to R. The application of Ohm's law to fluid dynamics is referred to as Darcy's law.
Figure 3 is a classic representation of the natural history of PAH and how the right heart starts to fail as the disease progresses. As the severity of the vascular disease progresses, the effective diameter of the blood vessels decreases, leading to a progressive and irreversible rise in PVR. While the right heart maintains its systolic function and cardiac output, there is a progressive increase in mPAP. As the PVR rises, the right heart eventually begins to fail and CO starts to drop. During severe decompensation with right heart failure (decrease in CO) the mPAP can, paradoxically, drop according to Ohm's law calculation with a very low CO and fixed high PVR. Patients at the highest risk of death are those with evidence of progressive right heart failure, elevated right atrial pressure, low CO, and episodes of systemic hypoperfusion with angina (chest pain) or syncope (sudden loss of consciousness). “Sudden death” can occur with a profound failure of the RV that leads to inadequate LV filling pressure, resulting in loss of blood flow to the brain, which is followed by cardiac asystole.
Figure 3. Natural history of PAH and right heart failure.
The normal pulmonary vasculature is a low-resistance, high-flow system. In the case of PAH, the small pulmonary arteries are progressively narrowed, leading to an increase in PVR and the pulmonary artery pressures. Right heart catheterization is the gold standard for diagnose of PAH. When the mPAP is elevated above 25 mm Hg and the PAOP is less than 15 mm Hg, PAH is diagnosed. The progressive increase in PVR and pulmonary pressures subsequently lead to reduced CO and right heart failure.
Because dyspnea or shortness of breath is primarily caused by an inability to increase CO rather than impaired alveolar gas exchange, the oxygen levels often do not drop until PAH is very advanced and mixed venous oxygen saturations drop. It should be noted that PH can also develop secondary to advanced lung diseases, such as interstitial lung diseases or chronic obstructive pulmonary disease, in which case gas exchange is impaired early in the course of disease and patients present with more significant hypoxia, such as cyanosis and digital clubbing. Recent mechanistic studies in rodent models with gas exchange imaging in the lung, actually suggest that oxygen transfer occurs to some extent across the pulmonary arterioles, suggesting that impaired gas exchange does indeed occur even in PAH without parenchymal lung disease.4
While PAH is characterized by right heart failure, progressive diastolic RV pressure overload can also adversely affect the LV diastolic filling and output. Displacement of the interventricular septum during the diastolic phase with right heart failure can compromise LV filling and volume (referred to as diastolic ventricular interaction or interdependence). A recent study explored this mechanism in non-severe PAH patients (mPAP 29 mm Hg), using invasive pressure-volume loop analysis during simultaneous atrial pacing to a heart rate of 120 beats per minute.5 Remarkably, even in these mildly affected patients, stroke volumes dropped by about 25% during pacing. When the IVC was occluded to transiently reduce the RV filling, the LV end diastolic volume increased by 7% and end-diastolic pressures dropped, resulting in an 11% increase in CO. This provided direct evidence that the heart rate dependent increase in the RV pressure/volume overload adversely impacts the LV cardiac filling and output, even in the setting of normal LV systolic function.
The genetic basis of PAH
While PAH clearly can be inherited in an autosomal dominant mode, it is much more likely that a patient has idiopathic disease, a spontaneous mutation, or PAH developing secondary to another co-existent disease. There are ways to diagnose familial PAH (FPAH, disease that occurs in multiple family members), which include molecular genetic testing for a most common causative of FPAH (bone morphogenetic protein receptor type II, BMPRII), excluding other known causes of PAH, and the presence of two or more family members with PAH.6 Certainly, more tests and carefully track the family medical history can provide accurate diagnosis.
FPAH may show genetic anticipation, with younger age of onset and death in subsequent generations, as well as incomplete penetrance, with only about 20% of individuals carrying the mutant gene develop the disease. Familial disease is related to germ-line mutations in genes encoding transforming growth factor β (TGF-β) receptor superfamily, namely endoglin 1, the activin-receptor-like kinase-1 (ALK 1), and the BMPRII.7-10 A rare new genetic mutation in a low conductance potassium channel KCNK3, which reduces potassium channel current, has recently been associated with FPAH and IPAH.11 Mutations in the BMPRII gene have been found in approximately 70% patients with FPAH.12 In addition, 10-40% of patients with sporadic IPAH have been found to carry similar mutations.13 Due to low penetrance of PAH, multiple generations may be unaffected in the family tree and show negative family history of the disease. FPAH and sporadic IPAH patients with identified genetic mutations are now referred as to heritable PAH (HPAH).
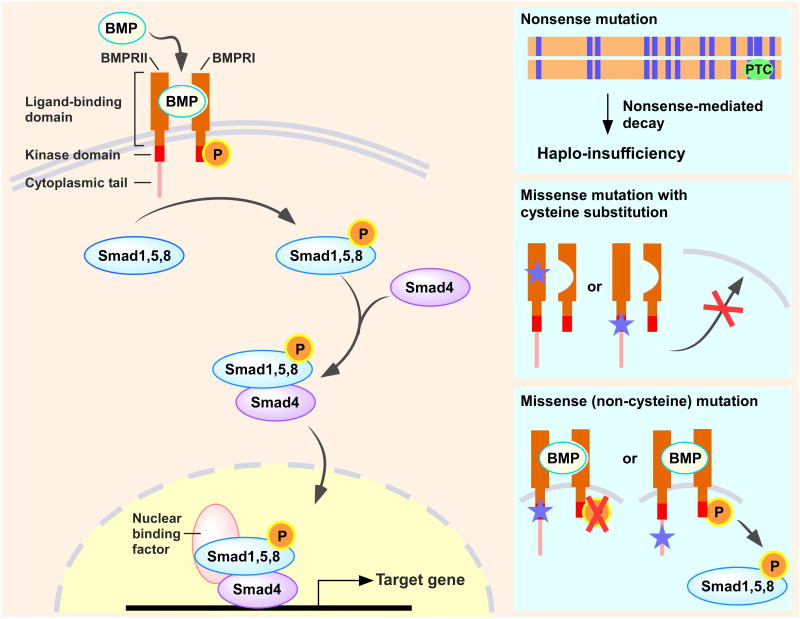
There are 144 distinct BMPRII mutations found in PAH patients, which affect multiple loci in the gene, including the ligand-binding domain, the serine-threonine kinase domain, or the cytoplasmic tail.14 About 70% of mutations are nonsense or frame-shift mutations, which result in nonsense-mediated mRNA decay of the BMPRII mutant transcripts, thus leading to haplo-insufficiency. Roughly 30% of mutations have missense cysteine substitutions within the ligand-binding or the kinase domain of BMPRII, which lead to impaired trafficking of BMPRII to the cell membrane.14 Noncysteine mutations in the kinase domain also affect receptor function. Moreover, BMPRII expression levels are often reduced in PAH patients independent of BMPRII mutations.15
BMP-BMPRII signaling
BMPs are multifunctional regulators that modulate cell proliferation, differentiation, and apoptosis in different tissues. Under normal conditions, BMPRII, the constitutively active serine-threonine kinase receptor, binds BMPs to form heterotetrameric complexes with one of the type I receptors (i.e., BMPRIA, BMPRIB, ALK1, or ALK2), which leads to phosphorylation and activation of the intracellular portion of the type I receptor. Subsequently, the type I receptor phosphorylates one or more of the intracellular signal-transduction molecules known as receptor-mediated Smads (R-Smads) 1, 5, and 8, which in turn form transcriptional complex with the co-Smad, Smad4. Once formed, the R-Smad/Smad4 complex translocates to the nucleus, where it binds to DNA, and interacts with other cofactors to regulate target gene transcriptional responses for either activation or repression (Figure 4).16
Figure 4. BMP-BMPRII signaling.
Following BMPs binding, BMPRII forms heterotetrameric complexes with BMPRI to generate and transduce a phosphorylation to receptor-mediated Smads (R-Smads) 1, 5, and 8, which in turn forms transcriptional complex with the co-Smad, Smad4. This complex then translocates to the nucleus to regulate target gene transcription (e.g., antiproliferative effects). Among the many BMPRII mutations have been identified in PAH patients, about 70% introduce a premature termination codon (PTC) and follow the process of nonsense-mediated decay, resulting in haplo-insufficiency. Cysteine residues substitutions in the ligand-binding or the kinase domain of BMPRII causes impaired trafficking of BMPRII to the cell membrane, as well as alters subcellular localization of BMPRI. Noncysteine mutations in the kinase domain reach the cell membrane but fail to activate BMP signaling. In contrast, BMPRII mutants in the cytoplasmic tail retain the ability to transduce BMP signals.
BMPRII and PAH
Loss of BMPRII in pulmonary artery endothelial cells increases susceptibility of endothelial cells to apoptosis,17 which leads to endothelial dysfunction and the subsequent development of PAH. Conditional knockout of BMPRII in pulmonary endothelial cells in mice is sufficient for PAH predisposition.18 Moreover, mice with a dominant negative BMPRII mutant in smooth muscle cells develop PAH with pulmonary arterial vascular remodeling.19
Improving BMPRII expression via targeting delivery of wild-type BMPRII to rat lung endothelium has been shown to attenuate PAH.20 Reduced BMPRII expression has also been linked to the activation of microRNAs, including miR-20a. Inhibition of miR-20a with a specific antagomiR-20a has been reported to restore BMPRII functional levels in pulmonary arteries and to prevent vascular remodeling in mice with hypoxia-induced PAH.21 Moreover, enhancement of the cell membrane trafficking of mutant BMPRII using chemical chaperones restores BMP-BMPRII signaling.22
How common is PAH?
