Abstract
Recent studies indicate that histone lysine methylation is subject to enzyme-catalyzed reversion, and jumonji C (JmjC) domain–containing proteins have been identified as one of the members of histone demethylases. Although an increasing number of histone demethylases have been identified and biochemically characterized, their biological functions are poorly characterized. To elucidate the physiological functions, we generated the knockout mouse model of dimethylated or monomethylated histone 3 lysine 9 (H3K9me2/1)–specific JmjC domain–containing histone demethylase 2A (JHDM2A; also known as JMJD1A and KDM3A) and showed that JHDM2A is essential for spermatogenesis. Jhdm2a-deficient mice exhibited impaired postmeiotic chromatin condensation, which caused infertility, even though the hormonal levels were maintained. Further molecular and biochemical analysis revealed that JHDM2A directly bound to the core promoter regions of transition nuclear protein 1 (Tnp1) and protamine 1 (Prm1) genes, and it induced the transcriptional activation of these genes by removing H3K9 methylation, which is known as a silencing marker of gene transcription. This work uncovered a role for JHDM2A in spermatogenesis and identified 2 downstream genes that are critical for sperm nuclear condensation. In addition, we also showed that JHDM2A plays a role in regulating fat metabolic gene expression in muscle and brown fat tissue, and the knockout mice exhibited obesity and hyperlipidemia. Thus, JHDM2A possesses organ/tissue-specific target genes, and impairment of this molecule cannot be compensated by other JmjC-containing histone demethylases, suggesting the importance of this molecule in vivo.
Keywords: Sperm, histone demethylation
Despite its relatively short history, histone methylation has become one of the best-studied research areas in epigenetics, mainly because of its importance in transcriptional regulation of gene expression in various organisms. In mammals in particular, many histone methyltransferases are known to be involved in human diseases and cancers through controlling transcription of downstream target genes, and studies using knockout mice have demonstrated that many histone methyltransferases possess indispensable function in vivo.
Histone methylation is described as “cellular memory,” because the modification is sometimes maintained over cell division (Peters and Schubeler, 2005). Therefore, the existence of histone demethylase(s) has been questioned until the first discovery of histone demethylase, lysine-specific demethylase 1 (LSD1), by Shi et al in 2004. The next identified histone demethylase, JmjC-containing histone demethylase/F-box and leucine-rich repeat protein 11 (JHDM1A/Fbxl11), consisted of a large family called JmjC domain–containing proteins (Tsukada et al, 2006). Because the JmjC domain is responsible for the catalytic activity, it was easy to predict that other JmjC domain–containing proteins are also histone demethylases. At present, nearly 20 JmjC family members have been identified as demethylases for distinct methylated lysine residues in H3 (Klose et al, 2006), and now the discovery of histone demethylases raises a new question: What is the physiological output to cancel “cellular memories”?
Histone 3 Lysine 9 Methylation and Spermatogenesis
During mammalian spermatogenesis, unique and dynamic genetic/epigenetic changes are observed, such as establishment of imprinting information in primordial germ cells (PGCs), meiotic chromosomal recombination and segregation, and histone removal followed by chromatin condensation in spermiogenesis. In these events, it has been elucidated that alteration of chromatin structure by histone modifications plays an important role (Rousseaux et al, 2005; Godmann et al, 2009), and among these modifications, dynamics of histone 3 lysine 9 (H3K9) methylation are one of the best-characterized modifications in the study of germ cell development.
For instance, genome-wide methylation of H3K9 catalyzed by G9a-like protein (G9a)/Euchromatic histone lysine N-methyltransferase 1 (Eu-HMTase1) occurs at the early to middle stages of PGC development (Seki et al, 2007). Mice carrying knockouts of G9a and suppressor of variegation 3–9 homolog (Suv39h), other H3K9 methyltransferases, exhibit impaired spermatogenesis due to meiotic defects, suggesting that H3K9 methylation is indispensable for meiosis (Peters et al, 2001; Tachibana et al, 2007). After meiosis, H3K9 methylation is accumulated in nuclei of round spermatids in a region corresponding to the chromocenter, and binding of chromodomain protein, Y-Like (Cdyl) to the methylated H3K9 is reported to be important for chromatin condensation in elongated spermatids (Lahn et al, 2002). Thus, the genome-wide level of H3K9 methylation is precisely regulated and maintained, implying the importance of H3K9 methylation at multiple stages of spermatogenesis (summarized in Figure 1).
