Abstract
Primary objective
The purpose of this study was to assess the effect of test duration on the visual-evoked potential (VEP) and related alpha power spectrum measures.
Design and methods
Two conditions (eyes-closed and eyes-open) were tested using four different durations: ten, twenty, forty-five, and sixty seconds. The Diopsys™ NOVA-TR system was used to obtain the visual-evoked potential (VEP) and extracted alpha-wave with its related power spectrum. 16 visually-normal, young-adult subjects (ages 22 to 25 years) participated in the experiment. The stimulus for the eyes-open condition consisted of a black-and-white, alternating checkerboard pattern with a small central fixation target. All trials were performed during one session.
Results
Regarding the VEP parameters, only variability of the VEP amplitude changed significantly with test duration: it decreased with increasing test duration, with the 45 and 60 second trials showing similarly low variability. Regarding the alpha wave parameters, test duration did not have a significant effect on either the mean alpha power or its variability across trials.
Conclusions
The findings demonstrate that forty-five second test durations are sufficient to minimize intra-session variability of the VEP amplitude and latency measurements, whereas several 10 second test durations may be sufficient for accurate measurement of the alpha wave. Optimization of test duration allows for repeatable measures with less total test time. This is especially important for special clinical populations.
Keywords: VEP, alpha wave, visual evoked potential, visual cortex, objective testing, test duration
Introduction
There are two specific desirable attributes for a useful clinical test. First, and foremost, it must be able to ascertain accurate information that varies little from trial to trial. That is, it should be repeatable. Secondly, it is desirable for the test to be performed in a relatively short amount of time, with the selected time deemed sufficient for the aforementioned accuracy and repeatability. Clinicians must often sacrifice test reliability for time-efficiency, and if time-efficiency is chosen inappropriately, the measures may be unreliable and of little clinical use.
The pattern visual-evoked potential (VEP) is a measurable electrical signal generated in response to a phase-alternated checkerboard stimulus. It's electrophysiological origins have been ascertained in both monkeys (thalamocortical cells in lamina 4C of the primary visual cortex [1]) and humans (multiple cortical areas including the striate cortex, extrastriate cortices, and parietal lobe [2]), thus allowing for a clearer understanding of the cortical basis of visual-perceptual processes. The pattern VEP results in a less variable waveform than that found for other related VEP stimuli (e.g., flash VEP) [3], but it is still often not included in general clinical vision test protocols due to claims of having a relatively long test duration with poor repeatability. These concerns become apparent with patients being monitored longitudinally for disease progression or response to treatment [4]. This perception may stem from intra- and/or inter-individual variability. Within an individual, test duration may impact upon the variability of visual-evoked potential parameters. For example, using a 20 second test duration with a short duration-transient VEP paradigm, Tello et al. [4] found very low and desirable coefficients of variation of only 0.03 and 0.15 for the P100 latency and VEP amplitude, respectively, within a single session. Test duration may also affect variability between sessions. However, Tello et al. [4] also found good agreement for latency and amplitude measurements for sessions as far apart as 1-2 months (e.g., intraclass correlation values of 0.71 and 0.81, respectively).
Across disease-free individuals in an age-matched sample, inter-individual VEP variability may result from differences in the anatomy of the primary visual cortex [5,6], influences of extrastriate cortices [7,8], gender [8] accommodative accuracy [9], and general and/or visual attention [9]. The latter two (accommodation and attention) may also change with time (i.e., test duration) [10-12], so this effect is important to consider as well: the VEP should be performed for no more, or less, time than necessary to obtain optimal responses.
Variability concerns have been noted in the EEG and alpha wave literature. This stems from the EEG being modeled as a random process [13,14], as well as differences in how it is recorded, filtered, and how artifact extraction is performed [15]. These concerns resonate with the alpha wave, which has been shown to contain the most variable band of frequencies [16,17], especially when measured over the occipital lobe [18]. Since past studies have used populations of different ages, as well as loosely defined and/or different conditions (e.g., eyes-open and eyes-closed), care must be taken when reviewing and comparing the literature. Two similar, well-designed studies may still produce somewhat different results secondary to task type, intra-individual variability, test-retest reliability, and the inter-individual variability inherent in EEG measures.
Task type itself may influence variability of the EEG measurement. Tasks in which the subject is instructed to achieve a specific but vague “state of mind” (e.g., gazing straight ahead with eyes open or relaxing with eyes closed) are expected to produce more variable results than that for more easily defined and controlled tasks, in which a cognitive or physical response is given to repetitive stimuli (e.g., pressing a button when a target stimulus is briefly presented) [18].
The intra-individual variability inherent in measurement of the alpha wave may be attributed to a subject's age at time of recording, arousal level, and cognitive state, as well as other factors [15]. Test duration is also a possible source of intra-individual variability. Several studies have found that the variability lowering benefit of longer epoch times and repeated within-session test measurements ceases to exist past a specific recording length [19-21]; however, there is no agreement on this critical duration.
In addition, the question remains as to how the visual-evoked potential and component alpha wave should be assessed with respect to temporality. One recording session may be sufficient for populations with stable intra- and inter-individual differences; however, averaging over weeks to months (“sharpening”) may be needed for populations lacking consistency in their VEP (e.g., TBI [22]) or EEG (e.g., Alzheimer's disease [23]). Most importantly, the clinician must know what is “normal” for a patient before a label of “abnormal” is applied [20]: although a one-time measurement may be sufficient, a definitive diagnosis cannot be made with a high degree of certainty until additional measurements are taken.
