Abstract
Glutamate signaling has been implicated in the regulation of social behavior. AMPA-glutamate receptors are assembled from four subunits (GluA1-4) of mainly GluA1/2 and GluA2/3 tetramers that form ion channels of distinct functional properties. Mice lacking GluA1 showed a reduced anxiety and male aggression. To understand the role of GluA3 in modulating social behavior, we investigated GluA3-deficient mice (Gria3 -/Y) on C57BL/6J background. Compared to wild type (WT) littermates (n=14), Gria3 -/Y mice (n=13) showed an increase in isolation-induced male aggression (p=0.011) in home cage resident-intruder test; an increase in sociality (p=0.01), and increase in male-male social interactions in neutral arena (p=0.005); an increase in peripheral activities in open field test (p=0.037) with normal anxiety levels in elevated plus maze and light-dark box; and minor deficits in motor and balance function in accelerating rotarod test (p=0.016) with normal grip strength. Gria3 -/Y mice showed no significant deficit in spatial memory function in Morris-water maze and Y-maze tests, and normal levels of testosterone. Increased dopamine concentrations in stratum (p=0.034) and reduced serotonin turnover in olfactory bulb (p=0.002) were documented in Gria3 -/Y mice. These results support a role of GluA3 in the modulation of social behavior through brain dopamine and/or serotonin signaling and different AMPA receptor subunits affect social behavior through distinct mechanisms.
Keywords: Glutamate receptor, GluA3, striatum, dopamine, serotonin, olfactory bulb, aggression, sociality, social interaction, mice
1. Introduction
Glutamate mediates the majority of excitatory neurotransmission in the central nervous system via a family of receptors with distinct substrate specificity [1, 2]. Glutamate receptors are widely expressed in brain structures that control emotion and behavior [3] suggesting a role of glutamate signaling in behavioral modulation. AMPA glutamate receptors are assembled from four subunits, GluA1-4, to tetrameric complexes [1-3] with a maximum of two different subunits present in each receptor complex [4, 5]. The subunit composition of AMPA receptors influences their ion permeability, rectification, and kinetics [1, 2, 6].
The roles of AMPA receptors in modulating behavioral phenotype have been studied in animal models. Two competitive antagonists for AMPA receptors, CNZX and NBQX, reduced the biting component of aggressive behavior in Turku Aggressive (TA) mice and GYKI, a non-competitive antagonist, suppressed all aggressive manifestations [7]. Mice lacking GluA1 [8] or carrying a mutation, R582Q, resulting in calcium-impermeable receptors [9] exhibited a reduced long-term potentiation, deficits in spatial working memory, reduced male aggression, and anxiety [10]. Mice-lacking GluA2 displayed an enhanced long-term potentiation, reduced exploratory activity, and impaired motor function [11]. Mice lacking GluA3 showed a significantly enhanced long term potentiation (LTP) and normal depotentiation after the establishment of LTP [12]. GluA3-deficient mice were also found to have a deficit in motor and balance, increased alcohol consumption after alcohol deprivation [13], a disturbance in non-rapid eye movement sleep and respiratory modulation, and an increased tendency of seizure [14]. A recent genome-wide scan of aggressive NZB/B1NJ and unaggressive A/J mice identified proximal chromosome X including Gria3 as one of the two quantitative trait loci for aggression [15].
Despite these studies, little is known about the contribution of GluA3 to social behavior. In this study, we investigated behavioral phenotype and brain monoamine levels of Gria3 -/Y mice on C57BL/6J background [16]. Gria3 -/Y mice were found to have an increase in isolation-induced male aggression, sociality, and male-male social interactions in neutral arena, elevated dopamine in striatum, and reduced serotonin turnover in olfactory bulb. The results support a role of GluA3 in the modulation of social behavior and implicate that different AMPA subunits influence social phenotype through distinct mechanisms.
2. Materials and Methods
2.1. Animals
Gria3 -/Y mice were generated by standard gene targeting method on a mixed C57BL/6J and 129 (129X1/SvJ × 129S1) strain background [16]. These mice have been back-crossed with C57BL/6J inbred strain (Jackson Laboratory, ME) for over 20 generations in order to achieve a homogenous strain background. Male Gria3 KO (Gria3 -/Y) and wild type (WT) littermate control mice (Gria3 +/Y) were generated by breeding WT C57BL/6J males with heterozygous (Gria3 +/−) females. Mice were genotyped using PCR of tail DNA following a published protocol [16]. Adult Gria3 -/Y (n=14) and Gria3 +/Y mice (n=13) between 4-9 months of age were studied using standard behavioral tests for rodent. Mice were housed in temperature-controlled rooms with 12 hr light/dark cycle (9:00 and 21:00) and had free access to water and standard mouse chow. Animal breeding and procedures were conducted in strict accordance with NIH Guide for Care and Use of Laboratory Animals. An animal study protocol for this project was approved by the Johns Hopkins University Animal Care and Use Committee.
2.2. Mouse behavioral testing
Mouse behavioral tests were conducted at Animal Behavioral Core of Johns Hopkins University School of Medicine following standard protocols from the Animal Behavior Core User Manual (http://www.brainscienceinstitute.org/index.php/cores) as described previously [17, 18]. The test order and age of mice for individual test (in parenthesis) are provided as follow: Open Field (4 month), Y-maze (4 month), elevated plus maze (5 month), resident-intruder test (5 month), social interaction and preference for social novelty (6 months), dyadic male-male interaction (6 month), rotarod (6 months), Morris water maze (7 month), light and dark box (9 month), grip strength (9 month), general olfaction (9 month), sample harvesting (9 month). For individual test, WT and knockout mice were always tested together to minimize potential variations. At least one week of rest was arranged between tests. Average ambient lighting (lux) for individual tests: open field (288-318), elevated plus maze (492), light-dark box (492 for light box and 0 for dark box, respectively), resident-intruder test, dyadic male-male interaction and sociability and social novelty (595).
2.2.1 Open-field test
Each individual test mouse was placed in a photo-beam (n=16 at equal spacing of 2.5 cm) equipped clear plastic chamber (45 × 45 cm and was allowed to explore free from interference for 30 minutes. The peripheral area (425 sq cm) was defined by the two side-photo beams, #1-2 and #15-16 while the central area (1,600 sq cm) was defined by photo beams #3-14 at each direction. Their movements were tracked using a SDI Photobeam Activity System (San Diego Instruments). Their patterns of ambulatory movement, fine movement, and rearing behavior at central and peripheral areas were recorded and analyzed.
