Abstract
Background
Apathy is a prominent feature of geriatric depression that predicts poor clinical outcomes and hinders depression treatment. Yet little is known about the neurobiology and treatment of apathy in late-life depression. This study examined apathy prevalence in a clinical sample of depressed elderly, response of apathy to selective serotonin reuptake inhibitor (SSRI) treatment, and neuroanatomical correlates that distinguished responders from nonresponders and healthy controls.
Methods
Participants included 45 non-demented, elderly with major depression and 43 elderly comparison individuals. After a 2-week single-blind placebo period, depressed participants received escitalopram 10mg daily for 12 weeks. The Apathy Evaluation Scale (AES) and 24-item Hamilton Depression Rating Scale (HDRS) were administered at baseline and 12 weeks. MRI scans were acquired at baseline for concurrent structural and diffusion tensor imaging of anterior cingulate grey matter and associated white matter tracts.
Results
35.5% of depressed patients suffered from apathy. This declined to 15.6% (p<0.1) following treatment, but 43% of initial sufferers continued to report significant apathy. Improvement of apathy with SSRI was independent of change in depression but correlated with larger left posterior subgenual cingulate volumes and greater fractional anisotropy of left uncinate fasciculi.
Limitations
modest sample size, no placebo control, post-hoc secondary analysis, use of 1.5T MRI scanner
Conclusions
While prevalent in geriatric depression, apathy is separable from depression with regards to medication response. Structural abnormalities of the posterior subgenual cingulate and uncinate fasciculus may perpetuate apathetic states by interfering with prefrontal cortical recruitment of limbic activity essential to motivated behavior.
Keywords: Apathy, Depression, Geriatric, SSRI, Cingulate, Uncinate
INTRODUCTION
Apathy is a common feature of late-life depression(Chase, 2011; Krishnan et al., 1995; Mehta et al., 2008). It afflicts 19–88% of those suffering from major depression, and is most prevalent in depressed older adults(Forsell et al., 1993; Lampe and Heeren, 2004; Mehta et al., 2008). The syndrome of apathy is defined as a primary motivational impairment that in depression results in diminished goal-oriented behavior, lack of intellectual interest, and indifference or flattening of affect(Marin, 1990). These clinical signs often translate into apathetic, depressed patients being poorly engaged in treatment, posing a greater burden to caregivers, and having increased risk of future functional and cognitive impairment(Holtta et al., 2012). Further, apathy is a predictor of poor response to antidepressants(Chaturvedi and Sarmukaddam, 1986; Levkovitz et al., 2011), and chronicity of depression(Lavretsky et al., 1999).
While selective serotonin reuptake inhibitors (SSRIs) are prescribed first-line for depression, apathy response to SSRIs is variable. Several case reports and case-control studies argue that SSRIs may actually cause or exacerbate apathy when used in the treatment of depression(Bolling and Kohlenberg, 2004; Fava, 2006; Hoehn-Saric et al., 1990; Kodela and Venkata, 2010; Padala et al., 2012; Sato and Asada, 2011; Wongpakaran et al., 2007). It is unclear to what extent apathy represents an SSRI side effect, a residual symptom not adequately treated by SSRIs alone, or both. To date, we lack an understanding of the neurobiology of apathy in depression and lack a consensus on its optimal treatment. As such, this study sought to investigate differences in neuroanatomical correlates that might explain the variable response of apathy to SSRI treatment in the context of depression.
Convergent findings from structural MRI, functional MRI, and neuropsychological studies implicate altered function of frontolimbic networks in late-life depression (Alexopoulos et al., 2012; Alexopoulos et al., 1997; Gunning-Dixon et al., 2009; Gunning-Dixon et al., 2008; Raz et al., 1997). Among the frontolimbic networks implicated in geriatric depression, the anterior cingulate cortex (ACC) plays a key role(Alexopoulos et al., 2008a). Based on cytoarchitecture and functional connectivity, the ACC is divided into dorsal (BA 24b'-c' and 32') and perigenual ACC (rostral BA 24a-c and 32 and subgenual BA 25 and 33) regions, which govern cognitive and emotional processes, respectively(Bush et al., 2000; Devinsky et al., 1995; Drevets et al., 2008; Vogt et al., 1992). While the dorsal ACC controls aspects of executive function (conflict detection, cognitive inhibition, and conflict resolution)(Carter et al., 1998; Carter and van Veen, 2007; Posner and DiGirolamo, 1998), the perigenual ACC assesses the salience of emotional input and regulates emotional responses(Devinsky et al., 1995; Etkin et al., 2006). In a previous analysis, our group described a pattern wherein smaller dorsal and rostral ACC volumes and decreased frontosubcortical white matter integrity predicted failure of depression to remit with SSRI treatment(Alexopoulos et al., 2010; Alexopoulos et al., 2002; Alexopoulos et al., 2008b; Gunning et al., 2009).
Given the association of apathy with poor depression response to antidepressants, we performed a post-hoc, secondary analysis to explore a potential relationship between structural characteristics of ACC and adjacent white matter tracts and apathy in late-life depression. Several clinical observations and neuroimaging studies support the notion that apathy may emerge from frontosubcortical network dysfunction and ACC abnormality. Apathy is common in elderly individuals with prominent vascular white matter lesions(Alves et al., 2009; Lavretsky et al., 2007) and focal frontal lobe and basal ganglia lesions(Chase, 2011; Levy and Dubois, 2006). Among various neurodegenerative diseases, apathy develops early and prominently in dementias with greater frontosubcortical pathology (Huntington's, Lewy Body, Parkinson's and HIV dementia)(Chase, 2011; Quaranta et al., 2012; Starkstein et al., 2006), and, in the case of Alzheimer's disease, apathy correlates with neurofibrillary tangle density in the ACC(Marshall et al., 2006) and reduced grey matter volume and metabolic activity of the ACC(Apostolova et al., 2007; Bruen et al., 2008; Marshall et al., 2007; Starkstein et al., 2009). In a prior study of late-life major depression, apathy was associated with reduced right ACC grey matter volumes(Lavretsky et al., 2007). Further dissecting the neural correlates of apathy in depression would afford an understanding of the brain circuitry underlying motivational states and thus inform future treatment approaches for apathetic depression.
