Abstract
Background
Mutational loss of tumor suppressor phosphatase and tensin homologue deleted on chromosome ten (PTEN) is associated with malignant progression in many cancers including colorectal cancer (CRC). PTEN is involved in negatively regulating the Phosphatidylinositol 3-kinase (PI3K)/AKT oncogenic signaling pathway and has been implicated in the metastatic colonization process. Few in vivo models are available to study CRC metastasis. The purpose of this study was to determine the effect of restoring PTEN activity on metastases in an orthotopic murine model.
Methods
GFP labeled TENN, a highly metastatic human colon cancer cell line with mutational loss of PTEN gene and TENN clones (with restoration of PTEN gene) tumors were orthotopically implanted onto the colons of BALB/c nude mice and allowed to develop primary and metastatic tumors. Seven weeks post-implantation, mice were euthanized and organs extracted for examination.
Results
Both TENN and TENN clone cell lines demonstrated 100% primary invasion. However, compared to the parental TENN cells, which demonstrated 62% metastases to both lungs and liver, TENN clone cells showed an approximately 50% reduction in metastasis, with only 31.6% liver metastasis and no metastasis to the lungs.
Conclusion
Our study shows that reactivation of PTEN tumor suppressor pathway leads to a 50% reduction in CRC metastasis without affecting primary tumor formation. Importantly, PTEN restoration also changed the organotropic homing from liver and lung metastasis to liver metastasis only. This in vivo study demonstrates that PTEN might act specifically as a metastasis suppressor and thus efforts to target the PI3K/PTEN pathway are legitimate.
Keywords: colorectal cancer, PI3Kinase, PTEN, metastases
Introduction
Colorectal cancer (CRC) is one of the most common human malignant tumors with an age-adjusted incidence of 46.3 per 100,000 (1). It remains the second leading cause of cancer related mortality in the United States with metastases to distant organs being the most frequent cause of cancer related deaths. Since treatment options for disseminated disease are severely limited, metastatic disease remains a significant clinical challenge (2, 3). Many critical molecular alterations in the development and progression of colorectal cancer have been identified in the past two decades following the seminal findings of Vogelstein and Fearon (4). Most of the alterations (either genetic and/or epigenetic) have been implicated in tumor progression (from mucosal hyperplasia to adenomas and carcinomas) as summarized by Markowitz and Bertagnolli (5). However, genes specifically involved in promoting or suppressing metastasis are still poorly understood. The identification and characterization of genes that govern the development and/or maintenance of CRC metastasis would lead to the understanding of a new paradigm for control of metastatic potential and would provide a basis for developing novel strategies for the treatment of CRC metastases. Therefore, the purpose of this study is to elicit the biological function of phosphatase and tensin homologue deleted on chromosome ten (PTEN), a tumor suppressor gene (6) involved in negatively regulating the Phosphatidylinositol 3-kinase (PI3K)/AKT oncogenic signaling pathways in an in vivo orthotopic implantation model of colorectal cancer metastases (2, 7, 8).
The Phosphatidylinositol 3-kinase (PI3K) signaling node has been linked to several critical functions in normal cellular growth and metabolism as well as in pathological conditions (9). The PI3K/AKT pathway is deregulated in several types of cancer including CRC and is involved in cancer progression and metastases through the regulation of its cell survival and proliferative functions (6). Thus, the PI3K/AKT signaling cascade has been extensively targeted for drug development (10). PTEN has been shown to be a natural inhibitor for PI3K at the 3-phosphate site and negatively regulates the AKT signaling pathway (6, 11, 12). In CRC, loss of PTEN led to an increased PI3K/AKT mediated intestinal mucosal tumors (11). PTEN, which is located at human chromosome 10q23.3 has been shown to be frequently inactivated in multiple advanced cancers (13, 14). Development of multi-organ tumors has been reported to be associated with PTEN heterozygotes, while embryonic lethality is caused by the homozygous deletion of the PTEN gene (15, 16). The frequent causes of PTEN loss of function are attributed to gene deletion, mutation at exon 7, 8 and 9 and promoter hypermethylation (13, 14) resulting in deregulation of several oncogenic factors (6). Aberrant alteration of PTEN facilitates cell proliferation and inhibits apoptosis (6, 11). PTEN loss has been positively correlated with malignant progression. In CRC, loss of nuclear PTEN was inversely correlated with liver metastasis, and a reduction in PTEN expression predicted local recurrence in CRC (17). Rychahou et al. have reported that loss of PTEN expression in approximately 83% of metastatic CRCs. PTEN inactivated was observed to be more frequent in association with microsatellite instability (11, 18, 19).
