Abstract
A chemical synthesis of the D-ring of mersacidin is reported. The synthetic route relied upon development of a method for late-stage introduction of an unusual S-[(Z)-2-aminovinyl]-(3S)-3-methyl-d-cysteine (AviMeCys) functional group via an oxidative decarbonylation/decarboxylation reaction.
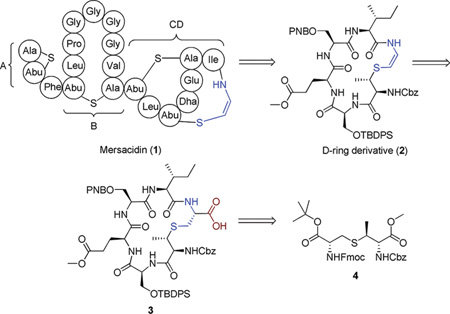
Mersacidin (1) is a 20-residue polycyclic lantibiotic peptide that possesses promising antibacterial activity against Gram-positive organisms. In addition, it has displayed good activity against problematic Gram-positive pathogens, in particular, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant entercocci (VRE), which have shown significant levels of resistance to commonly used members of the currently available antibiotic arsenal.1,2
Mersacidin is believed to inhibit the transglycosylation reaction in the bacterial cell wall biosynthetic pathway by forming a stoichiometric complex with lipid II, the final monomeric intermediate utilized by the bacterial cell for assembly of its cell wall. Sequestration of lipid II from the transglycosylases, enzymes that polymerize lipid II into the glycan strands that typify bacterial cell wall architecture, ultimately compromises the integrity of the bacterial cell wall and results in lysis and subsequent cell death.
Interestingly, mersacidin does not interact with the Lys-d-Ala-d-Ala portion of the stem peptide present in the lipid II intermediate and thus appears to target a site on lipid II that differs from the site utilized by vancomycin.3 Considering the serious public health threat posed by bacterial resistance to glycopeptide antibiotics, mersacidin may represent a promising avenue for development of new antibacterial therapeutics with activity versus problematic resistant organisms.
The CD bicyclic fragment is believed to have a major role in the binding of mersacidin with lipid II.4 However, the exact nature of this interaction is unknown. Structure–activity relationship studies (SAR) of mersacidin and its derivatives could reveal important insights into its mechanism of action and could contribute to the rational design of new lead compounds.5