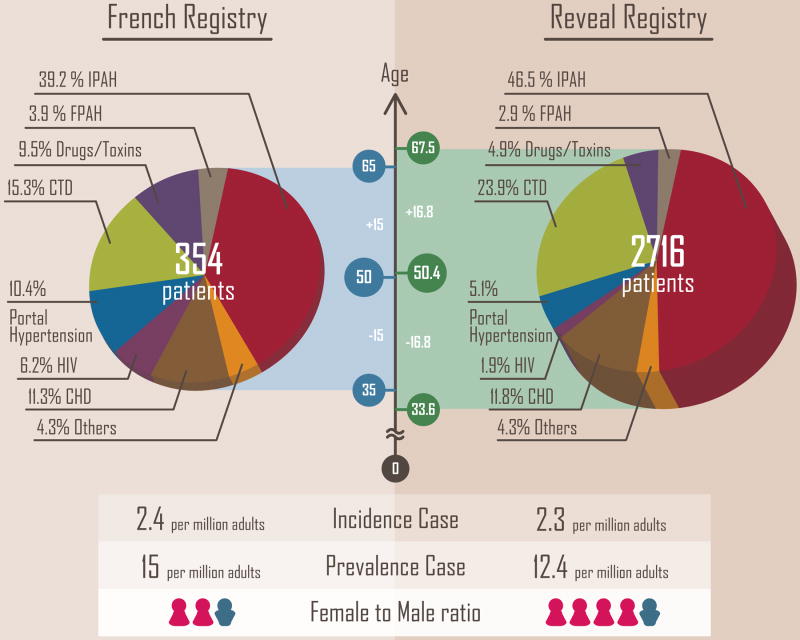
Data are available from two recent observational cohort studies of PAH, one based on 17 university hospitals across France during a 1-year period from 2002 to 2003 (the French Registry), and another one based on 54 centers in the United States (the REVEAL Registry), which enrolled approximately 3000 PAH patients from 2006 to 2007. The estimated incidence and prevalence of PAH are 2.4 and 15 cases per million adults in France, respectively.23 Within the cohort, 39.2% were classified as IPAH and 3.9% were classified as FPAH. In the US, the estimated incidence and prevalence of PAH are 2.3 and 12.4 cases per million adults, respectively,24 with 46.5% IPAH, and 2.9% of FPAH.25 Despite affecting over 3000 patients in the US, PAH is a rare disease.
PAH can develop in males or females at any age, but it is most common in females. The female-to-male ratio was 1.9:1 in the French Registry.23 Of note, 79% of the adult PAH patients in the REVEAL Registry were females, with a female-to-male ratio of 4.1:1.24, 25 The age of diagnosis is about 50 years and is similar in both French Registry and REVEAL Registry. The proportions among PAH subgroups in each Registry are shown in Figure 5.
Figure 5. Pie charts showing a comparison of PAH subgroups in the French Registry and REVEAL Registry.
Female gender and PAH
The role of female gender, and specifically estrogenic hormones, in the penetrance, severity and even treatment of PAH is the subject of active research. However, the findings are not congruent and in fact are paradoxical. For example, there is a female predominance in IPAH and increased penetrance of PAH in female members of families with mutations in the BMPRII gene.26 On the other hand, 2-methoxyestradiol (2-ME) therapy is protective in monocrotaline-induced PH and female rodents often develop less severe hypoxia-induced RV failure.27 These findings have been referred to as the “estrogen paradox” and the potential mechanisms are comprehensively reviewed.28 One possible explanation of increased penetrance of FPAH in female may relate to the estrogen-metabolizing enzyme, cytochrome p450 1B1 (CYP1B1).26 CYP1B1 is highly expressed in the lungs and it can hydroxylate estrogens into 2-hydroxyestrogen (2-OHE), an anti-mitogenic metabolite that can be further converted to 2-ME to exert protective effects. However, CYP1B1 expression levels are 10-fold lower in female BMPRII mutation carriers.29 Estrogens can be hydroxylated into 16α-hydroxyestrone (16α-OHE), a mitogenic metabolite that promotes pulmonary vascular remodeling, by other cytochrome p450 enzymes, lowering 2-OHE/16α-OHE ratio, thus increasing penetrance of FPAH.26 Further study is clearly required to explain the role of female gender and estrogen in PAH penetrance, prevalence and severity, as well as the role of 2-ME as a therapeutic.
Mortality and assessment of prognosis in PAH
Historically, the National Institutes of Health (NIH) Registry patients suffered an approximate 50% mortality rate at 3 years. Recent clinical trials study groups suggest better outcomes in the “modern management era”, with observed mortality rates of 20-30% at 3-5 years in the French Registry and 10-30% at 1-3 years in the REVEAL Registry. However, this may not be an accurate assessment. This is because less severe patients are enrolled in current clinical trials, with many more patients with a less severe WHO/NYHA functional class II at the time of enrollment. In addition, studies suggest that there are two groups of patients with PAH in such trials, each with very different outcomes. One group is described as incident cases. Those are the patients who present at the registration, screening or clinical trials center for the first time.30 These newly diagnosed patients actually have a mortality rate nearly as high as the original NIH Registry cohort patients. A second group is referred to as prevalent cases and these patients were diagnosed in the community and then referred to the registration, screening or clinical trials center.30 These patients have a much better survival rate.31 This may be explained by a lead time diagnosis bias for the second group: sicker patients are more likely to have died prior to referral in this group, thus selecting for a referred group with better survival. It is noteworthy that REVEAL Registry defined incident cases as patients newly diagnosed within 90 days prior to enrollment. This can further lead to an “immortal time bias” that overestimates a better outcome, since death could not have occurred during this time period.32
Hemodynamic values define mortality risk
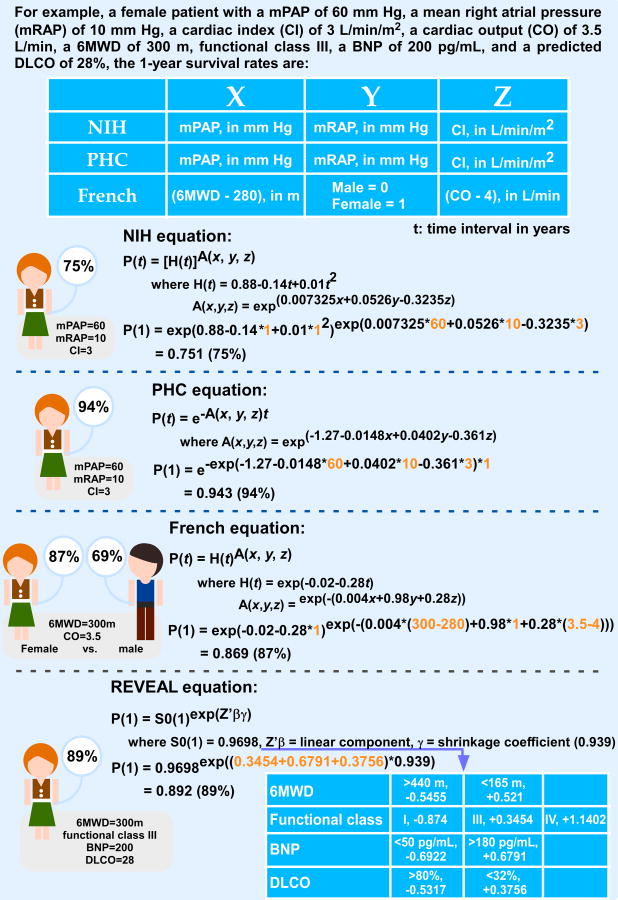
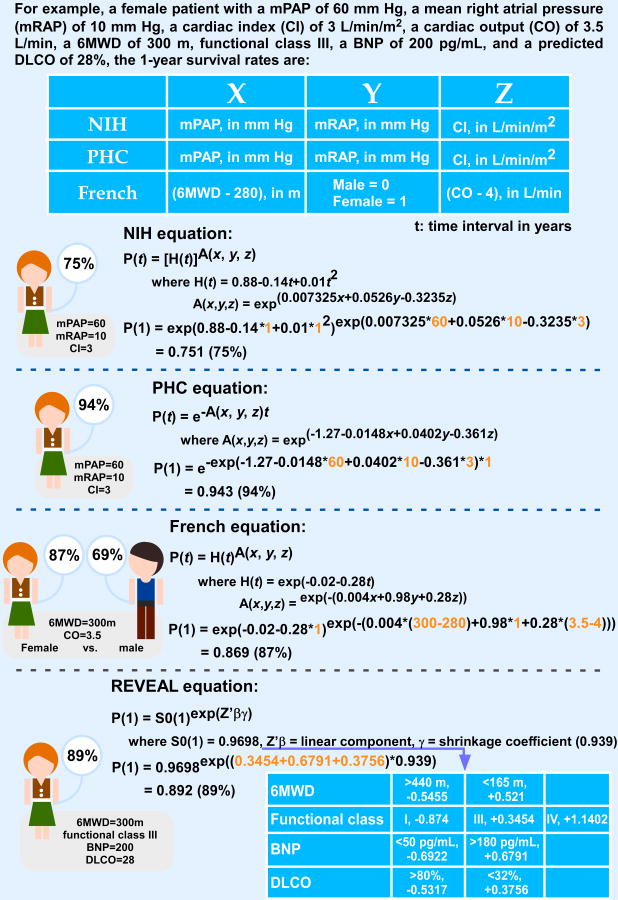
Four equations are available to predict a patient's likelihood of survival according to baseline hemodynamic measurements (Figure 6). The first one was developed in 1981 using the NIH Registry data. In this equation, mPAP, mean right atrial pressure, and cardiac index (CI) are important predictors of survival.33 Observed 1-, 3-, and 5-year survival rates for the total cohort were 68, 48, and 34%, respectively. Because the management and therapies of PAH have improved a lot since the mid-1980s, a second equation was developed based on the Pulmonary Hypertension Connection (PHC) Registry, which enrolled 576 PAH patients during 1991-2007. Observed 1-, 3-, and 5-year survival rates for the total cohort were 86, 69, and 61%, respectively, which were significantly higher than the predicted survival (65, 43 and 32%) based on the NIH Registry equation, further supporting that the NIH equation overestimates mortality rates in PAH.34 Of note, a patient's response to vasodilatory therapy is included as a survival predictor in the PHC equation. In the “modern management era”, a third equation was developed using the French Registry. Observed 1-, 2-, and 3-year survival rates (87, 76, and 67%, respectively) were improved by about 20% as compared to the classic NIH equation.30 Survival was better in the prevalent cohorts, as compared to the incident cohorts, as summarized earlier. In addition, better survival was observed in females and younger patients. Of note, 6-minute walk distance (6MWD), gender and CO are important parameters applied to the survival equation of the French Registry.30 The most recent equation/risk score calculator was developed based on REVEAL Registry, and a 1-year survival rate from the date of enrollment was 91%, whereas the 1- and 3-year survival rates from the time of PAH diagnosis were 88 and 72%, respectively. The equation is only available for 1-year survival prediction at this time; however, this equation has revealed key predictors of survival in the current treatment era. Three key predictors are associated with increased 1-year survival, which are 6MWD ≥ 440 m, serum brain natriuretic peptide (BNP) level < 50 pg/mL, and percent predicted carbon monoxide diffusing capacity (DLCO) ≥ 80%.25, 31
Figure 6. Comparison of survival rate for a PAH patient according to different Registries' survival equations.
