Abstract
The urogenital microbial infection in pregnancy is an important cause of maternal and neonatal morbidity and mortality. Uropathogenic Escherichia coli strains which express Dr fimbriae (Dr+) are associated with unique gestational virulence and they utilize cell surface decay accelerating factor (DAF or CD55) as one of the cellular receptor before invading the epithelial cells. Previous studies in our laboratory established that nitric oxide reduces the rate of E. coli invasion by delocalizing the DAF protein from cell surface lipid rafts and down-regulating its expression. The phosphoinositide 3-kinase/ protein kinase B (PI3K/Akt) cell signal pathway plays an important role in host-microbe interaction because many bacteria including E. coli activate this pathway in order to establish infection. In the present study we showed that the PI3K/Akt pathway negatively regulates the expression of DAF on the epithelial cell surface and thus inhibits the adhesion of Dr+ E. coli to epithelial cells. Initially, using two human cell lines Ishikawa and HeLa which differ in constitutive activity of PI3K/Akt we showed that DAF levels were associated with the PI3K/Akt pathway. We then showed that the DAF gene expression was up-regulated and the Dr+ E. coli adhesion increased after the suppression of PI3K/Akt pathway in Ishikawa cells using inhibitor LY-294002, and a plasmid which allowed the expression of PI3K/Akt regulatory protein PTEN. The down-regulation of PTEN protein using PTEN-specific siRNA activated the PI3K/Akt pathway, down-regulated the DAF and decreased the adhesion of Dr+ E. coli. We conclude that the PI3K/Akt pathway regulated the DAF expression in a nitric oxide independent manner.
Keywords: DAF, CD55, PI3K/Akt pathway, Escherichia coli, PTEN, Kinase, Ishikawa cells
Introduction
The urogenital microbial infection (Urinary tract infection or UTI) in pregnancy is an important cause of maternal and neonatal morbidity and mortality (1–3). The most common bacteria that colonize the urinary tract and cause UTI are the uropathogenic Escherichia coli (4). The uropathogenic E. coli bearing Dr fimbriae (Dr+) have been associated with various pathologies such as UTIs in young adult women, chronic diarrhea in children, and chronic pyelonephritis in pregnant women (5–7). The epithelial cell adhesion and invasion of Dr+ E. coli has been shown to be facilitated by glycosylphosphatidylinositol (GPI) anchored cell surface proteins of the host such as carcinoembryonic antigen (CEA)-related cell adhesion molecules (CEACAMs or CD66e) and decay accelerating factor (DAF or CD55) (8–10). Dr adhesins are a conserved family of gram negative bacterial adhesion proteins and they are assembled via chaperone-usher pathway (11). The biogenesis of Dr adhesins at the structural and molecular level is well known (12–15). However, the mechanisms of interactions between Dr+ E. coli and host cells that are crucial for bacterial pathogenesis are much less known. Previous studies in our laboratory have shown that nitric oxide (NO) down-regulates the DAF expression in a dose and time dependent manner in an endometrial epithelial cell line. The reduced DAF expression level was associated with reduced in vitro invasion of Dr+ E. coli suggesting that the down-regulation of DAF expression may be one of the mechanisms by which NO exhibits protective role against Dr+ E. coli infection during pregnancy (16).
DAF is a complement regulatory protein and is widely expressed on the surface of all the serum exposed cells including epithelial cells. DAF is bound to the plasma membrane by lipid raft associated GPI anchor (17). Apart from its complement regulatory role, DAF has been implicated in many physiological and pathophysiological conditions. Expression of DAF in endometrium was found to be up-regulated during secretory phase and implantation, suggesting a role in immune tolerance of the implanting conceptus (18;19). The DAF deficiency has been associated with various diseases such as paroxysmal nocturnal hemoglobinurea, luteal phase defect, malarial anemia, psoriatic lesions and apoptotic neutrophils (20–24). In contrast, higher levels of DAF expression are associated with tumorigenesis, decreased tumor cell lysis and metastasis of many types of cancers (25;26).
DAF expression levels may vary between tissues and under different physiological and pathophysiological conditions (27). The DAF gene expression is regulated by transcription factors such as Specificity protein 1 (Sp1) and cAMP response element-binding protein (CREB) (17;28). One of the major cellular signaling cascades that activate these transcription factors is the Phosphoinositide 3-kinase (PI3K) pathway (29;30). Therefore, we hypothesized that PI3K pathway may have a role in the regulation of DAF expression as well as adhesion of Dr+ E. coli to cells which express DAF.
The PI3K pathway is a major signaling cascade downstream of growth factor receptor tyrosine kinases. PI3K catalyzes the production of lipid second messengers such as phosphatidylinositol-3,4,5-triphosphate (PIP3) in the cell membrane. These phosphoinositides then activate phosphoinositide dependent kinases (PDK) that allows the membrane recruitment of protein kinase B (PKB or Akt), a serine-threonine kinase which is the central molecule in the PI3K signaling pathway. PKB is then activated by PDK dependent phosphorylation at two phosphorylation sites, amino acid residue threonine-308 and serine-473 (31). A lipid phosphatase, Tensin Homology Deleted on Chromosome Ten (PTEN) regulates the activity of PI3K/Akt by dephosphorylating phosphoinositides (32). In this report, we examined the role of PI3K/Akt pathway in the regulation of DAF expression and the cellular adhesion of Dr+ E. coli using two different human genital tract epithelial cell line models, endometrial Ishikawa and cervical HeLa cells. We have chosen these two cell lines for this study because of their expression of DAF and the difference in the constitutive activity of the PI3K/Akt pathway. We show here that PI3K/Akt pathway negatively regulates the DAF expression and cellular adhesion of Dr+ E. coli.
Materials and methods
Cell culture
Ishikawa cells are a human endometrial cell line derived from a well-differentiated endometrial adenocarcinoma that has been shown to mimic endometrial epithelial cells (33). Ishikawa cells were routinely cultured in Eagle’s minimum essential medium (MEM) containing 2 mM L-glutamine, 100 µg of penicillin-streptomycin/ml, and 10 mM HEPES supplemented with 10% fetal calf serum at 37 °C in a humidified 5% CO2 atmosphere. HeLa cells were cultured in MEM containing 100 µg of penicillin-streptomycin/ml, and 10 mM HEPES supplemented with 10% fetal calf serum at 37 °C in a humidified 5% CO2 atmosphere.
Antibodies and reagents
The DAF antibody (monoclonal antibody, cloneIH4) was a gift from D. M. Lublin, (Washington University School of Medicine, Seattle, WA, USA). The Akt (9272), pAkt-Thr-308 (9275), pAkt-Ser-473 (9271), PDK1(3062), pPDK1 (3485), PTEN (9552) and β-actin (4970) were purchased from Cell Signaling Technology (Danvers, MA, USA). The secondary anti-mouse antibody raised in goat was purchased from Molecular Probes (Grand Island, NY, USA) The horseradish-peroxidase conjugated anti-rabbit secondary antibody raised in donkey was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The LY294002 was purchased from Cayman Chemicals (Ann arbor, MI, USA). The L-N-G-Nitroarginine methyl ester (L-NAME, N5751) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Transient transfections of cells
The PTEN plasmid was purchased from addgene (28298, Cambridge, MA, USA). Ishikawa cells were grown in 12-well cell culture plates for 24 hours and the media were replaced by Opti-MEM reduced serum medium (31985070, Invitrogen, Carlsbad, CA, USA). The PTEN plasmid (7.8 µl) was diluted by mixing with 92.2 µl of Opti-MEM reduced serum medium to get a final concentration of 1 µg/ml. Similarly 2 µl of lipofectamine 2000 (11668019, Invitrogen) was diluted by mixing with 98 µl of Opti-MEM reduced serum medium and incubated at room temperature for 5 minutes. Diluted PTEN plasmid (50 µl) was then mixed with diluted lipofectamine 2000 (50 µl) and incubated at room temperature for 20 minutes. 100 µl of lipofectamine-PTEN plasmid mixture was added per well and the cells were incubated at 37 °C for 48 hours. For the controll cells diluted lipofectamine (100 µl) prepared as described above was used.