Figure 1.
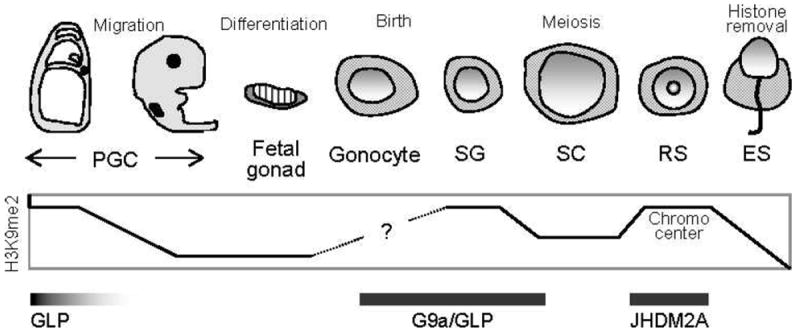
Dynamic change of genome-wide histone 3 lysine 9 (H3K9) dimethylation (H3K9me2) during male germ cell development. Timing of dimethyl H3K9 methyltransferases (GLP and G9a) and JmjC domain–containing histone demethylase 2A (JHDM2A) expression is also shown. PGC indicates primordial germ cell; SG, spermatogonium; SC, spermatocyte; RS, round spermatid; ES, elongated spermatid.
Spermiogenic Defect in the Jhdm2a Knockout Mouse
Before JmjC-containing histone demethylase 2a (JHDM2A), also known as JMJD1A or KDM3A, was identified as an H3K9 demethylase (for monomethylation and dimethylation) in 2005, it was originally cloned as a testis-specific gene transcript (Hoog et al, 1991; Yamane et al, 2006). Consistent with this previous report, immunohistochemical analysis using anti-JHDM2A antibody revealed an intense nuclear expression in round spermatids and a subnuclear distribution that was merged with the expression of RNA polymerase II, indicating that JHDM2A may contribute to transcriptional activation (Figure 2; Okada et al, 2007).
Figure 2.
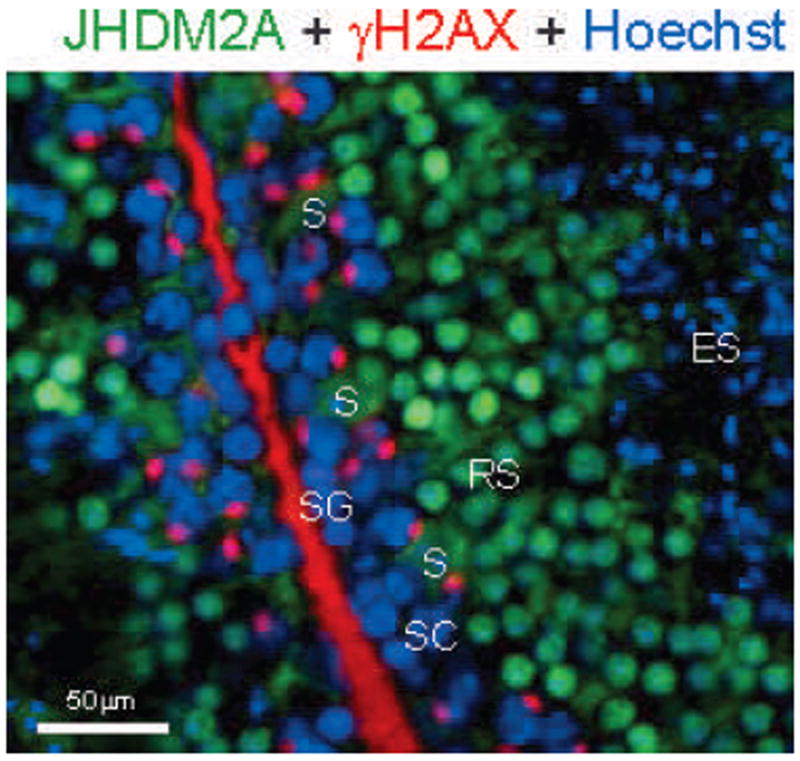
Immunohistochemical anaylsis of JmjC domain–containing histone demethylase 2A (JHDM2A) in mouse testis. JHDM2A (green) is highly expressed in round spermatids (RS) and not quite merged with gamma histone 2A variant X (γH2AX)-positive (red) spermatogonia (SG) and spermatocytes (SC). JHDM2A-positive signals disappear in elongated spermatids (ES). Cytoplasmic staining of JHDM2A is observed in Sertoli cells (S). Hoechst (DNA) staining is shown as blue.