The objectives of this study were to examine the prevalence and severity of apathy in a clinical sample of patients with late-life depression, to analyze the response of apathy to SSRI treatment, and to identify the neuroanatomical correlates that might explain the maintenance of an apathetic state. We included a non-depressed comparison group to examine the relative specificity of such neuroanatomical findings to apathetic versus depressed states. Guided by the above literature, this study focused on the role of ACC subregions and white matter tracts in the apathy of late-life depression. It hypothesized that depressed elders who suffer from persistent apathy despite SSRI treatment were more likely to possess structural abnormalities in perigenual, as opposed to dorsal, ACC and in white matter tracts connecting the perigenual ACC to structures related to mood regulation, such as the amygdala and ventral striatum.
METHODS
Participants
We studied 45 non-demented, elderly (>60 years) patients with non-psychotic major depression and 43 elderly, psychiatrically healthy participants. Subjects were recruited through radio and print advertisement in community radio stations and newspapers. Participants signed written informed consent approved by the Institutional Review Boards of Weill-Cornell Medical College and of the Nathan Kline Institute.
The depressed group met DSM-IV-TR criteria for unipolar major depression and had a score of ≥ 18 on the 24-item Hamilton Depression Rating Scale (HDRS)(Hamilton, 1960). Exclusion criteria for the depressed group were: (1) major depression with psychotic features; (2) history of other axis I psychiatric disorders prior to the onset of depression; (3) history of substance abuse; (4) severe medical illness (i.e., metastatic cancer, brain tumors, unstable cardiac, hepatic, or renal disease, myocardial infarction, or stroke) within the 3 months preceding the study; (5) neurological disorders (i.e., dementia, delirium, history of head trauma, Parkinson's disease, and multiple sclerosis); (6) medical illnesses often associated with depression (i.e., endocrinopathies other than diabetes, lymphoma, and pancreatic cancer); (7) drugs causing depression (i.e., steroids, α-methyl-dopa, clonidine, reserpine, tamoxifen, and cimetidine); (8) Mini-Mental State Examination (MMSE) score < 25(Folstein et al., 1975); (9) Mild Cognitive Impairment according to criteria described by Petersen et al. (Petersen et al., 1999) and (10) contraindications to MRI scanning. Exclusion criteria for non-depressed participants were the same as above, while inclusion criteria included absence of history of any psychiatric illness, and a HDRS score lower than 7.
Assessment
Depressive symptoms were assessed using the HDRS. Apathy was quantified using the self-rated Apathy Evaluation Scale (AES), a psychometrically validated instrument in older normal individuals and psychiatric patients (Clarke et al., 2007; Marin et al., 1991). For healthy comparison participants, the AES was administered at baseline. For depressed participants, the AES was administered at baseline (after the 2-week placebo lead-in/drug washout period) and again at the end of escitalopram treatment. An AES ≥ 36.5 was considered clinically significant apathy (Clarke et al., 2007). Overall cognitive impairment was examined in a clinical interview and was rated with the MMSE (Folstein et al., 1975) and the Dementia Rating Scale (DRS) (Mattis, 1988). Memory was rated with the Hopkins Verbal Learning Test-Revised (Brandt, 2001), response inhibition with the Stroop Color Word Test (Golden, 1978) and visual attention and task switching with Trails A and Trails B (Reitan, 1985).
Treatment
Depressed participants entering the study were informed that they would receive placebo at some point during their 14-week trial. They underwent a 2-week single-blind placebo lead-in and drug washout period in which patients were tapered off all antidepressant and anxiolytic medications, including benzodiazepines. Continued beta-blocker use was allowed. Participants who still met DSM-IV-TR criteria for major depression and had a HDRS ≥ 18 received treatment with escitalopram 10 mg every morning, daily for 12 weeks.
Depressed participants were assessed weekly throughout the treatment trial. Assessment consisted of a brief meeting with a research psychiatrist and ratings by a trained research assistant using the HDRS, a medication adherence questionnaire, and a vital signs form. The meeting with the research psychiatrist followed a medication clinical format focusing on psychiatric symptom and side effects evaluation. No participants received psychotherapy.
MRI procedures
MRI scans were acquired on a 1.5 T Siemens Vision System at the Center for Advanced Brain Imaging of the Nathan Kline Institute from depressed participants at the end of a 2-week single-blind placebo lead-in/drug washout phase of the treatment trial and from control participants at baseline. Patients received a magnetization prepared rapidly acquired gradient echo (MPRAGE) scan (TR=11.6ms, TE=4.9ms, matrix=256×256, FOV=320mm, NEX=1, slice thickness=1.25mm, 17s slices, no gap) and DTI scan (TR=600ms, TE=100ms, matrix=128×128, FOV=320mm, NEX=7, slice thickness=5mm, 10 slices, no gap). For the DTI scan, eight diffusion sensitization directions were used (with b=1000 s/mm2) along with an image with no diffusion weighting (b=0 s/mm2). The TSE and DTI scans were acquired in an oblique axial plane parallel to the anterior commisure-posterior commissure axis.
Volumetric image analysis
Images were displayed on a 21-inch monitor, and each region of interest (ROI) was traced manually. All structures were measured separately for each hemisphere. Manual tracing of anterior cingulate cortex subregions was performed on every coronal slice according to boundaries previously described by Gunning et al(Gunning et al., 2009), starting from the most anterior slice and moving posterior. An ROI was created by circling the entire gyrus, including white matter. To obtain grey matter volumes, each ROI was multiplied by individual subject whole brain grey matter masks that were created using FSL's Brain Extraction Tool (BET) and FMRIB's Automated Segmentation Tool (FAST) software (www.fmrib.ox.-ac.uk/fsl) (Zhang et al., 2001). Whole brain volume was calculated for each subject by summing total white matter and grey matter volumes that were obtained using FAST.
