Abstract
The paper by Nielsen et al in this journal reports the prevalence of dyspnea in 15 countries throughout the world as 27%. Dyspnea is a powerfully aversive sensation frequently overlooked despite its prevalence and the severity of distress it causes. Despite its ‘subjective’ nature, dyspnea is a powerful predictor of morbidity and mortality. We suggest that this is because the information provided by enteroceptors is so rich that it is as valuable as the more precise but relatively sparse information provided by clinical tests. Relatively simple measures of dyspnea, such as the Medical Research Council Breathlessness Scale used by Nielsen et al, can provide meaningful information at very little cost.
Dyspnea is defined as “breathing discomfort” [1]. The paper by Nielsen et al in this journal reports the prevalence of dyspnea in 15 countries throughout the world. There are few studies available of dyspnea in the general population, so this added information is indeed welcome. The overall prevalence of dyspnea in their study population was 27% - not far out of line with several other studies dating from 1964 to the present [2-4]. Some studies, resting on review of medical records, have reported much lower prevalence [5] – but patients may neglect to report dyspnea to their clinician because they think it does not reach a sufficient level of importance, or clinicians may fail to record what their patients say. Requiring patients to scale their symptoms can result in more uniform reporting because the patient does not have to decide how much is reportable, it takes very little time, and it facilitates uniform documentation. Busy clinicians may ask “is dyspnea worth documenting?” One powerful argument for routine assessment is the need to reduce suffering, as with pain. A second argument that is emerging is the predictive value of dyspnea in forecasting medical needs.
In addition to being prevalent, ‘dyspnea’ is a powerfully aversive sensation [e.g., 6], and patients deserve adequate management of this symptom, as passionately argued by Currow et al [7]. Effective interventions exist, and should be used [8-10]; assessing dyspnea is the first step in managing it. Dyspnea is frequently overlooked despite its prevalence and the severity of distress it causes [11]; for example, a majority of advanced cancer patients having dyspnea for months had not received any treatment for it [12]. Historically, funding for dyspnea research is a small fraction of funding for pain research, and the field is, not surprisingly, behind [13]. Yet in recent years, real progress has been made in understanding dyspnea – see, for example the report of a recent experts meeting in this issue of the Journal [14]. A great deal remains to be learned, but we now have a much better understanding of the neurophysiology underlying dyspnea, including several studies of brain activity [reviewed by 15].
Dyspnea is often dismissed as merely ‘subjective’ in contrast to the increasingly relied upon high-tech measurements that are assumed to yield more valuable ‘objective’ data. We know there is a wide variation among patients in the degree of discomfort reported for apparently similar objective pathophysiological impairment. Although large studies show a statistically significant relationship between dyspnea and airway obstruction (FEV1), a scatter plot of the data reveals a huge variance among patients; there are many patients with severe airways obstruction who report no dyspnea, and many others who report severe dyspnea without correspondingly severe pathophysiology [16]. Indeed, Nielsen found that a model incorporating about 20 demographic and clinical variables, including lung function measurements, explained only 13% of the individual variation in dyspnea. Other studies have reported weak correlation of dyspnea with objective measures such as FEV1 in COPD [17] and hemodynamic measures in heart failure [18]. Some of the variation in the dyspnea-pathophysiology relationship reflects differences in how individuals experience discomfort, some reflects differences in how individuals choose to report the discomfort they experience. One must also consider, however, the possibility that an important part of the variation in the relationship between dyspnea and pathophysiology reflects the inability of our ‘objective’ measures to accurately assess the most important features of pathophysiology. The body has been equipped by evolution with thousands of enteroceptors to detect problems in the crucial systems that support the gas exchange essential to life. When these enteroceptors detect malfunctioning gas transport systems, the message reaches consciousness as dyspnea. Perhaps, despite the degradation of information in the pathway from enteroceptors through conscious perception to patient report, the information from enteroceptors is so rich that even the degraded information is as valuable as the relatively sparse information available from “objective” clinical tests.