We hypothesize that restoration of PTEN in human CRC cells with PTEN loss may provide an increased pro-apoptotic environment leading to a decrease in PI3K/AKT mediated CRC metastasis. In this study, we show for the first time that the restoration of PTEN activity in an orthotopic colon cancer implantation model significantly decreases colon cancer metastasis to liver and lungs. The activation of PTEN in a CRC cell line exhibiting PTEN loss decreases the metastatic capability while changing the organotropic homing from primarily liver and lungs to liver only in an in vivo orthotopic model. These finding further establishes the clinical significance of tumor suppressor PTEN in preventing CRC metastasis.
Materials and Methods
Cell Culture and Reagents
TENN, HCT116 and DLD1 human colon cancer cell lines were established in tissue culture from a primary human colon cancer tumor as previously described (20). The TENN line was stably transfected with a full length PTEN cDNA creating the TENN clone. Both TENN and TENN clone cell lines were cultured in SM media supplemented with 10% fetal bovine serum as described previously (21). HCT116 and DLD1 cells were cultured in serum free medium consisting of McCoy’s 5A medium (Sigma, St. Louis, MO) supplemented with pyruvate, vitamins, amino acids, antibiotics, 10 ng/mL epidermal growth factor (R and D Systems, Minneapolis, MN), 20 mg/mL insulin (Sigma), and 4 mg/mL transferrin as described previously (21). Cells were maintained at 37 C in a humidified atmosphere of 5% CO2.
Green Fluorescence Protein Transfection
TENN and TENN clone cells were cotransfected with the plasmid encoding the VSVG envelope protein and the retroviral vector encoding green fluorescence protein (GFP) using FuGene (Invitrogen, Carlsbad, CA). Viruses were collected 48 hours later and used to infect all cell lines. After 48 hours, infected cells were selected with puromycin for 5 days.
Orthotopic Implantation
GFP-labeled TENN and TENN clone cells (7×106 cells) were inoculated subcutaneously onto the dorsal surfaces of separate BALB/c nude male mice. Once the xenografts were established, measuring approximately 500 mm3, they were excised and minced into 1 mm3 pieces. Two of these pieces were then orthotopically implanted into the colons of other male BALB/c nude mice as previously reported (23). Briefly, for operative procedures, animals were anesthetized with isoflurane inhalation. A 1 cm laparotomy was performed, and both the cecum and ascending colon were exteriorized. Using 7x magnification and microsurgical techniques, the serosa was disrupted in two different locations. The 1 mm3 pieces of xenograft were sub-serosally implanted using a 9-0 nylon suture at the two points of serosal disruption. The bowel was then returned to the peritoneal cavity and the abdomen was closed with interrupted 5-0 vicryl sutures. Similar operative procedures have been used for other orthotopic implantation studies (2, 21–23).
Imaging
Starting at 1 week post-implantation, animals were anesthetized with a 1:1 mixture of ketamine (10 mg/mL) and xylazine (1 mg/mL) by intraperitoneal injection (0.01 mL/mg) and weekly GFP fluorescence imaging was performed for up to 7 wk. Specifically, GFP fluorescence imaging was performed using a light box illuminated by fiberoptic lighting at 470 nm (Illumatool BLS; Lightools Research, Encinitas, CA) using a Retiga EXi color CCD camera (QImaging, Burnaby BC, Canada). High-resolution images consisting of 1360 31036 pixels were captured directly using a MS-Windows based PC. Images were visually optimized for contrast and brightness using commercial software (Adobe Photoshop, CS2; Adobe, San Jose, CA). Excitation of GFP in the light box facilitated identification of primary and metastatic disease by direct near-real time visualization of fluorescence in live animals.