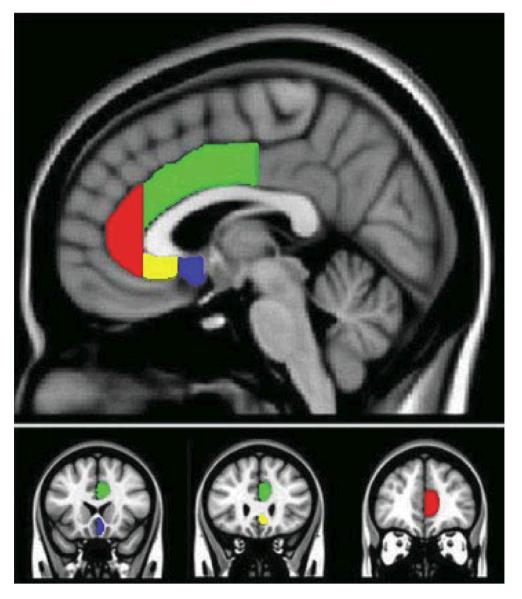
For posterior subgenual ACC: anterior boundary began where the putamen was first visible, posterior boundary was the coronal slice where the paraterminal gyrus was no longer present medially, superior boundary was identified where grey matter joined with white matter of the corpus callosum, inferior boundary was located at the inferior tip of the lower-most gyrus on the medial surface. The posterior subgenual ROI included BA25. Examples of traced ACC ROIs are depicted in Figure 1.
FIGURE 1.
Parcellation scheme for subregions of the Anterior Cingulate Cortex (ACC). Red region indicates rostral ACC; green region indicates dorsal ACC; yellow region indicates anterior subgenual ACC; blue region indicates posterior-subgenual ACC.
Fractional anisotropy (FA) analysis
FA was examined in specific ROIs using the Reproducible Object Quantification Scheme software to define the boundary of anatomically distinct white matter tracts in native space in a semi-automated manner as detailed by Alexopoulos et al(Alexopoulos et al., 2010). The following white matter ROIs were examined: centrum semiovale, cingulum, superior and anterior corona radiata, superior and inferior longitudinal fasciculi, the body, genu, and splenium of the corpus callosum, and the uncinate fasciculus.
Data analysis
Statistical analysis was performed with SPSS 19.0 (SPSS, Inc.). Mann-Whitney U, t-tests, paired sample t-tests, chi-square, and Fisher's exact tests were used to describe demographic and clinical aspects of the patient population, and to compare the prevalence of apathy between groups (non-depressed, depressed at baseline versus after treatment).
Bivariate analyses with Pearson or Spearman rank correlation were used to identify variables that significantly correlated with change in apathy between baseline and 12-week treatment time points. Candidate variables were assessed in a series of linear regression analyses. We constructed hierarchical linear regression models predictive of change in apathy in which we entered baseline AES, age, and whole brain volume followed by ROI measures. By including baseline AES, age, and whole brain volume in the first hierarchical level of analysis, we controlled for their effect on change in apathy. This allowed us to examine the specific impact of ROI parameters on change in apathy. Hierarchical linear regression model designs were guided by the hypothesis that specific white matter tracts and associated subregions of the ACC, possibly responsible for the communication between frontal and limbic regions implicated in motivational states, would be related to the persistence of apathy.
RESULTS
Depressed versus non-depressed subjects
A total of 84 depressed elderly participants fulfilled selection criteria, signed consent and entered the study. After the 2-week drug washout and single-blind placebo lead-in period, 63 continued to meet DSM-IV criteria for major depression, had a HDRS score equal to or greater than 18, and entered the escitalopram treatment phase. Of these participants, 53 had baseline apathy data and MRI scans collected, and 45 participants completed the 12-week treatment trial. Eight participants failed to complete the treatment trial: three exited because of worsening depression, two exited because they found the treatment ineffective, one withdrew because of medication side effect (hyponatremia), and two were lost to follow-up. We found no significant differences in apathy severity, depression severity, whole brain or ACC grey matter volumes or white matter burden between participants who did or did not complete the 12-week treatment trial. In addition to the 45 depressed participants, we studied 43 elderly normal comparison subjects. There were no significant differences in age, education, overall cognitive status (MMSE, DRS), memory (HVLT-R), and response inhibition (Stroop) between depressed and normal subjects. However, depressed subjects had significantly higher depression scores than normal subjects (HDRS mean: 22.0, SD 3.4 vs. 1.8, SD 1.6; Mann Whitney U, p<.001). Depressed subjects were divided into apathetic (AES ≥ 36.5) and non-apathetic (AES < 36.5) groups. There were no significant differences in demographics, depression severity, or cognitive function between apathetic and non-apathetic depressed subjects at baseline, with the exception of apathy scores (Table 1). The prevalence of clinically significant apathy (AES ≥ 36.5(Clarke et al., 2007)), was greater in the depressed (16 out of 45 participants) than non-depressed group (1 out of 43 subjects) at baseline (35.5% versus 2.3%, Fisher's exact test, p<.01).
Table 1.
