Abstract
Halogenated diarylacetylenes that possess fluorine or chlorine substituents in one aryl ring and N-methylamino or N,N-dimethylamino in the other aryl ring inhibit the proliferation of LS174T colon cancer cells through the repression of c-myc expression and induction of the cyclin-dependent kinase inhibitor-1 (i.e., p21(Wif1/Cip1)) and represent potentially useful antineoplastic agents.
In the course of developing new agents for the treatment of colorectal cancer, we identified a family of fluorinated N,N- dialkylaminostilbene analogs (FIDAS agents) that inhibit the expression of Wnt target genes, such as c-myc1, and repress colon cancer cell growth in vitro and in vivo2–4. Recently, we found that (E)-4-(2′,6′-difluorostyryl)-N,N-dimethylaniline (1) (Fig. 1) targeted exclusively the catalytic subunit3 of methionine S-adenosyltransferase-2 (MAT-2) that serves as a source of S-adenosylmethionine (SAM) in colorectal and liver cancers where MAT-2 is upregulated5–8. Presumably, neoplastic tissues make effective use of SAM from this isoform of MAT to manage crucial epigenetic modifications of histone proteins and thereby regulate gene expression. Interference with this process represented a new approach for developing potential antineoplastic agents, and consequently, we explored related compounds that might alter c-myc expression. In addition, we sought compounds that would avoid the facile E/Z-isomerizations that afflict the stilbenes and that complicate pharmacodynamic and pharmacokinetic studies.
Figure 1. Synthesis of halogenated diarylacetylenes 2.
Reagents: a, HC≡CHC6H4Y; 0.5% Pd(PPh3)4, 1% CuI, H2O, 75°C, 1–2 h; b, a, HC≡CHC6H4NH2; 0.5% Pd(PPh3)4, 1% CuI, H2O, 75°C, 1–2 h followed by CH3I, K2CO3, acetone, 5 h, 56°C.
Among the possible analogs that would avoid this isomerization problem, the diarylacetylenes 2 (Fig. 1) were an obvious choice. The only prior reports of acetylenic compounds as antineoplastic agents include monoalkylacetylenes from aquatic organisms9 and diarylacetylenic analogs of combrestatin10. The latter compounds showed cytotoxic activity against a murine leukemia cell line and one showed activity as an inhibitor of tubulin polymerization. The Sonogashira coupling11,12 of 4-(N,N-dimethylamino) phenylacetylene with various aryl iodides provided access to the desired diarylacetylenes 2 (Table 1). Prior work from our laboratories established that stilbenes with N-methylamino and N,N-dimethylamino groups in a para-orientation relative to the central double bond as well as 2,6-difluoro, 2-chloro-6-fluoro or 2,6-dichloro halogenation patterns in the other aromatic ring were the most potent analogs in the repression of c-myc expression and colon cancer cell LS174T proliferation2.
Table 1.
Halogenated N,N-diarylacetylenes 2 and their IC50 values in the inhibition of LS174T cell proliferation.