H and E Staining
At approximately 50 days post-implantation, the animals were euthanized. The colon, including any primary tumor, was harvested in addition to the liver and lungs. These organs were explanted, imaged, and immediately placed in 10% neutral buffered formalin fixative for 24 hours as described previously (22). The tissues were then processed and embedded in paraffin. Slides were then cut for hematoxylin and eosin (H and E) staining.
Results
Identification of TENN colon cancer cell line with PTEN mutation
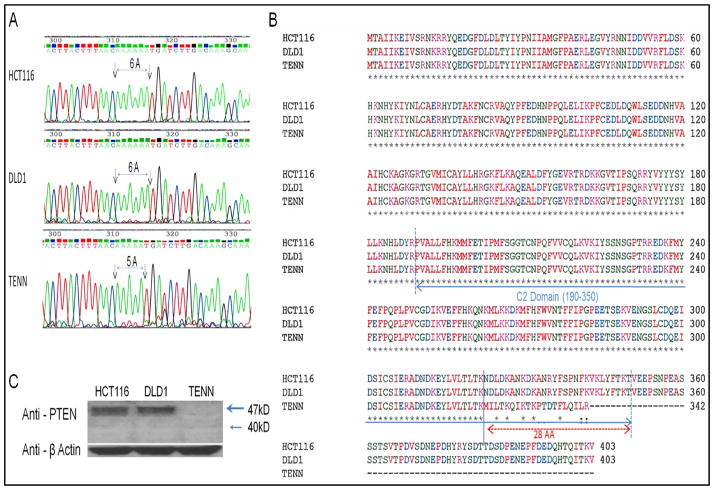
In an effort to identify CRC cells harboring PTEN mutation, eight colon cancer cell lines were analyzed by sequencing (data not shown). As shown in Fig 1A, mutation in PTEN gene was observed in TENN cells in which only one Adenine was deleted as compared to HCT116 and DLD1 cells harboring a wild type (WT) PTEN gene. It was further revealed that the predicted PTEN of TENN cells is shorter (342AA) than wild type (403AA) with the reading frame shifted in the end of C2 Domain and lost C-terminal tail of 61AA compared to HCT116 and DLD1 cells having full length PTEN gene (Fig 1B). Western blot analysis of the HCT116, DLD1 and TENN cell lines showed the absence of PTEN protein expression in TENN cells (Fig 1C).
Fig. 1. Identification of PTEN mutation in TENN colon cancer cells.
(A, B) Sequencing analysis of 3 colon cancer cell lines (HCT116, DLD1 and TENN) showing mutation in PTEN gene of TENN cells. (C) Western blot analysis showing a loss of PTEN in TENN cells. Anti-β Actin used as a loading control.
PTEN restoration leads to decreased metastases
It has been reported that the PI3K/AKT signaling plays a central role in CRC cell survival and metastasis. Loss of tumor suppressor activity of PTEN leads to increased pro-survival effects mediated through the activated PI3K/AKT signaling (6, 11). Parental TENN cell line was stably transfected with a full length PTEN cDNA creating TENN clone cell lines. Results from orthotopic implantation studies showed differential levels of liver and/or lung metastases on histological evaluation between the parental TENN and TENN clone cells as shown in Table 1. GFP imaging as well as histological examination indicated that all the animals had primary tumor invasion (Fig 3). In animals implanted with the parental TENN cells containing a PTEN mutation, 16/26 (62%) animals demonstrated liver and/or lung metastases. In contrast, on an average, 6/19 (31.6%) of PTEN clones showed only liver metastases whereas no animals showed any lung metastases on histological examination (Table 1, Fig 3 and Fig 4). This indicates that the restoration of PTEN activity in TENN cells resulted in 50% decreased in metastatic potential, as compared to parental and control lines.
