Abstract
Background
Carbon dioxide (CO2) hypersensitivity represents an individual difference response to breathing CO2 enriched air. People with a history of panic attacks or panic disorder are particularly prone to anxious response, suggesting that CO2 hypersensitivity is a robust risk marker of panic spectrum vulnerability.
Methods
Twin pairs (n = 346) from the general population-based Norwegian NIPH Mental Health Study completed a measure of anxiety before and after vital capacity inhalation of 35% CO2 air and before and after inhalation of regular air. Three hypotheses regarding genetic factors for CO2 hypersensitivity were examined: (1) a single set of genetic risk factors impacts anxiety before exposure to CO2 and these same genes constitute the only genetic influences on anxiety in response to CO2, (2) the genetic effects on pre-CO2 anxiety are entirely different from the genetic effects on anxiety in response to exposure to CO2 (i.e., new genetic effects), and (3) pre-CO2 anxiety influences anxiety in response to CO2 as well as unique genetic factors that become activated by respiratory stimulation.
Results
Our results support the latter hypothesis for response to 35% CO2, with additive genetic and unique environmental factors best fitting the data. Evidence of new genetic effects was observed, accounting for 20% unique variance in post 35% CO2 anxiety response. New genetic effects were not observed for anxiety ratings made post regular air where only preregular air anxiety ratings explained significant variance in this outcome.
Conclusions
These data suggest that there are distinct genetic factors associated with responsivity to respiratory stimulation via 35% CO2.
Keywords: panic, carbon dioxide sensitivity, twins, genetic, panic disorder, anxiety
INTRODUCTION
Carbon dioxide (CO2) reactivity reflects an enhanced physiologic and emotional response when breathing air containing increased concentrations of CO2. The 35% CO2 challenge is one of the most commonly used CO2 challenge methods and this task effectively and reliably produces enhanced subjective and physiologic responding in general population samples and provokes approximately 50–70% of persons with panic disorder (PD) to experience a panic attack.[1,2] By contrast, it is rare for healthy controls to develop panic attacks or a panic-like response after exposure to 35% CO2.[3,4] Response to 35% CO2 also demonstrates a level of specificity with PD in that persons with other anxiety disorders do not react with the same level of symptomatic intensity or panic attack rate.[1,5,7] Moreover, individuals with nonclinical panic (i.e. occasional, unexpected panic attacks) exhibit reactions to CO2 similar to those reported in persons with PD,[6] suggesting that CO2 hypersensitivity relates most robustly to panic spectrum liability.
Family studies of response to CO2 hypersensitivity indicate that healthy controls of PD probands react stronger to 35% CO2 than healthy controls without a positive family history of PD,[8,9] suggesting a familial influence. Although family studies provide valuable insight into the nature of panic, this design is unable to disaggregate genetic and common (shared) environmental contributions to phenotypic variation. Twin studies, however, allow for the dismantling of genetic, common (shared) environment, and unique (nonshared) environmental influences. To date, two twin studies[10,11] have examined response to CO2. One study observed a significantly higher concordance rate for CO2-induced panic among monozygotic (MZ) compared to dizygotic (DZ) twins (55.6% versus 12.5%, respectively;[9]), suggesting a robust genetic influence. The second study noted that the covariation between DSM-IV PD and panic-like anxiety elicited by 35% CO2 stimulation also was largely (73%) accounted for by additive genetic factors.[12,13] This observation is consistent with the results of a meta-analytic study,[14] where 30%–40% of the variance in liability to PD was attributed to additive genetic factors. Individual-specific environments best explained the residual variance in liability not accounted for by genetics, with no support observed for the role of common family environment in the etiology of PD. A more comprehensive model, which pooled family and twin studies, calculated a heritability estimate of 0.48. In line with this estimate, the observed heritability for 35% CO2 induced anxiety ranged between 0.42 and 0.57, depending on response definition (e.g. visual analogue score, number of DSM panic symptoms[12]). Collectively, genetically informed studies suggest that genetic factors explain a large portion of familial aggregation of PD as well as response to CO2. What we do not know is whether genetic contributions to CO2 response are related to a general, trait anxiety proneness, or to a unique, CO2 associated vulnerability.
The current study seeks to determine whether there are genetic risk factors associated with CO2 hypersensitivity that are expressly related to CO2 or whether genetic factors related to CO2 response are merely reflective of underlining trait anxiety. We use the term pre-CO2 anxiety as an index of trait anxiety proneness with the understanding that pre-CO2 anxiety is a complex combination of state and trait anxiety (e.g. anticipatory anxiety, anxiety sensitivity, etc.). In all, we seek to discriminate between the following three hypotheses that reflect differing genetically based associations: (1) a single set of genetic risk factors impacts anxiety before exposure to 35% CO2 and these same genetic factors constitute the only influence on anxiety in response to exposure to 35% CO2; (2) the genetic effects on pre-CO2 anxiety are entirely different from the genetic effects on anxiety in response to exposure to CO2; or (3) there are genetic effects related to anxiety experienced before breathing 35% CO2 enriched air that influence anxiety in response to CO2 as well as a unique genetic factor that becomes active or “turned on” in response to respiratory stimulation via CO2 enriched air.