The alpha wave has been demonstrated by many to reflect static (e.g., maintaining an attentional template [24] or ability to sustain attention during visual tasks [25]) and dynamic (e.g., shifting visual attention for discriminatory tasks [26,27]) aspects of attention. With this in mind, it may assist in monitoring the attentional state in normals and in special populations (e.g., ADHD, TBI, Alzheimer's disease, etc.), either in isolation or concurrent with the VEP measurement. For example, if there were increased VEP amplitude variability between test trials or sessions, the alpha wave recordings from these sessions could be analyzed to help explain this variability. For example, large alpha power with eyes-open (as during VEP testing) is suggestive of poor-visual attentional ability [25], and thus reflective of poor VEP quality. Along with subjective monitoring of the subject during testing, the alpha wave can help in the elimination of such erroneous trials.
Thus, with the above in mind with respect to special populations, the primary purpose of the present experiment was to determine the optimal VEP and power spectrum test-duration for which intra-session variability is the lowest. This optimal test duration is important during the course of a clinical vision examination, so that objective measures of the quality of visual information (VEP) and visual attentional state (alpha) are taken efficiently and accurately, with appropriate interpretation.
Methods
Subjects
Sixteen (4 male, 12 female), visually-normal graduate and optometry students from the State University of New York, State College of Optometry, participated in the study. Ages ranged from 22 to 25 years (X̄ = 23.94, SEM = 0.28). All were myopes having visual acuity of 20/20 at distance and near with refractive correction (X̄RE = −1.98, SEM = 0.47; X̄LE= −2.00, SEM = 0.47). None had a history of traumatic brain injury, ADHD, or any other attentional disorder. Exclusion criteria included the presence of amblyopia, strabismus, ocular or systemic disease, chronic or progressive neurological disease, cognitive deficit, or history of seizures. The Institutional Review Board (IRB) at the College of Optometry approved the study. Subjects provided their written, informed consent.
Apparatus
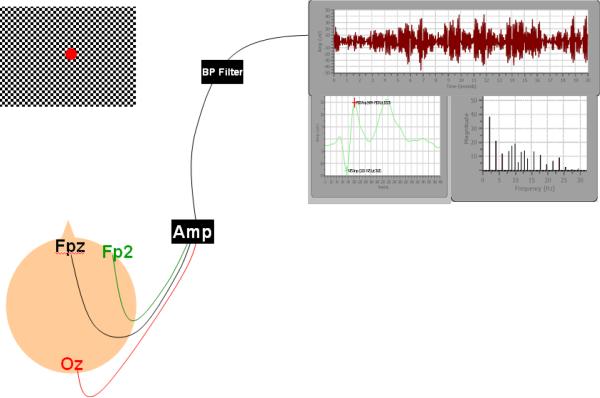
To record and analyze the VEP data, the Diopsys™ NOVA-TR system (Diopsys, Inc., Pine Brook, New Jersey, USA) was used [28,29]. The system consists of a 17H × 15V degree display monitor for stimulus presentation, a monitor for use by the experimenter for data viewing, and a computer for stimulus generation and graphical display (Figure 1). The Diopsys™ system is available commercially and is approved by the FDA for both research and clinical use. A Diopsys Enfant Amp 100 amplifier was used to increase the EEG signals.
Fig. 1.
Schematic diagram of Diopsys™ stimulus, electrodes, and experimenter display. The Diopsys™ system consists of a stimulus display screen, three electrodes, and a display screen for experimental observation of the dynamic VEP and alpha wave responses in real time
Three Grass (Grass Technologies, Astro-Med Inc., West Warwick, RI, USA) gold cup electrodes, each (active, ground, and reference) 1 cm in diameter, were used to record the VEP. The Diopsys™ E1M105 impedance meter was used to measure impedance to assure adequate electrical output. To clean the skin, NUPREP (Weaver and Co., Aurora, CO, USA) skin abrasive gel was used. Ten 20 (Weaver and Co., Aurora, CO, USA) conductive gel was used to adhere the electrodes to the scalp.
Procedures
All subjects were provided a vision screening. This included distance visual acuity, distance contrast sensitivity, and a cover test at near, all of which were clinically normal [30]. In addition, each subject confirmed that they had received a comprehensive optometric vision examination within the past year. They were also queried regarding their general ocular and medical health, especially as related to seizures, brain injury, and attentional problems.
The VEP amplitude, latency, and alpha-wave activity (8-13 Hz) from the primary visual cortex were measured using one Grass gold active-channel electrode, one reference electrode, and one ground electrode. The electrode placement was slightly modified from the International 10/20 system [3], as suggested by the manufacturer. The active electrode was placed at the Oz position, 2.5 cm above the inion. The reference electrode was placed at the Fpz position (approximately 10% of the distance from the nasion to the inion), and the ground electrode was positioned on the side of the forehead at Fp2. An elastic headband maintained the position of the electrodes on the scalp.