2.2.2. Sociability and preference for social novelty
The test was carried out in a 45 cm × 45 cm × 37.5 cm (H) clear plastic chamber divided equally into four quadrants. Two small mesh cages (10 cm in diameter, 15 cm high) were placed at the opposite corners of two quadrants. The test mouse was allowed to explore the chamber freely for 5 minutes with the small empty mesh cages before starting test trials. For trial 1, a wild-type stranger male mouse was placed inside one of the mesh cages and the test mouse was allowed to explore the chamber freely for 10 minutes. For trial 2, a second wild-type stranger mouse was placed in the other mesh cage and the test mouse was allowed to explore freely for another 10 minutes. The time that the test mouse spent in each of the four quadrants was measured during each of the 10 min sessions and analyzed.
2.2.3. Resident-intruder test
The test was carried out in the individual home cage of the testing male after seven days of isolation. On the eighth day, a young and unfamiliar intruder male was placed in the home cage of the test mouse. The mice were then allowed to interact free from interference for 10 min. The intruders were 2 month-old male mice of C57BL/6J strain without significant fighting experience. They were housed in separate cages from resident mice. Each intruder mouse was used only once in this test on any given test day. The entire interactions were video-recorded. Aggressive behavior (attacks and tail rattles) and nonaggressive social behavior (sniffing and following) were scored and analyzed by two independent observers.
2.2.4. Dyadic male-male interaction in neutral field
The test was carried out in a 45 cm × 45 cm × 37.5 cm (H) clear plastic box that is unfamiliar to both test mouse and stranger mouse. A test mouse and a stranger male mouse of same age and strain background were placed in the box that was separated by a divider. The mice were allowed to explore half of the box freely for 5 min. The divider was then removed and the mice were allowed to interact for 10 min free from interference. Aggressive behavior (attacks and tail rattles) and nonaggressive social behavior (sniffing and following) of the test mouse were video-recorded and analyzed.
2.2.5. Elevated-plus maze
Elevated plus maze tests anxiety-related behavior in rodents. The maze, made of stainless steel, consists of two closed arms measuring 48 cm (L) × 10 cm (W) × 38 cm (H) and two open arms measuring 48 cm (L) × 10 cm (W) (San Diego Instruments). The four arms were connected by a middle 10 cm × 10 cm platform. The test mouse was placed on the middle platform and remained in the maze during the 5 min session. The total time spent and number of entries into the closed and open arms were recorded and analyzed.
2.2.6. Light-dark box test
This test is for anxiety-related behavior in rodents. A test mouse was placed in the dark side of a light-dark box [35 cm (W) × 17.5 cm (D) × 3 cm (H), Coulbourn Instruments] and allowed to explore free from interference for 5 minutes. The time elapsed before the mouse entered the light side as well as the total time spent in each side of the box was recorded and analyzed.
2.2.7. Y-maze
The Y-maze for spatial working memory in rodents consists of three identical arms [46 cm (L) × 6.25 cm (W) × 2.5 cm (H)] radiating at 120-degree angles from a central platform. The test was done in three trials. During the first trial, the test mouse was placed at the end of one arm, chosen at random prior to the test, and remained in the maze free from interference for 5 minutes. The total number of spontaneous alternations divided by the number of total possible alternations was recorded and analyzed. The second and third trials were run seven days after the first trial. During the second trial, one of the arms, chosen randomly for each mouse prior to the test, was blocked. The test mouse was allowed to explore the two unblocked arms for 5 minutes followed by a resting period of 10 minutes. During the third trial, the test mouse was returned to the maze with all three arms open and allowed to explore for another 5 minutes. The third trial was analyzed for time spent in the arm blocked during the second trial. The data was analyzed for the first 2 minutes and full five minutes of trial 3.
2.2.8. Morris water maze
Morris Water Maze for spatial reference memory was tested in WT (n=9) and Gria3 KO (n=9) mice. A standard water maze (120 cm in diameter) containing deep, opaque water (25°C) was set up with a rescuing platform (10 cm × 10 cm) just below the water surface and marked with a cue (flag) and four large spatial cues outside the maze. On test day 1, mice were subjected to four, one-minute swimming trials in the maze to locate the platform. In each of the four trials, the platform was placed at a different quadrant of the maze. On test days 8-10, the platform cue was removed and the mice were subjected to three, one-minute trials to locate the hidden rescuing platform placed at a fixed quadrant of the maze. On test day 11, mice were subjected to one trial of three minutes of free swimming without platform. Their time spent in the quadrant where the platform was located during the hidden platform trials was determined and compared between WT and KO groups.
2.2.9. Rotarod test
A test mouse was placed on a rotating rod that is accelerated from 5 to 30 rpm during a 5 min session in a standard testing apparatus (Rotamex-5 from Columbus Instruments with mouse spindle). The performance was graded by the time a mouse stays on the rotating rod. Each mouse was tested under the same parameters three times each day for three days and a total of 9 trials. Data from all nine sessions was obtained and analyzed for each mouse.
2.2.10. Grip strength
This is to test forepaw grip strength in rodent. A grip strength meter was placed horizontally and mice, held by the tail, were allowed to grasp the pull bar with their front paws only. Mice were pulled backward, horizontally, and the peak force was recorded in pounds. This was done three times in a row during each of two training trials with one-hour in between. One hour after the training trial, a test trial was conducted with each mouse tested five times in a row. The highest and lowest values are removed and means and SEM for three middle values were calculated.
2.2.11. Olfaction test.
A test mouse was placed in a fresh, clean cage for this test. After five minutes of free exploration, three drops of vanilla extract and three drops of water were placed at the opposite corners of the cage. The time spent sniffing either vanilla or water was recorded over the next two minutes.
2.3. Plasma testosterone concentrations
Blood samples from were collected from individual WT and knockout mice between the time of 13:00-15:00. Plasma was isolated immediately by centrifugation at 2,000xg for 10 min at 4°C and stored at −80°C until testing. Plasma testosterone levels were measured using a Parameter™ Testosterone Assay kit (R&D Systems) following a standard protocol from the manufacturer. Briefly, a 96-well microplate coated with a goat anti-mouse antibody was first incubated with a testosterone-specific monoclonal antibody. After extensive washing using buffers supplied in the kit, 100μl of testosterone standards or diluted plasma samples from mice were added to each well along with HRP-conjugated testosterone supplied in the kit. The samples were incubated at room temperature for 3 hrs with slow rotation for competitive binding followed by extensive washing steps using buffers supplied in the kit. A colorimetric reaction for HRP-testosterone was conducted and optical density at 450nm for each well was determined using a SPECTRAmax™ GEMINI XS Dual-Scanning Microplate Spectrofluorometer (Molecular Devices). Testosterone concentrations of the study samples were calculated from a standard curve of testosterone controls provided in the kit. All samples were assayed in triplicates.