METHODS
SUBJECTS
Twin pairs in this study were originally enrolled in the Norwegian Twin Study on the Genetics of Personality and Mental Health (NIPH-MHS), which is a project of the Norwegian Institute of Public Health Twin Panel, a cohort sequential design research program using general population-based cohorts of twin.[15] A large sample of 6,349 twin pairs were invited to participate in the NIPH-MHS, with 34.3% being MZ twins, 33.3% being same-sex DZ twins, and 32.4% being opposite-sex DZ twins; 51.8% were female. All twins were born between 1967–1979 and were invited by postal mail to take part in the NIPH-MHS. Complete twin pairs (3,334) and 1,377 single responders (participation rates: 53% for pairs, 63% overall) responded to the mail invitation. Participation was higher for MZ female twin pairs (participation among monozygotic females (MZF) versus monozygotic males (MZM) pairs: 23.3% versus 15.8%, P =.01) and increased with age (Spearman r = .07, P < .001).
The present study examined response to 35% CO2–65% O2 and was interjected in the NIPH-MHS. That is, this study was based on the same population and recruitment measures, but was designed to include an a priori sample of approximately 350 twin pairs. To ascertain this subsample of twin pairs, 3,182 twins from the NIPH-MHS were invited consecutively to participate in the 35% CO2 challenge study in three waves of similar size. Participants were randomly ascertained in waves 1 and 3 and no significant differences emerged for participation rates across the multiple waves, with a 22.4% participation rate.
“Anxiety-prone” individuals were targeted to ensure a sufficient number of individuals who could be informative for the CO2 provocation test. Specifically, in wave 2, approximately 50% of participants were invited to participate if they met criteria suggestive of increased liability to panic disorder, agoraphobia, or social phobia, given evidence of increased sensitivity to CO2-enriched air among persons with these three anxiety disorders.[5,15–18] Criteria to define “anxiety-prone” subjects were based on five items (a) I feel a strong aversion when I socialize with many people at a time, for example, in stores, on the street, or in the movie theatre; (b) I often feel a strong aversion when I go out to eat or drink with people, for example, in a cafeteria, a café, or a restaurant; (c) I am afraid of blushing or trembling when people look at me; (d) I have had sudden attacks with palpitations, breathing trouble, and dizziness; (e) I can suddenly become very afraid, or I panic, without a specific reason. These items were selected from the original postal mail questionnaire.[10]
The final sample for the 35% CO2 study included 346 twin pairs. Table 1 presents demographic characteristics of twins, the prevalence of “anxiety-prone” subjects, and Visual Analogue Scale for Anxiety (VASA) response to the 35% CO2 challenge. Females were more likely to meet the operational definition of “anxiety-prone” and to be a “CO2-responder” relative to males. Differences in prevalence of positive response between MZM versus DZM or MZF versus DZF pairs were significant only for the positive response to 35% CO2 stimulation defined at the ∼90th percentile of the VASA scale (DZF > MZF). Liability thresholds could nonetheless be equated across zygosity groups within gender. Age was examined for each twin as an individual given that twins participated at different times and may not have been the same age at the time of study participation. There were no significant differences across the five zygosity groups (F(4, 699) = 0.247, P = .912). Smokers also reported higher post 35% CO2 subjective anxiety compared with nonsmokers (VASA: 12.4 ± 28.8 versus 5.36 ± 22.54, t(680) = 3.56, P = .001), but there were no differences in smoking rates across the five-zygosity groups. Sixteen participants were taking medication or receiving psychotherapy for anxiety-related difficulties. These 16 people also were evenly distributed across the five zygosity groups.
TABLE 1.
Sample demographics and characteristics during a 35%CO2–65%O2 challenge in 346 twin pairs
| Twins as individuals
|
Twins by zygosity group
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total sample | Females | Males | χ2 | MZF | MZM | DZF | DZM | DZOS | χ2 | |
| No. of subjects | 692 | 444 | 248 | — | 220 | 118 | 152 | 58 | 144 | — |
| Age (years ± SD) | 30.95 ± 3.6 | 30.97 ± 3.6 | 30.91 ± 3.6 | — | 30.9 ± 3.8 | 30.9 ± 3.3 | 31.2 ± 3.4 | 31.0 ± 3.7 | 30.9 ± 3.9 | — |
| Percentage of “anxiety-prone” subjectsa | 20.52 | 24.32 | 13.71 | 10.35* | 21.36 | 13.56 | 28.28 | 12.07 | 20.14 | 11.78* |
| Percentage of VASA post-CO2 ∼75th percentile | 24.45 | 28.57 | 17.07 | 10.69* | 23.85 | 16.24 | 32.24 | 15.79 | 27.27 | 12.23* |
| Percentage of VASA post-CO2 ∼90th percentile | 10.16 | 12.69 | 5.65 | 7.89* | 7.8 | 3.39 | 18.42 | 5.17 | 12.59 | 21.12* |
MZF, monozygotic females; MZM, monozygotic males; DZF, dizygotic females; DZM, dizygotic males; DZOS, dizygotic opposite sex.
P < .05.