| Compound | X1 | X2 | X3 | X4 | X5 | Y | Inhibition of LS174T Cell Proliferation IC50 (nM) |
|---|---|---|---|---|---|---|---|
| 1 | F | F | N(CH3)2 | 59±7.5 | |||
| 2a | F | F | NH2 | 55±7.8 | |||
| 2b | F | F | NHCH3 | 23±10.3 | |||
| 2c | N(CH3)2 | >3000 | |||||
| 2d | F | N(CH3)2 | 39±6.0 | ||||
| 2e | Cl | N(CH3)2 | 31±3.1 | ||||
| 2f | F | N(CH3)2 | >3000 | ||||
| 2g | F | N(CH3)2 | >3000 | ||||
| 2h | F | F | N(CH3)2 | 119±4.6 | |||
| 2i | F | F | N(CH3)2 | 56±8.1 | |||
| 2j | F | F | N(CH3)2 | >3000 | |||
| 2k | F | F | N(CH3)2 | >3000 | |||
| 2l | F | F | N(CH3)2 | 55±6.0 | |||
| 2m | F | F | N(CH3)2 | 23±6.0 | |||
| 2n | F | Cl | N(CH3)2 | 19±5.0 | |||
| 2o | Cl | Cl | N(CH3)2 | 52±7.1 |
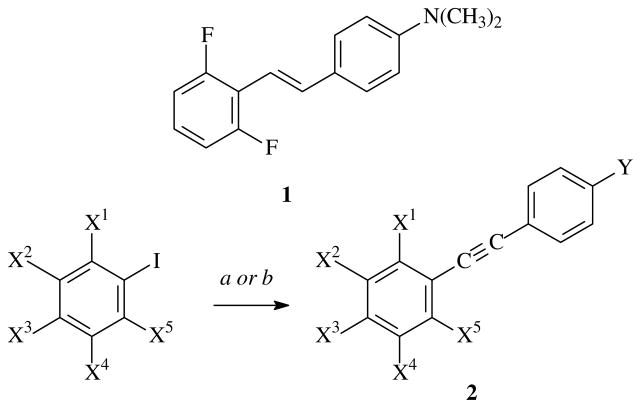
As a consequence, we limited SAR studies to those diarylacetylenes 2 that possessed fluorine or chlorine substituents in one aryl ring and N-methylamino or N,N-dimethylamino in the other aryl ring. We reported previously that stilbenes repressed colon cancer cell proliferation by inhibiting c-myc expression and inducing the cell cycle inhibitor, p21(Wif1/Cip1)2. The similarity of the diarylacetylenes to the stilbenes prompted an in silico modeling study of the binding of (E)-4-(2′,6′-difluorostyryl)-N,N-dimethylaniline (1) and 4-((2,6-difluorophenyl)ethynyl)-N,N-dimethylaniline (2m). Using a computationally constructed open-state model of the homodimer of MAT2A (see Supplementary Data for details), we observed that 1 and 2m bound to the same active site (Fig. 2) and that the diarylacetylene 2m inhibited MAT2 at concentrations comparable to that of the stilbene 1 (data not shown). Hydrophobic residues I139, F272, I274, and I344 define the boundaries of the binding pocket, and van der Waals contact between F272 and the halogen substituents accounted for the increased activity of ortho-halogens in 1 or 2m over the corresponding analogs with meta- or para-halogens. In addition, a cleft formed by residues K203, S269, I274, and D280 accommodated the shape of these particular ligands (Fig. 2).
Figure 2. Docked structures of MAT2A binding with stilbene 1 (Panel A) and diarylacetylene 2m (Panel B).
The letters A and B following the amino acid designations refer to the individual oligomers in the MAT2A homodimer.
Variability in the MAT2A inhibition assay made the measurement of the levels of c-myc expression a preferred analytical tool for assessing the potency of diarylacetylenes. We tested the effect of these diarylacetylenes 2 on the proliferation of LS174T colon cancer cells. The expression of c-myc and p21(Wif1/Cip1) was analyzed by western blotting (Fig. 3). The most active diarylacetylenes 2 inhibited c-myc expression at 1 μM concentrations and as expected, induced p21(wif1/Cip1) at the same time. Consistent with prior results in the stilbene family, the diarylacetylenes 2 lacking halogen substituents (e.g., 2c) or possessing only one fluorine substituent at a meta-position relative to the acetylenic linkage (e.g., 2f) had very low potency (Table 1). Diarylacetylenes with one or two halogen substituents at ortho-positions relative to the acetylenic linkage (e.g., 2b, 2d, 2e, 2m and 2n) possessed potencies as inhibitors of LS174T cell proliferation that exceeded that of the related stilbene 1 with IC50 values less than 50 nM (Table 1). Isomers of these diarylacetylenes (e.g., 2f, 2g, and 2j) with halogens in meta- or para-positions were significantly less active than the diarylacetylenes with ortho-halogens. Once again, these results are in consistent with the SAR findings in the stilbene family that also repress c-myc expression2. Finally, the N-methylation pattern in the diarylacetylenes suggested that N-methyl and N,N-dimethylaniline subunits led to equipotent repression of c-myc expression (i.e., IC50 of 2b ≈ IC50 of 2m) but the desmethyl analog was considerably less active (IC50 of 2a = 55±7.8 nm).