2.4. Monoamine concentrations in selected brain regions
Olfactory bulb, cortical, striatal and hippocampal tissue samples (N=8 per group) were dissected from brains of Gria3 knockout and wild-type mice immediately after cervical dislocation. Brain samples were weighed, extracted with 0.01M perchloric acid, and centrifuged at 15,000g for 15 min. Concentrations of norepinephrine (NE), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) in brain tissue extracts were measured by HPLC with electrochemical detection as described earlier [19, 20]. The analytical column was SunFire C18 5 μm (4.6 × 150 mm) from Waters Corporation. The mobile phase consisted of 0.01 M sodium dihydrogenphosphate, 0.01 M citric acid, 2 mM sodium EDTA, 1 mM sodium octylsulfate, 10% methanol, pH 3.5 and was used at flow rate 1.0 ml/min and temperature 35°C. The installation consisted of 717 Plus Autosampler (Waters), 1525 Binary HPLC pump (Waters), and Coulochem III electrochemical detector (ESA). The glassy carbon electrode was used at +0.34 V. Concentrations of NE, DA, DOPAC, HVA, 5-HT, and 5-HIAA were calculated with Breeze software (Waters) and expressed as pg/mg of tissue weight. Ratios of 5-HIAA/5-HT, HVA/DA, and DOPAC/DA were also calculated.
2.5. Statistical analysis
Statistical analysis of behavioral data were performed using two-tailed t-test for comparison of the means of two independent samples and two-way ANOVA followed by t-test post hoc for multiple comparisons. Statistical analysis of monoamine concentrations was performed using two-tailed t-test and one-way ANOVA followed by Fisher's protected least significant difference (PLSD). Data were presented as mean ± SEM; p<0.05 was considered statistically significant.
3. Results
3.1. Growth, general appearance, and olfaction
Gria3 -/Y and Gria3 +/Y mice on C57BL/6J strain background were confirmed by PCR-based genotyping. Gria3 -/Y mice had no detectable GluA3 expression in brain cortex and cerebellum (Fig.S1), and normal levels of GluA1 and GluA2 in whole brain tissues [16]. Gria3 -/Y mice were born following Mendelian ratios with no apparent congenital malformation. General physical growth, fur color, and home cage nesting behavior of Gria3 -/Y mice were indistinguishable from their wt littermates (Fig.S2). No statistical difference was detected in general olfactory function between Gria3 +/Y mice and Gria3 -/Y mice (Table 1). Female heterozygote for Gria3 KO (Gria3 ±) exhibited no apparent difference in physical growth, mating behavior, litter size and sex ratio, and maternal and mating behavior as compared to WT C57BL/6J female mice.
Table 1.
Additional behavioral characteristics of Glua3 -/Y and WT littermate control mice
| Wild Type | Glua3 -/Y | |
|---|---|---|
| Open Field Behavior | ||
| Total Activities (s) | 3148.0 ± 136.6 | 3147.9 ± 174.2 |
| Center Activities (s) | 2904.7 ± 129.8 | 2807.0 ± 171.1 |
| Peripheral Activities (s) | 243.3 ± 14.2 | 340.9 ± 43.4* |
| GRIP strength test | ||
| Forepaw pull strength (gm) | 71.8 ± 3.3 | 69.5 ± 3.6 |
| Olfaction test | ||
| Time sniffing vanilla (s) | 16.1 ± 1.5 | 12.6 ± 1.0 |
| Light/dark box | ||
| Time in light box (s) | 1006.8 ± 57.8 | 918.6 ± 63.5 |
| Time in dark box (s) | 1993.3 ± 57.7 | 2081.4 ± 63.5 |
| % Time in dark box | 66.4 ± 1.9 | 69.4 ± 2.1 |
| Elevated plus Maze | ||
| Total arm entries | 20.0 ± 1.1 | 15.9 ± 1.5 |
| Time in closed arms (s) | 168.1 ± 7.1 | 181.9 ± 11.5 |
| % Time in closed arms | 56.0 ± 2.4 | 60.6 ± 3.8 |
| Morris Water Maze | ||
| Time in right quadrant (s) | 86.0 ± 8.2 | 77 ± 4.4 |
| Y-Maze (spontaneous alternations) | ||
| Total entries | 16.0 ± 0.7 | 16.3 ± 0.8 |
| Completed alternations | 9.6 ± 0.8 | 9.8 ± 0.8 |
| % alternation / entries | 70.6 ± 6.6 | 69.4 ± 3.8 |
| Y-Maze (novel arm entries) | ||
| % Time in novel arms (first 2 min) | 34.2 ± 2.8 | 30.7 ± 5.1 |
| % Time in novel arms (total 5 min) | 32.4 ± 2.2 | 32.8 ± 2.7 |
Data are presented as means ± SEM. Genotype-based comparisons were conducted using Student's t test for independent samples
p < 0.05.
3.2. Motor coordination, balance, and grip strength
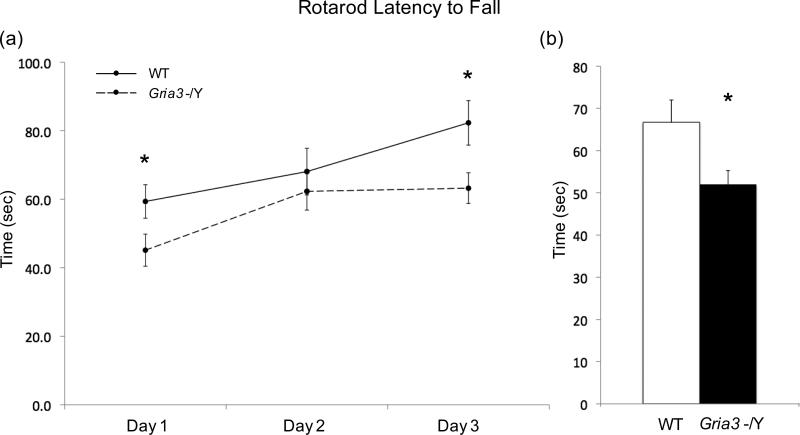
Motor coordination and balance of Gria3 -/Y mice were studied using accelerating rotarod test. Gria3 -/Y mice demonstrated an ability of motor learning but exhibited a mild deficit during day 1 and day 3 of the test compared to WT (Fig.1a). Overall, there is a mild deficit in motor coordination and/or balance as reflected by a shorter stay on accelerating rotarod compared to WT (t(23)=2.6; p=0.016) (Fig.1b). The poor performance of Gria3 -/Y mice in rotarod test was not associated with a significant defect in motor strength as demonstrated by comparable forepaw grip strength to WT (Table 1).
Figure 1.
Motor and Balance Function of Gria3 -/Y and WT Control Mice in the Rotarod Test. Motor learning and latency to fall of WT (n=13) and Gria3 -/Y (n=12) mice were tested individually on accelerating rotarod. Three trials were conducted each day on three consecutive days for a total of 9 trials. (a) Motor learning: means and SEM of three trials each day for the latency to fall were plotted for the three-day test. Note both WT and Gria3 -/Y mice show motor learning as reflected by longer latency to fall over the three-day test period. Gria3 -/Y exhibited significantly shorter stay on the rotarod during day 1 and day 3 of the test. (b) Latency to fall. Means and SEM for a total of 9 trials were shown WT and Gria3 -/Y cohorts. Note that Gria3 -/Y mice had shorter time of stay on the rotarod as compared to WT. (t-test, * p<0.05).