As part of our effort directed toward the total synthesis of mersacidin, we initially focused our attention on the development of an efficient methodology that would introduce the synthetically challenging S-[(Z)-2-aminovinyl]-(3S)-3-methyl-d-cysteine (AviMeCys) into the polypeptide structure of a D-ring derivative. Thus, we report herein the synthesis of the D-ring derivative (2) containing the Avi-MeCys unit.
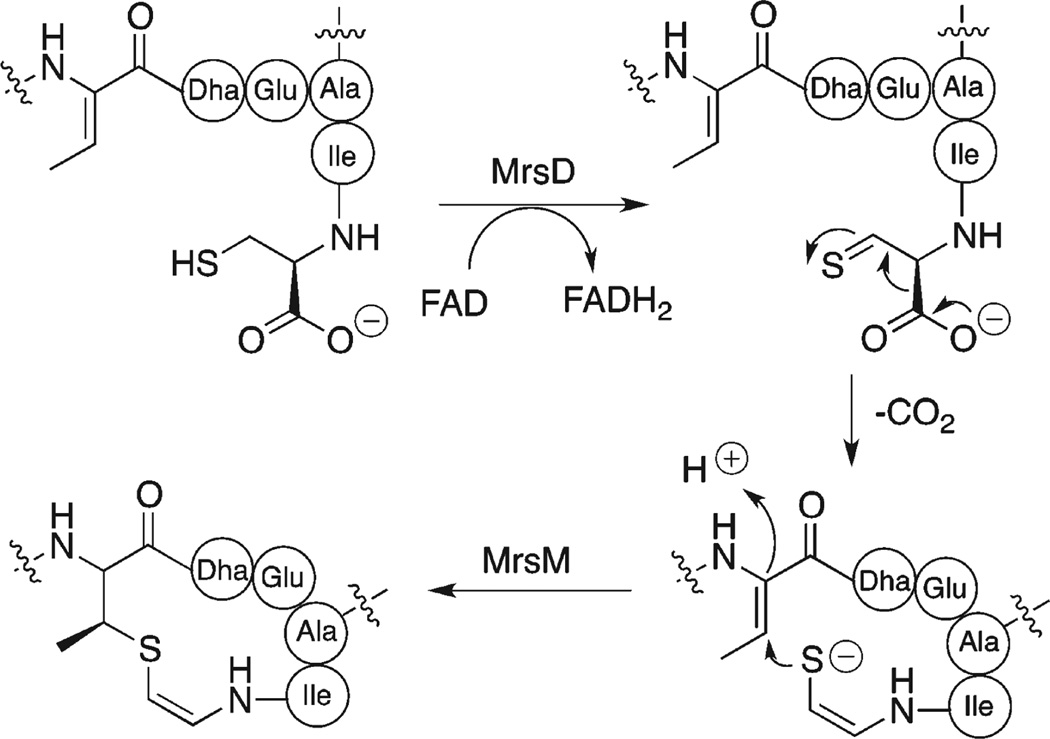
The (Z)-aminovinyl methyl sulfide bond found in mersacidin is the result of posttranslational modification mediated by the enzyme MrsD.6 The proposed mechanism is believed to involve the formation of a thioaldehyde intermediate, which spontaneously decarboxylates to an enethiolate. This enethiolate intermediate subsequently carries out a 1,4-conjugate addition reaction to an adjacent dehydroamino acid to install the vinyl sulfide bond (Figure 1).7
Figure 1.
Biosynthetic pathway for introduction of AviMeCys.
Inspired by this mechanism, we set out to test different routes that would leverage a carboxyl handle for late-stage introduction of the enamide linkage from a readily available peptide precursor. Previous reports in the literature have described similar strategies for the synthesis of enamides. These include the oxidative decarbonylation of activated carboxylic acids8 and the oxidative decarboxylation of amino acids using lead tetraacetate.9 To the best of our knowledge, however, these methods have not been tested on cysteine containing peptides as would be required for the AviMeCys subunit of mersacidin.10 In order to evaluate the potential utility of these methods for late-stage introduction of the AviMeCys subunit on a fully functionalized (C)D-ring precursor, a model study was carried out on a simplified β-methyllanthionine (MeLan) derivative 8.
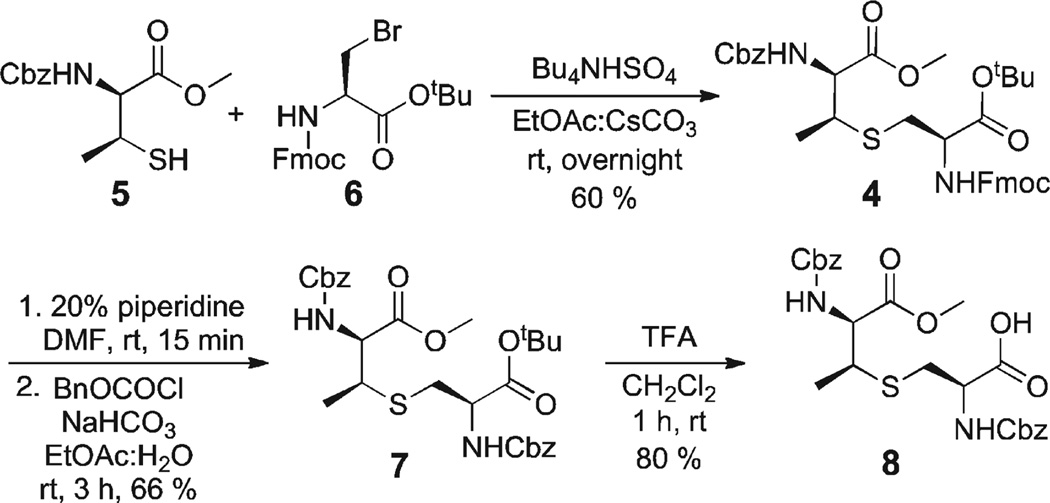
The synthesis of protected 8 relied on the alkylation of β-MeCys 5 with bromoalanine 611 following methodology previously developed in our laboratory12 (Scheme 1). Cleavage of the Fmoc group under standard conditions (20% piperidine, DMF), followed by reprotection of the free amine with Cbz (BnOCOCl, NaHCO3, EtOAc, H2O), provided diester 7. Finally, cleavage of the tert-butyl ester (TFA, CH2Cl2) provided the target MeLan derivative 8.