Demographic and clinical characteristics of older patients with major depression with high and low apathy scores and normal elders
| Variable | Apathetic depressed (N=16) Mean (SD) | Non-apathetic depressed (N=29) Mean (SD) | Normal elders (N=43) Mean (SD) |
|---|---|---|---|
| Age (years) | 71.6 (5.0) | 69.1 (5.8) | 70.6 (6.4) |
| Education (years) | 16.3 (3.2) | 16.1 (3.5) | 16.5 (2.6) |
| Gender (% Female) | 9 (56%) | 19 (65%) | 27 (63%) |
| Age of onset (years) | 53.8 (18.7) | 57.2 (15.9) | |
| Number of prior episodes | 2.6 (1.4) | 2.3 (1.3) | |
| Mini Mental Status Exam | 28.1 (1.9) | 28.5 (1.6) | 28.4 (1.1) |
| DRS totala | 134.8 (5.3) | 136.6 (5.6) | 137.1 (3.5) |
| Baseline HDRSb | 23.4 (4.3) | 21.3 (2.7) | 1.8 (1.6)*** |
| Baseline AESc | 47.9 (7.8)* | 29.4 (5.4) | 23.0 (4.4)** |
| Stroop color word | 33.8 (10.3) | 36.5 (8.3) | 35.9 (9.0) |
| Trails A | 38.1 (7.8) | 36.6 (9.5) | 32.5 (10.1) |
| Trails B | 100.7 (45.4) | 98.6 (62.5) | 89.9 (45.4) |
| HVLT-Rd immediate recall | 23.1 (5.5) | 24.1 (4.5) | 24.9 (4.2) |
| HVLT-R delayed recall | 8.6 (3.4) | 8.6 (2.8) | 8.9 (2.8) |
Mann Whitney U comparisons:
Apathetic depressed vs. Non-apathetic depressed p<.001
Normal elders vs. Non-apathetic depressed p<.001, Normal vs. Apathetic depressed p<.001
Normal vs. Apathetic depressed p<.001, Normal vs. Non-apathetic depressed p<.001
Dementia Rating Scale
24-item Hamilton Depression Rating Scale
Apathy Evaluation Scale, patient self-rated
Hopkins Verbal Learning Test - Revised
Escitalopram effects on depression and apathy
The percentage of subjects with clinically significant apathy declined within the depressed patient group after treatment with escitalopram (35.5% versus 15.6%, χ2 test, p<.01). However, 7 participants out of the 16, equivalent to 43% of the depressed patients who initially presented with apathy, continued to suffer from clinically significant, moderate to severe apathy. Severity of apathy decreased in the depressed patient group after escitalopram treatment (AES mean: 36.0, SD: 10.9 versus 27.5, SD: 8.1; Mean difference in AES: 8.5, SD: 9.8, t=5.75, df=44, paired t-test, p<.001), but to a lesser extent than did depression severity (HDRS mean: 22.0, SD: 8.6 versus 8.7, SD: 6.8; Mean difference in HDRS: 13.4, SD: 8.3, t=10.7, df=44, paired t-test, p<.001) (Figure 2). Of the 45 participants who completed 12 weeks of escitalopram treatment, only two patients reported an increase in apathy and, in both cases, this was a minor increase of two AES points.
FIGURE 2.
Apathy in depressed patient group before and after antidepressant treatment. Mean Apathy Evaluation Scale (AES) and 24-item Hamilton Depression Rating Scale (HDRS) scores with corresponding standard errors of the mean are depicted. *12 week versus baseline scale score, paired t-test, p < .001
Relationship between depression and apathy response
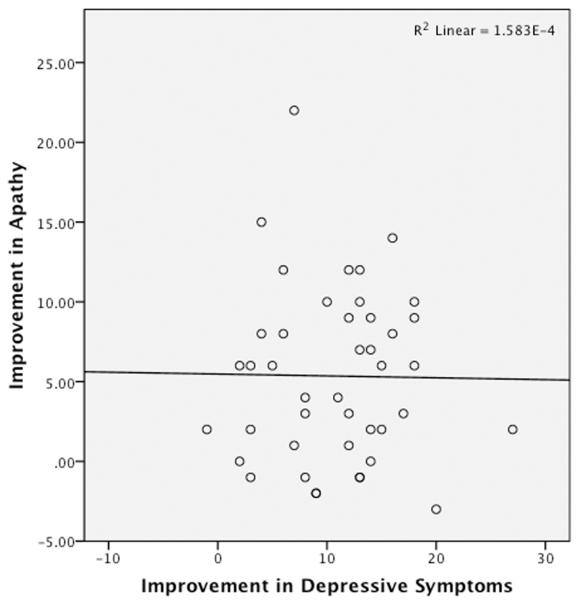
To analyze the relationship between response of apathy to SSRI and response of the rest of the depressive syndrome symptoms, HDRS scores were then modified to omit 4 items shown previously to be sensitive to the presence of apathy: diminished work/interest, psychomotor retardation, anergy, and lack of insight(Feil et al., 2003; Marin et al., 1993). The change in apathy across participants was independent of change in this modified HDRS score, in response to escitalopram treatment (Bivariate analysis, Pearson correlation between Improvement in AES and Improvement in HDRS-corrected, R=0.0001, p = 0.652) (Figure 3). Multiple regression analysis confirmed that change in this HDRS-corrected did not significantly predict change in apathy scores (Supplemental Figure).
FIGURE 3.
Analysis of relationship between change in apathy and change in non-apathy depressive symptoms following SSRI treatment. Scatterplot depicting independence between change in apathy and change in non-apathy, depression symptoms in response to SSRI treatment. (R=0.0001; p=0.652; df=44; x-axis represents difference between pre- and post-escitalopram Hamilton Depression Rating Scale (HDRS) score modified to omit four apathy-sensitive items, as discussed in Methods and Results; y-axis represents difference between pre- and post-escitalopram Apathy Evaluation Scale score).
Neuroanatomical predictors of persistent apathy
Given that 43% of the patients who had apathy at baseline continued to suffer from significant apathy after SSRI treatment, we next investigated the neuroanatomical differences that predicted persistence of apathy. To this end, we conducted a hierarchical regression in which we first entered baseline AES score, age, and whole brain volume, followed by fractional anisotropy and volume of ACC ROIs. After controlling for baseline AES, age, and whole brain volume, both greater left uncinate fasciculus fractional anisotropy and larger left posterior subgenual cingulate volumes were statistically significant predictors of greater improvement in AES score following escitalopram treatment (Table 2). The final model, with all independent variables entered into the equation, predicted 61% of the variance in change in apathy scores (Adjusted R2=0.61, F(4,39)=8.73, p<.001). We found a similar relationship between improvement in apathy and measures of the right uncinate fasciculus and right posterior subgenual cingulate, but these findings did not reach statistical significance.
Table 2.