Anxiety prone subjects as determined by responses to the postal mail questionnaire.
35% CO2 CHALLENGE
Subjects were asked to refrain from alcohol for at least 36 hr, from caffeine-containing beverages for at least 8 hr, from smoking for at least 6 hr, and from eating for at least 2 hr before the challenge. After complete description of exclusion criteria (i.e. cardiocirculatory/respiratory disorders; personal or familial history of aneurysm, hypertension, pregnancy, epilepsy; and history of alcohol, benzodiazepine, or other drug dependence), informed consent was obtained. The Regional Committee for Medical Research Ethics, an authorized agent of the Norwegian Government, approved study procedures.
A 35% CO2–65% O2 single-breath challenge was used. The apparatus, procedures, and method to evoke and rate the anxious response to the challenge are described in details elsewhere.[10] Briefly, two gas mixtures were used including compressed air and a mixture of 35% CO2. Both gases were inspired through the same self-administration mask connected to a Mark 20 Wright respirometer to measure vital capacity (calculated on the basis of the mean of three full inspirations) and the gas volume delivered with each inhalation. Subjects were informed before the challenge that they would inhale two different harmless gas mixtures containing different concentrations of O2 and CO2 and that the breathing task might produce sensations of discomfort ranging from a few physical symptoms to a clear sensation of anxiety or panic. After vital capacity was measured, subjects inhaled one vital capacity of 35% CO2 mixture followed by inhalation of compressed air, with an interval of 30 min between the two inhalations. Thus, the hypercapnic stimulus always preceded regular air inhalation. The 35% CO2 test is considered valid if the subject inhales at least 80% of their vital capacity.[19]
To measure response to CO2 inhalation and regular air, subjects were asked to rate themselves on a 0 (no anxiety at all) to 100 (the worst anxiety ever imaginable) VASA[20] immediately before and after inhalation of the two air mixtures, creating four scores (pre and post 35% CO2 and pre- and postregular air). These scales have been used in several studies of panic provocation[11,19,21,22] with good test–retest reliability.[21]
Pre and post ratings of response to the 35% CO2 challenge exhibited a skewed, L-type distribution.[23] Simulation studies show that in presence of L-shaped distributions, even after attempts to reduce skewness and kurtosis by transformation, there is risk for bias in the estimates based on normal theory maximum likelihood (ML) of genetic, unique environmental, and particularly, shared environmental influences.[24] For this reason, we decided to take a categorical approach to data analysis, which reduces statistical power, but also diminishes bias in parameters’ estimates.[24] It also has been shown that a continuous distribution of latent liability is feasible even in the presence of skewed distributions.[25] Thus, VASA scores pre- and postregular air and 35% CO2 were converted to an ordinal scale (0 = no anxiety, 1 = mild anxiety, or 2 = substantial anxiety) with two thresholds at the 75th and 90th percentile.
STATISTICAL ANALYSES
We used bivariate Cholesky decomposition to address questions regarding the magnitude of genetic and environmental influences between pre and post CO2 anxiety. Two or more traits may be correlated because they share common genes and/or common environmental influences. MZ and DZ twins measured on multiple traits allows for the covariation between traits to be disaggregated into its genetic and environmental components. The Cholesky decomposition is a saturated model that imposes a structure of stratification in shared latent factors.[26] For N phenotypes, there is a main factor that loads on all N, followed by another that only loads on the last N – 1, and so forth, until the Nth factor, which loads only on the last phenotype. The full Cholesky decomposition does not differentiate between common factor and specific factor variance and only estimates a specific factor effect for the last variable in the model.
Figure 1 provides an illustration of the relations between the additive genetic component of twin 1 and twin 2 for phenotype 1 (pre 35% CO2 anxiety) and phenotype 2 (post 35% CO2 anxiety). Correlations between twins for additive genetic factors were fixed at 1 for MZ twin pairs, as they share 100% of their genes, and 0.5 for DZ pairs as they share an average of 50% of their genes identical by descent. Common environment correlations between co-twins were fixed at 1 for both MZ and DZ pairs based on the rigorous and frequent testing that has supported the assumption that environments for MZ and DZ twins are comparable. By definition, nonshared environment is uncorrelated in twins. The expression of new genetic influences is reflected by the square of the “a22” path estimate in Fig. 1. The total additive genetic effect is reflected by the “a21” and “a22” path coefficients and is calculated as (a22 × a22) + (a21 × a21).
Figure 1.
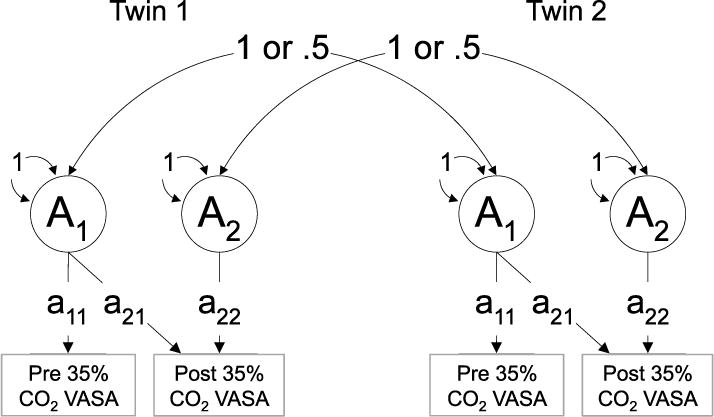
Diagram illustrating additive genetic effects for twin 1 and twin 2. This model also applies to common, shared (C) and unique (E) environmental factors. Path “a21” reflects the additive genetic contribution of the pre-CO2 VASA score on the post-CO2 VASA score whereas path “a22” represents new/unique genetic effects associated with the post-CO2 VASA score.