Scheme 1.
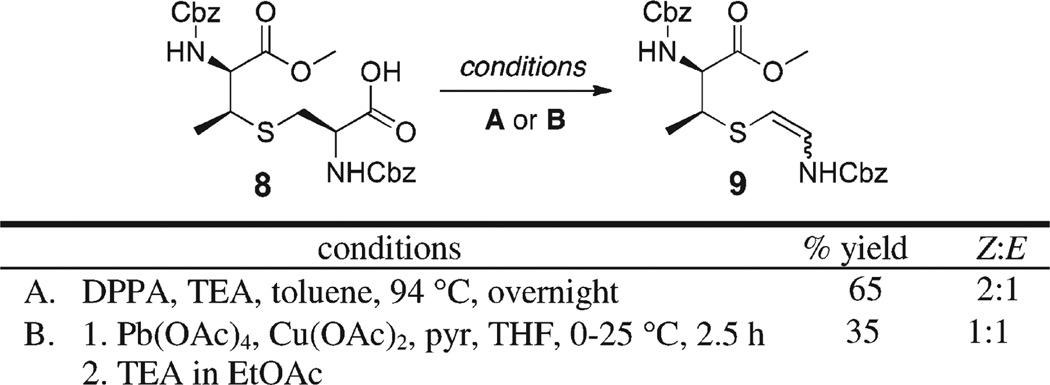
Our initial results are summarized in Scheme 2. Exposure of 8 to the oxidative decarbonylation conditions (2.2 equiv of diphenylphosphoryl azide (DPPA), 2.2 equiv of TEA, toluene) resulted in the formation of protected AviMeCys 9 as a mixture of stereoisomers in good yields. The same occurred when subjecting 8 to 1 equiv of Pb(AcO)4 in the presence of 1 equiv of Cu(OAc)2 under oxidative decarboxylation conditions. Although, the Avi-MeCys derivative was obtained as a mixture of Z and E isomers, we were encouraged by these results and were hoping that conformational constraints imposed by the cyclic peptide substrate would facilitate preferential formation of the desired (Z)-AviMeCys moiety upon subjecting a suitably protected D-ring precursor to the optimized oxidative decarboxylation conditions.
Scheme 2.