Hierarchical regression model predicting improvement of apathy from baseline left uncinate fasciculus fractional anisotropy (FA) and left posterior subgenual cingulate cortex volume
| Variables | B | SE | β | F | P | |
|---|---|---|---|---|---|---|
| Model 1: Adjusted R2 = .37 ΔR2 = .45 | 5.728 | .00 | ||||
| AESbaseline | .27 | .09 | .45 | .01 | ||
| Age | .22 | .15 | .24 | .17 | ||
| Whole brain volume | .02 | .00 | .45 | .01 | ||
|
| ||||||
| Model 2: Adjusted R2 = .50 ΔR2 = .13 | 6.98 | .00 | ||||
| AESbaseline | .28 | .08 | .47 | .00 | ||
| Age | .33 | .14 | .37 | .03 | ||
| Whole brain volume | .02 | .00 | .36 | .03 | ||
| Left uncinate FA | 54.41 | 21.57 | .41 | .02 | ||
|
| ||||||
| Model 3: Adjusted R2 = .61 ΔR2 = .12 | 8.73 | .00 | ||||
| AESbaseline | .28 | .07 | .47 | .00 | ||
| Age | .32 | .12 | .36 | .02 | ||
| Whole brain volume | .01 | .00 | .25 | .09 | ||
| Left uncinate FA | 51.15 | 18.91 | .39 | .01 | ||
| Left posterior subgenual cingulate volume | .01 | .00 | .36 | .01 | ||
A comparison between control and all depressed participants revealed significantly larger control group grey matter volumes of bilateral dorsal and rostral ACC subregions, but no difference in subgenual cingulate volumes. Group comparisons revealed greater fractional anisotropy of the splenium of the corpus callosum in controls than in depressed (Mann Whitney U test, p=0.04), but there were no significant group differences in uncinate fasciculi FA measures (Table 3). Greater left and right uncinate FA did distinguish control group from patients who were both depressed and apathetic at baseline (Mann Whitney U test, p=0.01 for both left and right comparisons). There were no other significant differences in white matter FA when this three-group comparison between controls, non-apathetic depressed, and apathetic depressed was performed. Bilateral dorsal and rostral ACC volumes distinguished control from apathetic depressed (Mann Whitney U test, p<0.01 for each comparison) and control from non-apathetic depressed (Mann Whitney U test, p<0.05 for each comparison). There were no other significant differences in ACC volumes from this three-group comparison.
Table 3.
Anterior cingulate cortical (ACC) volumes and white matter tract fractional anisotropy (FA) in depressed and normal elders
| Variable | Depressed (N=45) Mean (SD) | Normal elders (N=43) Mean (SD) | P-value |
|---|---|---|---|
| Grey matter volumes | |||
| Dorsal ACC, left | 2.52 (0.50) | 4.11 (1.15) | 0.00** |
| Dorsal ACC, right | 2.60 (0.66) | 4.39 (0.95) | 0.00** |
| Rostral ACC, left | 1.98 (0.67) | 3.09 (1.20) | 0.00** |
| Rostral ACC, right | 1.90 (0.65) | 2.47 (1.12) | 0.04* |
| Anterior subgenual, left | 0.73 (0.23) | 0.74 (0.24) | 0.79 |
| Anterior subgenual, right | 0.74 (0.24) | 0.74 (0.19) | 0.79 |
| Posterior subgenual, left | 0.53 (0.15) | 0.58 (0.20) | 0.16 |
| Posterior subgenual, right | 0.53 (0.17) | 0.58 (0.20) | 0.12 |
| White Matter FA | |||
| Uncinate fasciculus, left | 0.36 (0.04) | 0.38 (0.05) | 0.06 |
| Uncinate fasciculus, right | 0.35 (0.04) | 0.37 (0.05) | 0.07 |
| Corpus callosum, total | 0.44 (0.05) | 0.44 (0.05) | 0.93 |
| Corpus callosum, genu | 0.51 (0.07) | 0.52 (0.05) | 0.34 |
| Corpus callosum, splenium | 0.61 (0.08) | 0.64 (0.07) | 0.04* |
| Superior corona radiata, left | 0.43 (0.04) | 0.42 (0.05) | 0.08 |
| Superior corona radiata, right | 0.41 (0.04) | 0.40 (0.04) | 0.07 |
| Anterior corona radiata, left | 0.32 (0.05) | 0.33 (0.05) | 0.25 |
| Anterior corona radiata, right | 0.31 (0.06) | 0.31 (0.05) | 0.28 |
| Superior longitudinal fasciculus, left | 0.38 (0.04) | 0.39 (0.05) | 0.26 |
| Superior longitudinal fasciculus, right | 0.37 (0.04) | 0.38 (0.04) | 0.16 |
| Inferior longitudinal fasciculus, left | 0.40 (0.06) | 0.41 (0.04) | 0.38 |
| Inferior longitudinal fasciculus, right | 0.40 (0.06) | 0.41 (0.04) | 0.18 |
| Centrum semiovale, left | 0.40 (0.07) | 0.40 (0.04) | 0.83 |
| Centrum semiovale, right | 0.38 (0.04) | 0.38 (0.05) | 0.58 |
Unadjusted mean grey matter volumes are presented in cm3. Mann Whitney U comparisons:
Depressed vs. Normal elders p<.05
Depressed vs. Normal elders p<.01
DISCUSSION
The principal finding of this study is that depressed elderly patients who continued to suffer from apathy after treatment with escitalopram had greater white matter abnormalities of the uncinate fasciculus and smaller volumes of the posterior subgenual cingulate cortex than depressed patients whose apathy improved. This is the first study, to our knowledge, to identify a relationship between the persistence of apathy after SSRI treatment with frontolimbic white and grey matter abnormalities.
These results suggest that apathy is a common feature of late-life depression yet is also a clinical entity distinct from the rest of the depressive syndrome—with neurobiological underpinnings that may be insufficiently addressed by SSRI treatment alone. Our observation that 2.3% of normal and 35.5% of non-demented, depressed elderly individuals suffered from apathy is consistent with prior reports of apathy prevalence. A study of cognitively normal, community-based adults over age sixty-five found a 1.4% prevalence of apathy(Onyike et al., 2007), and 19–88% of individuals with major depression reportedly suffer from apathy(Chase, 2011). No large epidemiological studies on the prevalence of apathy in late-life depression have been published. However two studies demonstrate a 43% (N=51) (Groeneweg-Koolhoven et al., 2014) and 53% (N=30) (Marin et al., 1994) prevalence of apathy in non-demented, elderly depressed. We observed that 15.6% of elderly, depressed individuals treated with escitalopram continued to suffer from significant apathy, consistent with prior reports that 18.6% and 16.1% of depressed patients who complete a course of SSRI treatment report persistent apathy and loss of ambition, respectively(Bolling and Kohlenberg, 2004).