3.3. Explorative function and anxiety-related behavior
Gria3 -/Y mice showed a similar total exploratory and central activities (Table 1) but slightly increased peripheral activities (t(25)=2.2; p=0.037) compared to WT in open field test (Table 1). To determine whether the increased peripheral activities in Gria3 -/Y mice are associated with any change in their anxiety levels, we studied these mice using two standard tests for anxiety in rodent: elevated plus maze (EPM) and light-dark box. Gria3 -/Y mice exhibited no significant change in anxiety as reflected by similar time spent in the open and closed arms as compared to WT (Table 1). Gria3 -/Y mice exhibited no significant change in anxiety as reflected by similar time spent in light and dark boxes compared to WT (Table 1).
3.4. Aggressive and nonaggressive social interactions
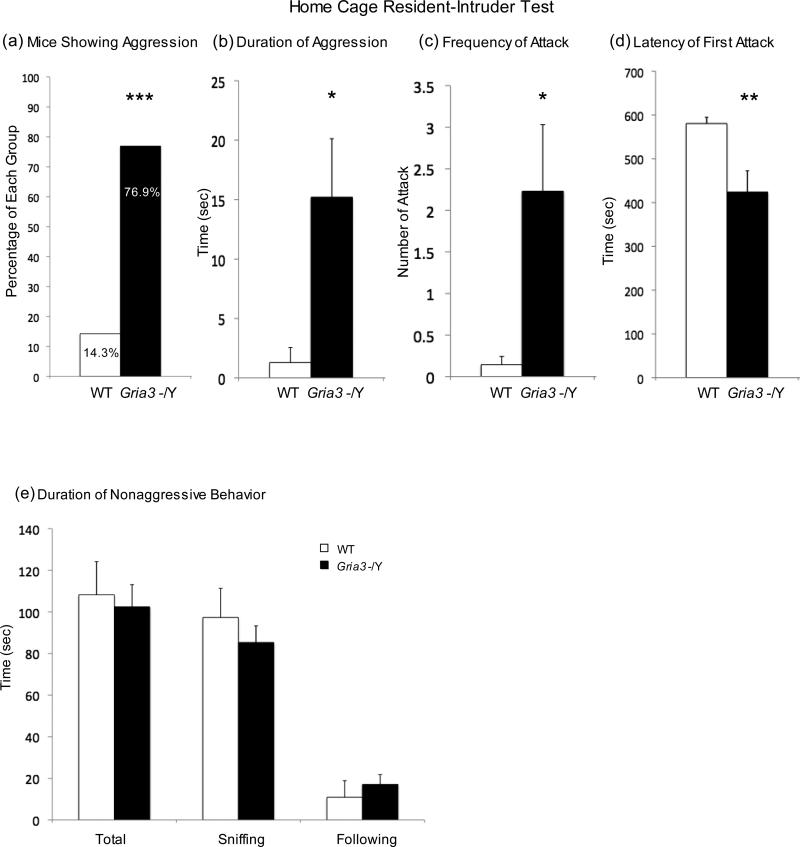
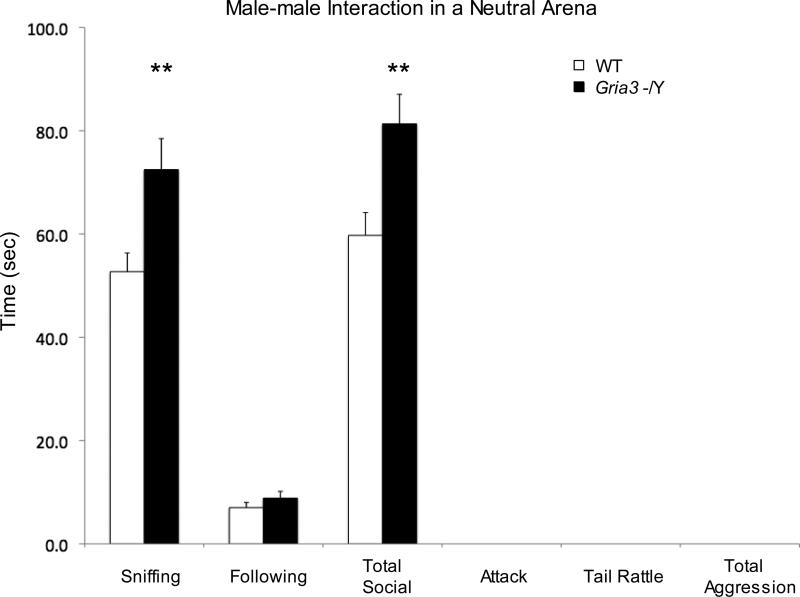
Two well-established tests for male aggression in rodents, home cage resident-intruder test and dyadic male-male interaction in neutral arena, were used to evaluate aggression-related social behavior in Gria3 -/Y mice. The resident-intruder test was carried out in the individual home cage of the test mouse for its interactions with a younger, unfamiliar intruder male for 10 min. Active aggression (attacks) and passive aggression (tail rattles) and nonaggressive social interactions (sniffing and following) were recorded. Compared to WT, Gria3 -/Y mice are more likely to show aggressive behavior (76.9%, 10 out of 13 for Gria3 -/Y; 14%, 2 out of 14 for WT; chi square, p < 0.001) (Fig.2a). These mice showed a significant increase in total number of attacks (t(25)=2.61; p=0.015), duration exhibiting aggressive behavior (t(25)=2.73; p=0.011), and a shorter latency to initial first attack (t(25)=3.19; p=0.0038) (Fig. 2b-2d). No significant change in nonaggressive social interactions (sniffing and following) was detected (Fig.2e). A dyadic male-male interaction test in neutral arena was carried out in an open chamber unfamiliar to both test and stranger mice. Gria3 -/Y mice exhibited a significant increase in nonaggressive social behavior (sniffing, t(25)=2.88; p=0.008; total social interaction, t(25)=3.11; p=0.0046) (Fig.3).
Figure 2.
Aggressive and Nonaggressive Social Behavior of Gria3 -/Y and WT Control Mice in Home Cage Resident Intruder Test. (a) Percentage of mice showing aggressive behavior in Gria3 -/Y (n=13) and WT (n=14) cohorts. (b) means and SEM of duration showing aggression in Gria3 -/Y and WT mice. (c) means and SEM of frequencies of attack in Gria3 -/Y and WT mice (d) means and SEM of the latency to first attack in Gria3 -/Y and WT mice. (e) means and SEM of the duration showing nonaggressive social interaction (sniffing and following) in Gria3 -/Y and WT mice. Chi square test for panel a; student's t-test for panels b-e. * p<0.05; ** p<0.01; *** p<0.001
Figure 3.