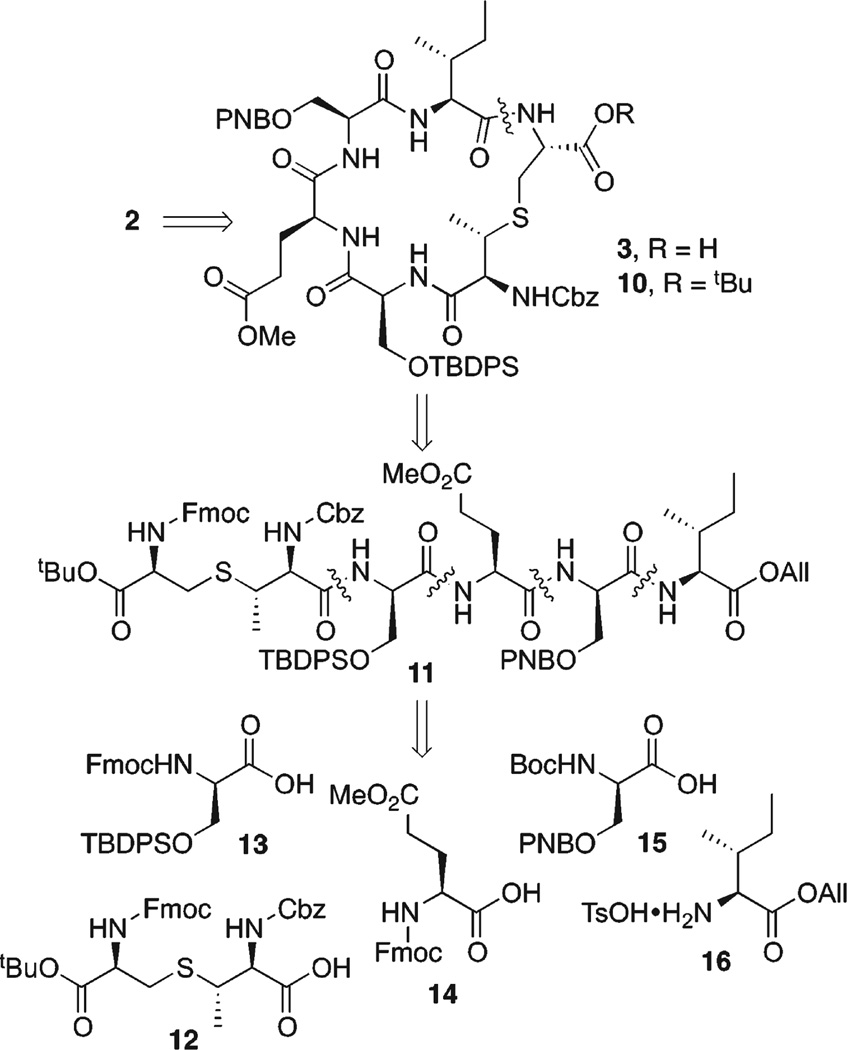
Cyclic peptide 3 was selected as the precursor for introduction of the AviMeCys subunit. Since the AviMeCys unit is acid-sensitive, the side-chain protecting groups were selected in order to achieve the dual goals of mutual orthogonality while maintaining nonacidic cleavage conditions. Additionally, orthogonal protection for each of the serine residues is required since subsequent conversion into a CD-ring system requires that one must be converted into a didehydroalanine, while the other will be incorporated into the C-ring lanthionine bridge.
Our retrosynthetic analysis for 2 is shown in Scheme 3. Our macrocyclization precursor was selected with Ile at the C-terminus. This site was selected for amide bond formation since the Ile is less prone to epimerization than Ser or Glu. In addition, this site is proximate to the site at which MrsD catalyzes the cyclization reaction in mersacidin (Figure 1), thus presenting the possibility that, although in protected form, the peptide might exist in a conformation to facilitate macrocyclization.
Scheme 3.
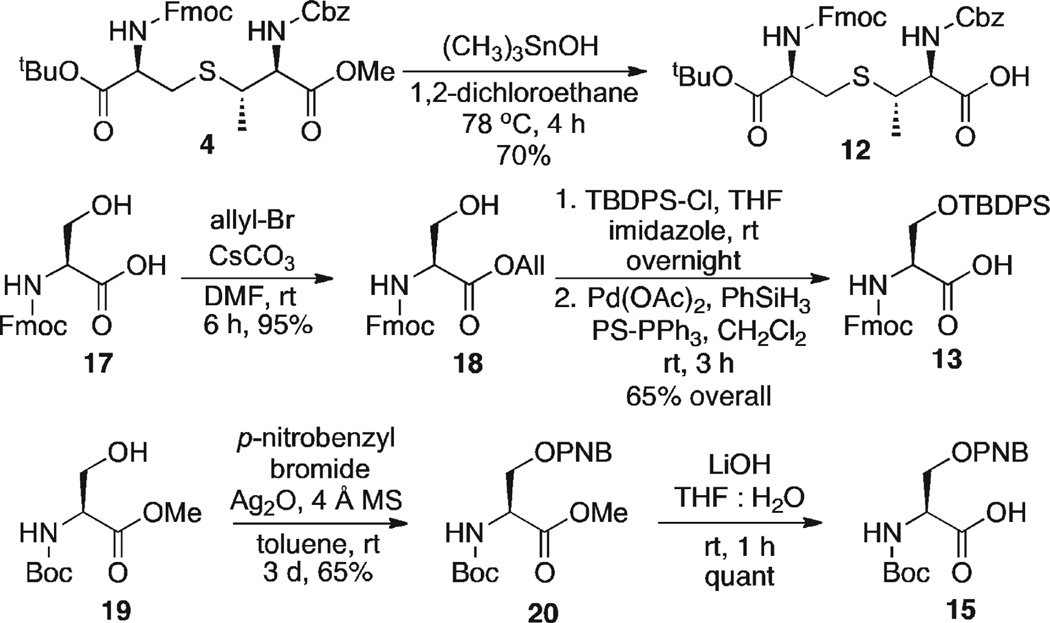