The PTEN specific siRNA (signal silence PTENsiRNA I) was purchased from cell signaling technology (6251). The HeLa cells were grown in 12-well cell culture plates for 24 hours and the media were replaced by Opti-MEM reduced serum medium. The PTEN siRNA (11.16 µl) was diluted by mixing with 88.84 µl of Opti-MEM reduced serum medium to get a final concentration of 1 µg/ml. Similarly 4 µl of lipofectamine 2000 was diluted by mixing with 94 µl of Opti-MEM reduced serum medium and incubated at room temperature for 5 minutes. Diluted PTEN siRNA (50 µl) was then mixed with diluted lipofectamine 2000 (50 µl) and incubated at room temperature for 20 minutes. 100 µl of lipofectamine-PTEN siRNA mixture was added per well and the cells were incubated at 37 °C for 48 hours. For the controll cells PTEN siRNA was replaced by a scrambled siRNA. After the transfection, cells were harvested by detergent lysis followed by scraping using a cell scraper for western blot analysis.
Western blotting
Harvested cells were lysed on ice for 30 minutes with 200 µl lysis buffer (9803, Cell Signalling Technology) containing 5 µl each of Phosphatase inhibitor Coctail1, Phosphatase Inhibitor Coctail 2 and Protease Inhibitor (Sigma-Aldrich) supplemented with Pefablock SC to 1 mM. Lysates were collected by centrifugation (14,000g) at 4 °C for 10 minutes. Protein concentrations in the cell lysates were determined by Pierce BCA protein assay kit (22660, Thermo Scientific, Rockford, IL, USA), against BSA standards. Proteins were seperated by polyacrylamide gel electrophoresis using NuPAGE 4–12% Bis-Tris mini gels (Invitrogen) with MOPS SDS as running buffer at 200V for 50 minutes. Electrophoresis was carried out under reducing conditions except for DAF, for which non-reducing conditions were employed because the IH4 antibody used recognizes its epitope under non-rducing condition. The protein bands of interest were identified by Western blotting and visualized on a blue sensitive autoradiography film using supersignal west pico chemiluminiscence substrate (32132, Thermo Scientific) according to the instructions provided by the manufacturer. The films were scanned and the protein band densitometric analysis was performed using the AlphaEase Flurochem 8000 software (Alpha Innotech, Santa Clara, CA, USA).
RNA isolation and real time quantitative PCR
Total RNA was isolated from the cells using RNeasy mini kit (74104, Qiagen, Valencia, CA, USA) according to the procedure recommended by the manufacturer. RNA extraction was followed by DNase I (Qiagen) treatment to remove DNA contamination. Total RNA of 1 µg was reverse transcribed into complementary DNA using avian myeloblastosis virus reverse transcriptase (M5101, Promega, Madison, WI, USA) and random oligonucleotide hexamers (N8080127, Invitrogen). Quantitative real time PCR was carried out using CFX96 system and SYBR green master mix (Biorad, Hercules, CA, USA). A comparative cycle of threshold fluorescence (CT) method was used with housekeeping gene as internal control.
Proximity Ligation Assay (PLA)
The PLA is a PCR based immuno-technique for the visualization of insitu protein expression, protein-protein interactions and posttranslational protein modifications. The insitu expression of DAF proein on the cell surface was visualized and quantified by PLA. The Duolink II kit used for the PLA was purchased from Olink Bioscience (Uppsala, Sweden). The HeLa or Ishikawa cells sparsley grown in 16-well lab-Tek chamber slides (Electron Microscopy Sciences, Hatfield, PA, USA) were transiently transfected with PTEN-specific siRNA and PTEN plasmid respectively for 24 hours. A scrambled siRNA or tranfection medium served as controls. The cells were washed twice with phosphate buffered saline and then fixed using 4% paraformaldehyde. The PLA was then performed according to the instructions provided by the duolink II kit manufacturer.
Bacterial adhesion to the cells
For E.coli adhesion study we used a recombinant strain (the laboratory E. coli K-12 strain carrying plasmid pBJN406) (34). The Ishikawa and HeLa cells were grown in 8-well lab-Tek chamber slides (Electron Microscopy Sceinces) and transiently transfected with PTEN plasmid and PTEN siRNA respectively for 24 hours. The media were removed and the cells were rinsed with phosphate buffered saline (PBS). Then, bacterial suspensions in 500 µl of PBS, prepared as described previously (35;36) for the adherence assay, were added to the monolayers of Ishikawa and HeLa cells, incubated at 37 °C for 3 hours. The cells were then rinsed with PBS three times, and the bacteria adhered to the cells were visualized by gram staining.
Statistical analysis
GraphPad Prism (version6) software was used for statistical analysis. Comparisons between the groups were made by t-test. Statistical significance (p < 0.05) between the groups was determined by Bonferroni’s multiple comparison method.
Results
Adhesion of E. coli expressing Dr+ adhesin to epithelial cells was associated with constitutive activity of PI3K-Akt pathway
The PI3K/Akt pathway is constitutively active in endometrial epithelial cell line Ishikawa because of a truncated mutation in PTEN gene (37). In cervical epithelial cell line HeLa, functional PTEN is expressed and therefore, the PI3K/Akt pathway is regulated (38). Therefore, we used these two cell lines to evaluate the role of PI3K/Akt pathway in the expression of DAF protein and the adhesion of E. coli that express Dr+ adhesin. Initially we confirmed the expression levels of PTEN in HeLa and Ishikawa cells and then analyzed the expression levels of DAF, PDK1, Akt, phosphor-PDK1 (pPDK1) and phospho-Akt [pAkt (Ser473) and pAkt (Thr308)] proteins by western blotting. The PTEN was expressed only in HeLa cells whereas the total PDK1 and total Akt were expressed in both the Ishikawa and the HeLa cells (Figure 1a). However, Western blotting followed by densitometric analysis revealed that pPDK1 levels were significantly higher in the Ishikawa cells compared to that of HeLa cells (Figure 1b and c). The pAkt (Ser473) and pAkt (Thr308) were detected only in the Ishikawa cells (Figure 1d). Taken together these results confirmed that the PI3K/Akt pathway was regulated in HeLa cells and constitutively active in Ishikawa cells. The western blot analysis of DAF protein levels revealed that its expression was dramatically higher in the HeLa cells compared to that of Ishikawa cells suggesting an association between the PI3K/Akt pathway and the expression of DAF protein (Figure 1e). This result led us to analyze the difference in adhesion of Dr+ E. coli to the HeLa and the Ishikawa cells. When Dr+ E. coli were allowed to bind to the monolayers of HeLa and Ishikawa cells in vitro, we found that the number of bacteria adhered to individual HeLa cells was significantly higher compared to that of Ishikawa cells (Figure 1f). These data suggested a possible association between PI3K/Akt regulated DAF expression and Dr+ E. coli adhesion to the epithelial cells.
Figure 1.