Tests to evaluate prognosis
There are a number of functional, laboratory, and hemodynamic tests that can be performed to further refine the risk and prognosis. These include simple clinical tests such as the 6MWD and heart rate recovery, and biomarker tests such as the BNP or NT-proBNP levels. Imaging studies such as echocardiographic and cardiac MRI are also helpful in prognostication. Measures of right ventricular glycolytic activity may provide new methods to assess clinical risk and are undergoing validation for utilization at this time.
The six-minute walk test (6MWT) measures simply the distance one can walk in six minutes. This test has traditionally served as the “work-horse” efficacy endpoint in clinical trials. A normal 6MWD is greater than 600-700 meters. A distance less than 300-350 meters predicts worse outcome in patients with PAH, and a value of less than 165 meters reflects extremely severe limitation.25, 35 While the 6MWD improves with therapy and is typically used as a primary endpoint for clinical trials of new drugs, the magnitude of improvement in walk distance with therapy that is clinically meaningful remains controversial. In a retrospective analysis of data from the Pulmonary Hypertension Response to Tadalafil (PHIRST) trial, Mathai et al used distributional and anchor-based methodologies to conclude that a distance of greater than 33 meters is associated with improvement in quality-of-life measures.36 These results were consistent with a more extensive analysis of ten randomized clinical trials of PAH-targeted therapy, which found that an increase of over 42 meters best predicted a reduction in the time to clinical worsening.37 It is also possible that threshold levels, for example achieving a walk distance of great than 380 meters, are more important than a small improvement over baseline.38 However, a recent study found that 6MWT in PAH did not accurately demonstrate clinical benefit in outcomes related to active treatments with clear survival benefits.39 Consideration of other parameters such as heart rate recovery (HRR) after completion of the 6MWT, defined as the difference in heart rate at the end of 6MWT and at 1 minute after completion of the test, may also be useful to predict outcomes. Another study showed that a HRR of less than 16 beats per minute predicted clinical worsening in 75 consecutive PAH patients.40 In this study, HRR was a better predictor of clinical worsening than 6MWT, but the sample size was very small.
Brain natriuretic peptide (BNP) is a hormone, which is released in response to cardiomyocyte stretch, and high levels of which reflect right atrial/ventricular volume and pressure overload. Prognostic importance of BNP has been demonstrated in several cardiovascular disorders.41, 42 BNP is primarily secreted by the cardiac ventricles as a pre-pro hormone that is successively cleaved into the N-terminal fragments (NT-proBNP) and the active hormone BNP, both of which are now measured clinically.41 BNP hormone mediates natriuresis, vasodilation and down-regulates the renin-angiotensin-aldosterone axis. Elevated BNP levels predict diminished exercise tolerance and poor prognosis in patients with left ventricular failure.42, 43 Recently, it has been demonstrated that baseline NT-proBNP can directly correlate with 6MWD.44 This provides an additional evaluation to be utilized in clinical trials for PAH. BNP levels are also increased, albeit to lower levels, in patients with primary and severe secondary PH; the degree of elevation reflects the patient's clinical and hemodynamic status.45, 46
Doppler-Echocardiography provides a non-invasive assessment of the RV structure and function, and it is used to monitor progression and response to therapy.47 There are a number of measurements that can be performed using Doppler-echocardiography, including RV performance index, RV systolic pressure and right atrial pressure (RAP), pericardial effusion, indexed right atrial area, the degree of septal shift toward the LV in diastole, and tricuspid annular plane systolic excursion (TAPSE).48 The RV performance index serves as a measure of the RV function and was found to be a strong predictor of adverse outcome.49 The tricuspid regurgitant jet is generally used to estimate the RV systolic pressure by the Bernoulli equation: 4v2, where v is the maximum jet velocity of the tricuspid valve. RAP is estimated based on the collapsibility of the IVC, and this measure is added to the peak systolic pressure calculated by the peak tricuspid regurgitant flow in order to quantify the RV systolic pressure. This measurement approximates the pulmonary artery systolic pressure, assuming there is no evidence of pulmonary valve stenosis or obstruction. The mPAP can be estimated by measuring the early diastolic component of the pulmonary valve insufficiency jet; however the accuracy of this measure is low as compared to more invasive measures. Echocardiography can also be used to estimate PVR by the ratio of the tricuspid regurgitant jet velocity to the acceleration time of the RV ejection into the PA. Other echocardiographic variables that would suggest the presence of PH independently of tricuspid regurgitation velocity include increased dimensions of right heart chambers, abnormal shape and function of the intraventricular septum, increased RV wall thickness, and a dilated main pulmonary artery. As the RV dilates and the septal wall becomes flattened and eventually bows, the LV is compressed and paradoxical ventricular septal wall motion is observed.
Cardiac MRI has now become the gold standard for assessment of the RV size, volume, CO, PA distensibility, and function.50 RV mass, which is difficult to quantify by other methods, can also be calculated by multiplying the RV volume by the specific gravity of the myocardium (1.05 g/cm3).51 Moreover, conventional MRI myocardial tagging and fast-strain-encoded (SENC) imaging allow direct and accurate measurement of RV remodeling.52
Angiography is a tool to assess the degree of peripheral vascular pruning seen as the hallmark of obliterative remodeling of PAH.51 This direct visualization of the branching pattern and vasculopathy illustrates severity of the disease process, but can also be used to evaluate response to therapy. Injection of gadolinium during cardiac MRI broadens the scope of the test to include magnetic resonance angiography (MRA). This angiogram shows filling defects as seen with thrombi, an important exclusion necessary in the initial classification of PAH which would otherwise require a CT scan with an intravenous contrast and a VQ scan.
Computed tomography-positron emission tomography (CT-PET) is a promising new approach that may help in evaluation the severity of PAH and response to therapy. CT-PET measures a shift from oxidative phosphorylation to glycolytic metabolism using PET imaging of 18F-labeled deoxyglucose (FDG) uptake. FDG uptake via the glucose transporter-1 (Glut1) is increased in the RV of PAH patients and in cultured pulmonary vascular cells.53 Marsboom et al evaluated the relationship between FDG uptake, cellular glycolytic activity and PH severity in monocrotaline and sugen/hypoxia rat models.54 FDG uptake increased in the pulmonary vasculature as well as the RV and the signal in the vasculature was related to increased cellular glycolytic activity in proliferating smooth muscle and endothelial cells. This was characterized by increased Glut1 expression, inhibition of pyruvate dehydrogenase activity by pyruvate dehydrogenase kinase, and reductions in mitochondrial oxidative phosphorylation. Increase in glycolytic activity correlated closely with disease progression and response to two therapies, inhibition of pyruvate dehydrogenase kinase by dichloroacetate, and inhibition of tyrosine kinase by imatinib. Hypoxia-inducible factor-1α (HIF-1α) was also implicated in the development of the glycolytic phenotype. Clinical utility of measurements of FDG uptake in IPAH remains to be determined.