Ile 16 and Glu 14 derivatives were commercially available, but the serine residues (13 and 15) and MeLan 12 had to be synthesized (Scheme 4). MeLan 12 was prepared by selective deprotection of the methyl ester of 4 using Nicolaou conditions ((CH3)3SnOH, 1,2-dichloroethane).13 For the synthesis of 13, Fmoc-Ser-OH 17 was converted to its allyl ester in excellent yields (allyl-Br, CsCO3, DMF).14 Protection of the side chain hydroxyl group of 18 as a TBDPS ether (TBDPS-Cl, imidazole, THF) followed by cleavage of the allyl ester (Pd(OAc)2, PS-PPh3, PhSiH3, CH2Cl2) afforded the acid in 65% overall yield. Finally, serine derivative 15 was prepared by protection of alcohol 19 as a p-nitrobenzyl ether (Ag2O, p-nitrobenzyl bromide, toluene) followed by cleavage of the methyl ester under standard conditions (LiOH, THF, H2O).
Scheme 4.
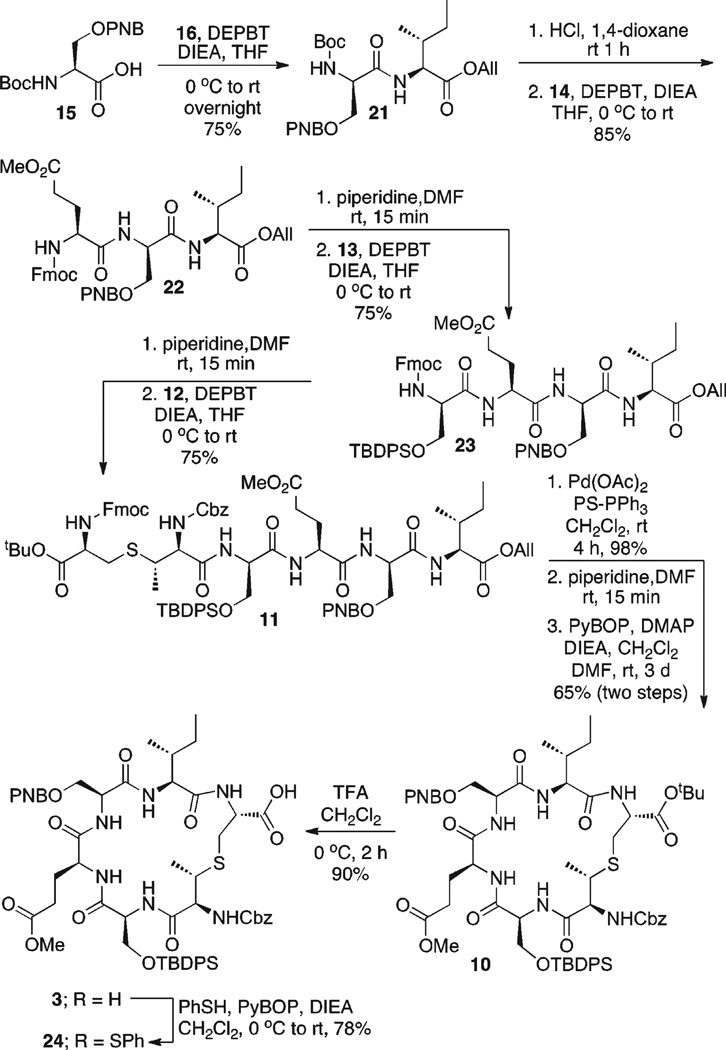
Our synthesis of 3 began with the coupling of 16 and 15, under standard peptide coupling conditions (DEPBT, DIEA, THF), affording 21 in 75% yield (Scheme 5). DEPBT (3-(diethoxyphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one) was the coupling reagent of choice since its ability to suppress racemization at the adjacent stereocenter of activated carboxylates has been demonstrated.15
Scheme 5.