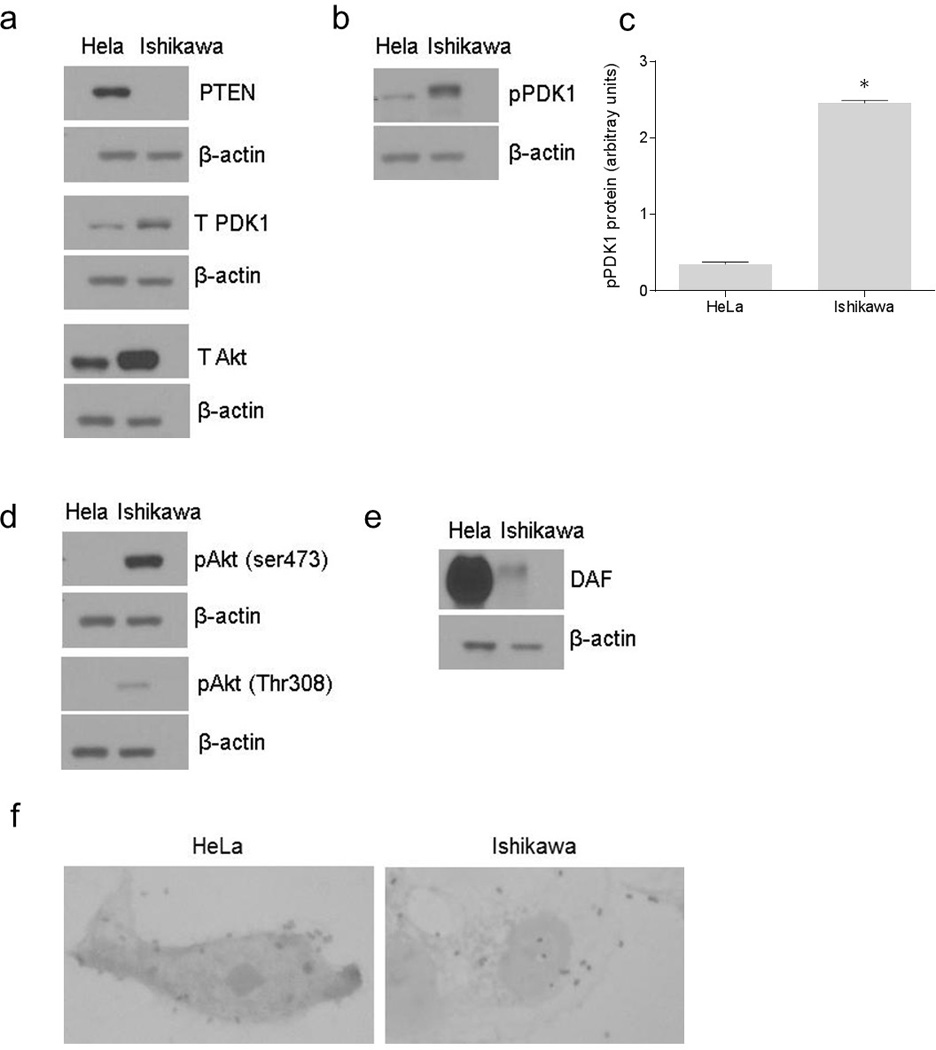
The association between constitutive activity of PI3K/Akt pathway and adhesion of Dr+ E. coli to epithelial cells. The expression of PI3K/Akt pathway proteins in HeLa and Ishikawa cell grown as monolayers for 24 hours was examined by western blot. The E. coli K-12 strain carrying the plasmid pBJN406, encoding the production of Dr fimbriae was allowed to bind to HeLa and Ishikawa cells for three hours and then the number of bacteria adhered to individual cells was examined by gram staining. (a) Western blot for PTEN, total PDK1 and total Akt proteins in HeLa and Ishikawa cells. (b) Western blot for pPDK1 protein in HeLa and Ishikawa cells. (c) Densitometric analysis of pPDK1 protein levels. * p < 0.0001 versus HeLa. (d) Western blot for activated forms of Akt, pAkt(ser473) and pAkt(Thr308). (e) Western blot for DAF. (f) Gram staining to visualize E. coli K-12 strain carrying plasmid pBJN406 adhered to HeLa and Ishikawa cells. Data are representative images or expressed as mean values ± SEM of each group from three separate experiments.
Pharmacological inhibitor of PI3K/Akt pathway up-regulated the expression of DAF mRNA and protein
In order to estbliash a direct evidence for the link between PI3K/Akt pathway and the DAF gene expression, we Inhibited PI3K/Akt pathway in Ishikawa cells using pharmacological inhibitor LY294002. The western blot followed by densitometric analysis of Akt and its phosporylation after treating Ishikawa cells with LY294002 for 24 hours revealed that level of phosphorylation at both Ser473 and Thr308 reduced siginficantly compared to that of untreated cells (Figure 2a, b and c). Then we analysed the effect of LY294002 on the expression of DAF gene in Ishikawa cells. As shown in Figure 2d, the real time quantitative PCR analysis revealed that DAF mRNA expression increased significantly in LY294002 treated cells compared to that of control cells. Further, western blot and densitometric analysis showed an increase in DAF protein expression after LY294002 treatment compared to controll cells (Figure 2e and f). These data suggested a direct inverse relation between PI3K/Akt pathway and the DAF gene expression.
Figure 2.
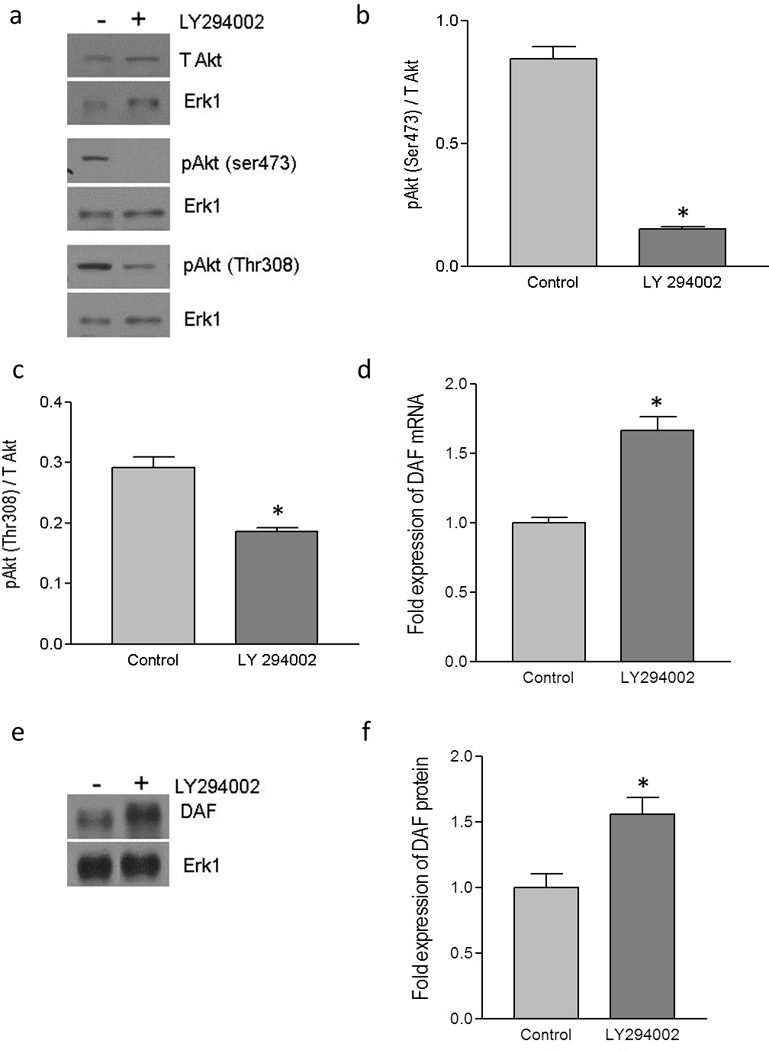
Up-regulation of DAF by pharmacological inhibitor of PI3K/Akt pathway. The monolayer of Ishikawa cells was treated with LY 294002 (50 µM) dissolved in DMSO or DMSO alone (control) for 24 hours. (a) Western blot for total Akt and its activated forms, pAkt(ser473) and pAkt(Thr308). (b) Density analysis of pAkt(ser473) western blot expressed as ratio between total Akt and pAkt(ser473). * p < 0.0001 versus control. (c) Density analysis of pAkt(Thr308) western blot expressed as ratio between total Akt and pAkt(Thr308). * p < 0.01 versus control. (d) Fold increase in the expression of DAF mRNA as assessed by real time qPCR after treating the Ishikawa cells with the LY294002. * p < 0.001 versus control. (e) Western blot for DAF protein (f) Density analysis of DAF western blot expressed as fold increase in the expression after treating Ishikawa cells with LY294002. p < 0.03 versus control. Data are representative images or expressed as mean values ± SEM of each group from three separate experiments.
Introduction of a plasmid expressing PTEN into Ishikawa cells up-regulated DAF expression and increased Dr+ E. coli adhesion
To further confirm the role of PI3K/Akt in DAF gene expression and Dr+ E. coli adhesion, we sought to manipulate the PI3K/Akt pathway by altering the expression of PTEN in Ishikawa and HeLa cells. Initially we introduced a mammalian expression plasmid for PTEN into the Ishikawa cells. The introduction of PTEN plasmid allowed the Ishikawa cells to over-express PTEN protein (Figure 3a and b).
Figure 3.