However, as described above, the genome-wide H3K9 methylation level is continuously maintained during spermatogeneis. So, how does the demethylase play a role? To further elucidate the importance of JHDM2A during spermatogenesis, Jhdm2a-deficient mice were generated (Okada et al, 2007). Although the Jhdm2a knockout mice were viable, males exhibited smaller testes, and they were functionally infertile. Histologically, spermatids of the knockout mice failed to elongate because of impaired chromatin condensation (Figure 3). Unexpectedly, genome-wide H3K9 methylation was unaltered. In addition, levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone were also maintained in the knockout mice, although JHMD2A was reported to function as a transcriptional coactivator of the androgen receptor in a human prostate cancer cell line (Yamane et al, 2006). If neither genome-wide hypomethylation of H3K9 nor impaired hormonal regulation is the cause, what is the underlying molecular mechanism of the infertility? According to the subnuclear distribution that was similar to that of RNA polymerase II, it was speculated that JHDM2A was involved in transcriptional activation of gene(s) that must be essential for sperm chromatin condensation. In fact, reverse transcription–quantitative polymerase chain reaction analysis revealed decreased expression of 2 testis-specific basic proteins, transition protein 1 (Tnp1) and protamine 1 (Prm1), in round spermatids of the knockout mice. Chromatin immunoprecipitation (ChIP) assays further demonstrated that JHDM2A was recruited to the core promoter regions of both Tnp1 and Prm1 in round spermatids, whereas the recruitment of JHDM2A was not observed in the knockout mice. In addition, the methylation levels of H3K9 in these promoter regions were significantly higher in round spermatids of Jhdm2a knockout mice compared with those of the wild-type control. Thus, we propose a model in which JHDM2A contributes to spermatogenesis by directly controlling expression of Tnp1 and Prm1, which are both essential for sperm chromatin condensation. One of the questions that remain is how JHDM2A is specifically recruited to the Tnp1 and Prm1 promoters but not to other genes. JHDM2A itself does not contain a defined DNA-binding motif, and it appears no consensus DNA sequence consistently exists in the promoter regions of the target genes, including not only Prm1 and Tnp1 but also peroxisome proliferator–activated receptor alpha (Ppara) and uncoupling protein 1 (Ucp1), which are described in the next paragraph. Therefore, it can be argued that other JHDM2A-binding proteins are responsible for the DNA targeting.
Figure 3.
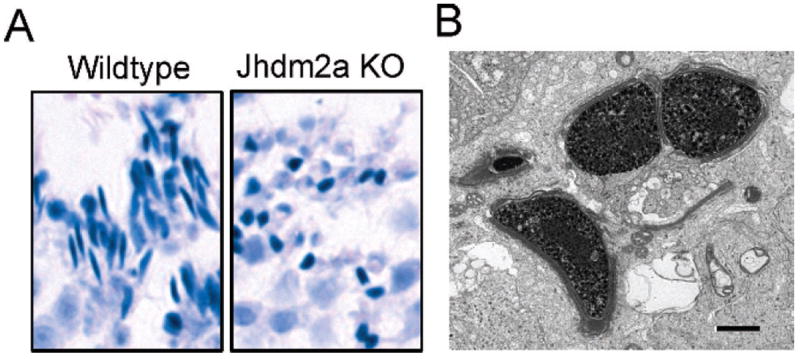
Impaired spermiogenesis in JmjC domain–containing histone demethylase 2A (Jhdm2a) knockout mice. (A) Abnormal spermatids at step 15 of spermiogenesis. Smaller, round-shaped spermatids are observed in the knockout mice. (B) Ultrastructurally, chromatin condensation is incomplete in the knockout spermatids. Bar = 1 μm.