Figure 3. Repression of c-myc expression and induction of p21(Wif1/Cip1) by diarylacetylenes 2 in colon cancer cells.
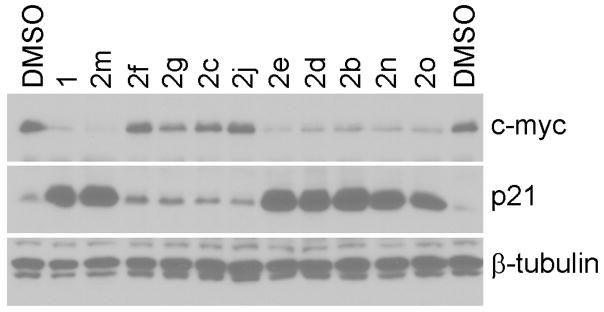
LS174T cells were treated with 1 μM of each diarylacetylenes 2 for 36 h. DMSO and 1 were used as control. Cell lysates were analyzed by western blotting with β-tubulin as a loading control.
In summary, diarylacetylenes 2 have a dramatic effect on the proliferation of LS174T colon cancer cells by altering the expression of c-myc and thereby inducing p21(Wif1/Cip1). These results are consistent with similar findings using halogenated stilbenes2 and suggest that diarylacetylenes and stilbenes repress colon cancer proliferation through similar mechanisms. Examining this question in detail is the subject of on-going investigations.
Supplementary Material
Acknowledgments
CL and DSW were supported by R21 CA139359 and CA172379 from the NIH. CGZ was supported by CHE-1111761 from the NSF. DSW was supported by the Office of the Dean of the College of Medicine and by NIH Grant Number P20 RR020171 from the National Institute of General Medical Sciences to L. Hersh, PI. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. Clin Cancer Res. 2012;18:5546. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Sviripa V, Kril LM, Chen X, Yu T, Shi J, Rychahou P, Evers BM, Watt DS, Liu C. J Med Chem. 2011;54:1288. doi: 10.1021/jm101248v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Sviripa V, Chen X, Shi J, Yu T, Hamza A, Ward ND, Kril LM, Vander Kooi CW, Zhan CG, Evers BM, Watt DS, Liu C. ACS Chem Biol. 2013;8:796. doi: 10.1021/cb3005353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watt D, Liu C, Sviripa VM, Zhang W. 8,664,276. US Patent. 2014 Mar 4;
- 5.Cai J, Sun WM, Hwang JJ, Stain SC, Lu SC. Hepatology. 1996;24:1090. doi: 10.1002/hep.510240519. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Xia M, Lin M, Yang H, Kuhlenkamp J, Li T, Sodir NM, Chen YH, Josef-Lenz H, Laird PW, Clarke S, Mato JM, Lu SC. Gastroenterology. 2007;133:207. doi: 10.1053/j.gastro.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 7.Ito K, Ikeda S, Kojima N, Miura M, Shimizu-Saito K, Yamaguchi I, Katsuyama I, Sanada K, Iwai T, Senoo H, Horikawa S. Surg Today. 2000;30:706. doi: 10.1007/s005950070081. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q, Chen J, Liu L, Zhang J, Wang D, Ma L, He Y, Liu Y, Liu Z, Wu J. J Biol Chem. 2011;286:17168. doi: 10.1074/jbc.M110.167783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dembitsky VM, Levitsky DO, Gloriozova TA, Poroikov VV. Nat Prod Commun. 2006;1:773. [Google Scholar]
- 10.Hadfield JA, McGown AT. Synth Commun. 1998;28:1421. [Google Scholar]
- 11.Bhattacharya S, Sengupta S. Tetrahedron Lett. 2004;47:8733. [Google Scholar]
- 12.Okuro K, Furuune M, Enna M, Miura M, Nomura M. J Org Chem. 1993;58:4716. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.