The impedance of each electrode was measured using the Diopsys™ impedance meter. Each electrode had to have an impedance of <5 kilo-ohms, per the standards of the International Society for Clinical Electrophysiology of Vision (ISCEV) [3]. The electrodes were then connected to the Diopsys™ Enfant Amp 100 amplifier, with an amplification factor of 10,000 to increase the very small analog signals. An electronic band-pass filter (0.5-100 Hz) filtered any noise. An artifact detector in the provided software eliminated undesirable EEG signals produced by either blinks or saccadic gaze shifts. The sampling rate was 1024 samples per second.
After attaching the electrodes, subjects were instructed to place their head in a chinrest/headrest assembly, and then to gaze carefully at the center of the monitor. The chinrest was used to ensure that subjects remained in a constant position and viewing distance throughout testing. Seat height was adjusted, so that the screen was at eye level and centered along the midline at a test distance of 1m. The VEP measurements were recorded with the subjects viewing binocularly with their distance refractive correction in place. Testing was performed in a darkened room (38 lux) with natural pupils. Subjects had rest periods between test conditions, as needed.
Data were obtained under two test conditions: (1) eyes-open, and (2) eyes-closed, with five trials per condition [31]. Each condition was performed for four different test durations: ten, twenty, forty-five, and sixty seconds, so that there were forty trials per subject. The eight conditions (eyes-open and eyes-closed at four durations) were counterbalanced, so that each condition followed the other at least one time [32]. First, a practice trial was performed to familiarize subjects with the stimulus. In the eyes-open condition, a standard full-field 64X64 (17 H X 15 V degrees), black-and-white checkerboard pattern (20.59 min arc check size at 1 meter) was displayed on the monitor to determine the conventional baseline VEP and eyes-open alpha power. The checkerboard pattern had a Michelson contrast of 85% and a mean luminance of 64 cd/m2. For each trial, the checkerboard pattern had a temporal frequency of 1 Hz (two reversals per second), per the Diopsys™ system standard software. To maintain fixation and visual attention, the Diopsys™ display contains a small, central, red, rotating central fixation target (0.5 deg diameter), which was presented at all times. In the eyes-closed condition, subjects closed their eyes, relaxed, and imagined that they were staring straight ahead where the central red fixation target had been presented. Additionally, subjects were instructed to “clear their mind” and sit back in the chair to achieve a “completely relaxed” state. This ensured that maximum alpha wave activity was elicited [33]. Sixty seconds were provided before the data collection to ensure adequate time for such relaxation to achieve this “completely relaxed” state.
Data Analysis
The Diopsys™ software was used to quantify and analyze the VEP and power spectrum data. The difference in amplitude (microvolts, μv) between the N75 and P100 components was taken as the VEP amplitude (delta). The N75 and P100 latencies were also recorded (milliseconds). Values for the VEP amplitude and latency were initially derived automatically with a cursor on the screen, with a resolution of approximately 0.1 μv and 0.04 msec, respectively. However, in less than 5% of the cases, when the waveform was slightly flattened, the automatic placement resulted in slight offset of the cursors from the trough-and-peak of the waveform. In these situations, manual adjustment was used to slightly alter the cursors, and thus allow better centering and optimization of their locations for more accurate quantitative assessment. Alpha-wave characteristics were extracted from the eyes-open VEP trials and derived using Fourier transformation and power spectrum [34]. The power spectrum values were read directly from the display screen, with a resolution of approximately 1 μv. As 9, 10, and 11 Hz showed the largest power and similar variability in a previous study [31], only these frequencies were analyzed for the power spectrum. Peak frequency (fp) was calculated for these specific frequencies using the equation provided by Klimesch [35] to ensure that all subjects had a similar alpha bandwidth. As in a previous study from this laboratory [31], the alpha attenuation ratio was defined as the alpha power (μv2) during the eyes-closed condition divided by the alpha power during the eyes-open condition [AAR2:1]; AAR2:1 values greater than 1.0 suggest attenuation, as the alpha power is largest with the eyes-closed condition (Figure 3). An AAR2:1 value of 2.0 or greater was considered to represent considerable attenuation ability [31]. The coefficient of variation (CV=σ/|μ|, when σ = the standard deviation of the multiple trials for each condition, and μ = the mean of these multiple trials) was used to assess repeatability of all measures taken [36] at the four different test durations. It represents the intra-individual variability [18]: the smaller the value, the greater the repeatability.
Fig. 3.
Mean alpha power across conditions summed across test durations. Power changes evident when eyes are open and closed at three frequencies: 9, 10, and 11 Hz. Mean +1 SEM is plotted. Mean AAR2:1 value is shown in filled bars
Statistical analysis of all data was performed using GraphPad Prism 5 software. This included the ANOVA. When a comparison was not significant, the data were not shown.
Results
I. VEP Parameters
Mean summed across all durations
Group mean parameters of the visual-evoked potential were analyzed summed across all durations. VEP amplitude ranged from 7.07 μv to 38.02 μv (X̄ = 20.31, SEM = 2.17) (Table 1), N75 latency ranged from 71.27 ms to 81.07 ms (X̄ = 77.83, SEM = 0.68), and P100 latency ranged from 99.60 ms to 109.20 ms (X̄ = 105.57, SEM = 0.71).
Table 1.