Including male and female same sex twins allows a test of quantitative sex differences (i.e. whether the magnitudes of genetic and environmental effects differ in males and females) for a specific trait or disorder. Inclusion of opposite sex twins permits a test of qualitative sex differences, which determines whether different genetic and shared environmental effects are important for males and females. To test for quantitative sex effects, the genetic correlation for opposite sex twin pairs (rG) is freely estimated in the model as opposed to being fixed.[24]
For all models of anxiety response to 35% CO2 and regular air, full ACE1 models were tested first; these models included quantitative and qualitative sex effects. The Akaike Information Criterion[27] was used to determine the best fitting model. A lower Akaike Information Criterion (AIC) (i.e. more negative) value represents a better balance between goodness of fit and parsimony. Models with fewer parameters also are preferred if they do not result in a significant deterioration of fit. The best fitting ACE model was then simplified by successively eliminating parameters (i.e. submodels: AE, CE) to determine whether these factors contribute to phenotypic variance/covariance, resulting in improved model fit using the AIC statistic. Genetic modeling was performed using the statistical package Mx[28] using ML estimation.
RESULTS
All subjects inhaled at least 80% of their vital capacity of regular air and 35% CO2 and no response to regular air exceeded the 75th VASA percentile; Table 1 presents VASA scores. Since regular air always followed the 35% CO2 challenge this may have played a role in influencing the degree of anticipatory anxiety before regular air. To control for this possible bias, we examined bivariate correlations between of the VASA response post 35% CO2 and VASA preregular air, while controlling for VASA pre 35% CO2 (since VASA preregular air and VASA pre 35% CO2 were strongly correlated: r = .80, P = .001) both in the whole sample and only among “responders” (i.e. subjects who rated their post 35% CO2 VASA at ≥75th percentile). The correlations were 0.46 in the entire sample and 0.51 in the “responders” sample. After controlling for the role of the VASA prescores, the correlations were .15 and .19, respectively. These results suggest the VASA preregular air is not determined by the VASA post 35%CO2 level.
Results of the bivariate Cholesky decompositions for response to 35% CO2 and regular air are presented in Table 2. For all models, Model I always includes specified pathways from all latent variables (A1, C1, E1 and A2, C2, and E2), allows path coefficients to differ between the sexes (i.e. quantitative sex effects), and freely estimates the genetic correlation (rG; i.e. qualitative sex effects). Model II is the same as model I except rG is fixed to 1 (i.e. no qualitative sex effects). Model III is the same as Model I except that male and female parameters are equated (i.e. no quantitative sex effects). Although Model IV also includes all ACE parameters, male and female parameters are constrained to equality, and the rG is fixed at 1.
TABLE 2.
Results of the bivariate Cholesky decomposition for VASA scores assessed before and after one vital capacity breath of 35% CO2 and again before and after one vital capacity breath of regular air
| Model parameters | Sex effects (Quan/Qual) | −2LL | DF | AIC | ΔAIC |
|---|---|---|---|---|---|
| 35% CO2 | |||||
| I. ACE | +/+ | 1,990.89 | 1,388 | −785.11 | — |
| II. ACE | +/− | 1,990.89 | 1,389 | −787.11 | — |
| III. ACE | +/− | 1,999.61 | 1,395 | −790.39 | — |
| IV. ACE | −/− | 2,002.84 | 1,396 | −789.16 | — |
| Submodels | |||||
| V. AE* | −/− | 2,002.99 | 1,398 | −795.01 | −5.85 |
| VI. CE | −/− | 2,007.62 | 1,398 | −790.38 | −1.22 |
| Regular air | |||||
| I. ACE | +/+ | 1,536.39 | 1,383 | −1,229.61 | — |
| II. ACE | +/− | 1,540.89 | 1,384 | −1,227.11 | — |
| III. ACE | −/+ | 1,550.36 | 1,390 | −1,229.64 | — |
| IV. ACE* | −/− | 1,551.87 | 1,391 | −1,230.13 | — |
| Submodels | |||||
| V. AE | −/− | 1,553.76 | 1,394 | −1,234.24 | −4.11 |
| VI. CE | −/− | 1,553.09 | 1,394 | −1,234.91 | −4.78 |
Indicates best fitting model.
Qual, qualitative sex effects; Quan, quantitative sex effects; “+” indicates the presence of either qualitative or quantitative sex effects or “−” indicates their absence from the model. The AE and CE models are nested submodels of the ACE model.