This study suggests that apathy within depression warrants more focused study in order to improve apathy and depression outcome. The fact that 43% of the depressed individuals we studied continued to suffer from apathy despite 12 weeks of treatment corroborates the inadequacy of SSRIs alone for the treatment of depression that co-occurs with apathy(Kodela and Venkata, 2010; Padala et al., 2012; Sato and Asada, 2011; Wongpakaran et al., 2007). A recent multi-center, double-blind, randomized study of depression with apathy using SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) treatment documented the frequent persistence of apathy after an initial SSRI course and some improvement in apathy when a switch was made to treatment with either another SSRI or SNRI but without an appreciable difference between the two treatment arms(Raskin et al., 2012). On the other hand, escitalopram has been shown to be more effective than placebo in preventing new onset of apathy following stroke (Mikami et al., 2013).
The neuroanatomical findings of our study expand upon the existing model in which disrupted frontolimbic networks underlie late-life depression and may shed light on which individuals are more or less likely to have apathy that responds to SSRI treatment alone. Our results implicate the posterior subgenual cingulate cortex and its connections via the uncinate fasciculus to limbic structures in the persistence of apathy in geriatric depression. Note that the sample for the current study overlapped substantially with that reported on by Gunning et al. 2009 wherein remission of depression after escitalopram treatment correlated with dorsal and rostral anterior cingulate grey matter volumes(Gunning et al., 2009), but not with subgenual cingulate volumes. Similarly, in our current analysis, we found that differences in dorsal and rostral ACC, but not subgenual cingulate, volumes distinguished control from depressed patient groups. Differences in subgenual cingulate volume and uncinate fasciculus fractional anisotropy did not appear to distinguish healthy controls from depressed but were predictors of persistent apathy. We did observe lower FA in bilateral uncinate fasciculi of the depressed patient group compared to controls, however this difference failed to reach statistical significance. Work by others demonstrated lower FA of right uncinate fasciculi in depressed compared to healthy controls (Charlton et al., 2013) and less white matter hyperintensities in left uncinate fasciculi of depressed compared to healthy controls (Sheline et al., 2008). It is possible that our study was not adequately powered to identify this as a significant difference between depressed and control groups. On the other hand, Taylor and colleagues found no differences in FA of left and right uncinate fasciculi between elderly depressed and healthy controls but did find lower FA of left uncinate fasciculus in elders with early onset depression when compared to later onset and non-depressed groups. The authors discuss one explanation that repeated depressive episodes result in cumulative atrophy of white matter fibers (Taylor et al., 2007). Apathy is associated with chronicity of depressive episodes (Lavretsky et al., 1999), and it might therefore be interesting to assess how the persistence of apathy may have partially contributed to the FA differences in their early onset depression group.
Our observation that morphologic measures of uncinate fasciculus and posterior subgenual cingulate limited to the left hemisphere significantly predicted persistent apathy may be due to a small sample size. Of note, we observed a similar relationship between improvement in apathy and measures of the right uncinate fasciculus and right posterior subgenual cingulate. These findings approached but did not reach statistical significance.
One explanation of our current findings is that decreased integrity of the uncinate fasciculus and decreased volume of the posterior subgenual cingulate in some depressed, elderly individuals may interfere with prefrontal cortical communication with limbic regions needed to produce appropriate, motivated responses. This in turn may contribute both to a depressive and apathetic clinical picture. Prior work by Taylor et al. support a model in which functional uncoupling of the amygdala and subgenual cingulate, by compromised uncinate fasciculus integrity, contributes to depression (Anand et al., 2005; Taylor et al., 2007). As such, our findings identify neuroanatomical changes that contribute to persistent apathy within the context of depression but may not be sufficient to solely explain the mechanism of apathy. The uncinate fasciculus is a bidirectional ventral limbic pathway that links the subgenual cingulate with the amygdala (Ebeling and von Cramon, 1992; Klingler and Gloor, 1960; Petrides and Pandya, 2007; Thiebaut de Schotten et al., 2012) and may play a role in processing information about the emotional significance or salience of stimuli when generating emotional expression (Schmahmann and Pandya, 2006). Lindquist et al. point to the role of the amygdala in routinely orienting responses to motivationally salient stimuli and suggest that “the amygdala is most likely to be active when the rest of the brain cannot easily predict what sensations mean, what to do about them or what value they hold in a particular context”(Lindquist et al., 2012). A disconnect between prefrontal cortex and the amygdala through disruption of subgenual cingulate or uncinate fasciculus activity may contribute to both depressive states and to the persistence of an apathetic state within depression. This model may also substantiate our finding of the inadequacy of SSRIs as a treatment and suggest the utility of exploring treatments that would more adequately engage this dopamine-sensitive limbic system. Consistent with this notion, a recent MRI study of post-stroke depression concluded that affective versus apathetic symptoms of the depression were associated with different monoaminergic neuroanatomic pathways (serotonergic and dopaminergic, respectively) (Murakami et al., 2013). Also of note, our parcellation of posterior subgenual cingulate overlapped with BA25, which exhibits aberrant metabolic activity in treatment-resistant depression and is a focus of deep brain stimulation (Mayberg et al., 2005). This raises the question of whether apathy is a significant component of the cases of treatment-resistant depression that respond to deep brain stimulation of BA25.