Mean VEP Amplitude (μv)
| Mean VEP Amplitude For Each Duration | |||||
|---|---|---|---|---|---|
| 10 | 20 | 45 | 60 | MEAN (summed across durations) | |
| MEAN | 19.17 | 20.21 | 20.75 | 21.11 | 20.31 |
| SEM | 2.13 | 2.18 | 2.26 | 2.19 | 2.17 |
Effects of Duration
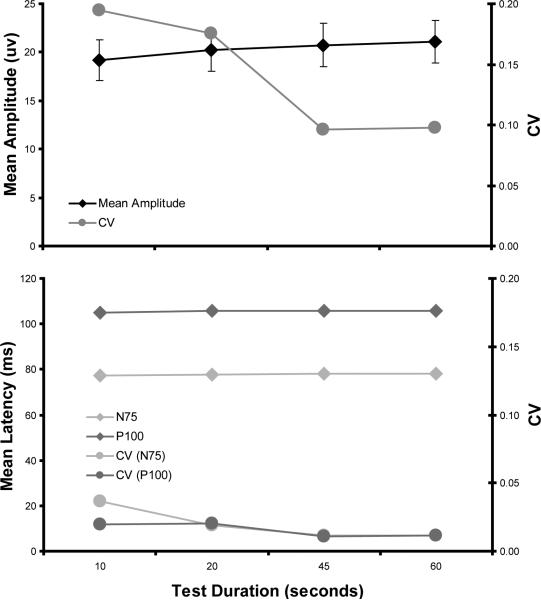
The mean VEP amplitude was examined for the effect of test duration (Figure 2). A one-way ANOVA comparing the VEP amplitude at different test durations revealed that this measure did not change significantly with test duration [F(3, 60) = 0.14, p = 0.9303]. In contrast, a one-way ANOVA comparing the VEP coefficient of variation measure revealed that amplitude variability did change significantly with test duration [F(3, 60) = 9.08, p < 0.0001] (Figure 2). Fisher's LSD post-hoc test revealed significant differences between the mean coefficient of variation at 10 and 45 seconds, 10 and 60 seconds, 20 and 45 seconds, and 20 and 60 seconds (all p <0.05), with the CV decreasing with increasing test duration. Among the sixteen subjects, CV was lowest for 9 subjects for the 45-second trials and lowest in 7 subjects for the 60-second trials (Table 2).
Fig. 2.
Mean (+/−1 SEM) values for VEP amplitude, N75 latency, and P100 latency with their respective coefficient of variation values for each test duration. The SEM error bars are smaller than symbol size for the mean latency and CV measurements
Table 2.
VEP Amplitude Coefficient of Variation Across Durations For Each Subject
| Duration | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|
| 10 | 0.14 | 0.42 | 0.28 | 0.14 | 0.22 | 0.29 | 0.21 | 0.14 |
| 20 | 0.13 | 0.19 | 0.21 | 0.26 | 0.24 | 0.19 | 0.05 | 0.20 |
| 45 | 0.08 | 0.13 | 0.10 | 0.12 | 0.09 | 0.19 | 0.04 | 0.24 |
| 60 | 0.03 | 0.16 | 0.06 | 0.04 | 0.06 | 0.30 | 0.10 | 0.11 |
| MEAN (subject) | 0.09 | 0.23 | 0.16 | 0.14 | 0.15 | 0.24 | 0.10 | 0.17 |
| Duration | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 |
|---|---|---|---|---|---|---|---|---|
| 10 | 0.16 | 0.14 | 0.11 | 0.16 | 0.10 | 0.22 | 0.16 | 0.23 |
| 20 | 0.19 | 0.14 | 0.25 | 0.07 | 0.27 | 0.09 | 0.13 | 0.21 |
| 45 | 0.10 | 0.06 | 0.10 | 0.07 | 0.08 | 0.03 | 0.07 | 0.06 |
| 60 | 0.11 | 0.10 | 0.07 | 0.05 | 0.11 | 0.06 | 0.10 | 0.13 |
| MEAN (subject) | 0.14 | 0.11 | 0.13 | 0.09 | 0.14 | 0.10 | 0.12 | 0.16 |
| 10 sec | 20 sec | 45 sec | 60 sec | |
|---|---|---|---|---|
| MEAN (group) | 0.195 | 0.175 | 0.096 | 0.098 |
Bolded number represents the lowest coefficient of variation for an individual subject across the four test durations.
Mean N75 and P100 latency were examined for effect of test duration (Figure 2). A one-way ANOVA comparing the N75 latency at the different test durations, and a one-way ANOVA comparing P100 latency at the different test durations, revealed that neither latency measure changed significantly with test duration [F(3, 60) = 0.17, p = 0.9146] and [F(3, 60) = 0.45, p = 0.7172], for N75 and P100, respectively. Additionally, variability of these measures did not change significantly with test duration [F(3, 60) = 1.26, p = 0.2955] and [F(3, 60) = 2.75, p = 0.0506], for N75 and P100, respectively.