Obesity in Jhdm2a Knockout Mouse
Other than male infertility, Jhdm2a knockout mice also exhibit an obese phenotype, such as increased body fat deposition and higher serum lipid content, as they age without affecting food intake (Figure 4; Tateishi et al, 2009). Microarray analysis revealed that the Jhdm2a deficiency affected the expression of metabolic genes, which caused impaired β-oxidation and glycerol release in skeletal muscle. Among these affected genes, JHDM2A directly targeted the peroxisome proliferator response element (PPRE) of the Ppara enhancer and resulted in demethylation of H3K9 at the enhancer region, followed by the transactivation of Ppara. In addition, defective adaptive thermogenesis of the Jhdm2a knockout mice also pinpointed a potential role for Jhdm2a in β-adrenergic signaling in brown adipose tissue (BAT). In fact, expression of several genes involved in mitochondrial functions, including Ppara, was decreased in the Jhdm2a knockout. In addition, analysis of UCP1, a key gene involved in β-adrenergic signaling–mediated thermogenesis in BAT, demonstrated that cold-induced Ucp1 up-regulation was completely abolished in the knockout mice. ChIP analysis further demonstrated that Ucp1 was also one of the downstream target genes of JHDM2A, and JHDM2A induced transactivation through removing H3K9 methylation from the promoter. Taken together, we propose that JHDM2A is involved in regulating systemic metabolic control, including Ppara and β-adrenergic signaling pathways, and its deficiency induces an obese phenotype in mice.
Figure 4.
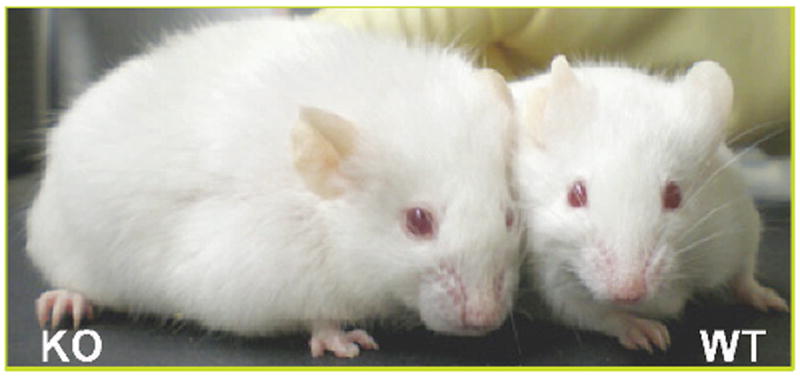
JmjC domain–containing histone demethylase 2A (Jhdm2a) knockout mouse (KO; left) exhibits obese phenotype.
Is There a Link Between Infertility and Obesity in Jhdm2a Knockout Mice?
A potential link between male infertility and obesity has been proposed in humans, and several causative factors have been suggested, such as hormonal abnormality and genetic mutation (Hammoud et al, 2006; Kasturi et al, 2008). However, genetic factors that contribute to obesity are often complicated and depend on multiple other genes and factors (Hammoud et al, 2006; Kasturi et al, 2008). Similarly, ablation of several genes leads to male infertility when they are disrupted in mice, but very few of them are mutated in human patients showing male infertility (O’Bryan and de Kretser, 2006). How about in the Jhdm2a-deficient mice? Unlike most human patients who show both male infertility and obesity, no abnormal hormonal changes were observed in the knockout mice. Despite the previous report that JHDM2A interacts with the androgen receptor in prostate cancer cells, both male hormones (androstenedione and testosterone) and female hormones (LH, FSH, and estradiol) in the knockout mice were maintained within a normal range, and so were other hormones related to fat metabolism (norepinephrine, epinephrine, T3, adiponectine, and corticosterone; Okada et al, 2007; Tateishi et al, 2009). However, LSD1, a histone demethylase which also catalyzes H3K9 demethylation, contributes to transactivation of estrogen receptor α (ERα) target genes pS2, suggesting that there might be a possibility that JHDM2A plays some role in the ERα pathway (Garcia-Bassets et al, 2007). Another possible factor(s) that can link these 2 phenotypes is expression of glucose metabolism–related genes expressed in testis, especially because the Jhdm2a knockout mice are diabetic. Furthermore, although the possibility might be low, searching for genetic mutations in the Jhdm2a gene has been under investigation in human patients (summarized in Figure 5).