Table 1.
Results of orthotopic implantation demonstrating the primary invasion and liver/lung metastases on histological evaluation.
| Primary invasion | Liver metastasis | Lung metastasis | Liver and/or lung metastases | |
|---|---|---|---|---|
| TENN | 26/26 (100%) | 11/26 (42%) | 13/26 (50%) |

|
| TENN Clone 1 | 9/9 (100%) | 3/9 (37.9%) | 0/9 (0%) | 3/9 (37.9%) |
| TENN Clone 2 | 10/10 (100%) | 3/10 (30%) | 0/10 (0%) | 3/10 (30%) |
| TENN Clone Avg | 19/19 (100%) | 6/19 (31.6%) | 0/19 (0%) |

|
Fig. 3.
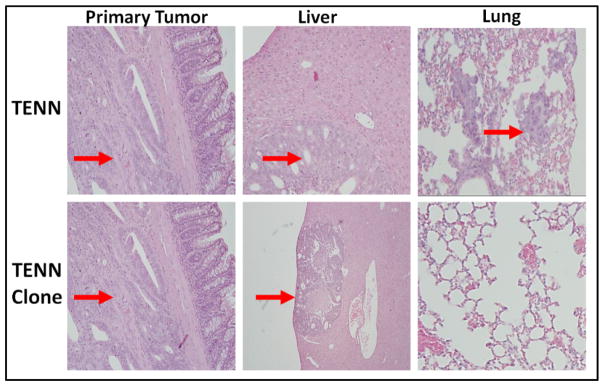
Histological comparison of primary tumors and liver/lung metastases by H&E staining in TENN and TENN clone in paraffin-embedded tissue sections.
Discussion
A major limitation for understanding the metastatic process is the absence of any in vivo models of metastatic CRC. The development of orthotopic xenograft transplants from human CRC cell lines in our laboratory that recapitulates the metastatic pattern of the disseminated disease in patients allow for the study of CRC metastasis in vivo (2, 8, 22, 23). The major goal of this study was to analyze the role of PTEN in the development of metastases in CRC in vivo. The tumor suppressor gene PTEN has been observed to be frequently mutated in different subtypes of CRC (11, 17, 19, 24). Recent studies using TMAs have shown that the genomic deletion of PTEN is strongly correlated with tumor progression (25). In breast cancer, comparative genetic hybridization (CGH) arrays used to compare patterns of chromosomal aberrations in primary breast tumors and brain metastases suggested a role of PTEN in brain metastases of breast cancer (26). Studies using targeted deep-sequencing analysis of the primary adenocarcinoma (CRC) and their respective liver metastasis have found genetic function-impairing variations in PTEN, in addition to KRAS, BRAF and PI3KCA (27). In a multivariate analysis, PTEN was significantly correlated with CRC liver metastasis (28). In contrast, Feng et al. showed that in pancreatic cancer, high level of PTEN expression is associated with low-grade liver metastasis and higher patient 5-year survival rate (29). Trotman et al. (30) demonstrated dose-dependent inverse correlation between PTEN inactivation and metastatic progression of prostate cancer using a series of engineered hypomorphic PTEN mutant with decreasing PTEN activity. Several mutations in the PI3K/AKT pathway have been reported in various types of cancer. Genetic loss of PTEN leads to increased PI3K/AKT mediated cell proliferation and decreased apoptosis at the site of metastasis (6). The nuclear PTEN inhibits the PI3K/AKT signaling axis, blocks cyclin D1 and induces cell cycle arrest (6). Studies from Evers laboratory identified an inverse correlation between AKT2 and PTEN. It was reported that AKT2 overexpression in WT PTEN CRC cells formed micro-metastases; however sustained metastases required PTEN loss (19). Furthermore, Bowen et al. showed that PTEN loss induced epithelial-to-mesenchymal transition (EMT) in human colon cancer cells (11).