II. Power Spectrum
Mean summed across all durations
Group mean power spectrum (PS) data were analyzed (Figure 3). Summed across all four test durations (10, 20, 45, and 60 sec) and two conditions (eyes-open and eyes-closed), 10 Hz had the largest power (X̄ = 25.35 μv2, SEM = 3.09), followed by 11 Hz (X̄ = 22.475 μv2, SEM = 3.35), and then 9 Hz (X̄ = 21.80 μv2, SEM = 3.20). A two-way ANOVA for the factors of PS frequency and test condition revealed that the PS frequency did not significantly contribute to variations in power among the three frequencies [F(3, 60) = 0.54, p = 0.5838], but that test condition did [F(3, 60) = 60.69, p < 0.0001]. Fisher's LSD post-hoc tested revealed differences between the mean power in the eyes-open and eyes-closed conditions at all frequencies (13.0 vs. 30.6 μv2 at 9 Hz, 17.5 vs. 33.2 μv2 at 10 Hz, and 13.0 vs. 32.0 μv2 at 11 Hz, all p < 0.0005). There was no interaction between the factors [F(2, 90) = 0.05, p = 0.9543].
A one-way ANOVA revealed no significant differences in the coefficient of variation among the three frequencies, with either the eyes-open [F(2, 45) = 1.74, p = 0.1860] or the eyes-closed [F(2, 45) = 0.33, p = 0.7172] conditions. Individual peak frequencies ranged from 9.81 Hz to 10.21 Hz (X̄ = 10.03 Hz, SEM = 0.02).
Effects of Duration
Eyes Open
A two-way ANOVA for the factors of frequency and test duration revealed that neither contributed significantly to the variance in power ([F(2, 180) = 1.81, p = 0.1653], [F(3, 180) = 2.34, p = 0.0739]), respectively. There was no interaction between the two variables [F(6, 180) = 0.17, p = 0.9856]. However, the power value progressively increased with increasing test duration. Furthermore, the difference between power measured at 10 and 60 seconds was always largest at each frequency: 3.638 μv2 at 9 Hz, 1.487 μv2 at 10 Hz, and 3.847 μv2 at 11 Hz.
A two-way ANOVA for the factors of frequency and test duration revealed that frequency did not significantly contribute to the variance in the coefficient of variation [F(2, 180) = 1.20, p = 0.3050], but that test duration did [F(3, 180) = 3.94, p = 0.0094]. Fisher's LSD post-hoc test revealed significant differences among relevant comparisons [10 (0.47) vs. 45 seconds (0.65), 10 (0.47) vs. 60 seconds (0.69), both at 9 Hz and p < 0.05] There was no interaction between the two variables [F(6, 180) = 0.74, p = 0.6175].
Eyes Closed
A two-way ANOVA for the factors of frequency and test duration revealed that neither contributed significantly to the variance in power ([F(2, 180) = 0.79, p = 0.4572], [F(3, 180) = 0.07, p = 0.9750]), respectively. There was no interaction between the two variables [F(6, 180) = 0.08, p = 0.9980].
A two-way ANOVA for the factors of frequency and test duration revealed that frequency did not significantly contribute to the variance in the coefficient of variation [F(2, 180) = 1.52, p = 0.2225], but that test duration did [F(3, 180) = 3.520, p = 0.0163]. Fisher's LSD post-hoc test revealed a significant difference among one relevant comparison [10 (0.41) vs. 45 seconds (0.58) at 10 Hz, p < 0.05], There was no interaction between the two variables [F(6, 180) = 0.45, p = 0.8457].
III. Alpha Attenuation
Mean summed across all durations
Mean alpha attenuation ratio (AAR2:1) data were analyzed summed across all durations. For 9, 10, and 11 Hz, the mean AAR2:1 was 2.09 (SEM = 0.25), 2.04 (SEM = 0.18), and 2.26 (SEM = 0.26), respectively. A one-way ANOVA revealed no significant differences between frequencies for the attenuation ratio magnitude [F(2, 45) = 0.36, p = 0.6976].
Effects of Duration
A two-way ANOVA for the factors of frequency and test duration revealed that neither contributed significantly to the variance in the alpha attenuation ratio ([F(2, 180) = 0.73, p = 0.4830], [F(3, 180) = 2.32, p = 0.0762]), respectively. There was no interaction between the two variables [F(6, 180) = 0.19, p = 0.9781].
Discussion
VEP
The results of the present study provide strong evidence for achieving temporal optimization of the VEP response. Although the mean VEP amplitude did not change with duration, the coefficient of variation did. Since the VEP is a time-averaged response, longer test-durations allow a stronger signal component, as compared to noise, to mature. The signal arises from responses of primary visual cortex cells, which were once thought to be highly variable [37-39]. However, their response variability has recently been demonstrated to be low under suprathreshold stimulus conditions (i.e., 85% contrast VEP) and high only at low contrast threshold stimulus levels [40]. Additionally, monkey studies of primary visual cortex have shown coefficient of variation values for cell response variability to decrease linearly with increasing stimulus strength (i.e., high firing rates) [41]. The lowest CV value, found for the highest signal strength [41], is similar to the CV values found in the present study for VEP amplitude at all durations. These findings suggest that the present study's stimulus configuration is likely to be eliciting maximal firing rates, with low variability, in cells from the primary visual cortex. It may also be concluded that the VEP response variability cannot be attributed to retinal ganglion cell and/or LGN cell responses: these cell populations have been shown to have similar [40] or lower [37-39] response variability than cells in the primary visual cortex.