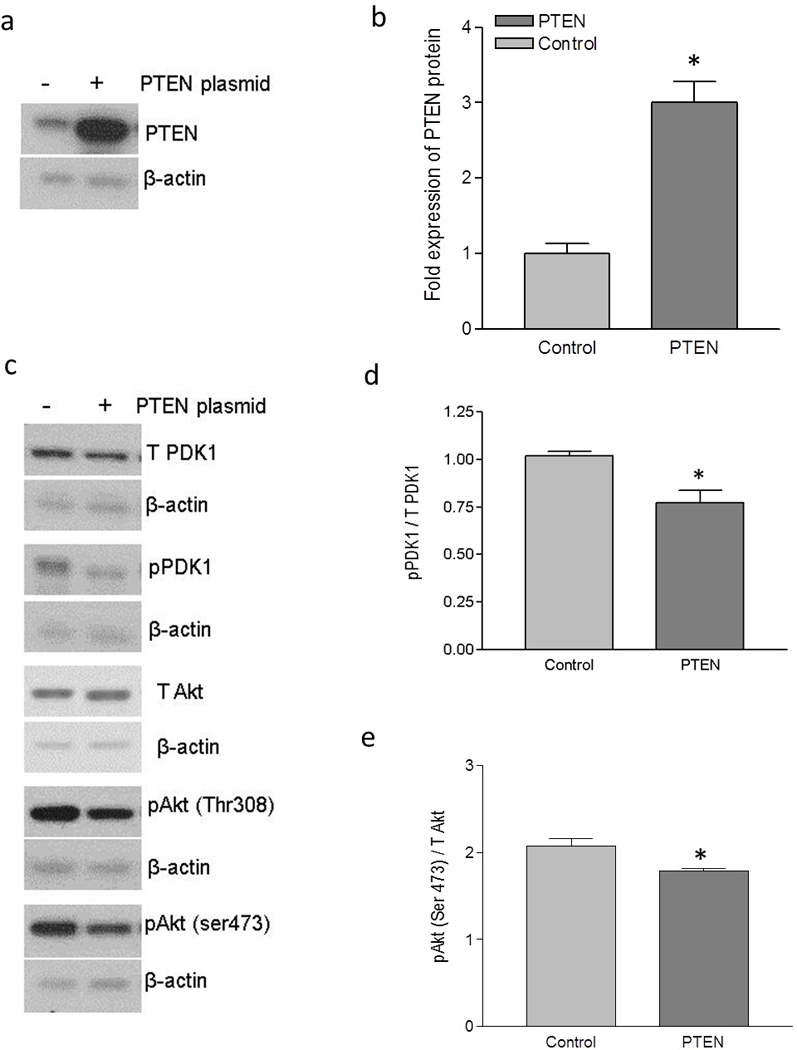
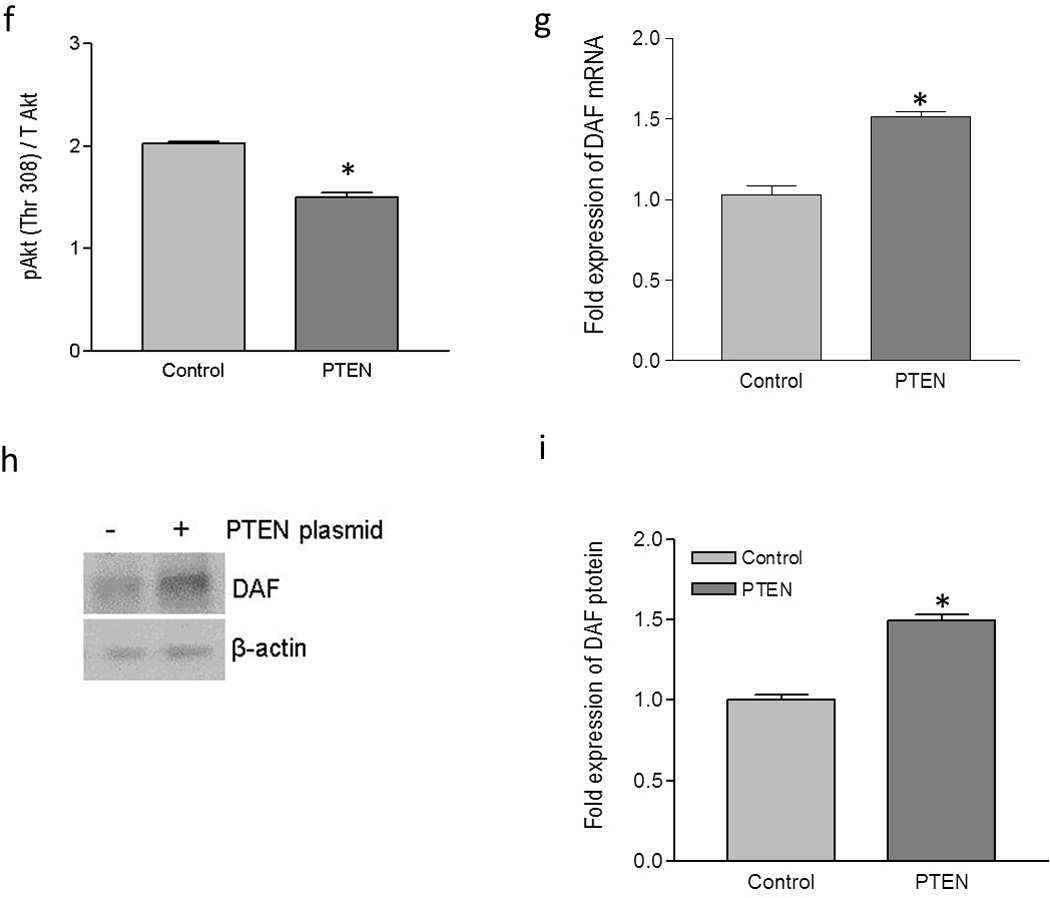
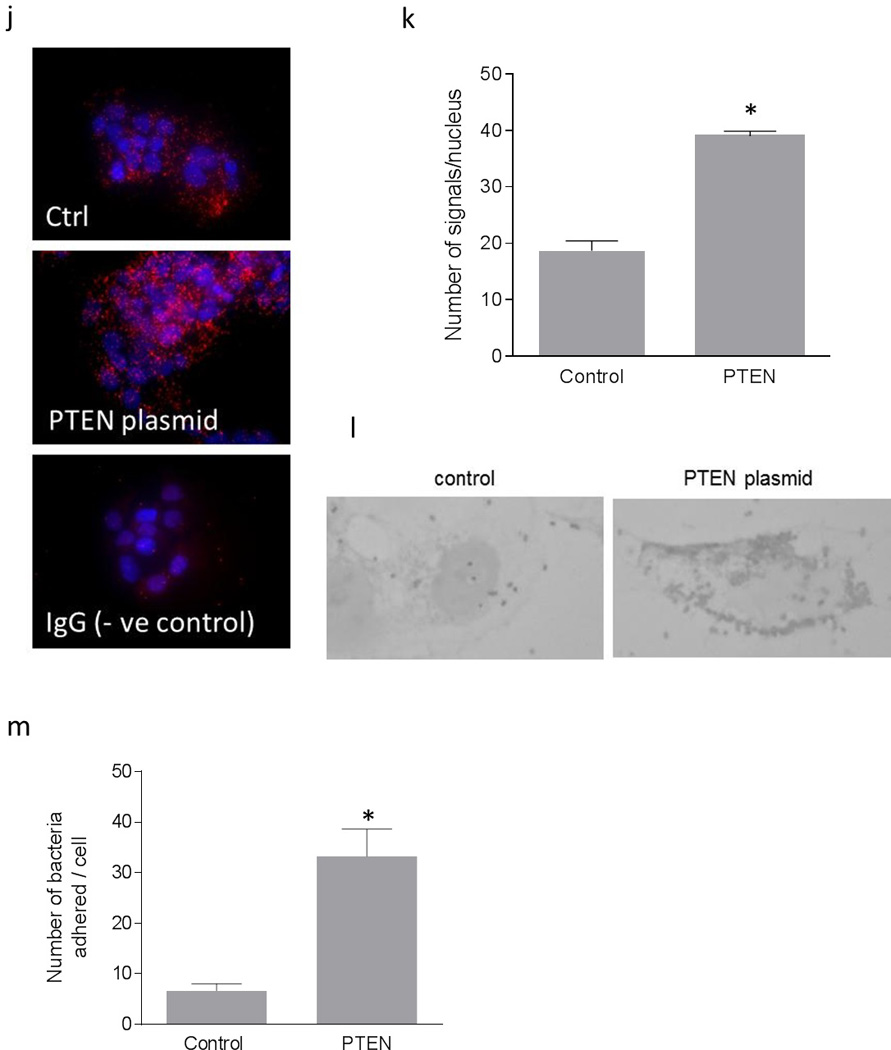
Up-regulation of DAF and increase in Dr+ E. coli adhesion after the expression of PTEN in Ishikawa cells. Ishikawa cells were transiently transfected with plasmid encoding functional PTEN gene. For control, cells were treated with equivalent amount of transfection regent lipofectamine 2000. After 24 hours of transfection, cells were either lysed for western blot and real time qPCR or E coli. K-12 strain carrying plasmid pBJN406, encoding the production of Dr fimbriae was allowed to bind to the cells for 3 hours. The number of bacteria adhered per cell was counted after the gram staining. Similarly, Ishikawa cells sparsely grown on 16-well lab-Tek chamber slides were transfected as mentioned above, fixed using formalin and proximity ligation assay was performed. (a) Western blot for PTEN expression. (b) Density analysis of western blot indicating the fold increase in the PTEN protein expression. * p < 0.05 versus control. (c) Western blot for total PDK1, total Akt and their activated forms, pPDK1, pAkt(ser473) and pAkt(Thr308). (d) Western blot density analysis of pPDK1 protein levels expressed as ratio between pPDK1 and total PDK1. * p < 0.05 versus control. (e) Western blot density analysis of pAkt(ser473) expressed as ratio between pAkt(ser473) and total Akt. * p < 0.05 versus control. (f) Western blot density analysis of pAkt(Thr308) expressed as ratio between pAkt(Thr308) and total Akt. * p < 0.001 versus control. (g) Fold increase in DAF mRNA as assessed by real time qPCR. * p < 0.0001 versus control. (h) Western blot for DAF protein expression. (i) Density analysis of western blot indicating the fold increase in the DAF protein expression. * p < 0.001 versus control. (j) PLA assay for the expression of DAF protein on the surface of Ishikawa cells. (k) The DAF protein levels on the surface of Ishikawa cells as assessed by PLA assay, expressed as number of signals per nucleus. * p < 0.01 versus control. (l) The adherence of E. coli K-12 strain carrying the plasmid pBJN406 to the Ishikawa cells as revealed by gram staining. (m) The average number of bacteria adhered per cell. * p < 0.01 versus control. Data are representative images or expressed as mean values ± SEM of each group from three separate experiments.