ANXIETY RESPONSE TO 35% CO2
The model containing qualitative sex effects, but not quantitative sex effects (Model III) and the model that did not include either qualitative or quantitative sex effects (Model IV) yielded similar fit indices. Thus, the more parsimonious model that did not contain either qualitative or quantitative sex effects was selected as best fitting. Next, two submodels (Models V and VI) associated with Model IV were examined. Model V includes the AE parameters only and determines whether dropping the C parameter (i.e. shared environment) results in an improvement or worsening of fit relative to the full ACE model (Model IV). Results indicated that Model V, the AE model, resulted in improved fit based on the AIC (ΔAIC = −5.85), whereas the model that included no genetic effects (CE; Model VI) resulted in only a very modest improvement in model fit (ΔAIC = −1.22).
Figure 2 provides the path coefficients for the best-fitting AE model. The parameter estimates for the pre and post 35% CO2 VASA scores can be calculated from the path coefficients. For the pre 35% CO2 VASA score, a2 was 0.41 (0.64 × 0.64) and e2 was 0.59 (0.77 × 0.77). For post 35% CO2 VASA scores, the total additive genetic contribution (i.e. a2) was 45% ((0.45 × 0.45 + 0.50 × 0.50) × 100), with the unique additive genetics (a2) associated with the post 35% CO2 VASA accounting for almost half of the variation ((0.45 × 0.45) × 100 = 20%) in this outcome and pre 35% CO2 anxiety accounting for the remaining 25% (0.50 × 0.50) × 100). For post 35% CO2 VASA scores, the unique environmental factor (e2) accounted for 55% ((0.65 × 0.65 + 0.36 × 0.36) × 100) of variance. The correlation between the pre and post 35% CO2 VASA scores was 0.60 (0.64 × 0.50 + 0.77 × 0.36).
Figure 2.
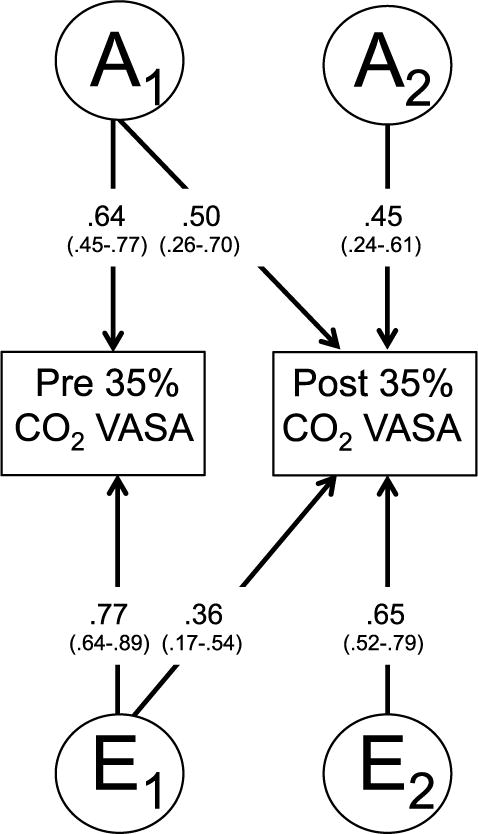
Path estimates from the best-fitting bivariate Cholesky model for pre and post 35% CO2 VASA scores, with 95% confidence intervals in parentheses. “A” represents additive genetic factors; “E” represents nonshared environmental factors.
ANXIETY RESPONSE TO REGULAR AIR
The VASA response to regular air was used as an index of anxiety in absence of CO2 exposure, against which we contrasted the anxious response to 35% CO2 inhalation. The same model-fitting procedure that we used to examine anxiety response to 35% CO2 was used to examine anxiety response to regular air. Models I–IV were the same as those described above. Similar to the previous analysis, Model III, which included qualitative sex effects, but not quantitative sex effects, and Model IV, which did not include either quantitative or qualitative sex effects, yielded similar fit as indexed by the AIC. Thus, we again selected Model IV, which is more parsimonious.
Next, two submodels (Models V and VI) associated with Model VI were examined. Model V includes the AE parameters whereas Model VI includes the CE parameters. Results indicated that Models V and VI yielded very similar AIC values indicating that we are not able to differentiate effects related to familial influences (i.e. A and C). For this reason, we interpret Model IV, which is the full ACE model.
Figure 3 shows the path coefficients for the ACE model for anxiety ratings in response to breathing regular air. Parameter estimates for the pre- and postregular air VASA scores were calculated using the path coefficients. For the preregular air VASA score, a2 was 18% ((0.43 × 0.43) × 100). For postregular air VASA scores, total additive genetic factors accounted for approximately 23% ((0.00 × 0.00 + 0.48 × 0.48) × 100) of the variance, with unique additive genetics associated with the postregular air VASA accounting for no variance in this outcome and preregular air anxiety accounting for 23% ((0.48 × 0.48) × 100). Common and unique environmental factors accounted for the largest portion of variance in ratings of anxiety assessed pre- and postregular air.
Figure 3.