Clinically, reduction of this noise with increasing test duration will result in less variable response amplitude measurements. However, there appears to be a point at which the benefit of a stronger signal ceases to exist: thus, there may be neurophysiological limitations. When presented with time-locked stimuli, neuronal populations still exhibit response time variability due to small inherent differences in response time for the individual neurons of the pool. Thus, there will always be noise present in the visual system [42]. Although there are cellular temporal averaging mechanisms to overcome such limitations [42], the question remains as to the time frame over which these mechanisms remain useful. The present study provides some insight into this critical question: there was no difference in the relatively higher CV values for trials run for 10 versus 20 seconds, or between the lower CV values in trials run for 45 versus 60 seconds. In fact, trials run for 45 seconds had the lowest mean coefficient of variation among all four test-durations. This suggests that for the most desirable results (i.e., the least variable), the VEP should be performed for more than twenty seconds, but not for more than forty-five, with several trials (e.g., n=4) being optimal for an accurate determination.
The question as to whether or not a thirty second test-duration would provide the same benefits as forty-five seconds remains to be tested in the future. In some recent pilot testing in our laboratory, test trials of 20 and 30 seconds revealed similar CV values (i.e., 45 second trials remained less variable than shorter test durations). This “optimal” test duration may reflect the effective physiological limit for temporal averaging in the visual system, and if so, it may provide additional insight into how the visual system filters and processes such information. As a whole, the coefficients of variation for VEP amplitude, N75, and P100 measurements (0.14, 0.04, and 0.02, respectively) were very low, and furthermore, they were similar to those found by Tello et al. [4]: they found values of 0.15 and 0.03 for the VEP amplitude and P100 measurements, respectively.
Other factors expected to increase intra-individual variability, specifically accommodative ability and attention, did not appear to have a significant effect. As decreases in either or both would be expected to decrease the quality of the visual information, a decrease in VEP amplitude would be predicted [9]. However, the present results revealed the opposite: the VEP amplitude progressively increased with increasing test duration. This small increase in amplitude may suggest that even if amplitude decreases secondary to defocus or inattention occur, a critical test duration yields stronger signals sufficient to overcome them to a great extent.
With regards to inter-individual variability among the normal population, and determining whether or not a subject falls outside this range, several suggestions can be drawn from our data. First, since the VEP is expected to have the greatest variability for the shortest test durations, those subjects who yield increasing variability with increasing test duration may have trouble sustaining attention throughout each trial or throughout the entire test session. Second, any change in amplitude with test duration may reflect normal variability in the population, since no significant trend was identified from the current study. Third, although an optimum test duration has been suggested, researchers and clinicians should use this information in conjunction with their own judgment when a spurious value is obtained.
Alpha Wave/Power Spectrum
As compared to the VEP measurements, the mean alpha wave coefficients of variation were expectedly larger and in agreement with those found in previous studies, for both the eyes-open [18] and the eyes-closed [43] conditions. Although suggested previously in the literature (e.g., [44]), there was no difference in variability among the eyes-open and eyes-closed conditions: this supports other previous studies (e.g., [15, 45]). However, the two conditions did provide insight into the notion that individual differences are often lost when the data are grouped [15]. The eyes-open condition, with a mean CV of 0.56, had values ranging from 0.44-0.70 among all frequencies and subjects. The eyes closed-condition, with a mean CV of 0.50, was less variable by comparison, but had a larger range of values (0.34-0.75). This suggests that observation of both the group and individual subject alpha data yields a more comprehensive picture than observation of either one alone.
Test duration did not have a significant effect on either the mean alpha wave responses or their variability. This initially appears to disagree with previous findings, which suggested that longer epoch durations may reduce variability of such EEG measurements [19-21]. However, comparison between the aforementioned studies is not direct: different epoch durations, and not truly test-durations per se, were used. Epochs were often chosen from the “best” (i.e., least noisy) recordings and pooled from and across any point in time, whereas the present study measured and analyzed all data collected during a trial. Since extensive filtering and selection is not likely to be performed in a clinical setting, the present findings may be more relevant.
With regards to a previous study in our laboratory [31], several findings were confirmed. First, a similar hierarchy of power magnitude was noted: 10 Hz provided the largest mean power, followed by 11 Hz and 9 Hz. Second, no frequency was significantly more variable than another. Third, peak frequency was again demonstrated to be a stable characteristic in a group of visually-normal, young-adults, and it may be a future metric for assessing EEG changes that may occur with age and/or disease. Lastly, alpha attenuation ratios of 2.0 or greater were found at all frequencies measured, thus suggesting that it too is a relatively stable and predictable physiologically-based metric under carefully controlled test conditions.
Conclusions
The visual-evoked potential and alpha wave are both physiological phenomenon pertinent to clinical practice. However, they are often not used due to their perceived response variability. Variability is present in all physiological measures, and thus it is up to the clinician to incorporate ways to minimize and interpret it. The results of the present study suggest that within-session variation in measurements of the VEP amplitude may be minimized by using several 45 second test-durations. Similarly, several 10 second test durations may be sufficient for accurate and repeatable measurement of the alpha wave. Furthermore, regardless of the VEP test duration used, the stability of alpha variation across test durations provides a window into the subject's attentional state.