The N-terminal Boc protecting group found in 21 was cleanly removed (4 N HCl, dioxane), and the resulting free amine was coupled with Fmoc-Glu(OMe)-OH 14 (DEPBT, DIEA, THF) to provide 22 in 85% yield. Cleavage of the N-terminal Fmoc protecting group of 22 (20% piperidine, DMF) followed by coupling with 13 (DEPBT, DIEA, THF) provided tetrapeptide 23 in 75% yield. Fmoc deprotection (20% piperidine, DMF) of 23 and coupling with MeLan 12 (DEPBT, DIEA, THF) provided 11 in 75%yield. Cleavage of the C-terminal allyl ester (Pd(OAc)2, PS-PPh3, PhSiH3, CH2Cl2) provided the free carboxylic acid in 98% yield. Deprotection of the N-terminal Fmoc protecting group (20% piperidine, DMF), followed by cyclization of the peptide (PyBOP, DMAP, DIEA, DMF, CH2Cl2), afforded D-ring precursor 10 in 65% overall yield.
In order to set the stage for investigation of reaction conditions for oxidative decarboxylation, the C-terminal tert-butyl ester of 10 was cleaved under standard conditions (50% TFA in CH2Cl2, 0 °C), affording carboxylic acid 3 in good yield. In addition, carboxylic acid 3 was easily converted to the corresponding thioester 24 under standard conditions (PhSH, PyBOP, DIEA, CH2Cl2, 0 °C to rt, 78%).
Our initial attempt to introduce the (Z)-AviMeCys subunit sought to take advantage of Ni(0)-mediated decarbonylation reactions as described in the preceding manuscript. Unfortunately, all efforts to facilitate oxidative decarbonylation of 24 under the optimized reaction conditions (1.2 equiv of Ni(PPh3)4, copper(I) 3-methylsalicylate (CuMeSal), dioxane) were unsuccessful as only complex product mixtures were obtained.16 In response to this disappointing result, we then turned our attention to oxidative decarbonylation/decarboxylation of carboxylic acid 3 via the reaction conditions described in Scheme 2.
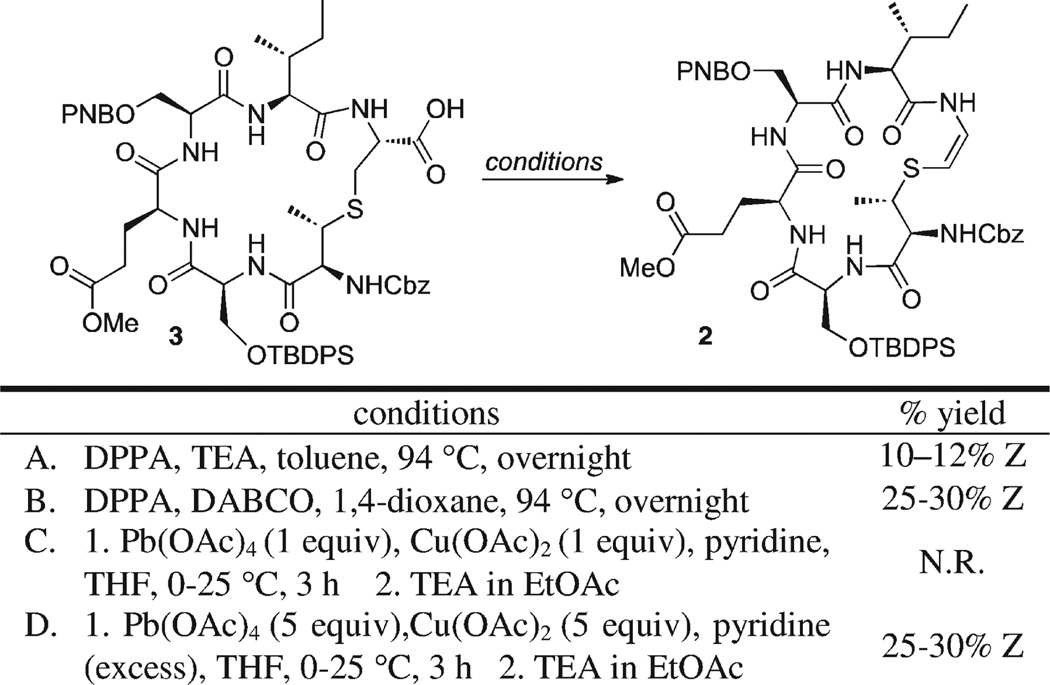