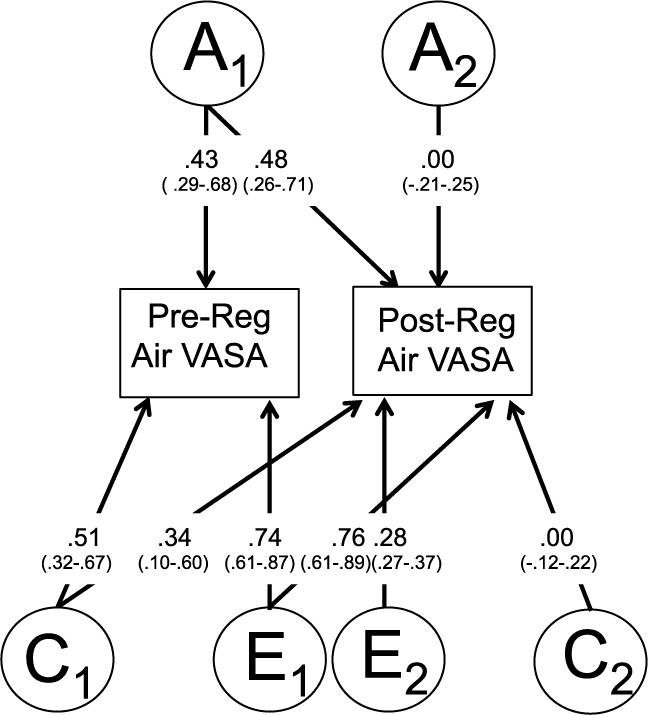
Path estimates from the best-fitting bivariate Cholesky model for pre and post regular room air VASA scores, with 95% confidence intervals in parentheses. “A” represents additive genetic factors; “C” common shared environment; “E” represents nonshared environmental factors.
DISCUSSION
The current study examined whether response to 35% CO2 represents a distinct genetic reaction, or is merely a function of an individual’s trait anxiety response, or is the product of both distinct CO2-induced anxiety and trait anxiety. Using a general population twin sample, we observed that total additive genetics associated with trait anxiety accounted for significant variance (i.e. 45%) in response to 35% CO2 and additive genetics specifically associated with CO2 precipitated anxiety explained significant incremental variance (i.e. 20%), suggesting the expression of new genetic effects. This finding is particularly impressive when compared with the results of response to regular air where only additive genetics associated with pre-CO2 anxiety predicted a significant amount of variance (i.e. 23%) whereas additive genetics specifically associated with postregular air rated anxiety did not account for any incremental variance. These results suggest that a significant amount of variation in anxious response to 35% CO2 is accounted for by a unique genetic liability that is not explained by trait anxiety.
This novel finding supports CO2 hypersensitivity as a biologic marker that taps into a pathophysiological mechanism distinct from that associated with pre-CO2 anxiety and dovetails with the extended suffocation alarm theory.[18,29] That is, our results suggest that respiratory stimulation via CO2 is associated with a unique genetic factor similar to Klein who maintains that the panic response is distinct from the general “flight or fight” fear response. Our results also are interesting in the context of the childhood separation anxiety disorder (SAD)—adult PD link[30,31] as both conditions are associated with heightened hypersensitivity to inhalation of CO2[32,33] and may reflect maladaptive conditions stemming from the same pathogenic process (e.g. opioidergic dysfunction[29]). For this reason, genetically informative developmental studies as well as studies using animal models may prove helpful in the study of the childhood separation anxiety, panic, and hypersensitivity to CO2.[34]
There also was a significant effect for individual-specific environment. In a previous analysis of this dataset, response to 35% CO2 was found to be influenced by a number of environmental factors including childhood parental loss, childhood separation anxiety, major life events, stressful life events, events of suffocative nature, and being female.[35] The significant role of nonshared environmental experiences stresses the importance of identifying putative environmental risk factors that may predispose individuals to development of PD.
For models examining anxious response to 35% CO2, the model containing additive genetic and unique environmental factors (AE) fit the data better relative to a model containing only common and unique environmental (CE) factors, but the difference between these two models was not substantial. This suggests that our study lacked sufficient power to unequivocally determine the contribution of common environmental influences on anxious response to 35% CO2.
Our results may be most supportive of psychosocial treatments that contain a specific focus on panic related anxiety versus a general anxiety focus. For example, panic control therapy,[36] which includes a therapeutic component that targets respiratory aberrations associated with panic anxiety, may yield greater improvement in panic symptomotology compared with the unified approach to targeting emotional disorders, which concentrates on negative emotionality and associated psychological difficulties as a singular psychological entity.[37] This is merely conjecture and empirical support is needed.
To our knowledge, this is the first study to examine the specificity of genetic response to CO2 as compared to general trait anxiety. Nonetheless, several limitations should be considered. Our sample is not fully representative of the general population because anxiety-prone individuals were intentionally oversampled, and the “anxiety-proneness” status was associated with a stronger response to CO2 (Wald |2 = 24.91; P = .001, Exp. B = 1.02,[10]). However, after carrying out a multivariate genetic analyses jointly with the “anxiety prone” screening variable, which was available for the entire sample of 3,334 complete twin pairs in the NIPHTP-MHS from which this sample was collected, we found that this ascertainment bias had a relatively small impact upon the reliability of structural equation modeling (SEM) calculations for CO2 responses.[10,12] Also, although this is probably the largest sample ever probed with the 35% CO2 challenge, the use of categorical outcomes coupled with the low prevalence of heightened response weakens the power of this study. Moreover, as noted previously,[10] the possible effect of concurrent therapy in twins was not controlled for in our analyses. Fortunately, only a very small percentage (i.e. 2.3%) of participants was in treatment and the number of persons receiving treatment was evenly distributed across the five zygosity groups. Finally, there is only limited support for the reliability of subjective anxiety response to the 35% CO2 challenge[21,38] and, therefore, a proportion of variance accounted for by environmental factors may be related to measurement error, which contributes to an underestimate of heritability/genetic factors.