What causes PAH to develop?
In all forms of PH, the progressive vasculopathy is a complex with a broad imbalance of vasodilators, such as nitric oxide (NO) and prostacyclin (PGI2), and vasoconstrictors, such as endothelin-1 (ET-1) and thromboxane A2 (TXA2). This condition likely precedes the development of secondary aberrant cellular proliferation. Classic vasodilator systems are dysregulated with decreases in endothelial NO synthase function (eNOS) caused by enzymatic “uncoupling”, decreases in production of prostacyclin (cyclooxygenase-2 dysfunction), and increased expression and activity of the vasoconstrictor and mitogenic ET-1 signaling system. A fundamental scientific understanding of the critical imbalances of these three major pathways, NO, PGI2, and ET-1, has led to the rapid clinical development of more than ten new major FDA-approved medications for the therapy of PAH, more will be discussed in other reviews in this compendium.
In addition to dysregulation of vasodilator and vasoconstrictor factors, there is a parallel induction of major oxidase enzymes, which will produce reactive oxygen species (ROS), such as superoxide, hydrogen peroxide (H2O2), and peroxynitrite. These oxidases are the subject of current active research and include the NADPH oxidase (Nox) family, xanthine and aldehyde oxidases, an “uncoupled” eNOS, and dysfunctional mitochondria.55, 56 For example, increased expression of NADPH oxidase isoform 4 (Nox4) in the vasculature of patients with PAH may disrupt canonical NO signaling through a number of pathways. ROS produced from Nox4 and other oxidases can critically alter the balance of NADP+/NADPH and tetrahydrobiopterin to dihydrobiopterin (BH4/BH2), which maintain cellular redox balance. An increase in BH2 levels (or decrease in BH4/BH2 ratios) results in an uncoupling of the eNOS enzyme, such that it produces superoxide rather than NO. Such an increase in oxidase expression and ROS generation will enhance vasoconstriction, mitogen activation, and cellular proliferation.
Nitric oxide
Evidence for a critical role of NO in regulating vasodilation through the primary activation of soluble guanylyl cyclase (sGC) lead to the award of Nobel Prize in Physiology and Medicine to Robert Furchgott, Louis Ignarro, and Ferid Murad in 1992, and provided the scientific backbone for rapid advances in our understanding of NO biology and related therapeutics in PAH. NO is produced in endothelium by eNOS, which catalyzes the oxidation of L-arginine to produce L-citrulline in the presence of oxygen, NAPDH, and essential cofactors such as BH4. Once formed, NO diffuses to the underlying vascular smooth muscle, where it binds avidly to sGC. sGC converts guanosine triphosphate (GTP) to cGMP, which in turn activates downstream cGMP-dependent protein kinase (PKG) to relax smooth muscle contractile filaments and promote vasodilation. While regulating smooth muscle relaxation, NO also inhibits vascular smooth muscle cell proliferation. In addition, NO decreases platelet aggregation and thrombosis in the blood vessel lumen. These vasodilatory, anti-proliferative, and anti-thrombotic signaling effects of NO maintain normal healthy vascular function.
While PAH is clearly associated with a reduction in NO bioavailability, this does not always correlate with low levels of eNOS. A general consensus is that patients with PAH have impaired NO production associated with diminished eNOS expression, thus promoting pulmonary vasoconstriction and excessive medial proliferation.57 Similarly, mice with eNOS deficiency are more susceptible to hypoxia-induced PAH.58 However, other studies found increased eNOS in the plexiform lesions, and suggested that this promoted apoptosis-resistant endothelial cell proliferation.59 Another recent study showed increased eNOS activation secondary to caveolin-1 deficiency that impaired NO signaling through PKG nitration, thus leading to PAH in mice.60 A potential solution to this paradox is that while eNOS protein levels may be normal, they exhibit impaired NO synthesis activity caused by eNOS uncoupling, a process by which eNOS produces superoxide instead of NO by transferring electrons from the NOS reductase domain to the oxygenase domain, which are then diverted to molecular oxygen rather than to L-arginine. NO production by eNOS correlates closely with the intracellular concentration of BH4. Without BH4, or a decrease in BH4 availability due to BH4 oxidization to BH2, NO production is reduced and superoxide generation is increased as a result of eNOS uncoupling.61 Reduced BH4 levels have been shown to impair eNOS function in ischemic hearts,62 and supplementation of BH4 decreases NOS-dependent generation of superoxide and corrects eNOS dysfunction.62, 63 In addition, mice that have BH4 deficiency predispose to hypoxia-induced PAH.64 Recent studies suggest that eNOS uncoupling is not simply a consequence of low amount of BH4, but rather results from a decreased ratio of BH4:BH2, or increased BH2 levels in endothelial cells.65, 66 While BH2 was generally considered as an inert product of BH4 oxidation, studies have demonstrated that BH2 can compete with BH4 at the binding site of eNOS with equal affinity and exacerbate eNOS uncoupling.65, 66 In addition, elevated BH2 levels were linked to the development of pulmonary hypertension in lambs.67
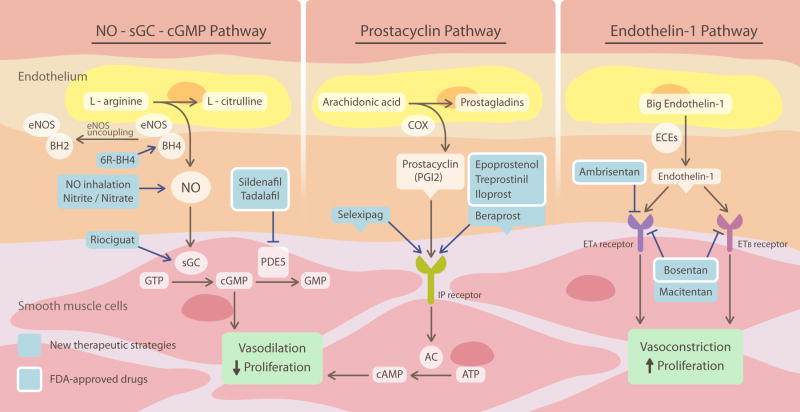
An increasing number of translational therapies targeting the NO-sGC-cGMP pathway have been shown effective in the treatment of PAH (Figure 7). These include inhalation of NO,68-70 which is FDA-approved for the treatment of persistent pulmonary hypertension of the newborn. More recently, inhalation of nitrite, a product of NO oxidation that can be reduced back in the lung to form NO, has been shown to reverse established PAH in pre-clinical models.71-73 Nitrite, as well as nitrate, which can be converted to nitrite by oral commensal bacteria, have been shown to inhibit PAH through NO-dependent signaling in pre-clinical studies.71, 73, 74 Currently, inhaled nitrite is in a multi-national phase II proof-of-concept clinical trial. Other agents, like BH4 analogues 6R-BH4 and L-arginine, have been evaluated in clinical trials with varying levels of success.75, 76
Figure 7. Classic vasodilator and vasoconstrictor systems and their translational therapies for PAH.
NO activates vasodilation and antiproliferation of smooth muscle cells via a cGMP-dependent mechanism. Inhalation of NO, administration of nitrite and/or nitrate, sGC stimulator (riociguat), PDE5 inhibitors (sidenafil and tadalafil) and BH4 analogue (6R-BH4) have been shown effective in the treatment of PAH. Prostacyclin activates vasodilation and inhibits proliferation of smooth muscle cells via a cAMP-dependent mechanism. Prostacyclin and its derivatives (epoprostenol, treprosinil, iloprost, and beraprost) and IP receptor agonist (selexipag) provide therapeutic benefit in PAH. Endothelin-1 stimulates vasoconstriction and proliferation via activation of both ETA and ETB receptors on smooth muscle cells. ETA blocker (ambrisentan) and dual endothelin-1 blockers (bosentan and macitentan) prove useful in the treatment of PAH.