Some interesting outcome studies suggest that patient-reported dyspnea is indeed valuable information. In COPD patients, dyspnea severity was a much stronger predictor of 5-year mortality than FEV1 [19] (See Figure 1); dyspnea predicted cardiac death better than angina in patients with suspected cardiac disease [20]; dyspnea was a stronger predictor of mortality than gastrointestinal symptoms in esophageal and gastric cancer patients [21, 22]; and dyspnea was a strong predictor of all-cause mortality in more general populations [4, 23, 24]. A growing body of evidence suggests that routine measurement and documentation of dyspnea would have clinical value disproportionate to the minimal effort needed to obtain the data. Our group has shown that routine nursing measurement of dyspnea in hospitalized patients is feasible, and that it may provide useful risk prediction [25-27]
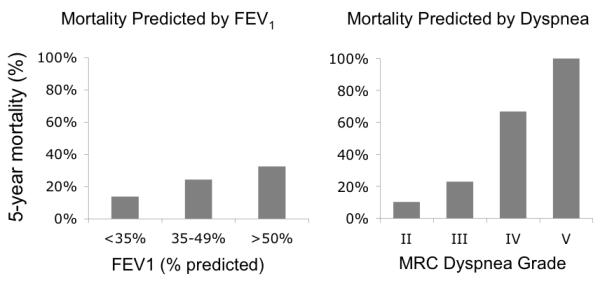
The left panel shows 5-year mortality rates of patients classified by pulmonary function test according to the ATS guidelines: Stage I, FEV1 > 50% of predicted; Stage II FEV1 = 35 to 49% of predicted; and Stage III, FEV < 35% of predicted. The right panel shows 5-year mortality of patients classified by dyspnea grade as measured with the Medical Research Council Breathlessness Scale (MRC): Grade II (short of breath when hurrying on the level or walking up a slight hill); Grade III (have to walk slower than most people on the level); Grade IV (have to stop for breath after walking about 100 yards (or after a few minutes) on the level); Grade V (too breathless to leave the house, or breathless after undressing). Data from Nishimura et al [19].
What is the best way to measure dyspnea? The only clear answer is that using some form of quantitative scale is more useful than the yes/no approach. Nielsen et al utilized a form of the ubiquitous MRC Breathlessness Scale (‘Breathlessness’ is most commonly used in this context in British English; ‘Shortness of Breath’ is more common in the USA; and the word ‘Dyspn(o)ea’ is not understood by most patients). The MRC scale was devised more than half a century ago to assess lung disease in coalminers, but has been widely used in many contexts [28]. The MRC scales dyspnea by asking which activities, ranging from vigorous exercise to minimal activities of daily living, are limited by dyspnea. The MRC has shown good utility in many studies. A major drawback of the MRC in some populations is the lack of a scale point for patients who experience dyspnea at rest, but it is easy to extend the scale to ask about dyspnea at rest. Several lengthier scales are also available [29, 30]. However, scales such as the MRC are indirect – they do not actually ask the patient how much dyspnea they experience. There are various scales used to assess dyspnea directly, including single-dimension scales of respiratory discomfort, and multidimensional scales that assess discomfort, modality of sensation, and emotional response [31-33]. The instrument used should suit the situation, for instance brevity may be more important than completeness for routine clinical use, and different scales may be appropriate for outpatients vs inpatients. But even a simple measurement is better than no measurement.
Nielsen et al have used mutually standardized measures to extend the observation of dyspnea prevalence and variance across several cultures and language groups. Their study found important differences in dyspnea reports among the 15 countries sampled, and between men and women across countries. We don’t know if these differences really reflect variation in pathophysiology that might be measured by hard outcomes such as morbidity and mortality, or whether they reflect differences in reporting rooted in gender, culture, language, etc. Such questions can be addressed, both in reductionist studies using highly controlled laboratory models of dyspnea, and in prospective population studies looking at hard outcomes.
Although dyspnea measures have been shown to be useful predictors at a population level (potentially enabling better management of health care resources), better understanding of the variation in reporting among individuals and among groups of individuals is needed to refine the use of dyspnea assessment at the individual prognostic level. Routine measurement in primary care and during hospitalization may help in overcoming uncertainty introduced by inter-individual variation – an individual’s dyspnea history is therefore likely to be more helpful than a snapshot in time. Despite the many still unanswered questions about dyspnea, we already know that simple measures in individual patients can be useful in tracking disease progress or treatment efficacy, can usefully supplement objective measures in diagnosis and prognosis, and are essential in targeting individual symptom management.