Acknowledgments
Preparation of this manuscript was supported by grant K01-MH-080953 from the National Institutes of Health/National Institute of Mental Health to the first author (RRN). The research study was supported by the National Alliance for Research in Schizophrenia and Depression (NARSAD), the Norwegian Foundation of Health and Rehabilitation, the Anna Villa and Felice Rusconi Foundation, and the LiberaMente Association; the Norwegian NIPH Study of Mental Health was supported by the Norwegian Research Council, The Foundation of Borderline Research, and The European Commission under the program “Quality of Life and Management of the Living Resources” of the 5th Framework Program (no. QLG2-CT-2002-01254; M.B.).
Footnotes
A = additive genetics; C = common (shared) environment; E = unique (non-shared) environment.
References
- 1.Perna G, Barbini B, Cocchi S, Bertani A, Gasperini M. 35% CO2 challenge in panic and mood disorders. J Affect Disord. 1995;33:189–194. doi: 10.1016/0165-0327(94)00088-q. [DOI] [PubMed] [Google Scholar]
- 2.Rassovsky Y, Kushner MG. Carbon dioxide in the study of panic disorder: issues of definition, methodology, and outcome. J Anxiety Disord. 2003;17:1–32. doi: 10.1016/s0887-6185(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 3.Perna G, Barbini B, Cocchi S, Bertani A, Gasperini M. 35% CO2 challenge in panic and mood disorders. J Affect Disord. 1995;33:189–194. doi: 10.1016/0165-0327(94)00088-q. [DOI] [PubMed] [Google Scholar]
- 4.Perna G, Bertani A, Arancio C, Ronchi P, Bellodi L. Laboratory response of patients with panic and obsessive-compulsive disorders to 35% CO2 challenges. Am J Psychiatry. 1995;152:82–89. doi: 10.1176/ajp.152.1.85. [DOI] [PubMed] [Google Scholar]
- 5.Verburg C, Griez E, Meijer J. A 35% carbon dioxide challenge in simple phobias. Acta Psychiatr Scand. 1994;90:420–423. doi: 10.1111/j.1600-0447.1994.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 6.Griez E, de Loof C, Pols H, Zandbergen J, Lousberg H. Specific sensitivity of patients with panic attacks to carbon dioxide inhalation. Psychiatry Res. 1990;31:193–199. doi: 10.1016/0165-1781(90)90121-k. [DOI] [PubMed] [Google Scholar]
- 7.Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry. 1993;150:1149–1157. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- 8.Coryell W, Fyer A, Pine D, Martinez J, Arndt S. Aberrant respiratory sensitivity to CO(2) as a trait of familial panic disorder. Biol Psychiatry. 2001;49:582–587. doi: 10.1016/s0006-3223(00)01089-1. [DOI] [PubMed] [Google Scholar]
- 9.Coryell W, Pine D, Fyer A, Klein D. Anxiety responses to CO2 inhalation in subjects at high-risk for panic disorder. J Affect Disord. 2006;92:63–70. doi: 10.1016/j.jad.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 10.Battaglia M, Ogliari A, Harris J, et al. A genetic study of the acute anxious response to carbon dioxide stimulation inman. J Psychiatr Res. 2007;41:906–917. doi: 10.1016/j.jpsychires.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Bellodi L, Perna G, Caldirola D, Arancio C, Bertani A, Di Bella D. CO2-induced panic attacks: a twin study. Am J Psychiatry. 1998;155:1184–1188. doi: 10.1176/ajp.155.9.1184. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia M, Pesenti-Gritti P, Spatola CA, Ogliari A, Tambs K. A twin study of the common vulnerability between heightened sensitivity to hypercapnia and panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:586–593. doi: 10.1002/ajmg.b.30647. [DOI] [PubMed] [Google Scholar]
- 13.Battaglia M, Pesenti-Gritti P, Medland SE, Ogliari A, Tambs K, Spatola CA. A genetically informed study of the association between childhood separation anxiety, sensitivity to CO(2), panic disorder, and the effect of childhood parental loss. Arch Gen Psychiatry. 2009;66:64–71. doi: 10.1001/archgenpsychiatry.2008.513. [DOI] [PubMed] [Google Scholar]
- 14.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 15.Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health Twin Panel: a description of the sample and program of research. Twin Res. 2002;5:415–423. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- 16.Gorman JM, Papp LA, Martinez J, et al. High-dose carbon dioxide challenge test in anxiety disorder patients. Biol Psychiatry. 1990;28:743–757. doi: 10.1016/0006-3223(90)90510-9. [DOI] [PubMed] [Google Scholar]