Patients with metastatic disease and wild type kras are candidates for combination therapies that include cetuximab, a monoclonal antibody to the epidermal growth factor receptor (EGFR). Chemo-resistance to EGFR targeted therapy with cetuximab, however, remains a concern for the treatment of CRC metastasis. Hsu et al., reported that a high expression of PTEN is positively correlated with high chemosensitivity to 5-FU and oxaliplatin in combination with cetuximab (6). Studies by Lin et al., showed that patients with PTEN mutations did not respond to cetuximab (32). However, response rates increased by 45% in patients with wild type PTEN tumors.
In conclusion, we have shown that mutational loss of PTEN increases the metastatic potential of CRC cells. However, overexpression of PTEN in TENN cell lines with mutational loss of PTEN led to a 50% decrease in metastasis. Importantly, primary tumor invasion was unaffected by the restoration of PTEN tumor suppressor activity. Similar findings were reported by Davies et al.(31) in a prostate cancer in vivo metastasis model showing that PTEN significantly suppressed metastasis without affecting primary tumorigenesis. This study corroborates the in vivo and clinical studies demonstrating a specific negative correlation between loss of PTEN expression and metastasis (11, 19) and thereby highlighting the possibility of PTEN being a metastasis suppressor gene and a potential marker for precision therapy.
Fig. 2. PTEN restoration leads to decreased metastatic colonization.
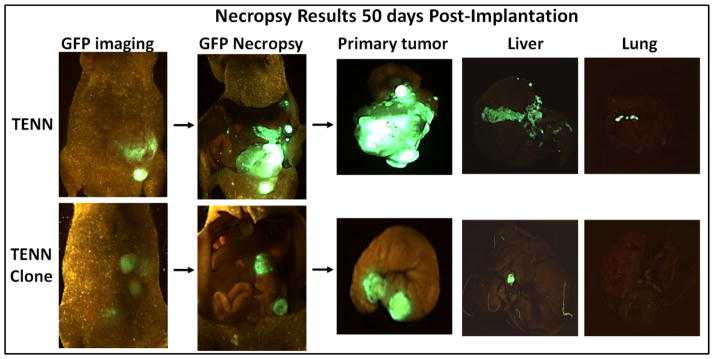
Comparison of GFP images of primary tumors and liver/lung metastases in TENN and TENN clone mice.
Synopsis.
PI3 kinase activity is correlated with tumor progression and metastases. Restoring PTEN activity to block PI3kinase activity decreases colorectal cancer metastases in an in vivo model.
References
- 1.Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) SEER web site. http://seercancergov/csr/1975_2009_pops09/2011.
- 2.Chowdhury S, Howell GM, Rajput A, et al. Identification of a novel TGFbeta/PKA signaling transduceome in mediating control of cell survival and metastasis in colon cancer. PLoS One. 2011;6:e19335. doi: 10.1371/journal.pone.0019335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu CP, Kao TY, Chang WL, Nieh S, Wang HL, Chung YC. Clinical significance of tumor suppressor PTEN in colorectal carcinoma. Eur J Surg Oncol. 2011;37:140–7. doi: 10.1016/j.ejso.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Rajput A, Koterba AP, Kreisberg JI, Foster JM, Willson JK, Brattain MG. A novel mechanism of resistance to epidermal growth factor receptor antagonism in vivo. Cancer Res. 2007;67:665–73. doi: 10.1158/0008-5472.CAN-06-2773. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Rajput A, Kan JL, et al. Knockdown of Ron kinase inhibits mutant phosphatidylinositol 3-kinase and reduces metastasis in human colon carcinoma. J Biol Chem. 2009;284:10912–22. doi: 10.1074/jbc.M809551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–42. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 43–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 11.Bowen KA, Doan HQ, Zhou BP, et al. PTEN loss induces epithelial--mesenchymal transition in human colon cancer cells. Anticancer Res. 2009;29:4439–49. [PMC free article] [PubMed] [Google Scholar]
- 12.Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 13.Bonneau D, Longy M. Mutations of the human PTEN gene. Hum Mutat. 2000;16:109–22. doi: 10.1002/1098-1004(200008)16:2<109::AID-HUMU3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Soria JC, Lee HY, Lee JI, et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8:1178–84. [PubMed] [Google Scholar]