Figure 5.
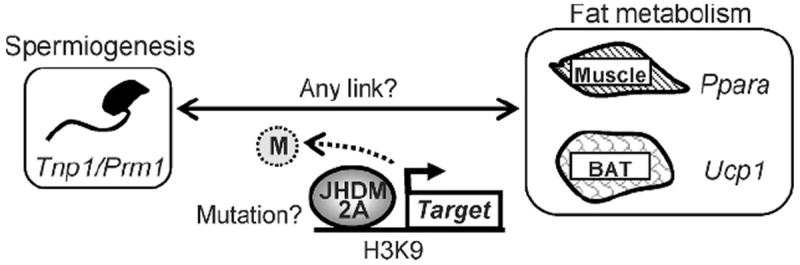
Summary of molecular events promoted by JmjC domain–containing histone demethylase 2A (JHDM2A).
Acknowledgments
Generation of the knockout mice was performed by Dr Yuji Mishina and the Knockout Mouse Core Facility at National Institutes of Health, National Institute of Environmental Health Sciences.
Supported by the National Institutes of Health (Y.Z.). Y.Z. is an Investigator of the Howard Hughes Medical Institute.
References
- Garcia-Bassets I, Kwon YS, Telese F, Prefontaine GG, Hutt KR, Cheng CS, Ju BG, Ohgi KA, Wang J, Escoubet-Lozach L, Rose DW, Glass CK, Fu XD, Rosenfeld MG. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128:505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godmann M, Lambrot R, Kimmins S. The dynamic epigenetic program in male germ cells: its role in spermatogenesis, testis cancer, and its response to the environment. Microsc Res Tech. 2009;72(8):603–619. doi: 10.1002/jemt.20715. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Gibson M, Peterson CM, Hamilton BD, Carrell DT. Obesity and male reproductive potential. J Androl. 2006;27:619–626. doi: 10.2164/jandrol.106.000125. [DOI] [PubMed] [Google Scholar]
- Hoog C, Schalling M, Grunder-Brundell E, Daneholt B. Analysis of a murine male germ cell-specific transcript that encodes a putative zinc finger protein. Mol Reprod Dev. 1991;30:173–181. doi: 10.1002/mrd.1080300302. [DOI] [PubMed] [Google Scholar]
- Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29:251–259. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- Lahn BT, Tang ZL, Zhou J, Barndt RJ, Parvinen M, Allis CD, Page DC. Previously uncharacterized histone acetyltransferases implicated in mammalian spermatogenesis. Proc Natl Acad Sci U S A. 2002;99:8707–8712. doi: 10.1073/pnas.082248899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryan MK, de Kretser D. Mouse models for genes involved in impaired spermatogenesis. Int J Androl. 2006;29:76–89. doi: 10.1111/j.1365-2605.2005.00614.x. discussion 105–108. [DOI] [PubMed] [Google Scholar]
- Okada Y, Scott G, Ray MK, Mishina Y, Zhang Y. Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature. 2007;450:119–123. doi: 10.1038/nature06236. [DOI] [PubMed] [Google Scholar]
- Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Peters AH, Schubeler D. Methylation of histones: playing memory with DNA. Curr Opin Cell Biol. 2005;17:230–238. doi: 10.1016/j.ceb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Rousseaux S, Caron C, Govin J, Lestrat C, Faure AK, Khochbin S. Establishment of male-specific epigenetic information. Gene. 2005;345:139–153. doi: 10.1016/j.gene.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, Saga Y, Tachibana M, Shinkai Y, Saitou M. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development. 2007;134:2627–2638. doi: 10.1242/dev.005611. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Nozaki M, Takeda N, Shinkai Y. Functional dynamics of H3K9 methylation during meiotic prophase progression. EMBO J. 2007;26:3346–3359. doi: 10.1038/sj.emboj.7601767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, Okada Y, Kallin EM, Zhang Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature. 2009;458:757–761. doi: 10.1038/nature07777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]