To date, the most successful therapeutic approach has been to inhibit phosphodiesterase 5 (PDE5), the enzyme responsible for the hydrolysis of cGMP. This enzyme provides a constant “brake” on the vasodilatory and antiproliferative effects of NO in pulmonary vascular smooth muscle cells. Selective PDE5 inhibitors, such as sildenafil and tadalafil, are FDA-approved oral therapies for the treatment of PAH patients who are functional class II-III. Randomized clinical trials have demonstrated that both sildenafil and tadalafil improve hemodynamics and 6MWD.77, 78
Prostacyclin
In 1982, Nobel Prize was awarded to Sune Bergström, Bengt Samuelsson, and John Vane for their discovery of prostacyclin, a potent vasodilator produced from arachidonic acid by the cyclooxygenase (COX) pathway and prostacyclin synthase in endothelial cells. Prostacyclin binds to its specific I-prostanoid (IP) receptor in the underlying smooth muscle cells to promote relaxation and subsequent vasodilation by activating adenylyl cyclase (AC) and increasing intracellular cAMP levels, which in turn activates protein kinase A (PKA).79 Through the same pathway, prostacyclin attenuates vascular smooth muscle cells proliferation, inhibits platelet aggregation, and exerts anti-inflammatory and anti-thrombotic effects.80
The critical role of prostacyclin in the development of PAH is supported by the evidence that mice with PGI2 receptor deficiency develop severe PAH and vascular remodeling after chronic hypoxia exposure.81 In addition, patients with PAH have reduced production of prostacyclin.82 Furthermore, the expression of prostacyclin receptor and synthase are reduced in PAH patients.81, 83
The use of prostacyclin and its analogues is one of the most successful translational therapies for PAH. Continuous intravenous infusion of epoprostenol, a synthetic prostacyclin, decreases PVR, increases CO, and improves exercise capacity and life expectancy.84 It was the first PAH drug, and is approved for the treatment of PAH patients with functional class III-IV. Despite its therapeutic efficacy, the shortcomings of epoprostenol, including poor stability, high cost, and the need for continuous intravascular infusion, have urged the development of analogues of prostacyclin. These include treprostinil, iloprost, and beraprost. Clinical trials have demonstrated that both subcutaneous and intravenous of treprostinil improve exercise capacity and hemodynamics.85, 86 Inhaled iloprost improves functional class by at least one level and exercise capacity by at least 10%.87 Clinical trials of beraprost have shown a significant improvement in 6MWD in Europe.88 However, a subsequent randomized control trial in the US failed to show improvement in 6MWD.89
Endothelin-1
Endothelin-1 is a 21 amino acid vasoactive peptide, which is one of the most potent vasoconstrictor molecules in biology.90 It is produced from a 39 amino acid precursor, big ET-1, through endothelin-converting enzymes (ECEs) on the endothelial cell membrane.90 ET-1 acts at two different G-protein-coupled receptors: ETA and ETB. ETA receptors are located predominantly on vascular smooth muscle cells and mediate vasoconstriction, as well as proliferation, hypertrophy, cell migration, and fibrosis. ETB receptors are found on both endothelial and smooth muscle. ETB activation on smooth muscle cells produces vasoconstriction; however, ETB activation on endothelial cells leads to vasodilation and antiproliferation by increasing NO and PGI2 production.55
As PAH progresses, the cellular distribution of the ET-1 receptors changes, with increased expression of both constrictive ETA and ETB on smooth muscle cells and decreased expression of vasodilatory endothelial ETB.55 In animals and patients with PAH, it was reported that ET-1 levels were increased in lungs and in circulation.91, 92 In addition, plasma levels of big ET-1 were elevated in patients with PAH.93
Antagonists of ET-1 receptors, such as ambrisentan (ETA-selective) and bosentan (dual ETA and ETB), are approved treatments for PAH patients with functional class III-IV. Clinical trials have shown that both of ambrisentan and bosentan decrease mPAP and PVR, increase cardiac index, and improve exercise endurance.94, 95 However, bosentan can produce hepatotoxicity.96 Regular monitoring of liver function is required during therapy. Additionally, inhibition of ET-1 production with endothelin-converting enzyme inhibitor, daglutril, may provide therapeutic benefit in PAH, although data for its use in PAH are limited.97
It is clear that new drugs targeting the major pathways associated with NO, PGI2 and ET-1 remain the most likely candidates to enter clinical practice in the near future. The use of an oral prostanoid (selexipag), a new dual endothelin antagonist (macitentan) and a soluble guanylate cyclase stimulator (riociguat), led to positive clinical results in 2012.98-100
Reactive oxygen species and oxidases in PAH pathogenesis
NADPH oxidase isoforms
Accumulating evidence indicates that increased production of ROS is associated with endothelial dysfunction and subsequent development of PAH.67 All members of Nox family are transmembrane proteins, which generate superoxide by transferring electrons from NADPH in the cytosol to oxygen in the extracellular or intracellular space (depending on the isoform). In the pulmonary vasculature, superoxide generated by Nox enzymes, particularly Nox2 and Nox4, are involved in vascular dysfunction and/or vascular remodeling induced by hypoxia.101, 102 In a mouse model of hypoxia-induced PAH, Nox2 deficiency reduced vasoconstrictor activity as well as vascular remodeling.103 In addition, siRNA-mediated knockdown of Nox4 or Nox4 inhibitor GKT137831 attenuated vascular remodeling.104, 105 A crosstalk between ET-1 and Nox has been reported, which may be explained by the fact that ET-1 stimulates the proliferation of pulmonary arterial smooth muscle cells via activation of Nox-catalyzed ROS production.106 Moreover, superoxide produced by Nox has been shown to impair NO signaling by inducing eNOS uncoupling.107 Understanding of the role of Nox isoforms in PAH pathogenesis may present new opportunities for specific inhibitors, such as the peptidic Nox2 inhibitor Nox2ds.108
Xanthine oxidase
ROS may be produced by other oxidases, such as xanthine oxidase (XO). Under basal conditions, XO exists primarily in its dehydrogenase form (XOR), catalyzing the final two steps of purine degradation (hypoxanthine-xanthine-uric acid). Under conditions of hypoxia or inflammation, oxidation of critical cysteine residues or proteolytic cleavage converts XOR back to the oxidase form, which in turn transfers substrate-derived electrons to oxygen, generating superoxide and H2O2. It has been shown that XO can be activated in endothelial cells, in the arteries of patients with PAH, and in rodent models of PAH induced by hypoxia.109-111 It was also found that treatment of XO inhibitors normalized pulmonary pressure and limited vascular remodeling in these rodent models.110, 111 New highly potent XO inhibitors, such as febuxostat, have been developed for the therapy of gout,112 and may now be tested in human PAH.
Mitochondrial ROS
Mitochondria normally generate ROS as by-products of aerobic metabolism by the electron transport chain (ETC) enzyme complexes, with diversion of approximately 3% of net electron flux to oxygen to form superoxide. Most of the electrons flowing through the respiratory chain are ultimately reduced to water; however, some electrons “leak” from complex I and III and react with molecular oxygen to produce superoxide.113 Superoxide is then converted to H2O2 by superoxide dismutase 2 (SOD2) in mitochondrial matrix. H2O2 can be further reduced to water by catalase, or can permeate membranes to regulate downstream signaling pathways and to activate redox-sensitive voltage-gated channel, Kv 1.5. In the case of hypoxic pulmonary vasoconstriction and related hypoxic pulmonary hypertension, two competing hypotheses for mitochondrial ROS have been advanced. One hypothesis by the Schumacher group suggests that increased mitochondrial ROS production at complex III induces intracellular Ca2+ release, which in turn opens Ca2+ channels in the plasma membrane and activates vasoconstriction.114 This is supported by recent studies in which complex III activity is blocked by conditional deletion of complex III Rieske iron-sulfur protein (RISP), which inhibits hypoxia related increases in mitochondrial ROS and impairs pulmonary vasoconstriction.114 On the other hand, Archer and colleagues have suggested that reduced, not increased, mitochondrial ROS levels in pulmonary artery smooth muscle cells in hypoxia inhibit Kv1.5 channel, which causes membrane depolarization, increases intracellular Ca2+, and subsequently stimulates vasoconstriction and smooth muscle cells proliferation.115 While the role and levels of mitochondrial ROS in PAH remain controversial, ROS levels do appear to increase in mitochondrial at lower oxygen levels, and decreased expression levels of SOD2 and Kv1.5 channel have been observed in PAH patients.116, 117 In addition, activation of Kv1.5 channel by the treatment of dichloroacetate decreases smooth muscle cells proliferation in animals with PAH.118 Finally, it is becoming increasingly clear that mitochondrial function is impaired in PAH and is associated with enhanced cellular glycolytic activity, suggesting a central role for mitochondrial dysfunction in disease pathobiology. For a more detailed recent review by our group, see ref55 as well as discussions in this review series.
Cellular changes
In addition to imbalance of vasoactive mediators, dysfunctional endothelium also releases factors (e.g., serotonin, and FGF-2) that initiate abnormal vascular proliferation and remodeling in the pulmonary arteries. Abnormal smooth muscle cell proliferation is the earliest pathobiological feature of vascular remodeling,119 which leads to muscularization of peripheral pulmonary arteries and medial hypertrophy in the pulmonary muscular arteries (Figure 8).120 Increased proliferation and progressive migration of smooth muscle cells, which may be derived from stem cells, fibrocytes, or endothelial cells, into the space between endothelium and the internal elastic lamina, termed intimal fibrosis, also leads to occlusive changes in the small pulmonary arteries.120 In the late stage of disease progression, disorganized proliferation of apoptosis-resistant endothelial cells, smooth muscle cells, fibroblasts, and macrophages leads to formation of a plexiform lesion, a complex lumen-obliterating lesion that has been found frequently in severe PAH patients.121 Additionally, platelet activation, continuous intravascular coagulation, and the development of in situ thrombus further occlude the vessel lumen. Endothelial cells apoptosis in early phase of PAH has been linked to the loss of small pulmonary arteries.120 Moreover, lung vascular remodeling can be associated with abnormal proliferation and differentiation of fibroblasts, increased extracellular matrix deposition, and chronic inflammatory events in the adventitia.122 Together, these uncontrolled and unpredictable events narrow/obstruct/obliterate pulmonary arteries, thus leading to PAH.
Figure 8. Cellular changes in PAH.