Overexpression of PTEN protein significantly reduced the level of phosphorylation in PDK1 and Akt (Figure 3c, d, e and f). To assess whether this negative regulation of Akt activity due to the overexpression of PTEN protein had an effect on DAF gene expression, we performed real time quantitative PCR and western blot followed by densitometric analysis. As shown in Figure 3g, h and i, the expression of DAF mRNA and protein increased significantly after the overexpression of PTEN protein in Ishikawa cells.
Although the qPCR and western blot provided the information on expression levels of DAF, we wanted to know the changes in cellular localization of DAF protein. The DAF protein is a membrane bound protein which is anchored to the cell surface through GPI molecule and thus it is available on the cell surface for the bacterial ligand to bind before establishing the infection. Therefore, we sought to determine whether up-regulation of DAF protein increased DAF protein levels on the cell surface by performing proximity ligation assay (PLA). The PLA revealed that the cell surface expression of DAF significantly increased in the Ishikawa cells transfected with PTEN plasmid compared to that of control cells (Figure 3j and k). Further, when the Dr+ E. coli were allowed to bind to Ishikawa cells for 3 hours, their adhesion to individual Ishikawa cells significantly increased after the introduction of PTEN plasmid (Figure 3l and m). Overall, theses data indicated that introduction of a plasmid vector for the expression of PTEN into Ishikawa cells up-regulated DAF expression and increased Dr+ E. coli adhesion.
Down-regulation of PTEN using siRNA in HeLa cells down-regulated DAF expression and decreased Dr+ E. coli adhesion
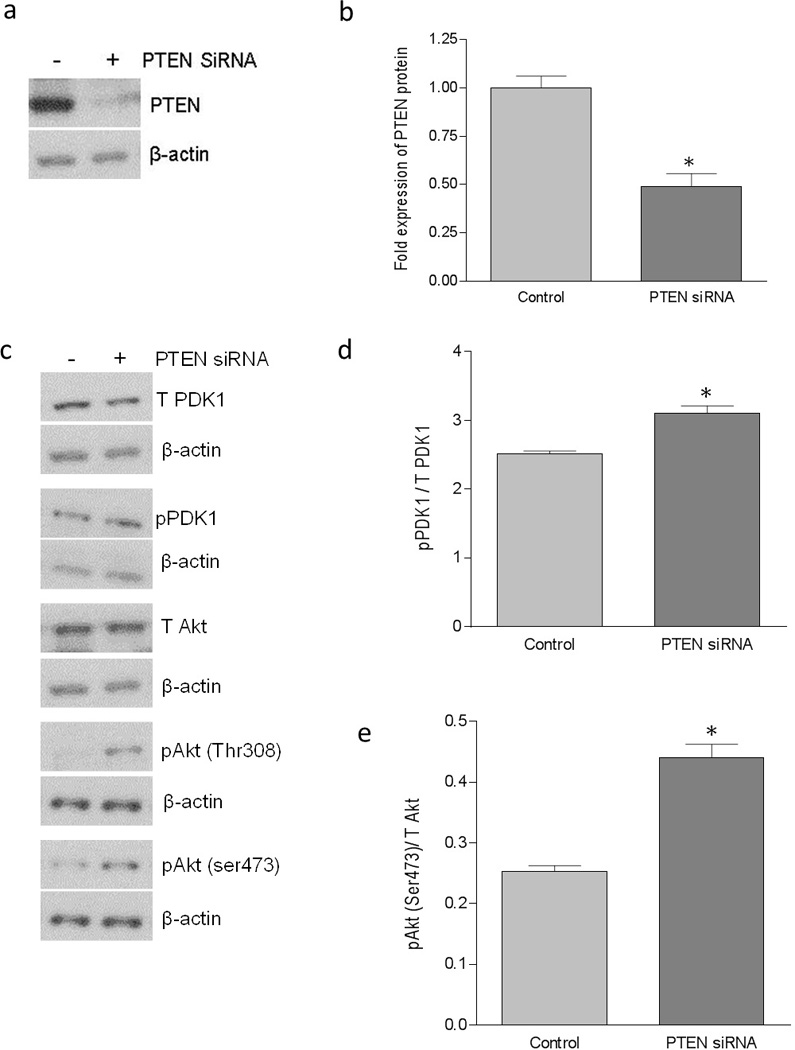
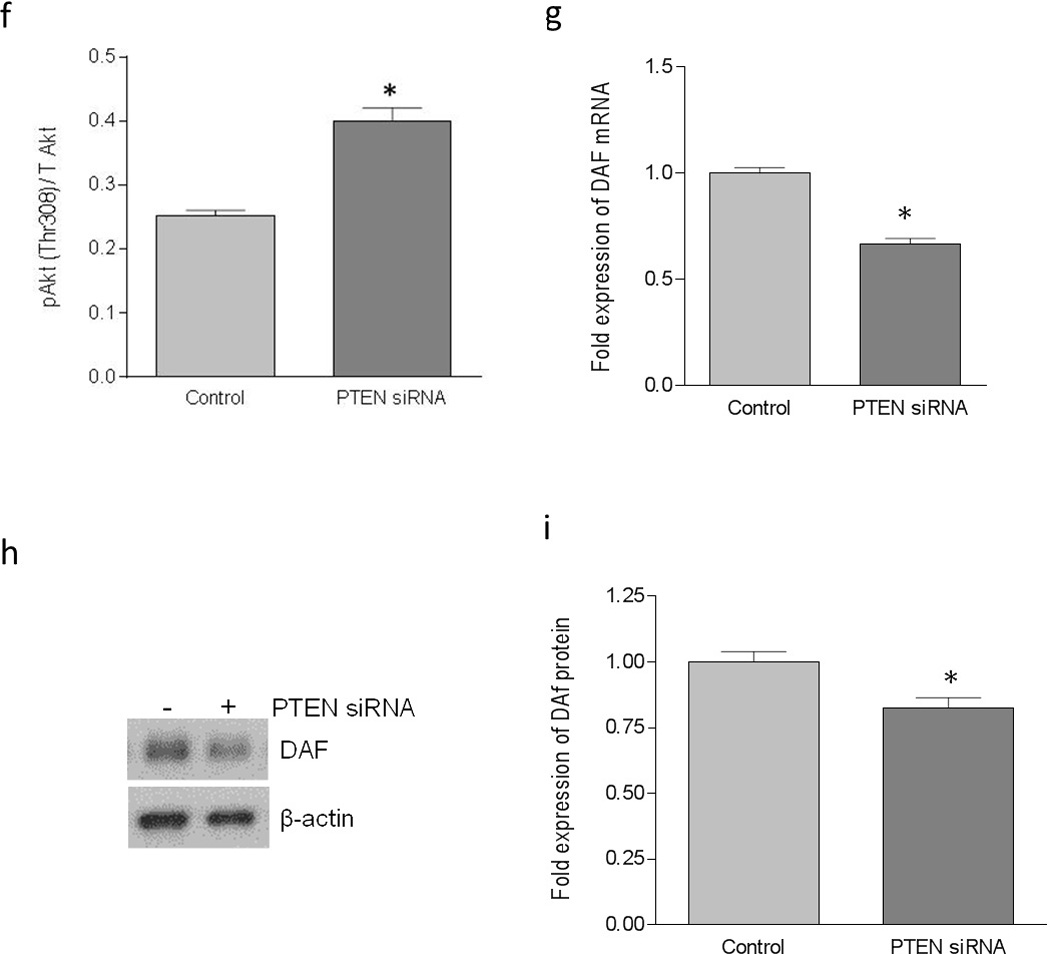
We further confirmed the inverse association between PI3K/Akt pathway and the DAF expression by using a PTEN specific siRNA. When HeLa cells were transfected with PTEN specific siRNA, expression of PTEN protein was reduced significantly (Figure 4a and b). The suppression of PTEN expression led to an increase in the phosphorylation of PDK1 and Akt (Figure 4c, d, e, and f) compared to the control cells. As shown in Figure 4g, h and i, PTEN down-regulation also induced a decreased expression of DAF mRNA and protein compared to the control cells. Further, the PLA showed a decrease in the DAF protein levels on the cell surface after the siRNA mediated PTEN suppression in the HeLa cells (Figure 4j and k). The adhesion of Dr+ E. coli to HeLa cells also decreased significantly after the transfection with PTEN-specific siRNA (Figure 4i and m). Taken together, these results confirmed that PI3K/Akt pathway negatively controls the expression of DAF on the cell surface and thus plays an important role in the adhesion of Dr+ E. coli.