Take home message.
Dyspnea is easy to measure, prevalent, and predicts risk of mortality and morbidity. We urge simple quantitative dyspnea assessment in all patients.
Acknowledgments
Supported by NIH grants NR10006 and NR12009
References
- 1.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O’Donnell DE. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammond E. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. American Journal of Public Health. 1964;54(1):11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy [see comments] Arch Intern Med. 1990;150(8):1685–1689. doi: 10.1001/archinte.150.8.1685. [DOI] [PubMed] [Google Scholar]
- 4.Frostad A, Soyseth V, Andersen A, Gulsvik A. Respiratory symptoms as predictors of all-cause mortality in an urban community: a 30-year follow-up. J Intern Med. 2006;259(5):520–529. doi: 10.1111/j.1365-2796.2006.01631.x. [DOI] [PubMed] [Google Scholar]
- 5.Kroenke K, Mangelsdorff AD. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med. 1989;86(3):262–266. doi: 10.1016/0002-9343(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 6.O’Driscoll M, Corner J, Bailey C. The experience of breathlessness in lung cancer. Eur J Cancer Care (Engl) 1999;8(1):37–43. doi: 10.1046/j.1365-2354.1999.00129.x. [DOI] [PubMed] [Google Scholar]
- 7.Currow DC, Abernethy AP, Ko DN. The active identification and management of chronic refractory breathlessness is a human right. Thorax. 2014;69(4):393–394. doi: 10.1136/thoraxjnl-2013-204701. [DOI] [PubMed] [Google Scholar]
- 8.Currow D, Johnson M, White P, Abernethy A. Evidence-based intervention for chronic refractory breathlessness: practical therapies that make a difference. Br J Gen Pract. 2013;63(616):609–610. doi: 10.3399/bjgp13X674611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bausewein C, Booth S, Gysels M, Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2008;(2):CD005623. doi: 10.1002/14651858.CD005623.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Booth S, Farquhar M, Gysels M, Bausewein C, Higginson IJ. The impact of a breathlessness intervention service (BIS) on the lives of patients with intractable dyspnea: a qualitative phase 1 study. Palliat Support Care. 2006;4(3):287–293. doi: 10.1017/s1478951506060366. [DOI] [PubMed] [Google Scholar]
- 11.Gysels M, Higginson IJ. Access to services for patients with chronic obstructive pulmonary disease: the invisibility of breathlessness. J Pain Symptom Manage. 2008;36(5):451–460. doi: 10.1016/j.jpainsymman.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Roberts DK, Thorne SE, Pearson C. The experience of dyspnea in late-stage cancer. Patients’ and nurses’ perspectives. Cancer Nurs. 1993;16(4):310–320. [PubMed] [Google Scholar]
- 13.Max MB. How to move pain and symptom research from the margin to the mainstream. J Pain. 2003;4(7):355–360. doi: 10.1016/s1526-5900(03)00719-3. [DOI] [PubMed] [Google Scholar]
- 14.Laviolette L, Laveneziana P. Dyspnoea: A Multidimensional and Multidisciplinary Approach. Eur Respir J. 2014 doi: 10.1183/09031936.00092613. In Press. [DOI] [PubMed] [Google Scholar]
- 15.Evans KE, Banzett RB. Neuroimaging of Dyspnea. In: Mahler DA, O’Donnell DE, editors. Dyspnea: Mechanisms, Measurement, and Management. 3 rd ed CRC Press; 2014. p. 256. [Google Scholar]