Healthy endothelium regulates the balance between vasodilation and vasoconstriction and inhibits smooth muscle cell proliferation to maintain a low-resistance pulmonary vasculature. In PAH, dysfunctional endothelium alters vasodilator/vasoconstrictor balance to increase contractility of pulmonary arteries. In addition, abnormal proliferation of smooth muscle cells, the earliest pathobiological features of vascular remodeling, leads to muscularization of peripheral pulmonary arteries and medial hypertrophy in pulmonary muscular arteries. Increased proliferation and progressive migration of smooth muscle cells further results in intimal fibrosis. In the late stage of disease progression, formation of plexiform lesions and in situ thrombus occlude the vessel lumen. These dysregulated events lead to progressive reducing of the blood flow, thus causing PAH.
Summary
PAH is characterized by progressive endothelial dysfunction with low NO and prostacyclin bioavailability, increased ET-1 signaling, enhanced oxidative stress, increased smooth muscle contractility, oxidase activation, mitochondrial dysfunction, and ultimate cellular proliferation and pathological remodeling of the lung vasculature. The changes in PVR subsequently lead to decreased cardiac output and right heart failure. A loss of blood flow/oxygen delivery due to reduced cardiac output is the primary cause of shortness of breath, rather than impaired alveolar gas exchange and arterial oxygen levels. While PAH can be inherited (with a higher penetrance in women), the prevalence of the disease is higher for IPAH, spontaneous BMPRII mutations, or PAH secondary to co-existent diseases. According to the French Registry, female patients have about 18% lower mortality rates than males with the same hemodynamic measurements. Although there is still no cure for this disease, prognosis and survival in the present-day have improved over that two decades ago. Finally, current therapies targeting endothelial function and vasodilation/antiproliferation have shown efficacy in decreasing pulmonary pressures, increasing CO and right heart function, augmenting functional class, and improving exercise capacity and quality of life of PAH patients. Moreover, new therapeutic strategies further enhancing NO signaling, reducing oxidative stress and enhancing mitochondrial function are being developed.
Supplementary Material
A Patient Asks Questions….
Why am I really so short of breath? My oxygen levels are not that low…
This is a common point of confusion for patients. It is true that in most lung diseases, which affect the part of the lung in which the exchange of oxygen to carbon dioxide takes place, the oxygen levels drop. This easily explains the shortness of breath, reflecting the lack of oxygen in the body. In PAH, this part of the lung tissue is not primarily affected. PAH affects the blood vessels that go through the lung and in most cases the oxygen levels do not decrease. What happens is that the obstruction of the blood vessels by the overgrowth of the cells in the vessel wall, poses a strain in the right ventricle, i.e. the heart chamber that “pushes” the blood through the lung. This chamber is thin-walled and quickly fails in response to the “extra work”. As a result, its pumping efficiency drops and the amount of blood that the right chambers eject with each contraction (i.e. the “ejection fraction of the right ventricle”) decreases. The right ventricle pushes the blood to the lung but is still a part of the whole circuit of the blood circulation. Therefore the decreased pumping efficiency of the right ventricle results in a decreased amount of blood circulating in the whole body. Thus patients with PAH get short of breath not because the oxygen levels in the blood drop, but because the amount of the blood (which carries the oxygen) decreases with each heart contraction. The tissues sense the lack of oxygen because they receive less blood.
This is more prominent during increased activity (exercise) when the tissues need more oxygen/blood to cover the increased need for energy (which is offered by the oxygen). This is why patients with PAH are mostly short of breath with activity and only at the very advanced/late stages of the disease they get short of breath at rest. Although oxygen generally makes most people “feel better” oxygen supplementation is not critical for the management of PAH, in contrast to most other lung diseases. This also explains why patients may pass out during exercise. The decrease in the amount of blood sent to the brain drops significantly during exercise, causing a transient loss of consciousness (i.e. syncope). This concept is important for the patients to understand. It also makes it clear that PAH is essentially a disease of “heart failure”; only that in contrast to the left chambers affected in the more common forms of heart failure, in PAH only the right chambers are affected.
How long do I have to live?
This is almost an impossible question to answer in Medicine, yet a very important one, bothering most patients. Based on the discussion above it is clear that the function of the right chambers of the heart are the most important in terms of symptoms (like shortness of breath) in PAH patients. We now know that the function of the right ventricle is most critical for the survival of PAH patients as well. However, we do not know what makes the right chambers transition from a state of compensation to a state of failure. We do recognize that there are probably genetic and other reasons that may explain why the right ventricle in one patient may fail earlier than in another patient. Thus overall it is premature to claim that we can predict how long a patient has to live, assuming he or she is offered all the available therapies at this point.
However, studies in large numbers of patients with PAH have allowed scientists to develop mathematical equations to predict survival, based on certain parameters, like the blood pressure in the lung, the cardiac output (i.e. the amount of blood ejected from the right chambers) and others. These equations were developed at different points of time, when the management of PAH patients was different, explaining some differences in the results from these formulas. The authors present in Table 3 the results for our patient (as described in the introductory paper by Dr Michelakis) based on several of these formulas. Using the specific information for our patient, one can see how the one-year survival is calculated based on formulas developed in the 1980s (like the “NIH formula”) to more recent ones that included additional parameters in the formula. Thus although the calculation from the NIH formula would have predicted a 75% chance of our patient to survive one year, more recent formulas predict a higher chance, around 90%. It is important for the patients to understand the efforts of physicians and scientists in the field to provide tools to help in these complicated questions, providing answers that are as specific and individualized as possible.
Acknowledgments
The authors thank Sergei Snovida for helpful comments on the manuscript, Marta Bueno for analysis of survival equations and calculations, and Elfy Chiang for assistance and production of figures.
Sources of funding: Dr. Gladwin receives research support from NIH grants RO1HL098032, RO1HL096973, and PO1HL103455, the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Non-standard Abbreviations and Acronyms
- 2-ME
2-methoxyestradiol
- 2-OHE
2-hydroxyestrogen
- 6MWD/6MWT
six-minute walk distance/test
- 16α-OHE
16α-hydroxyestrone
- BH4/BH2
tetrahydrobiopterin/dihydrobiopterin
- BMPRII
bone morphogenetic protein receptor type II
- BNP
brain-type natriuretic peptide
- CI
cardiac index
- CO
cardiac output
- COX
cyclooxygenase
- CT
computed tomography
- CTEPH
chronic thromboembolic pulmonary hypertension
- CT-PET
computed tomography-positron emission tomography
- CXR
chest x-ray
- CYP1B1
cytochrome p450 1B1
- DLCO
diffusing capacity of the lung for carbon monoxide
- ECG
electrocardiogram
- eNOS
endothelial NO synthase
- ET-1
endothelin-1
- FDG
18F-labeled deoxyglucose
- FPAH
familial pulmonary arterial hypertension
- Glut1
glucose transporter-1
- H2O2
hydrogen peroxide
- HRR
heart rate recovery
- IPAH
idiopathic pulmonary arterial hypertension
- IVC
inferior vena cava
- LFTs
liver function tests
- LV
left ventricle/ventricular
- mPAP
mean pulmonary artery pressure
- NIH
National Institutes of Health
- NO
nitric oxide
- Nox
NADPH oxidase
- NT-proBNP
N-terminal fragments of pro-brain-type natriuretic peptide
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PAOP
pulmonary artery occlusion pressure
- PCWP
pulmonary capillary wedge pressure
- PDE5
phosphodiesterase 5
- PFTs
pulmonary function tests
- PGI2
prostacyclin
- PH
pulmonary hypertension
- PHC Registry
the Pulmonary Hypertension Connection registry
- PVH
pulmonary venous hypertension
- PVR
pulmonary vascular resistance
- RA/RAP
right atrium/right atrial pressure
- REVEAL Registry
the Registry to Evaluate Early and Long-Term PAH Disease Management
- ROS
reactive oxygen species
- RV
right ventricle/ventricular
- SOD2
superoxide dismutase 2
- TAPSE
tricuspid annular plane systolic excursion
- TXA2
thromboxane A2
- VQ
ventilation-perfusion
- XO
xanthine oxidase
- XOR
xanthine oxidoreductase
Footnotes
In April 2014, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.38 days.
Disclosures: Dr. Gladwin is listed as a co-inventor on a NIH government patent for the use of nitrite salts in cardiovascular diseases. Dr. Gladwin consults with Aires Pharmaceuticals on the development of a phase II proof of concept trial using inhaled nitrite for PAH.