Dyadic Male-Male Interactions in Neutral Arena for Gria3 -/Y and WT Control Mice. Means and SEM for duration showing aggressive behavior (attacks, tail rattles, and total) and nonaggressive social behavior (sniffing, following, and total) were presented for WT (n=13) and Gria3 -/Y (n=14) mice during 5 min free interaction period. Note the increase in nonaggressive social interaction in Gria3 -/Y mice compared to WT (t-test, ** p<0.01).
3.5. Sociality and preference for social novelty
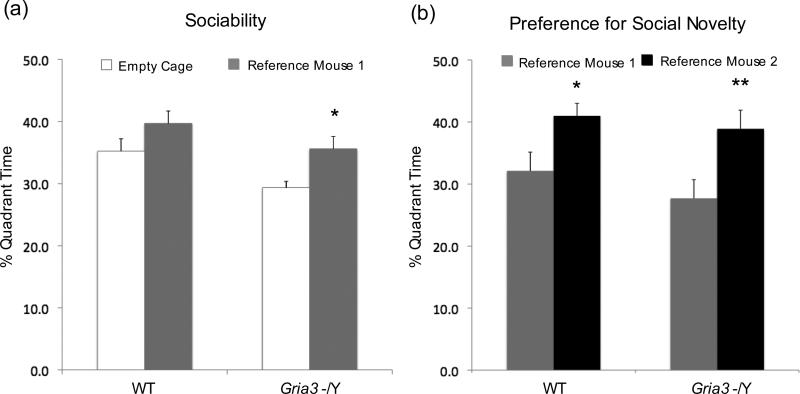
To further explore changes in social behavior associated with GluA3 deficiency, we performed sociality and preference for social novelty tests on Gria3 -/Y mice and WT control mice. In the sociality test, the test mouse was first allowed a free exploration of an open chamber with two small empty wire cages at opposite corners. A stranger male (reference 1) was then put in one of wire cages. The test mouse was given a choice of spending time with this stranger male versus an object (empty cage). Preference for social novelty test was followed immediately after sociality test. A novel stranger male (reference 2) was put into the empty wire cage. The test mouse was given a choice to spend time with novel stranger male (reference 2) versus the already-investigated and now-familiar mouse (reference 1). For sociality test, we first performed a t-test within the same genotype. The results showed that Gria3 -/Y spent more time in the quadrant with the wire cage containing the first stranger (reference 1) mouse versus that with the empty cage (t(22) = 2.55, p = 0.018) (Fig.4a). We next performed two-way ANOVA test and the p values indicate a genotype effect (Ft(1,24)=7.198, p= 0.01). To understand this difference, we performed a planned t-test post-hoc indicating that Gria3 -/Y mice have a moderate increase in sociality compared to WT (Gria3 -/Y, t(11)=2.313, p=0.02; WT, t(13)=1.784, p=0.05). For preference for social novelty, we first performed a t-test within the same genotype. Both WT and Gria3 -/Y mice spent more time with the novel mouse (reference 2) than with the familiar mouse (reference 1) (WT, t (26)=2.13, p=0.043; Gria3 -/Y, t(22)=2.9, p=0.008) (Fig.4b). However, two-way ANOVA did not indicate significant difference in preference for social novelty between WT and Gria3 -/Y groups. All together, these results suggest a moderate increase in sociality but not in preference for social novelty for Gria3 -/Y mice compared to WT.
Figure 4.
Sociality and Preference for Social Novelty of Gria3 -/Y and WT Mice. (a) Sociality. The percentage of total time for a test mouse spent in the quadrant with an empty mesh cage versus the quadrant with a stranger mouse (reference mouse 1) in a mesh cage during 10 min was shown for WT (n=14) and Gria3 -/Y (n=12). (b) Preference for social novelty. The percentage of total time for a test mouse spent in the quadrant with familiar mouse (reference 1) in a mesh cage versus the quadrant with a novel mouse (reference 2) in a mesh cage during 10 min was shown for WT (n=14) and Gria3 -/Y (n=12). Statistical analysis within the same genotype was calculated using t-test (* p<0.05; ** p<0.01). For comparisons between the groups, two-way ANOVA followed by planned t-test post hoc were utilized.
3.6. Spatial working and spatial reference memory
Y-maze of spontaneous alternation and reentry into previously blocked novel arm were used to test hippocampus-dependent spatial working memory in WT and Gria3 -/Y mice. Gria3 -/Y mice demonstrated no significant deficit in spatial working memory function in both trials of Y-maze tests (Table 1). Morris Water Maze was used to test hippocampus-dependent spatial reference memory of Gria3 -/Y mice. The test consists of two training trials using a visible and a hidden platform, followed by a probe trial in absence of the platform. Both WT and Gria3 -/Y mice showed similar progression of learning during the training trials (data not shown). No significant difference was detected between WT (n=9) and Gria3 -/Y (n=9) mice in the time spent probing the quadrant where the platform was located during the hidden platform trial, suggesting the mutant mice had no significant deficit in spatial reference memory in this test (Table 1).
3.7. Plasma testosterone concentration
Testosterone levels in males are known to influence aggressive behavior during puberty [21, 22]. We therefore measured plasma testosterone levels from Gria3 -/Y mice and controls. The study identified no significant difference in plasma testosterone levels between these two groups (ng/ml of mean ± SEM; control: 1.79± 0.11, n=6; Gria3 -/Y: 1.97± 0.23, n=8; (t(12)=0.62; p=0.54).
3.8. Monoamine concentrations in selected brain regions
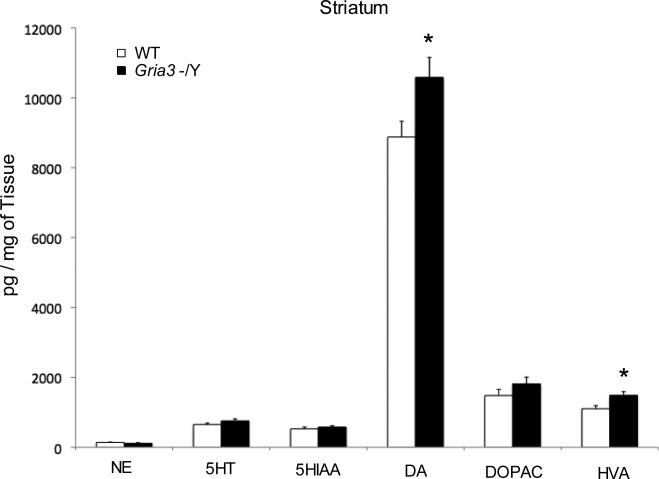
Norepinephrine (NE), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) concentrations were determined in striatum, olfactory bulb, frontal cortex, and hippocampus of adult Gria3 -/Y mice (n=8) and WT (n=8) since serotonin, dopamine, and GABA signaling are known to influence aggressive behavior [23]. In striatum, we found a significant elevation of DA in Gria3 -/Y mice compared to WT (pg/mg of tissue; mean ± SEM; control: 8,879 ± 451; Gria3 -/Y: 10,584 ± 572; t(14)=2.34; p=0.035) and HVA (pg/mg of tissue; mean ± SEM; control: 1,103 ± 90; Gria3 -/Y: 1,486 ± 106; t(14)=2.74; p=0.016) (Fig.5; Table 2). No change in DOPAC concentrations or ratios of DOPAC/DA and HVA/DA were detected in striatum of Gria3 -/Y mice compared to WT (Table 2). In olfactory bulb, we found a significant reduction in 5-HIAA/5-HT ratio (Control, 0.75±0.05; Gria3 -/Y, 0.55±0.02; t(14)=3.76, p=0.0022) whereas similar concentrations of 5-HT were found between Gria3 -/Y and WT mice (pg/mg of tissue; mean ± SEM; control: 361 ± 17; Gria3 -/Y: 411 ± 30, t(14)=1.44, p=0.17) (Table 2). In the rest of the tested brain regions, we found no significant change in the monoamine concentrations including NE in olfactory bulb and 5-HT in striatum between Gria3 -/Y and WT mice (Table 2).