Figure 4.
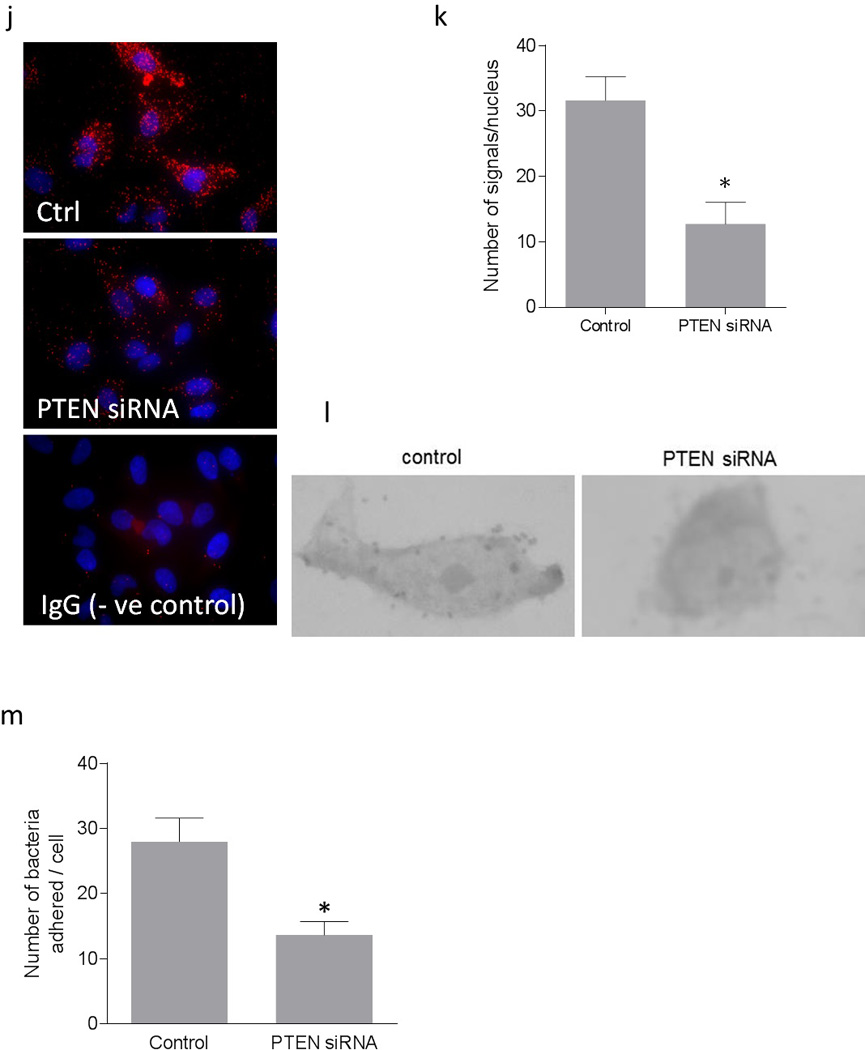
Down-regulation of PTEN using PTEN-specific siRNA down-regulated DAF expression and decreased Dr+ E. coli adhesion in HeLa cells. The HeLa cells were transiently transfected with PTEN-specific siRNA. For control, cells were treated with a scrambled siRNA. After 24 hours of transfection, cells were either lysed for western blot and real time qPCR or E coli. K-12 strain carrying plasmid pBJN406, encoding the production of Dr fimbriae was allowed to bind to the cells for 3 hours. The number of bacteria adhered per cell was counted after the gram staining. Similarly, HeLa cells sparsely grown on 16-well lab-Tek chamber slides were transfected as mentioned above, fixed using formalin and proximity ligation assay was performed. (a) Western blot for PTEN expression. (b) Density analysis of western blot indicating the fold decrease in the PTEN protein expression. * p < 0.01 versus control. (c) Western blot for total PDK1, total Akt and their activated forms, pPDK1, pAkt(ser473) and pAkt(Thr308). (d) Western blot density analysis of pPDK1 protein levels expressed as ratio between pPDK1 and total PDK1. * p < 0.05 versus control. (e) Western blot density analysis of pAkt(ser473) expressed as ratio between pAkt(ser473) and total Akt. * p < 0.001 versus control. (f) Western blot density analysis of pAkt(Thr308) expressed as ratio between pAkt(Thr308) and total Akt. * p < 0.001 versus control. (g) Fold decrease in DAF mRNA as assessed by real time qPCR. * p < 0.01 versus control. (h) Western blot for DAF protein expression. (i) Density analysis of western blot indicating the fold decrease in the DAF protein expression. * p < 0.05 versus control. (j) PLA assay for the expression of DAF protein on the surface of HeLa cells. (k) The DAF protein levels on the surface of HeLa cells as assessed by PLA assay, expressed as number of signals per nucleus. * p < 0.01 versus control. (l) The adherence of E. coli K-12 strain carrying the plasmid pBJN406 to the HeLa cells as revealed by gram staining. (m) The average number of bacteria adhered per cell. * p < 0.05 versus control. Data are representative images or expressed as mean values ± SEM of each group from three separate experiments.