Supplementary Material
Acknowledgements
We would like to thank Diopsys™ for the use of their equipment.
References
- 1.Schroeder CE, Tenke CE, Givre SJ, Arezzo JC, Vaughan HG. Striate cortical contribution to the surface-recorded pattern-reversal VEP in the alert monkey. Vis Res. 1991;11:1143–1157. doi: 10.1016/0042-6989(91)90040-c. [DOI] [PubMed] [Google Scholar]
- 2.Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visually evoked potential. Hum Brain Mapp. 2001;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Tormene AP, Vaegan ISCEV standard for clinical visual-evoked potentials (2009 update). Doc Ophthalmol. 2009;120:111–119. doi: 10.1007/s10633-009-9195-4. [DOI] [PubMed] [Google Scholar]
- 4.Tello C, DeMoraes CGV, Prata TS, Derr P, Patel J, Siegfried J, Liebmann JM, Ritch R. Repeatability of short-duration transient visual evoked potentials in normal subjects. Doc Ophthalmol. 2010;120:219–228. doi: 10.1007/s10633-010-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rademacher J, Caviness VS, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993;3:313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- 6.Aine CJ, Supek S, George JS, Ranken D, Lewine J, Sanders J, Best E, Tiee W, Flynn ER, Wood CC. Retinotopic organization of human visual cortex: departures from the classical model. Cereb Cortex. 1996;6:354–361. doi: 10.1093/cercor/6.3.354. [DOI] [PubMed] [Google Scholar]
- 7.Ossenblok P, Spekreijse H. The extrastriate generators of the EP to checkerboard onset: a source localization approach. Electroencephalogr Clin Neurophysiol. 1991;80:181–193. doi: 10.1016/0168-5597(91)90120-m. [DOI] [PubMed] [Google Scholar]
- 8.Klistorner AI, Graham SL. Electroencephalogram-based scaling of multifocal visual evoked potentials: effect on intersubject amplitude variability. Invest Ophthalmol Vis Sci. 2001;42:2145–2152. [PubMed] [Google Scholar]
- 9.Mezer E, Bahir Y, Leibu R, Perlman I. Effect of defocusing and of distracted attention upon recordings of the visual evoked potential. Doc Ophthalmol. 2004;109:229–238. doi: 10.1007/s10633-004-8055-5. [DOI] [PubMed] [Google Scholar]
- 10.Ciuffreda KJ. Nearwork-induced transient myopia: basic and clinical aspects. Optom Vis Dev. 1999;30:5–20. [Google Scholar]
- 11.Chase C, Tosha C, Borsting E, Ridder W. Visual discomfort and objective measures of static accommodation. Optom Vis Sci. 2009;86:883–889. doi: 10.1097/OPX.0b013e3181ae1b7c. [DOI] [PubMed] [Google Scholar]
- 12.Tosha C, Borsting E, Ridder WH, Chase C. Accommodation response and visual discomfort. Ophthalmic Physiol Opt. 2009;29:625–633. doi: 10.1111/j.1475-1313.2009.00687.x. [DOI] [PubMed] [Google Scholar]
- 13.Dumermuth G. Numerical spectral analysis of the electroencephalogram. In: Remond A, editor. Handbook of electroencephalography and clinical neurophysiology, 5A. Elsevier; Amsterdam: 1973. pp. 33–60. [Google Scholar]
- 14.Gasser T. General characteristics of the EEG as a signal. In: Remond A, editor. EEG Informatics. A didactic review of methods and applications of EEG data processing. Elsevier; Amsterdam: 1977. pp. 37–55. [Google Scholar]
- 15.Oken BS, Chiappa KH. Short-term variability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol. 1988;69:191–198. doi: 10.1016/0013-4694(88)90128-9. [DOI] [PubMed] [Google Scholar]
- 16.Matousek M, Petersen I. Frequency analysis of the EEG in normal children and adolescents. In: Kellaway P, Petersen I, editors. Automation of clinical electroencephalography. Raven Press; New York: 1973. pp. 75–102. [Google Scholar]
- 17.Burgess A, Gruzelier J. Individual reliability of amplitude distribution in topographical mapping of EEG. Electroencephalogr Clin Neurophysiol. 1993;86:219–223. doi: 10.1016/0013-4694(93)90101-z. [DOI] [PubMed] [Google Scholar]
- 18.Maltez J, Hyllienmark L, Nikulin VV, Brismar T. Time course and variability of power in different frequency bands of EEG during resting conditions. Clin Neurophysiol. 2004;34:195–202. doi: 10.1016/j.neucli.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Lynch J, Paskewitz DA, Orne MT. Inter-session stability of human alpha rhythm densities. Electroencephalogr Clin Neurophysiol. 1974;36:538–540. doi: 10.1016/0013-4694(74)90211-9. [DOI] [PubMed] [Google Scholar]
- 20.Van Dis H, Corner M, Dapper R, Hanewald G, Kok H. Individual differences in the human electroencephalogram during quiet wakefulness. Electroencephalogr Clin Neurophysiol. 1979;47:87–94. doi: 10.1016/0013-4694(79)90035-x. [DOI] [PubMed] [Google Scholar]
- 21.Mocks J, Gasser T. How to select epochs of the EEG at rest for quantitative analysis. Electroencephalogr Clin Neurophysiol. 1984;58:89–92. doi: 10.1016/0013-4694(84)90205-0. [DOI] [PubMed] [Google Scholar]