Figure 5.
Striatal Monoamine Concentrations of Gria3 -/Y and WT mice. Striatum tissues were dissected from brain of adult Gria3 -/Y and WT control mice. Concentrations of norepinephrine (NE), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) were determined using HPLC, calculated with Breeze software (Waters) and expressed as pg/mg of tissue weight. The means and SEM for WT (n=8) and Gria3 -/Y (n=8) were shown. Statistical analysis was performed using Student's t test and ANOVA followed by Fisher's protected least significant difference (PLSD). Data were presented as mean ± SEM; p<0.05 was considered statistically significant.
Table 2.
Monoamine concentrations in four brain regions of adult Glua3 -/Y and WT littermate control mice
| Tissue | Genotype | Number (n) | NE (pg / mg of protein) | 5HT (pg / mg of protein) | 5HIAA (pg / mg of protein) | 5HIAA/5HT (pg / mg of protein) | DA (pg / mg of protein) | DOPAC (pg / mg of protein) | HVA (pg / mg of protein) | DOPAC/DA (pg / mg of protein) | HVA/DA (pg / mg of protein) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Olfactory Bulb | WT | 8 | 279.5 ± 11.2 | 361.2 ± 17.2 | 268.4 ± 19.6 | 0.75 ± 0.05 | 301.5 ± 28.8 | 109.3 ± 13.2 | 242.3 ± 20.6 | 0.37 ± 0.04 | 0.82 ± 0.06 |
| Gria3 -/Y | 8 | 334.3 ± 24.8 | 411.1 ± 30.0 | 222.9 ± 13.2 | 0.55 ± 0.02 | 316.3 ± 16.9 | 99.0 ± 7.2 | 260.3 ± 10.7 | 0.31 +0.02 | 0.83 ± 0.04 | |
| t-test, p value | 0.063 | 0.172 | 0.075 | 0.002 | 0.666 | 0.506 | 0.452 | 0.272 | 0.848 | ||
| Frontal Cortex | WT | 8 | 306.3 ± 25.1 | 522.1 ± 18.3 | 509.5 ± 45.7 | 0.98 ± 0.09 | 76.8 ± 23.5 | 28.2 ± 12.7 | 83.1 ± 23.8 | 0.45 ± 0.19 | 1.15 ± 0.29 |
| Gria3 -/Y | 8 | 346.3 ± 47.7 | 523.0 ± 25.6 | 467.6 ± 44.1 | 0.89 ± 0.06 | 52.3 ± 14.1 | 7.1 ± 1.6 | 74.1 ± 15.3 | 0.25 ± 0.10 | 2.38 ± 0.81 | |
| t-test, p value | 0.455 | 0.978 | 0.520 | 0.424 | 0.411 | 0.149 | 0.767 | 0.420 | 0.182 | ||
| Hippocampus | WT | 8 | 405.2 ± 34.1 | 474.5 ± 26.6 | 600.8 ± 39.5 | 1.28 ± 0.09 | -- | -- | -- | -- | -- |
| Gria3 -/Y | 8 | 448.3 ± 28.4 | 524.7 ± 20.3 | 612.8 ± 34.6 | 1.18 ± 0.08 | -- | -- | -- | -- | -- | |
| t-test, p value | 0.347 | 0.156 | 0.822 | 0.405 | -- | -- | -- | -- | -- | ||
| Striatum | WT | 8 | 139.6 ± 9.9 | 652.0 ± 42.1 | 528.6 ± 51.7 | 0.86 ± 0.15 | 8879.2 ± 450.7 | 1481.6 ± 176.7 | 1103.9 ± 90.0 | 0.16 ± 0.02 | 0.12 ± 0.01 |
| Gria3 -/Y | 8 | 114.8 ± 17.6 | 756.6 ± 58.6 | 578.7 ± 41.2 | 0.78 ± 0.06 | 10584.8 ± 572.4 | 1816.2 ±193.6 | 1486.6 ± 106.6 | 0.17 ± 0.02 | 0.14 ± 0.01 | |
| t-test, p value | 0.240 | 0.169 | 0.461 | 0.638 | 0.034 | 0.222 | 0.016 | 0.714 | 0.197 |
Data are presented as means ± SEM. Genotype-based comparisons were conducted using Student's t test for independent samples (p < 0.05).
Norepinephrine (NE), 5-hydroxytryptamine (5HT), 5-Hydroxyindoleacetic acid (5-HIAA), Dopamine (DA), 3,4-Dihydroxyphenyl-acetic acid (DOPAC), Homovanillic acid (HVA)
4. Discussion
To understand the role of GluA3 in the modulation of social behavior, we characterized behavioral phenotype of Gria3 KO mice on C57BL/6J background and WT littermate controls. We back-crossed Gria3 KO mice with inbred C57BL/6J inbred mice for over 20 generations to minimize strain background as a confounding factor in behavioral phenotype. Consistent with previous reports on a different line of Gria3 KO mice [13], we detected a moderate deficit in motor and/or balance in Gria3 -/Y mice using rotarod test. This deficit appears not to associate with a change in the grip strength or general motor activities. We also found a small increase in peripheral activities of Gria3 -/Y mice in open field test. This increase appears not to associate with any significant change in anxiety levels in light-dark box and elevated plus maze.