- 16.Mullerova H, Lu C, Li H, Tabberer M. Prevalence and burden of breathlessness in patients with chronic obstructive pulmonary disease managed in primary care. PLoS One. 2014;9(1):e85540. doi: 10.1371/journal.pone.0085540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guglin M, Patel T, Darbinyan N. Symptoms in heart failure correlate poorly with objective haemodynamic parameters. Int J Clin Pract. 2012;66(12):1224–1229. doi: 10.1111/j.1742-1241.2012.03003.x. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 20.Abidov A, Rozanski A, Hachamovitch R, Hayes SW, Aboul-Enein F, Cohen I, Friedman JD, Germano G, Berman DS. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med. 2005;353(18):1889–1898. doi: 10.1056/NEJMoa042741. [DOI] [PubMed] [Google Scholar]
- 21.Djarv T, Metcalfe C, Avery KN, Lagergren P, Blazeby JM. Prognostic value of changes in health-related quality of life scores during curative treatment for esophagogastric cancer. J Clin Oncol. 2010;28(10):1666–1670. doi: 10.1200/JCO.2009.23.5143. [DOI] [PubMed] [Google Scholar]
- 22.Healy LA, Ryan AM, Moore J, Rowley S, Ravi N, Byrne PJ, Reynolds JV. Health-related quality of life assessment at presentation may predict complications and early relapse in patients with localized cancer of the esophagus. Dis Esophagus. 2008;21(6):522–528. doi: 10.1111/j.1442-2050.2008.00814.x. [DOI] [PubMed] [Google Scholar]
- 23.Tessier JF, Nejjari C, Letenneur L, Filleul L, Marty ML, Barberger Gateau P, Dartigues JF. Dyspnea and 8-year mortality among elderly men and women: the PAQUID cohort study. Eur J Epidemiol. 2001;17(3):223–229. doi: 10.1023/a:1017977715073. [DOI] [PubMed] [Google Scholar]
- 24.Tinetti ME, McAvay G, Chang SS, Ning Y, Newman AB, Fitzpatrick A, Fried TR, Harris TB, Nevitt MC, Satterfield S, Yaffe K, Peduzzi P. Effect of chronic disease-related symptoms and impairments on universal health outcomes in older adults. J Am Geriatr Soc. 2011;59(9):1618–1627. doi: 10.1111/j.1532-5415.2011.03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker K, Barsamian J, Leone D, Donovan BC, Williams D, Carnevale K, Lansing R, Banzett R. Routine dyspnea assessment on unit admission. Am J Nurs. 2013;113(11):42–49. doi: 10.1097/01.NAJ.0000437112.43059.a0. quiz 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banzett R, Howell M, O’Donnell C, Lansing R, Schwartzstein R, Barsamian J, Leone D, Donovan B, Williams D, Carnevale K, Baker K. Dyspnea Prevalence and Risk of Adverse Event in a General Hospital Population. Am J Resp Crit Care Med. 2013;187:A2508. [Google Scholar]
- 27.Baker K, Stevens J, Anderson L, Bernstein H, O’Donnell C, Howell M, Banzett R. Dyspnea Assessed By Nurses Every Shift - Prevalence And Risk Prediction In A Pilot Study. Am J Resp Crit Care Med. 2013;189:A1787. [Google Scholar]
- 28.Stenton C. The MRC breathlessness scale. Occup Med (Lond) 2008;58(3):226–227. doi: 10.1093/occmed/kqm162. [DOI] [PubMed] [Google Scholar]
- 29.Bausewein C, Booth S, Higginson IJ. Measurement of dyspnoea in the clinical rather than the research setting. Curr Opin Support Palliat Care. 2008;2(2):95–99. doi: 10.1097/SPC.0b013e3282ffafe8. [DOI] [PubMed] [Google Scholar]
- 30.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med. 2007;21(3):177–191. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]
- 31.Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax. 2010;65(1):21–26. doi: 10.1136/thx.2009.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lansing RW, Gracely RH, Banzett RB. The multiple dimensions of dyspnea: Review and hypotheses. Respir Physiol Neurobiol. 2009;167:53–60. doi: 10.1016/j.resp.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meek PM, Banzett R, Parshall MB, Gracely RH, Schwartzstein RM, Lansing R. Reliability and validity of the multidimensional dyspnea profile. Chest. 2012;141(6):1546–1553. doi: 10.1378/chest.11-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]