- 15.Podsypanina K, Ellenson LH, Nemes A, et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A. 1999;96:1563–8. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–11. [PubMed] [Google Scholar]
- 17.Sawai H, Yasuda A, Ochi N, et al. Loss of PTEN expression is associated with colorectal cancer liver metastasis and poor patient survival. BMC Gastroenterol. 2008;8:56. doi: 10.1186/1471-230X-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nassif NT, Lobo GP, Wu X, et al. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene. 2004;23:617–28. doi: 10.1038/sj.onc.1207059. [DOI] [PubMed] [Google Scholar]
- 19.Rychahou PG, Kang J, Gulhati P, et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:20315–20. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brattain MG, Levine AE, Chakrabarty S, Yeoman LC, Willson JK, Long B. Heterogeneity of human colon carcinoma. Cancer Metastasis Rev. 1984;3:177–91. doi: 10.1007/BF00048384. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Kuropatwinski K, Hauser J, et al. Colon carcinoma cells harboring PIK3CA mutations display resistance to growth factor deprivation induced apoptosis. Mol Cancer Ther. 2007;6:1143–50. doi: 10.1158/1535-7163.MCT-06-0555. [DOI] [PubMed] [Google Scholar]
- 22.Ongchin M, Sharratt E, Dominguez I, et al. The effects of epidermal growth factor receptor activation and attenuation of the TGFbeta pathway in an orthotopic model of colon cancer. J Surg Res. 2009;156:250–6. doi: 10.1016/j.jss.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Rajput A, Dominguez San Martin I, Rose R, et al. Characterization of HCT116 human colon cancer cells in an orthotopic model. J Surg Res. 2008;147:276–81. doi: 10.1016/j.jss.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Parsons DW, Wang TL, Samuels Y, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 25.Krohn A, Diedler T, Burkhardt L, et al. Genomic Deletion of PTEN Is Associated with Tumor Progression and Early PSA Recurrence in ERG Fusion-Positive and Fusion-Negative Prostate Cancer. Am J Pathol. 2012 doi: 10.1016/j.ajpath.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Wikman H, Lamszus K, Detels N, et al. Relevance of PTEN loss in brain metastasis formation in breast cancer patients. Breast Cancer Res. 2012;14:R49. doi: 10.1186/bcr3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermaat JS, Nijman IJ, Koudijs MJ, et al. Primary colorectal cancers and their subsequent hepatic metastases are genetically different: implications for selection of patients for targeted treatment. Clin Cancer Res. 2012;18:688–99. doi: 10.1158/1078-0432.CCR-11-1965. [DOI] [PubMed] [Google Scholar]
- 28.Wu JH, Tian XY, Hao CY. The significance of a group of molecular markers and clinicopathological factors in identifying colorectal liver metastasis. Hepatogastroenterology. 2011;58:1182–8. doi: 10.5754/hge11380. [DOI] [PubMed] [Google Scholar]
- 29.Feng C, Yao R, Huang F, Liu X, Nie W. High level of PTEN expression is associated with low-grade liver metastasis and satisfactory patient survival in pancreatic cancer. Arch Med Res. 2011;42:584–8. doi: 10.1016/j.arcmed.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Trotman LC, Niki M, Dotan ZA, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies MA, Kim SJ, Parikh NU, Dong Z, Bucana CD, Gallick GE. Adenoviral-mediated expression of MMAC/PTEN inhibits proliferation and metastasis of human prostate cancer cells. Clin Cancer Res. 2002;8:1904–14. [PubMed] [Google Scholar]
- 32.Lin JK, Lin AJ, Lin CC, et al. The status of EGFR-associated genes could predict the outcome and tumor response of chemo-refractory metastatic colorectal patients using cetuximab and chemotherapy. J Surg Oncol. 2011;104:661–6. doi: 10.1002/jso.21993. [DOI] [PubMed] [Google Scholar]