- 17.Griez E, Zandbergen J, Pols H, de Loof C. Response to 35% CO2 as a marker of panic in severe anxiety. Am J Psychiatry. 1990;147:796–797. doi: 10.1176/ajp.147.6.796. [DOI] [PubMed] [Google Scholar]
- 18.Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- 19.Griez E, van den Hout MA. CO2 inhalation in the treatment of panic attacks. Behav Res Ther. 1986;24:145–150. doi: 10.1016/0005-7967(86)90085-9. [DOI] [PubMed] [Google Scholar]
- 20.Wolpe J. The Practice of Behavior Therapy. Elmsford, NY: Pergamon Press Inc; 1973. [Google Scholar]
- 21.Battaglia M, Bertella S, Ogliari A, Bellodi L, Smeraldi E. Modulation by muscarinic antagonists of the response to carbon dioxide challenge in panic disorder. Arch Gen Psychiatry. 2001;58:114–119. doi: 10.1001/archpsyc.58.2.114. [DOI] [PubMed] [Google Scholar]
- 22.Coryell W, Arndt S. The 35% CO2 inhalation procedure: test-retest reliability. Biol Psychiatry. 1999;45:923–927. doi: 10.1016/s0006-3223(98)00241-8. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia M, Perna G. The 35% CO2 challenge in panic disorder: optimization by receiver operating characteristic (ROC) analysis. J Psychiatr Res. 1995;29:111–119. doi: 10.1016/0022-3956(94)00045-s. [DOI] [PubMed] [Google Scholar]
- 24.Derks EM, Hudziak JJ, van Beijsterveldt CE, Dolan CV, Boomsma DI. A study of genetic and environmental influences on maternal and paternal CBCL syndrome scores in a large sample of 3-year-old Dutch twins. Behav Genet. 2004;34:571–583. doi: 10.1007/s10519-004-5585-2. [DOI] [PubMed] [Google Scholar]
- 25.van den Oord EJ, Pickles A, Waldman ID. Normal variation and abnormality: an empirical study of the liability distributions underlying depression and delinquency. J Child Psychol Psychiatry. 2003;44:180–192. doi: 10.1111/1469-7610.00112. [DOI] [PubMed] [Google Scholar]
- 26.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dodrecht, the Netherlands: Klüver; 1992. [Google Scholar]
- 27.Akaike H. Factor-analysis and AIC. Psychometrica. 1987;52:317–332. [Google Scholar]
- 28.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. Richmond, VA: Virginia Commonwealth University, Medical College of Virginia; 2003. [Google Scholar]
- 29.Preter M, Klein DF. Panic, suffocation false alarms, separation anxiety and endogenous opioids. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:603–612. doi: 10.1016/j.pnpbp.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein RG. Is panic disorder associated with childhood separation anxiety disorder? Clin Neuropharmacol. 1995;18:7–14. [Google Scholar]
- 31.Aschenbrand SG, Kendall PC, Webb A, Safford SM, Flannery-Schroeder E. Is childhood separation anxiety disorder a predictor of adult panic disorder and agoraphobia? A seven-year longitudinal study. J Am Acad Child Adolesc Psychiatry. 2003;42:1478–1485. doi: 10.1097/00004583-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Pine DS, Klein RG, Roberson-Nay R, et al. Response to 5% carbon dioxide in children and adolescents: relationship to panic disorder in parents and anxiety disorders in subjects. Arch Gen Psychiatry. 2005;62:73–80. doi: 10.1001/archpsyc.62.1.73. [DOI] [PubMed] [Google Scholar]
- 33.Roberson-Nay R, Klein DF, Klein RG, et al. Carbon dioxide hypersensitivity in separation-anxious offspring of parents with panic disorder. Biol Psychiatry. 2010;67:1171–1177. doi: 10.1016/j.biopsych.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Amato FR, Zanettini C, Lampis V, et al. Unstable maternal environment, separation anxiety, and heightened CO2 sensitivity induced by gene-by-environment interplay. PLoS One. 2011;6:e18637. doi: 10.1371/journal.pone.0018637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogliari A, Tambs K, Harris JR, et al. The relationships between adverse events, early antecedents, and carbon dioxide reactivity as an intermediate phenotype of panic disorder: a general population study. Psychother Psychosom. 2010;79:48–55. doi: 10.1159/000259417. [DOI] [PubMed] [Google Scholar]
- 36.Verburg K, Pols H, de Leeuw M, Griez E. Reliability of the 35% carbon dioxide panic provocation challenge. Psychiatry Res. 1998;78:207–214. doi: 10.1016/s0165-1781(98)00009-2. [DOI] [PubMed] [Google Scholar]
- 37.Turovsky J, Barlow DH. Albany panic control treatment (PCT) for panic disorder and agoraphobia. Clin Psychologist. 1995;48:5–6. [Google Scholar]
- 38.Barlow DH, Allen LB, Choate ML. Toward a unified treatment of emotional disorders. Behav Ther. 2004;35:205–230. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]