Effect of PI3K/Akt pathway on DAF expression was not mediated by NO
Earlier studies in our laboratory demonstrated that NO down-regulates the expression of DAF. Therefore, we sought to examine whether negative regulation of DAF expression by PI3K/Akt pathway was mediated through NO. In order to study the possible role of NO, we inhibited the endogenous synthesis of NO in PTEN knock-down HeLa cells by treating the cells with NOS inhibitor L-N-G-Nitroarginine methyl ester (L-NAME) for 24h. The transfection with PTEN specific siRNA reduced the expression of DAF mRNA in HeLa cells compared to that of control cells as revealed by real time quantitative PCR. However, inhibition of NO syntehesis using L-NAME did not reverse the effect of PI3K/Akt activation (Figure 5a) suggesting that NO was not mediating the effect of PI3K/Akt on DAF expression.
Figure 5.
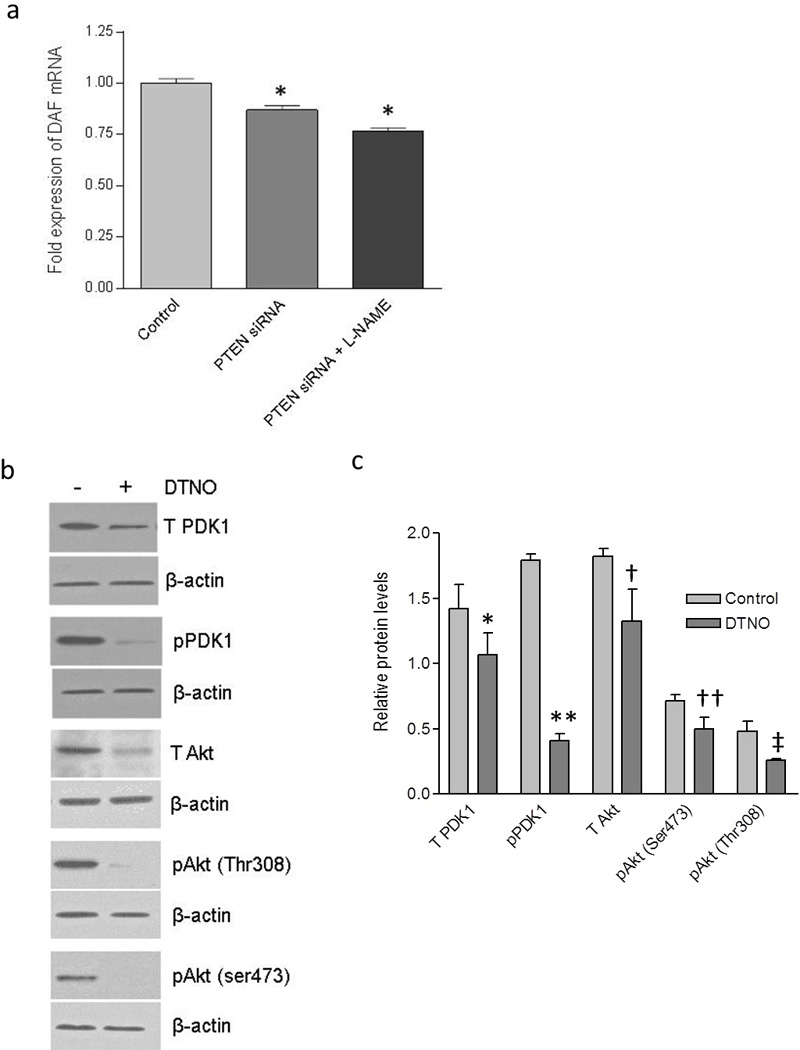
The effect of PI3K/Akt pathway on DAF expression was not mediated by NO. The HeLa cells were transiently transfected with PTEN-specific siRNA or a scrambled siRNA (control) for 24 hours and then treated with either nitric oxide synthase inhibitor L-NAME or NO donor DETANONOate for 24 hours followed by cell lysis for real time qPCR and western blot respectively. (a) Fold change in the expression of DAF mRNA in HeLa cells. * p < 0.0001. (b) Western blot for total PDK1, total Akt and their activated forms, pPDK1, pAkt(ser473) and pAkt(Thr308) in Ishikawa cells. (c) Western blot density analysis for total PDK1, total Akt and their activated forms, pPDK1, pAkt(ser473) and pAkt(Thr308) in Ishikawa cells. * p < 0.05 versus control, ** p < 0.001 versus control, † p < 0.05 versus control, †† p < 0.05 versus control, ‡ p < 0.05 versus control. Data are representative images or expressed as mean values ± SEM of each group from three separate experiments.
Then we asked whether the NO can trigger the PI3K/Akt pathway in Ishikawa cells to down-regulate the DAF because, NO activates PI3K/Akt pathway in bovine and human endothelial cells (39). Previously we observed that in Ishikawa cells, NO donor Diethylenetriamine (DETA) NONOate (DTNO) down-regulated the expression of DAF at a concentration of 0.1 mM or higher (16). Therefore, in order to examine the effect of NO on the PI3K/Akt pathway, we treated the Ishikawa cells with 1 mM of DTNO for 24 hours. As shown in the Figure 5b and c, the western blot followed by densitometric analysis revealed that total PDK1 and total Akt protein expressions decreased significantly after DTNO treatment. Correspondingly, the levels of pPDK1 and pAkt also decreased significantly in DTNO treated cells compared to that of control cells. Together, these data demonstrated that NO reduced the PI3K/Akt signaling by down-regulating the pathway proteins, PDK1 and Akt.
Inhibition of NO synthesis using NOS inhibitor, L-NAME failed to reverse the effect of PI3K/Akt activation in HeLa cells, prompting us to accept that NO was not mediating the effect of PI3K/Akt pathway on the DAF protein expression. Instead, we observed down-regulation of PDK1 and Akt by NO, which could reduce the signaling and consequently ought to up-regulate the DAF expression. However, our previous in vitro and in vivo experiments showed a significant down-regulation of DAF by NO (16;40). This contradiction could be due to a more direct effect of NO on DAF gene expression because, NO decreased the DAF mRNA transcription and half-life by inhibiting the binding of transcription factor Sp1 to 5'-untranslated region of DAF gene and HuR protein to 3'-UTR of DAF mRNA respectively (41).
Discussion
The adherence of bacteria to mucosal surfaces is an important step in the pathogenesis of most infections in humans and animals (42). The pathogenic E. coli strains express on their surfaces adhesins and invasins which are responsible for the recognition and binding of specific membrane-bound host molecules acting as receptors. Among pathogenic E. coli strains, uropathogenic and diarrheagenic E. coli express Dr-family of adhesins such as Afa, Dr and F1845 (43). The major receptors on the host cells recognized by these adhesins are type IV collagen, DAF and CEACAMs (43;44). In this report, we have shown that PI3K/Akt pathway negatively regulated the expression of DAF in the epithelial cells and thus restricted the attachment of E. coli that expressed Dr adhesin.
Although, bacterial adhesins can bind to a number of receptors on the host cell, DAF specific pathogens are known to cause chronic recurrent infections. The Dr/Afa family of adhesins includes at least 13 E. coli adhesins involved in human diseases (43). Some of these adhesins exhibit narrow specificity towards the host receptors. Except AfaE-VII and AfaE-VIII, all other Afa/Dr adhesins bind to DAF (45–47). However, collagen binding phenotype is unique to the Dr adhesins whereas only a subfamily of Afa/Dr adhesins designated Afa/Dr-I (Afa/DrCEA) bind to the CECAM family of proteins (48;49). Although binding of Dr adhesin to type IV collagen is critical for the development of persistent renal infection, role of type IV collagen in the development of intestinal infection is not clear. The DAF rather than type IV collagen is present at the apical surface of the polarized intestinal epithelial cells where E. coli tend to colonize (49;50). DAF is expressed in various parts of digestive, urogenital, and respiratory tracts (49;51). In the digestive tract, Helicobacter pylori, which causes chronic gastritis attaches to the DAF protein on gastric epithelial cells (52;53). Coxsackieviruses and Enteroviruses also utilize DAF as receptor on the host cell surface (49;54–56). Therefore, pathogenicity due to microbial or viral ligands and DAF interactions appear to be involved in the development of protracted often subclinical bacterial and viral diseases.