We documented an increase in the isolation-induced male aggression in Gria3 -/Y mice. In their home cage after isolation, Gria3 -/Y mice are more likely to develop aggressive behavior, have increased frequency of attack and longer duration of aggression, and a shorter latency to initiate the first attack. The increase in aggressive behavior in Gria3 -/Y mice appears to be isolation-induced as they do not exhibit an increase in aggression in male-male interaction in a neutral arena. In this test environment, we noted an increase in sociality and nonaggressive social behavior (sniffing, following) in Gria3 -/Y mice. It is tempting to speculate that the increase in social behavior contribute to the increase in aggressive behavior in these mice. However, these data suggest that a lack of GluA3 affects a broad spectrum of social behavior in rodent. The distinct social behaviors in Gria3 -/Y are not associated with changes in general exploratory activities, anxiety, spatial learning and memory function, or plasma testosterone concentrations.
Aggression is a complex social behavior and is commonly associated with psychiatric disorders and intellectual disability[24, 25]. Previous studies have implicated a strong genetic contribution and multiple neural circuits involving dopamine, GABA, serotonin, norepinepherine, and nitric oxide in the modulation of aggressive behavior in humans and animals[26, 27].
To understand the mechanisms that underlie social and aggressive behavior in Gria3 -/Y mice, we determined monoamine levels in four brain regions in these mice. Interestingly, Gria3 -/Y mice showed a significant increase in levels of striatal dopamine and its metabolite, HVA, suggesting an excessive dopaminergic signaling in this region. We found reduced 5-HIAA/5-HT ratio with normal 5-HT levels in olfactory bulb suggesting a reduction of serotonin turnover in this region in Gria3 -/Y mice. Increased brain dopamine concentrations have been reported to associate with increased aggression, which can be blocked by dopamine antagonists[28, 29]. Intra-cerebral dopamine levels were found to be higher in aggressive rodents than controls [30]. Socially dominant and aggressive male lizard Anolis carolinensis exhibited higher dopamine levels in striatum, nucleus accumbens, and AN/VTA [31]. Serotonin levels and turnover in brain regions were studied in two strains of mice with isolation-induced aggression [32]. Decreased serotonin turnover but not its levels were found in amygdala, lateral hypothalamus, and pons of C57 strain and lateral hypothalamus and pons of DBA strain. Furthermore, increased 5-HT levels were reported in the olfactory-bulbectomized (Obx) mice, an established murine model of aggression [33]. Data from our studies support a potential role of the disturbance in glutamate and/or serotonin signaling in the modulation of social and aggressive behavior in Gria3 -/Y mice. However, the current study could not establish a causal relationship between the changes in brain monoamine concentrations and social behavior. Additionally, it would be interesting to investigate the role of other brain regions that are known to modulate aggressive behavior, such as hypothalamus[34], in the observed social and aggressive behavior in Gria3 -/Y mice.
The mechanism of how GluA3-mediated glutamate signaling modulates social behavior is not known. AMPA receptors form heteromeric and homomeric receptor complexes assembled from four distinct subunits, GluA1-4. In adult hippocampus, heteromeric GluA1/2 and GluA2/3 complexes are the major forms while homomeric GluR1 complex accounts for 8% of the total pool. Studies showed that the GluA2/3 subunits, as a “constitutive receptor pool”, are essential for activity-independent receptor delivery and recycling and responsible for stable basal synaptic transmission while GluA1/2 subunits, as a “reserve receptor pool”, are important for activity-induced enhancement of synaptic transmission at hippocampal pyramidal neurons. In GluA1-deficient mice, the majority of AMPA receptor complexes are expected to be GluA2/3 while in GluA3 deficient mice, the majority of AMPA receptor complexes are expected to be GluA1/2. Interestingly, GluA1-deficient mice present with reduced aggression while GluA3 deficient mice show increased aggression. We speculate that different electrophysiological properties of the expected receptor complexes in GluA1-deficient and GluA3-deficient mice play a role in the distinct social behavioral phenotype.
Striatum receives prominent glutamatergic input from cerebral cortex and thalamus via corticostriatal and thalamostriatal projections. Dopaminergic neurons at substantia nigra and ventral tegmental areas innervating striatum also receive glutamatergic afferents and play a role in addiction, aggression, and processing reward information [35]. Consistent with these inputs, striatal neurons are rich in glutamate receptors [36-38]. Immunohistochemical and in situs hybridization studies indicate that GluA1-4 are differentially localized on subpopulations of striatal neurons and interneurons with type-specific differences in subunit composition in rodents [36, 38, 39] and in primate [40, 41]. Differential expression of GluA1-4 subunits in striatal projection neurons and interneurons may also explain the differences of these neurons in AMPA-mediated responses to glutamate or cortical excitation [37, 42].
We hypothesize that lack of GluA3 potentially result in predominant GluA1/2 complexes that are responsible for the majority of activity-induced enhancement of synaptic transmission in neuronal circuits that innervate striatum and other brain regions that control social behavior. The disturbance in glutamate signaling with the associated changes in dopamine and/or serotonin metabolism contributes to the observed social behavioral phenotype in Gria3 -/Y mice. Further studies are needed to test this hypothesis and delineate the neuronal circuits that are disturbed in striatum and other brain regions of Gria3 -/Y mice.
Supplementary Material
Highlights.
Behavioral phenotype of GluA3-deficient mice was studied > increased social and aggressive behavior was observed > elevated dopamine levels were found in striatum > a role of GluA3 in modulating social behavior is implicated.
Acknowledgement
This study was supported in part by research grants from a Basil O’Connor Award of the March of Dimes Foundation (to T.W.) and NICHD (HD044789 and HD052680, to T.W.); a postdoctoral fellowship from Ministry of Education and Science of Spain (to R.M.); RH is an Investigator with the Howard Hughes Medical Institute.