Our findings are limited in several respects. This study was a post-hoc secondary exploratory analysis. Moreover, as this study was concerned with apathy in late-life depression, our analysis focused on subregions of the anterior cingulate cortex and associated white matter tracts previously implicated in late-life depression. Our study did not include other regions such as basal ganglia that likely play a role in the apathy of stroke and Parkinson's disease. Our neuroanatomical findings are therefore limited to a discussion of apathy in late-life depression. These findings add to the growing knowledge of the neuroanatomy that can perpetuate apathetic states in depression, but they are likely only part of a more complex constellation of brain changes that explains the etiology of apathy. Our study was also limited to cognitively intact, depressed elders. In studies of elders with lower cognitive function and Alzheimer's dementia, apathy is typically more profound and associated with more diffuse brain changes that include decreased anterior cingulate volume but also differences in caudate, putamen, thalamus, and orbitofrontal and temporal cortices (van Reekum et al., 2005). Levy and Dubois discuss how apathy may evolve from a combination of “emotional-affective”, “cognitive”, and “auto-activation” processes (Levy and Dubois, 2006) that may be differentially affected in the apathy of depression versus the apathy of dementia. Other limitations of our study include the absence of randomization to a placebo control group. Thus, we cannot rule out that the observed improvement in apathy score may have derived from the natural course of illness and not escitalopram treatment. We attempted to address the contribution of placebo effect by including a two-week placebo lead-in phase that allowed for the early exclusion of participants prone to placebo effect. Because this is a study of a relatively small number of participants, our findings should be viewed as preliminary.
In a previously published study, we demonstrated that apathetic depressed patients had lower functional connectivity of the dorsal anterior cingulate cortex with dorsolateral and prefrontal cortices and lower functional connectivity of the nucleus accumbens with the amygdala and thalamus than non-apathetic depressed patients (Alexopoulos et al., 2013). Our current findings along with our prior report corroborate a model of apathetic depression that involves altered functional and structural connectivity between prefrontal cortical and limbic regions. The neuroanatomic structures implicated thus far set the stage for a future study exploring a role for Salience and Central Executive Networks in the apathy of late-life depression.
In conclusion, this study indicates that smaller posterior subgenual cingulate volumes and decreased uncinate fasciculus fractional anisotropy increase the likelihood of SSRI-treatment resistant apathy in elderly, depressed patients. Structural abnormalities of the posterior subgenual cingulate and uncinate fasciculus may impede adequate treatment of apathy by interfering with prefrontal cortical recruitment of limbic activity essential to motivated behavior. The clinical and structural neuroanatomy findings detailed in this study highlight apathy as an important and frequently persistent symptom in depression that warrants further study.
Supplementary Material
REFERENCES
- Alexopoulos GS, Glatt CE, Hoptman MJ, Kanellopoulos D, Murphy CF, Kelly RE, Jr., Morimoto SS, Lim KO, Gunning FM. BDNF val66met polymorphism, white matter abnormalities and remission of geriatric depression. Journal of affective disorders. 2010;125:262–268. doi: 10.1016/j.jad.2010.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Murphy CF. Anterior cingulate dysfunction in geriatric depression. International journal of geriatric psychiatry. 2008a;23:347–355. doi: 10.1002/gps.1939. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of affective disorders. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, Gunning FM. Functional connectivity in apathy of late-life depression: a preliminary study. Journal of affective disorders. 2013;149:398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. The American journal of psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. `Vascular depression' hypothesis. Archives of general psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural white matter abnormalities and remission of geriatric depression. The American journal of psychiatry. 2008b;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Alves GS, Alves CE, Lanna ME, Ericeira-Valente L, Sudo FK, Moreira D, Engelhardt E, Laks J. Clinical characteristics in subcortical ischemic white matter disease. Arquivos de neuropsiquiatria. 2009;67:173–178. doi: 10.1590/s0004-282x2009000200001. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov ID, Toga AW, Cummings JL, Thompson PM. Structural correlates of apathy in Alzheimer's disease. Dementia and geriatric cognitive disorders. 2007;24:91–97. doi: 10.1159/000103914. [DOI] [PubMed] [Google Scholar]
- Bolling MY, Kohlenberg RJ. Reasons for quitting serotonin reuptake inhibitor therapy: paradoxical psychological side effects and patient satisfaction. Psychotherapy and psychosomatics. 2004;73:380–385. doi: 10.1159/000080392. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Psychological Assessment Resources. Inc, Lutz; FL: 2001. The Hopkins Verbal Learning Test-Revised. [Google Scholar]
- Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain : a journal of neurology. 2008;131:2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, affective & behavioral neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Lamar M, Zhang A, Yang S, Ajilore O, Kumar A. White-matter tract integrity in late-life depression: associations with severity and cognition. Psychological medicine. 2013:1–11. doi: 10.1017/S0033291713001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase TN. Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment. Neurotoxicity research. 2011;19:266–278. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- Chaturvedi SK, Sarmukaddam SB. Prediction of outcome in depression by negative symptoms. Acta psychiatrica Scandinavica. 1986;74:183–186. doi: 10.1111/j.1600-0447.1986.tb10603.x. [DOI] [PubMed] [Google Scholar]
- Clarke DE, Reekum R, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: an examination of the psychometric properties of the apathy evaluation scale. The Journal of neuropsychiatry and clinical neurosciences. 2007;19:57–64. doi: 10.1176/jnp.2007.19.1.57. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain : a journal of neurology. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta neurochirurgica. 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fava M. Pharmacological approaches to the treatment of residual symptoms. J Psychopharmacol. 2006;20:29–34. doi: 10.1177/1359786806064325. [DOI] [PubMed] [Google Scholar]
- Feil D, Razani J, Boone K, Lesser I. Apathy and cognitive performance in older adults with depression. International journal of geriatric psychiatry. 2003;18:479–485. doi: 10.1002/gps.869. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forsell Y, Jorm AF, Fratiglioni L, Grut M, Winblad B. Application of DSM-III-R criteria for major depressive episode to elderly subjects with and without dementia. The American journal of psychiatry. 1993;150:1199–1202. doi: 10.1176/ajp.150.8.1199. [DOI] [PubMed] [Google Scholar]
- Golden CJ. The Stroop Color and Word Test: A Manual for Clincial and Experimental Uses. Stoelting Co; Chicago, IL: 1978. [Google Scholar]