References
- 1.Archer SL, Djaballah K, Humbert M, Weir KE, Fartoukh M, Dall'ava-Santucci J, Mercier JC, Simonneau G, Dinh-Xuan AT. Nitric oxide deficiency in fenfluramine- and dexfenfluramine-induced pulmonary hypertension. American journal of respiratory and critical care medicine. 1998;158:1061–1067. doi: 10.1164/ajrccm.158.4.9802113. [DOI] [PubMed] [Google Scholar]
- 2.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, et al. Primary pulmonary hypertension. A national prospective study. Annals of internal medicine. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 3.Perez VA, Haddad F, Zamanian RT. Diagnosis and management of pulmonary hypertension associated with left ventricular diastolic dysfunction. Pulmonary circulation. 2012;2:163–169. doi: 10.4103/2045-8932.97598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabuchi A, Mertens M, Kuppe H, Pries AR, Kuebler WM. Intravital microscopy of the murine pulmonary microcirculation. Journal of applied physiology. 2008;104:338–346. doi: 10.1152/japplphysiol.00348.2007. [DOI] [PubMed] [Google Scholar]
- 5.Kasner M, Westermann D, Steendijk P, Drose S, Poller W, Schultheiss HP, Tschope C. Left ventricular dysfunction induced by nonsevere idiopathic pulmonary arterial hypertension: A pressure-volume relationship study. American journal of respiratory and critical care medicine. 2012;186:181–189. doi: 10.1164/rccm.201110-1860OC. [DOI] [PubMed] [Google Scholar]
- 6.Loyd JE, Phillips JA. Heritable pulmonary arterial hypertension. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. Genereviews. Seattle (WA): 1993. [Google Scholar]
- 7.Harrison RE, Flanagan JA, Sankelo M, et al. Molecular and functional analysis identifies alk-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. Journal of medical genetics. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaouat A, Coulet F, Favre C, Simonneau G, Weitzenblum E, Soubrier F, Humbert M. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax. 2004;59:446–448. doi: 10.1136/thx.2003.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene pph1) is caused by mutations in the bone morphogenetic protein receptor-ii gene. American journal of human genetics. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International PPHC. Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in bmpr2, encoding a tgf-beta receptor, cause familial primary pulmonary hypertension. Nature genetics. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 11.Ma L, Roman-Campos D, Austin ED, et al. A novel channelopathy in pulmonary arterial hypertension. The New England journal of medicine. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cogan JD, Pauciulo MW, Batchman AP, Prince MA, Robbins IM, Hedges LK, Stanton KC, Wheeler LA, Phillips JA, 3rd, Loyd JE, Nichols WC. High frequency of bmpr2 exonic deletions/duplications in familial pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2006;174:590–598. doi: 10.1164/rccm.200602-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G, Galie N, Manes A, Corris P, Simonneau G, Humbert M, Morrell NW, Trembath RC. Bmpr2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Human mutation. 2006;27:212–213. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- 14.Machado RD, Aldred MA, James Vea. Mutations of the tgf-beta type ii receptor bmpr2 in pulmonary arterial hypertension. Human mutation. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type ii bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 16.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes & development. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 17.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: Implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circulation research. 2006;98:209–217. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 18.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the bmpr2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118:722–730. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative bmprii gene in smooth muscle. Circulation research. 2004;94:1109–1114. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds AM, Xia W, Holmes MD, Hodge SJ, Danilov S, Curiel DT, Morrell NW, Reynolds PN. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. American journal of physiology Lung cellular and molecular physiology. 2007;292:L1182–1192. doi: 10.1152/ajplung.00020.2006. [DOI] [PubMed] [Google Scholar]
- 21.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, Huber LC. Antagomir directed against mir-20a restores functional bmpr2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. European heart journal. 2012 doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 22.Sobolewski A, Rudarakanchana N, Upton PD, Yang J, Crilley TK, Trembath RC, Morrell NW. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: Potential for rescue. Human molecular genetics. 2008;17:3180–3190. doi: 10.1093/hmg/ddn214. [DOI] [PubMed] [Google Scholar]
- 23.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in france: Results from a national registry. American journal of respiratory and critical care medicine. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 24.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: Baseline characteristics from the reveal registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 25.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: Insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (reveal) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 26.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, Wheeler LA, Parl FF, Loyd JE, Phillips JA., 3rd Alterations in oestrogen metabolism: Implications for higher penetrance of familial pulmonary arterial hypertension in females. The European respiratory journal. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tofovic SP, Salah EM, Mady HH, Jackson EK, Melhem MF. Estradiol metabolites attenuate monocrotaline-induced pulmonary hypertension in rats. Journal of cardiovascular pharmacology. 2005;46:430–437. doi: 10.1097/01.fjc.0000175878.32920.17. [DOI] [PubMed] [Google Scholar]
- 28.Umar S, Rabinovitch M, Eghbali M. Estrogen paradox in pulmonary hypertension: Current controversies and future perspectives. American journal of respiratory and critical care medicine. 2012;186:125–131. doi: 10.1164/rccm.201201-0058PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West J, Cogan J, Geraci M, Robinson L, Newman J, Phillips JA, Lane K, Meyrick B, Loyd J. Gene expression in bmpr2 mutation carriers with and without evidence of pulmonary arterial hypertension suggests pathways relevant to disease penetrance. BMC medical genomics. 2008;1:45. doi: 10.1186/1755-8794-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humbert M, Sitbon O, Yaici A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. The European respiratory journal. 2010;36:549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 31.Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD. The reveal registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin VV, Suissa S. Prognosis of pulmonary arterial hypertension: The power of clinical registries of rare diseases. Circulation. 2010;122:106–108. doi: 10.1161/CIRCULATIONAHA.110.963983. [DOI] [PubMed] [Google Scholar]
- 33.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Annals of internal medicine. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 34.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: A reappraisal of the nih risk stratification equation. The European respiratory journal. 2010;35:1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, Nakanishi N, Miyatake K. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. American journal of respiratory and critical care medicine. 2000;161:487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 36.Mathai SC, Puhan MA, Lam D, Wise RA. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2012;186:428–433. doi: 10.1164/rccm.201203-0480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabler NB, French B, Strom BL, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation. 2012;126:349–356. doi: 10.1161/CIRCULATIONAHA.112.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin LJ. The 6-minute walk test in pulmonary arterial hypertension: How far is enough? American journal of respiratory and critical care medicine. 2012;186:396–397. doi: 10.1164/rccm.201206-1137ED. [DOI] [PubMed] [Google Scholar]
- 39.Savarese G, Paolillo S, Costanzo P, D'Amore C, Cecere M, Losco T, Musella F, Gargiulo P, Marciano C, Perrone-Filardi P. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. Journal of the American College of Cardiology. 2012;60:1192–1201. doi: 10.1016/j.jacc.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 40.Minai OA, Gudavalli R, Mummadi S, Liu X, McCarthy K, Dweik RA. Heart rate recovery predicts clinical worsening in patients with pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2012;185:400–408. doi: 10.1164/rccm.201105-0848OC. [DOI] [PubMed] [Google Scholar]
- 41.Mukoyama M, Nakao K, Saito Y, Ogawa Y, Hosoda K, Suga S, Shirakami G, Jougasaki M, Imura H. Increased human brain natriuretic peptide in congestive heart failure. The New England journal of medicine. 1990;323:757–758. doi: 10.1056/NEJM199009133231114. [DOI] [PubMed] [Google Scholar]
- 42.Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton C, Turner J, Crozier IG, Yandle TG. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–2792. doi: 10.1161/01.CIR.0000070953.76250.B9. [DOI] [PubMed] [Google Scholar]
- 43.Kruger S, Graf J, Kunz D, Stickel T, Hanrath P, Janssens U. Brain natriuretic peptide levels predict functional capacity in patients with chronic heart failure. Journal of the American College of Cardiology. 2002;40:718–722. doi: 10.1016/s0735-1097(02)02032-6. [DOI] [PubMed] [Google Scholar]
- 44.Frantz RP, McDevitt S, Walker S. Baseline nt-probnp correlates with change in 6-minute walk distance in patients with pulmonary arterial hypertension in the pivotal inhaled treprostinil study triumph-1. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:811–816. doi: 10.1016/j.healun.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, Kuribayashi S, Hamada S, Kakishita M, Nakanishi N, Takamiya M, Kunieda T, Matsuo H, Kangawa K. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. Journal of the American College of Cardiology. 1998;31:202–208. doi: 10.1016/s0735-1097(97)00452-x. [DOI] [PubMed] [Google Scholar]
- 46.Leuchte HH, Holzapfel M, Baumgartner RA, Ding I, Neurohr C, Vogeser M, Kolbe T, Schwaiblmair M, Behr J. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. Journal of the American College of Cardiology. 2004;43:764–770. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 47.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (esc) and the european respiratory society (ers), endorsed by the international society of heart and lung transplantation (ishlt) European heart journal. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 48.Bossone E, D'Andrea A, D'Alto M, Citro R, Argiento P, Ferrara F, Cittadini A, Rubenfire M, Naeije R. Echocardiography in pulmonary arterial hypertension: From diagnosis to prognosis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013;26:1–14. doi: 10.1016/j.echo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Yeo TC, Dujardin KS, Tei C, Mahoney DW, McGoon MD, Seward JB. Value of a doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. The American journal of cardiology. 1998;81:1157–1161. doi: 10.1016/s0002-9149(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 50.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. American heart journal. 2004;147:218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: State of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 52.Shehata ML, Lossnitzer D, Skrok J, Boyce D, Lechtzin N, Mathai SC, Girgis RE, Osman N, Lima JA, Bluemke DA, Hassoun PM, Vogel-Claussen J. Myocardial delayed enhancement in pulmonary hypertension: Pulmonary hemodynamics, right ventricular function, and remodeling. AJR American journal of roentgenology. 2011;196:87–94. doi: 10.2214/AJR.09.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, Chen CT, Archer SL. Lung (1)(8)f-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2012;185:670–679. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free radical biology & medicine. 2012;52:1970–1986. doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki YJ, Steinhorn RH, Gladwin MT. Antioxidant therapy for the treatment of pulmonary hypertension. Antioxidants & redox signaling. 2013;18:1723–1726. doi: 10.1089/ars.2013.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. The New England journal of medicine. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 58.Fagan KA, Fouty BW, Tyler RC, Morris KG, Jr, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous enos-null mice is hyperresponsive to mild hypoxia. The Journal of clinical investigation. 1999;103:291–299. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, Polak JM. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. The Journal of pathology. 1998;185:313–318. doi: 10.1002/(SICI)1096-9896(199807)185:3<313::AID-PATH93>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 60.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent enos activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through pkg nitration. The Journal of clinical investigation. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 62.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (bh4) oxidation with impaired endothelial function ameliorated by bh4. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bevers LM, Braam B, Post JA, van Zonneveld AJ, Rabelink TJ, Koomans HA, Verhaar MC, Joles JA. Tetrahydrobiopterin, but not l-arginine, decreases no synthase uncoupling in cells expressing high levels of endothelial no synthase. Hypertension. 2006;47:87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- 64.Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2126–2133. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- 65.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in no vs. Superoxide production by enos. American journal of physiology Heart and circulatory physiology. 2008;294:H1530–1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: An epr spin trapping study. The Biochemical journal. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: Role of nadph oxidase and endothelial no synthase. American journal of physiology Lung cellular and molecular physiology. 2006;290:L1069–1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 68.Channick RN, Newhart JW, Johnson FW, Williams PJ, Auger WR, Fedullo PF, Moser KM. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: An ambulatory delivery system and initial clinical tests. Chest. 1996;109:1545–1549. doi: 10.1378/chest.109.6.1545. [DOI] [PubMed] [Google Scholar]
- 69.Hampl V, Tristani-Firouzi M, Hutsell TC, Archer SL. Nebulized nitric oxide/nucleophile adduct reduces chronic pulmonary hypertension. Cardiovascular research. 1996;31:55–62. [PubMed] [Google Scholar]
- 70.Lam CF, Van Heerden PV, Blott J, Roberts B, Ilett KF. The selective pulmonary vasodilatory effect of inhaled deta/no, a novel nitric oxide donor, in ards-a pilot human trial. Journal of critical care. 2004;19:48–53. doi: 10.1016/j.jcrc.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive no-dependent selective pulmonary vasodilator. Nature medicine. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 72.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 73.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 74.Baliga RS, Milsom AB, Ghosh SM, Trinder SL, Macallister RJ, Ahluwalia A, Hobbs AJ. Dietary nitrate ameliorates pulmonary hypertension: Cytoprotective role for endothelial nitric oxide synthase and xanthine oxidoreductase. Circulation. 2012;125:2922–2932. doi: 10.1161/CIRCULATIONAHA.112.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robbins IM, Hemnes AR, Gibbs JS, Christman BW, Howard L, Meehan S, Cabrita I, Gonzalez R, Oyler T, Zhao L, Du RH, Mendes LA, Wilkins MR. Safety of sapropterin dihydrochloride (6r-bh4) in patients with pulmonary hypertension. Experimental lung research. 2011;37:26–34. doi: 10.3109/01902148.2010.512972. [DOI] [PubMed] [Google Scholar]
- 76.Morris CR, Kuypers FA, Lavrisha L, Ansari M, Sweeters N, Stewart M, Gildengorin G, Neumayr L, Vichinsky EP. A randomized, placebo-control trial of arginine therapy for the treatment ofchildren with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica. 2013 doi: 10.3324/haematol.2013.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil Use in Pulmonary Arterial Hypertension Study G. Sildenafil citrate therapy for pulmonary arterial hypertension. The New England journal of medicine. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 78.Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ. Pulmonary Arterial H, Response to Tadalafil Study G. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 79.Namba T, Oida H, Sugimoto Y, Kakizuka A, Negishi M, Ichikawa A, Narumiya S. Cdna cloning of a mouse prostacyclin receptor. Multiple signaling pathways and expression in thymic medulla. The Journal of biological chemistry. 1994;269:9986–9992. [PubMed] [Google Scholar]
- 80.Smith WL. The eicosanoids and their biochemical mechanisms of action. The Biochemical journal. 1989;259:315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoshikawa Y, Voelkel NF, Gesell TL, Moore MD, Morris KG, Alger LA, Narumiya S, Geraci MW. Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. American journal of respiratory and critical care medicine. 2001;164:314–318. doi: 10.1164/ajrccm.164.2.2010150. [DOI] [PubMed] [Google Scholar]
- 82.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. The New England journal of medicine. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 83.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. American journal of respiratory and critical care medicine. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 84.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The New England journal of medicine. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 85.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ Treprostinil Study G. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: A double-blind, randomized, placebo-controlled trial. American journal of respiratory and critical care medicine. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 86.Tapson VF, Gomberg-Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman A, Barst RJ. Safety and efficacy of iv treprostinil for pulmonary arterial hypertension: A prospective, multicenter, open-label, 12-week trial. Chest. 2006;129:683–688. doi: 10.1378/chest.129.3.683. [DOI] [PubMed] [Google Scholar]
- 87.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. The New England journal of medicine. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 88.Galie N, Humbert M, Vachiery JL, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled trial. Journal of the American College of Cardiology. 2002;39:1496–1502. doi: 10.1016/s0735-1097(02)01786-2. [DOI] [PubMed] [Google Scholar]
- 89.Barst RJ, McGoon M, McLaughlin V, et al. Beraprost therapy for pulmonary arterial hypertension. Journal of the American College of Cardiology. 2003;41:2119–2125. doi: 10.1016/s0735-1097(03)00463-7. [DOI] [PubMed] [Google Scholar]
- 90.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 91.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. The New England journal of medicine. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 92.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: Marker or mediator of disease? Annals of internal medicine. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 93.Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, Hoeffken G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120:1562–1569. doi: 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 94.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: A randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 95.Galie N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, Frost AE, Zwicke D, Naeije R, Shapiro S, Olschewski H, Rubin LJ. Ambrisentan therapy for pulmonary arterial hypertension. Journal of the American College of Cardiology. 2005;46:529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 96.Eriksson C, Gustavsson A, Kronvall T, Tysk C. Hepatotoxicity by bosentan in a patient with portopulmonary hypertension: A case-report and review of the literature. Journal of gastrointestinal and liver diseases : JGLD. 2011;20:77–80. [PubMed] [Google Scholar]
- 97.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: New concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlocai K, Galie N, Degano B, Bonderman D, Kurzyna M, Efficace M, Giorgino R, Lang IM. Selexipag: An oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. The European respiratory journal. 2012;40:874–880. doi: 10.1183/09031936.00137511. [DOI] [PubMed] [Google Scholar]
- 99.Tse MT. Trial watch: Phase iii success for first-in-class pulmonary hypertension drug. Nature reviews Drug discovery. 2012;11:896. doi: 10.1038/nrd3906. [DOI] [PubMed] [Google Scholar]
- 100.Mittendorf J, Weigand S, Alonso-Alija C, et al. Discovery of riociguat (bay 63-2521): A potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem. 2009;4:853–865. doi: 10.1002/cmdc.200900014. [DOI] [PubMed] [Google Scholar]
- 101.Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. British journal of pharmacology. 2006;148:714–723. doi: 10.1038/sj.bjp.0706779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mittal M, Roth M, Konig P, et al. Hypoxia-dependent regulation of nonphagocytic nadph oxidase subunit nox4 in the pulmonary vasculature. Circulation research. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 103.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: Role of superoxide and nadph oxidase (gp91phox) American journal of physiology Lung cellular and molecular physiology. 2006;290:L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 104.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. Nox4 regulates ros levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxidants & redox signaling. 2008;10:1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 105.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The nox4 inhibitor gkt137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. American journal of respiratory cell and molecular biology. 2012;47:718–726. doi: 10.1165/rcmb.2011-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wedgwood S, Dettman RW, Black SM. Et-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. American journal of physiology Lung cellular and molecular physiology. 2001;281:L1058–1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- 107.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. The Journal of clinical investigation. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 b-loop peptide, nox2ds, specifically inhibits the nadph oxidase nox2. Free radical biology & medicine. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Partridge CA, Blumenstock FA, Malik AB. Pulmonary microvascular endothelial cells constitutively release xanthine oxidase. Archives of biochemistry and biophysics. 1992;294:184–187. doi: 10.1016/0003-9861(92)90155-p. [DOI] [PubMed] [Google Scholar]
- 110.Hoshikawa Y, Ono S, Suzuki S, Tanita T, Chida M, Song C, Noda M, Tabata T, Voelkel NF, Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. Journal of applied physiology. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- 111.Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. American journal of physiology Lung cellular and molecular physiology. 2008;294:L233–245. doi: 10.1152/ajplung.00166.2007. [DOI] [PubMed] [Google Scholar]
- 112.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. The New England journal of medicine. 2005;353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 113.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex iii triggers acute responses to hypoxia in the pulmonary circulation. American journal of respiratory and critical care medicine. 2013;187:424–432. doi: 10.1164/rccm.201207-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circulation research. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 116.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: A basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated k+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 118.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circulation research. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 119.Rubin LJ. Primary pulmonary hypertension. The New England journal of medicine. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 120.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. The Journal of clinical investigation. 2008;118:2372–2379. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. The American journal of pathology. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 122.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-c antisense prevents progression, of vascular disease. The Journal of clinical investigation. 2000;105:21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.