Abbreviations
- AMPA
2-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- GluA3
AMPA glutamate receptor 3
- NE
Norepinephrine
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- 5-HT
serotonin
- 5-HIAA
5-hydroxyindoleacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seeburg P. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 2.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 3.Dingledine R, Borges K, Bowie D, Traynelis S. The glutamte receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 4.Ayalon G, Stern-Bach Y. Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron. 2001;31:103–113. doi: 10.1016/s0896-6273(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 5.Mansour M, Nagarajan N, Nehring R, Celements J, Rosenmund C. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–853. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 6.Jonas P, Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 7.Vekovischeva O, Aitta-aho T, Verbitskaya E, SandnabbaE Korpi K. Acute effects of AMPA-type glutamate receptor antagonists on intermale social behavior in two mouse lines bidirectionally selected for offensive aggression. Pharmacol Biochem behav. 2007;87:241–249. doi: 10.1016/j.pbb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser K, Koster H, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly P, Sommer B, Andersen P, Seeburg P, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 9.Burnashev N, Monyer H, Seeburg P, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 10.Vekovischeva O, Aitta-aho T, Echenko O, Kankaanpaa A, Seppala T, Honkanen A, Sprengel R, Korpi E. Reduced aggression in AMPA-type glutamate receptor GluR-! subunit-deficient mice. Genes, Brain and Behavior. 2004;3:253–265. doi: 10.1111/j.1601-1848.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 11.Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna F, Velumian A, MacDonald J, Carlen P, Abramow-Nweerly W, Roder J. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 12.Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- 13.Sanchis-Segura C, borchardt T, Vengeliene V, Zhghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26 doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenland H, Kim S, Zhuo M. GluR3 subunit regulates sleep, breathing and seizure generation. Eur J Neurosci. 2008;27:1166–1173. doi: 10.1111/j.1460-9568.2008.06078.x. [DOI] [PubMed] [Google Scholar]
- 15.Brodkin E, Goforth S, Keene A, FosseliaL Silver J. Identification of quantitative trait loci that affect aggressive behavior in mice. J Neurosci. 2002;22:1165–1170. doi: 10.1523/JNEUROSCI.22-03-01165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner S, Takamiya K, Xia J, Yu S, Suh J, Huganir R. Calcium permeable AMPA receptor plasticity is mediated by subunit specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Pletnikov M, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov M, Huang H, Mori S, Moran T, Ross C. Indicible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- 18.Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova I, Sawa A, Margolis R, Cadet J, Mori S, Vogel M, RossM Pletnikov C. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence of neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2011;16:293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasnova I, Betts E, Dada A, efferson A, Ladenheim B, Becker K, Cadet J, Hohmann C. Neonatal dopamine depletion induces changes in morphogenesis and gene expression in the developing cortex. Neurotox Res. 2007;11:107–130. doi: 10.1007/BF03033390. [DOI] [PubMed] [Google Scholar]
- 20.Krasnova I, Hodges A, Ladenheim B, Rhoades R, Phillip C, Cesena A, Ivanova E, Hohmann C, Cadet J. Methamphetamine treatment causes delayed decrease in novelty-induced locomotor activity in mice. Neurosci Res. 2009;65:160–165. doi: 10.1016/j.neures.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banks T, Dabbs J. Salivary testosterone and cortisol in a deliquent and violent urban subculture. J Soc Psychol. 1996;136:49–56. doi: 10.1080/00224545.1996.9923028. [DOI] [PubMed] [Google Scholar]
- 22.Lucion A, de Almeida R, da Silva R. Territorial aggression, body weight, carbohydrate metabolism and testosterone levels of wild rats maintained in laboratory colonies. Braz J Med Biol Res. 1996;29:1657–1662. [PubMed] [Google Scholar]
- 23.Miczek K, Fish E, de Bold J, de Almeida R. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and GABA systems. Psychopharmacology. 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- 24.Appelbaum P. Violence and mental disorders: data and public policy. Am J Psychiatry. 2006;163:1316–1321. doi: 10.1176/ajp.2006.163.8.1319. [DOI] [PubMed] [Google Scholar]
- 25.Jensen P, Youngstrom E, Steiner H, Findling R, Meyer R, Malone R, Carlson G, Coccaro E, Aman M, Blair J, Dougherty D, Ferris C, Flynn L, Green E, Hoagwood K, Jutchinson J, Laughren T, Leve L, Novins DD, Vitiello B. consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications formedication studies. J Am Acad Child Adolescent Psychiatry. 2007;46:309–322. doi: 10.1097/chi.0b013e31802f1454. [DOI] [PubMed] [Google Scholar]
- 26.Nelson R, Trainor B. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 27.Neumann I. V AHD Beiderbeck, Aggression and anxiety: social context and neurobiological links. Front Behavioral neurosci. 2010;4:1–16. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudryavtseva N, Lipina T, Koryakina L. Effects of haloperidol on communicative and aggressive behavior in male mice with different experiences of aggression. Pharmacol Biochem Behav. 1999;63:229–236. doi: 10.1016/s0091-3057(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 29.Sokolov B, Cadet J. Methamphetamine causes alterations in the MPA kinase-related pathways in the brains of mice that display increased aggressiveness. Neuropsychopharmacology. 2006;31:956–966. doi: 10.1038/sj.npp.1300891. [DOI] [PubMed] [Google Scholar]
- 30.van Erp A, Miczek K. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korzan W, forster G, Watt M, Summers C. Dopaminergic activity modulation via aggression, status, and a visual social signal. Behav Neurosci. 2006;120:93–102. doi: 10.1037/0735-7044.120.1.93. [DOI] [PubMed] [Google Scholar]
- 32.Kempf E, Puglisi-Allegra S, Cabib S, Schleef C, Mandel P. Serotonin levels and turnover in different brain areas of isolated aggressive or non-aggressive strains of mice. Prog. Neuro-Psychopharmacol & Biol. Psychiat. 1984;8:365–371. [PubMed] [Google Scholar]
- 33.Garris D. Aggression-associated changes in murine olfactory tubercle bioamines. Brain Res. 2003;963:150–155. doi: 10.1016/s0006-8993(02)03963-x. [DOI] [PubMed] [Google Scholar]
- 34.Lin D, Boyle M, Dollar P, Lee H, Lein E, Perona P, Anderson D. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wise R. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Q, Veenman C, Reiner A. Cellular expression of ionotropic glutamate receptor subunits on specific striatal neuron types and its implication for striatal vulnerability in glutamate receptor-mediated excitotoxicity. Neuroscience. 1996;73:715–731. doi: 10.1016/0306-4522(96)00011-5. [DOI] [PubMed] [Google Scholar]
- 37.Gotz T, Kraushaar U, Geiger J, Lubke J, Berger T, Jonas P. Functional propertiew of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci. 1997;17:204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Y, Xie J, Wang H, Lei W, Chen Q, Reiner A. Differential localizationo f the GluR1 and GluR2 subunits of the AMPA-type glutamate receptor among striatal neuron types in rats. J Chem Neuroanat. 2007;32:167–192. doi: 10.1016/j.jchemneu.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwok K, Tse Y, Wong R, Yung K. Cellular localization of GluR1, GluR2/3, and GluR4 glutamate receptor subunits in neurons of the rat neostriatum. Brain Res. 1997;778:43–55. doi: 10.1016/s0006-8993(97)00950-5. [DOI] [PubMed] [Google Scholar]
- 40.Paquet M, Smith Y. Differential localization of AMPA glutamate receptor subunits in the two segments of teh globus pallidus and the substantia nigra pars reticulata in the squirrel mondey. Eur J Neurosci. 1996;8:229–233. doi: 10.1111/j.1460-9568.1996.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 41.Deng Y, Shelby E, Reiner A. Immunohistochemical localization of AMPA-type glutamate receptor subunits in the striatum of rhesus monkey. Brain Res. 2010;1344:104–123. doi: 10.1016/j.brainres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calabresi P, Centonze D, Pisani A, Sancesario G, Gubellini P, Marfia G, Bernardi G. Striatal spiny neurons and cholinergic interneurons express differential ionotropic glutamatergic responses and vulnerability: implications for ischemia and Huntington's disease. Ann. Neurol. 1998;43:586–597. doi: 10.1002/ana.410430506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.