- Groeneweg-Koolhoven I, de Waal MW, van der Weele GM, Gussekloo J, van der Mast RC. Quality of life in community-dwelling older persons with apathy. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2014;22:186–194. doi: 10.1016/j.jagp.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Gunning FM, Cheng J, Murphy CF, Kanellopoulos D, Acuna J, Hoptman MJ, Klimstra S, Morimoto S, Weinberg J, Alexopoulos GS. Anterior cingulate cortical volumes and treatment remission of geriatric depression. International journal of geriatric psychiatry. 2009;24:829–836. doi: 10.1002/gps.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. International journal of geriatric psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V, Majcher-Tascio M, Hrabe J, Ardekani BA, Alexopoulos GS. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2008;16:255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. Journal of clinical psychopharmacology. 1990;10:343–345. [PubMed] [Google Scholar]
- Holtta EH, Laakkonen ML, Laurila JV, Strandberg TE, Tilvis RS, Pitkala KH. Apathy: prevalence, associated factors, and prognostic value among frail, older inpatients. Journal of the American Medical Directors Association. 2012;13:541–545. doi: 10.1016/j.jamda.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. The Journal of comparative neurology. 1960;115:333–369. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- Kodela S, Venkata PD. Antidepressant induced apathy responsive to dose reduction. Psychopharmacology bulletin. 2010;43:76–79. [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Tupler LA, George LK, Blazer DG. Clinical and phenomenological comparisons of late-onset and early-onset depression. The American journal of psychiatry. 1995;152:785–788. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- Lampe IK, Heeren TJ. Is apathy in late-life depressive illness related to age-at-onset, cognitive function or vascular risk? International psychogeriatrics / IPA. 2004;16:481–486. doi: 10.1017/s1041610204000766. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A. Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2007;15:386–394. doi: 10.1097/JGP.0b013e3180325a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 1999;7:309–316. [PubMed] [Google Scholar]
- Levkovitz Y, Sheer A, Harel EV, Katz LN, Most D, Zangen A, Isserles M. Differential effects of deep TMS of the prefrontal cortex on apathy and depression. Brain stimulation. 2011;4:266–274. doi: 10.1016/j.brs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. The Behavioral and brain sciences. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin RS. Differential diagnosis and classification of apathy. The American journal of psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry research. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. The sources of convergence between measures of apathy and depression. Journal of affective disorders. 1993;28:117–124. doi: 10.1016/0165-0327(93)90040-q. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. Group differences in the relationship between apathy and depression. The Journal of nervous and mental disease. 1994;182:235–239. doi: 10.1097/00005053-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of apathy in Alzheimer's disease. Dementia and geriatric cognitive disorders. 2006;21:144–147. doi: 10.1159/000090674. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Archives of neurology. 2007;64:1015–1020. doi: 10.1001/archneur.64.7.1015. [DOI] [PubMed] [Google Scholar]
- Mattis S. Psychological Assessment Resources. Odessa, FL: 1988. Dementia Rating Scale: Professional Manual. [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mehta M, Whyte E, Lenze E, Hardy S, Roumani Y, Subashan P, Huang W, Studenski S. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young-old and old-old. International journal of geriatric psychiatry. 2008;23:238–243. doi: 10.1002/gps.1868. [DOI] [PubMed] [Google Scholar]
- Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, Small SL, Fonzetti P, Hegel MT, Robinson RG. Prevention of poststroke apathy using escitalopram or problem-solving therapy. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21:855–862. doi: 10.1016/j.jagp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Murakami T, Hama S, Yamashita H, Onoda K, Kobayashi M, Kanazawa J, Yamawaki S, Kurisu K. Neuroanatomic pathways associated with poststroke affective and apathetic depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21:840–847. doi: 10.1016/j.jagp.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Onyike CU, Sheppard JM, Tschanz JT, Norton MC, Green RC, Steinberg M, Welsh-Bohmer KA, Breitner JC, Lyketsos CG. Epidemiology of apathy in older adults: the Cache County Study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2007;15:365–375. doi: 10.1097/01.JGP.0000235689.42910.0d. [DOI] [PubMed] [Google Scholar]
- Padala PR, Padala KP, Monga V, Ramirez DA, Sullivan DH. Reversal of SSRI-associated apathy syndrome by discontinuation of therapy. The Annals of pharmacotherapy. 2012;46:e8. doi: 10.1345/aph.1Q656. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The attentive brain. MIT Press; Cambridge, Mass: 1998. p. xii.p. 577. [Google Scholar]
- Quaranta D, Marra C, Rossi C, Gainotti G, Masullo C. Different apathy profile in behavioral variant of frontotemporal dementia and Alzheimer's disease: a preliminary investigation. Current gerontology and geriatrics research. 20122012:719250. doi: 10.1155/2012/719250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin J, George T, Granger RE, Hussain N, Zhao GW, Marangell LB. Apathy in currently nondepressed patients treated with a SSRI for a major depressive episode: outcomes following randomized switch to either duloxetine or escitalopram. Journal of psychiatric research. 2012;46:667–674. doi: 10.1016/j.jpsychires.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Sato S, Asada T. Sertraline-induced apathy syndrome. The Journal of neuropsychiatry and clinical neurosciences. 2011;23:E19. doi: 10.1176/jnp.23.1.jnpe19. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Uncinate Fasciculus. Fiber pathways of the brain. Oxford University Press; Oxford ; New York: 2006. p. xviii.p. 654. [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, Wilkins CH, Snyder AZ, Couture L, Schechtman K, McKinstry RC. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. The American journal of psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer's disease. Journal of neurology, neurosurgery, and psychiatry. 2006;77:8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD. Neuroimaging correlates of apathy and depression in Alzheimer's disease. The Journal of neuropsychiatry and clinical neurosciences. 2009;21:259–265. doi: 10.1176/jnp.2009.21.3.259. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Gerig G, Krishnan RR. Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatric disease and treatment. 2007;3:669–674. [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex; a journal devoted to the study of the nervous system and behavior. 2012;48:82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- van Reekum R, Stuss DT, Ostrander L. Apathy: why care? The Journal of neuropsychiatry and clinical neurosciences. 2005;17:7–19. doi: 10.1176/jnp.17.1.7. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wongpakaran N, van Reekum R, Wongpakaran T, Clarke D. Selective serotonin reuptake inhibitor use associates with apathy among depressed elderly: a case-control study. Annals of general psychiatry. 2007;6:7. doi: 10.1186/1744-859X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE transactions on medical imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.