As shown in Scheme 6 when 3 was exposed to the oxidative decarbonylation conditions (DPPA, TEA, toluene), the desired (major) product possessing the (Z)-olefin geometry was obtained, albeit in poor yield. The vicinal coupling constant found for the olefinic hydrogens was consistent with coupling constant values for 1,2-disubstituted double bonds of (Z)-geometry (J = 8 Hz). A 2D-NOESY experiment (see Supporting Information) also provided support for the cis geometry of the double bond.
Scheme 6.
Various bases and solvents were screened in an effort to improve the reaction yield. The combination of DPPA, DABCO, and 1,4-dioxane gave the best results, providing D-ring derivative 2 containing the desired (Z)-AviMeCys in 25–30% yield.
Acid 3 was also exposed to the oxidative decarboxylation conditions utilizing lead tetraacetate. However, when using 1 equiv of both Pb(OAc)4 and Cu(OAc)2, the reaction produced none of the desired product.Wefelt that this resultmay be due to the greater number of Lewis basic sites present in 3 relative to those inMeLan 8, our test substrate. Thus, when 5 equiv of each reagent were added under the same conditions, the desired product 2 was formed in 25–30% yield.
In summary, we were able to synthesize the D-ring of mersacidin that contains the unusual amino acid S-[(Z)-2-aminovinyl]-(3S)-3-methyl-d-cysteine (AviMeCys). Our strategy took advantage of a C-terminal carboxyl group that enabled late-stage introduction of the enamide subunit via an oxidative decarbonylation reaction promoted by DPPA or an oxidative decarboxylation promoted by Pb(OAc)4. These reactions produced the desired (Z)-enamide predominantly; only minor amounts of the (E)-enamide were detected. The methodology reported above is currently being applied toward the synthesis of the CDring system of mersacidin, with the goal of gaining a better understanding of how this subunit interacts with lipid II. The results of these studies will be presented in due course.
Supplementary Material
Acknowledgment
Financial support for this work was provided by the NIH (AI 059327). Helpful discussions with William Turner (Indiana University) and Pablo Garcia-Reynaga (Indiana University) are gratefully acknowledged.