- 22.Freed S, Fishman Hellerstein L. Visual electrodiagnostic findings in mild traumatic brain injury. Brain Inj. 1997;11:25–36. doi: 10.1080/026990597123782. [DOI] [PubMed] [Google Scholar]
- 23.Soininen H, Partanen J, Laulumaa V, Helkala E-L, Laakso M, Riekkinen PJ. Longitudinal EEG spectral analysis in early stage of Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1989;72:290–297. doi: 10.1016/0013-4694(89)90064-3. [DOI] [PubMed] [Google Scholar]
- 24.Tineke G, Boehler CN, Kenemans JL, Woldorff MG. Differential functional roles of slow-wave and oscillatory alpha activity in visual sensory cortex during anticipatory visual-spatial attention. Cereb Cortex. 2011;21:2204–2216. doi: 10.1093/cercor/bhq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller P. Attention and the EEG alpha rhythm in learning disabled children. J Learn Disabil. 1978;11:303–312. doi: 10.1177/002221947801100507. [DOI] [PubMed] [Google Scholar]
- 26.Kelly SP, Gomez-Ramirez M, Foxe JJ. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. Eur J Neurosci. 2009;30:2224–2234. doi: 10.1111/j.1460-9568.2009.06980.x. [DOI] [PubMed] [Google Scholar]
- 27.van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav NK, Ludlam DP, Ciuffreda KJ. Effect of different stimulus configurations on the visual-evoked potential (VEP). Doc Ophthalmol. 2012;124:177–196. doi: 10.1007/s10633-012-9319-0. [DOI] [PubMed] [Google Scholar]
- 29.Ciuffreda KJ, Yadav NK, Ludlam DP. Effect of binasal occlusion (BNO) on the visual-evoked potential (VEP) in mild traumatic brain injury (mTBI). Brain Inj. doi: 10.3109/02699052.2012.700088. in press. [DOI] [PubMed] [Google Scholar]
- 30.Benjamin WJ. Borish's Clinical Refraction. 2nd Ed Butterworth-Heinemann; St Louis: 2006. [Google Scholar]
- 31.Willeford KT, Ciuffreda KJ, Yadav NK, Ludlam DP. Objective assessment of human visual attention. Doc Ophthalmol. doi: 10.1007/s10633-012-9357-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley JV. Complete counterbalancing of immediate sequential effects in a latin square design. J Amer Statist Assoc. 1958;53:525–528. [Google Scholar]
- 33.Gomarus HK, Wijers AA, Minderaa RB, Althaus M. Do children with ADHD and/or PDD-NOS differ in reactivity of alpha/theta ERD/ERS to manipulations of cognitive load and stimulus relevance? Clin Neurophysiol. 2009;120:73–79. doi: 10.1016/j.clinph.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Dumermuth G. Spectral analysis of the EEG. Neuropsychobiology. 1987;17:85–99. doi: 10.1159/000118345. [DOI] [PubMed] [Google Scholar]
- 35.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 36.Salkind N, editor. Encyclopedia of research design. Sage; Thousand Oaks: 2010. [Google Scholar]
- 37.Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 1983;23:775–785. doi: 10.1016/0042-6989(83)90200-6. [DOI] [PubMed] [Google Scholar]
- 38.Swindale NV, Mitchell DE. Comparison of receptive field properties of neurons in area 17 of normal and bilaterally amblyopic cats. Exp Brain Res. 1994;99:399–410. doi: 10.1007/BF00228976. [DOI] [PubMed] [Google Scholar]
- 39.Kara P, Reinagel P, Reid RC. Low response variability in simultaneously recorded retinal, thalamic, and cortical neurons. Neuron. 2000;27:635–646. doi: 10.1016/s0896-6273(00)00072-6. [DOI] [PubMed] [Google Scholar]
- 40.Gur M, Snodderly DM. High response reliability of neurons in primary visual cortex (V1) of alert, trained monkeys. Cereb Cortex. 2006;16:888–895. doi: 10.1093/cercor/bhj032. [DOI] [PubMed] [Google Scholar]
- 41.Gur M, Beylin A, Snodderly DM. Response variability of neurons in primary visual cortex (V1) of alert monkeys. J Neurosci. 1997;17:2914–2920. doi: 10.1523/JNEUROSCI.17-08-02914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faisal AA, Selen LPJ, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–203. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salinsky MC, Oken BS, Morehead L. Test-retest reliability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol. 1991;79:382–392. doi: 10.1016/0013-4694(91)90203-g. [DOI] [PubMed] [Google Scholar]
- 44.Gasser T, Bacher P, Steinberg H. Test-retest reliability of spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol. 1985;60:312–319. doi: 10.1016/0013-4694(85)90005-7. [DOI] [PubMed] [Google Scholar]
- 45.Pollock VE, Schneider LS, Lyness SA. Reliability of topographic quantitative EEG amplitude in healthy late-middle-aged and elderly subjects. Electroencephalogr Clin Neurophysiol. 1991;79:20–26. doi: 10.1016/0013-4694(91)90152-t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.