Footnotes
Supporting Information Available. Experimental procedures describing the synthesis of all new compounds as well as the characterization data are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- 1.Chatterjee S, Chatterjee DK, Rajendra HJ, Blumbach J, Ganguli BN, Klesel N, Limbert M, Seibert G. J. Antibiot. 1992;45:839. doi: 10.7164/antibiotics.45.839. [DOI] [PubMed] [Google Scholar]
- 2.(a) Chatterjee C, Paul M, Xie L, van der Donk WA. Chem. Rev. 2005;105:633. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]; (b) Willey JM, van der Donk WA. Annu. Rev. Microbiol. 2007;61:477–501. doi: 10.1146/annurev.micro.61.080706.093501. [DOI] [PubMed] [Google Scholar]
- 3.(a) Brotz H, Bierbaum G, Markus A, Molitor E, Sahl HG. Antimicrob. Agents Chemother. 1995;39:714.3. doi: 10.1128/AAC.39.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brotz H, Bierbaum G, Reynolds PE, Sahl HG. Eur. J. Biochem. 1997;246:193.3. doi: 10.1111/j.1432-1033.1997.t01-1-00193.x. [DOI] [PubMed] [Google Scholar]; (c) Brotz H, Bierbaum G, Leopold K, Reynolds PE, Sahl HG. Antimicrob. Agents Chemother. 1998;42:154. doi: 10.1128/aac.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu S-TD, Breukink E, Bierbaum G, Sahl HG, de Kruijff B, Kaptein R, van Nuland NAJ, Bonvin AM. J. Biol. Chem. 2003;278:13110. doi: 10.1074/jbc.M211144200. [DOI] [PubMed] [Google Scholar]
- 5.(a) Szekat C, Jack RW, Skutlarek D, Farber H, Bierbaum G. Appl. Environ. Microbiol. 2003;69(7):3777–3783. doi: 10.1128/AEM.69.7.3777-3783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Appleyard AN, Choi S, Read DM, Lightfoot A, Boakes S, Hoffmann A, Chopra I, Bierbaum G, Rudd BAM, Dawson MJ, Cortes J. Chem. Biol. 2009;16:490–498. doi: 10.1016/j.chembiol.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Levengood MR, Knerr PJ, Oman TJ, van der Donk WA. J. Am. Chem. Soc. 2009;131:12024–12025. doi: 10.1021/ja903239s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majer F, Schmid DG, Altena K, Bierbaum G, Kupke T. J. Bacteriol. 2002;184:1234. doi: 10.1128/JB.184.5.1234-1243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For a recent review of AviCys-containing lantibiotics, see: Sit CS, Yoganathan S, Vederas JC. Acc. Chem. Res. 2011;44:261–268. doi: 10.1021/ar1001395.
- 8.(a) Martin-Lopez MJ, Bermejo-Gonzalez F. Tetrahedron Lett. 1994:4235. [Google Scholar]; (b) Martin-Lopez MJ, Bermejo-Gonzalez F. Tetrahedron Lett. 1994;35:8843. [Google Scholar]
- 9.Wang X, Porco JA. J. Org. Chem. 2001;66:8215. doi: 10.1021/jo0158027. [DOI] [PubMed] [Google Scholar]
- 10.For a review on recent developments in lantibiotic synthesis, see: Tabor AB. Org. Biomol. Chem. 2011;9:7606–7628. doi: 10.1039/c1ob05946g.
- 11.Bromoalanine 6 was prepared following reported procedures. Ginisty M, Gravier-Pelletier C, Le Merrier Y. Tetrahedron: Asymmetry. 2006;17:142. Zhu X, Schmidt RR. Chem.–Eur. J. 2004;10:875. doi: 10.1002/chem.200305163.
- 12.Narayan RS, VanNieuwenhze MS. Org. Lett. 2005;7:2655. doi: 10.1021/ol0507930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolaou KC, Estrada AA, Zak M, Lee SH, Safina BS. Angew. Chem. Int. Ed. 2005;44:1378. doi: 10.1002/anie.200462207. [DOI] [PubMed] [Google Scholar]
- 14.Ficht S, Payne RJ, Guy RT, Wong CH. Chem.–Eur. J. 2008;14:3620. doi: 10.1002/chem.200701978. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Jiang X, Ye Y-h, Fan C, Romoff T, Goodman M. Org. Lett. 1999;1:91. doi: 10.1021/ol990573k. [DOI] [PubMed] [Google Scholar]
- 16.Spectroscopic examination of the reaction product mixture appeared to show evidence of thiol elimination (described in the preceding manuscript) as well as a product(s) arising from loss of the p-nitrobenzyl protecting group. See also the preceding manuscript: García-Reynaga P, Carrillo AK, VanNieuwenhze MS. Org. Lett. 2012;14 doi: 10.1021/ol203399x.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.