Bacterial pathogens manipulate several host cell kinase signaling such as nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), mitogen activated protein kinase (MAPK) and PI3K/Akt to exploit down-stream effector functions in order to establish intracellular infection (57). PI3K/Akt pathway is an essential signaling pathway in the process of phagocytosis and therefore, that allows bacterial pathogens to hijack PI3K/Akt signaling either to induce their uptake by host cells through a process similar to phagocytosis or to inhibit their phagocytosis by immune cells such as macrophages. Yersinia species inject a phosphatase to disrupt focal adhesion complex mediated activation of PI3K/Akt pathway in macrophages and thus inhibit their phagocytosis (58). Shigella flexneri and Pseudomonas aeruginosa activate the host cell PI3K/Akt pathway to induce their epithelial uptake by inducing actin reorganization in host cells (58). Adherent E. coli strains also have been reported to manipulate PI3K/Akt pathway in order to establish infection. Peifer et al, reported that in an invitro intestinal cell model, a clinically isolated strain of E. coli C 1845 that expressed F1845 adhesin induced F-actin reorganization in a PI3K/Akt dependent manner (59). However, role of host cell PI3K/Akt pathway in the protection against bacterial invasion is not clear. In this report we have provided the evidence to show that PI3K/Akt pathway could be involved in restricting the attachment of Dr+ E. coli to epithelial cells. PI3K/Akt pathway is essential for pathogenic E. coli to gain entry in to host cells whereas PI3K/Akt signaling activated by ligand-receptor interactions in host cells down-regulates DAF and restricts their attachment as proposed in Figure 6. The regulation of PI3K/Akt signaling in the physiological context is complex and therefore, further studies are required to establish overall role of PI3K/Akt pathway in Dr+ E. coli infection. However, we suggest that restriction of DAF expression could contribute to limit the level of virulence of Dr+ E. coli and therefore, may direct infection process towards chronic subclinical infection in contrast to acute inflammatory diseases caused by other pathogenic bacteria such as shigella species.
Figure 6.
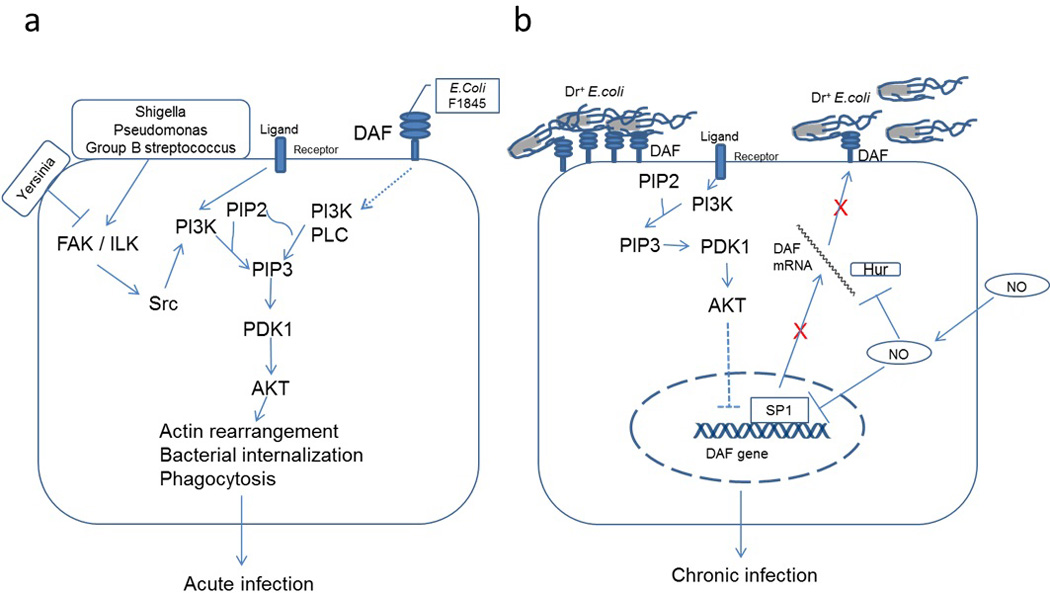
Pictorial representations for the role of PI3K/Akt pathway in Dr+ E. coli infection. (a) Pathogenic bacteria such as Shigella, Pseudomonas and Group B streptococcus bind to host cells and activate PI3K/Akt pathway that allows them to induce actin reorganization resulting in their internalization. Yersinia species inhibit PI3K/Akt pathway and thus restrict their phagocytosis by macrophages (58). E. coli bearing F1845 adhesins bind to DAF on host cell surface and activate PI3K/Akt pathway by an unknown mechanism causing actin reorganization and their internalization (59). The induced internalization of these pathogenic bacteria produces an acute infection process. (b) The activity of PI3K/Akt pathway is regulated by several ligands such as hormones through different transmembrane receptors. The expression of DAF gene is negatively regulated by PI3K/Akt pathway. The NO also down-regulates DFA expression by inhibiting its transcription and destabilizing mRNA (41). Thus both PI3K/Akt pathway and NO reduce the expression of DAF on host cell surface resulting in restricted binding of Dr+ E. coli. Restricted binding of E. coli may help in establishing a symbiotic type of relation where both are protected from a lethal outcome thus leading to a chronic type of infection.
In summary, the present study provides evidence for the association between the PI3K/Akt mediated variations in the DAF expression levels on the epithelial cells and the adhesion of Dr+ E. coli to epithelial cells. The involvement of two independent host systems, NO and PI3K/Akt in the down-regulation of DAF in the context of Dr+ E. coli pathogenesis appears to be consistent with the host-pathogen strategy to achieve well controlled limited infection level. Restricted DAF expression may therefore, lead to chronic instead of acute infection thus leading to a symbiotic host-pathogen status, protecting both from a lethal outcome.
Acknowledgements
This work was supported by the National Institute of Health [grant number HL72620, HD57013].
Footnotes
Statement of Author Contributions
MB, PG and CY participated in the design, interpretation of the data, MB and DL conducted the experiments, MB wrote, CY and BJN reviewed the manuscript, BJN and SN provided the plasmids pBJN406 and protocol for the preparation of bacteria for adhesion assay.
Reference List
- 1.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995;172:1598–1603. doi: 10.1016/0002-9378(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 3.Krohn MA, Hillier SL, Nugent RP, Cotch MF, Carey JC, Gibbs RS, Eschenbach DA. The genital flora of women with intraamniotic infection. Vaginal Infection and Prematurity Study Group. J Infect Dis. 1995;171:1475–1480. doi: 10.1093/infdis/171.6.1475. [DOI] [PubMed] [Google Scholar]
- 4.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(Suppl 1A):14S–19S. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 5.Pham T, Kaul A, Hart A, Goluszko P, Moulds J, Nowicki S, Lublin DM, Nowicki BJ. dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect Immun. 1995;63:1663–1668. doi: 10.1128/iai.63.5.1663-1668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giron JA, Jones T, Millan-Velasco F, Castro-Munoz E, Zarate L, Fry J, Frankel G, Moseley SL, Baudry B, Kaper JB. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991;163:507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- 7.Foxman B, Zhang L, Tallman P, Palin K, Rode C, Bloch C, Gillespie B, Marrs CF. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J Infect Dis. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 8.Selvarangan R, Goluszko P, Popov V, Singhal J, Pham T, Lublin DM, Nowicki S, Nowicki B. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect Immun. 2000;68:1391–1399. doi: 10.1128/iai.68.3.1391-1399.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowicki B, Hart A, Coyne KE, Lublin DM, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guignot J, Peiffer I, Bernet-Camard MF, Lublin DM, Carnoy C, Moseley SL, Servin AL. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect Immun. 2000;68:3554–3563. doi: 10.1128/iai.68.6.3554-3563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zav'yalov V, Zavialov A, Zav'yalova G, Korpela T. Adhesive organelles of Gram-negative pathogens assembled with the classical chaperone/usher machinery: structure and function from a clinical standpoint. FEMS Microbiol Rev. 2010;34:317–378. doi: 10.1111/j.1574-6976.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KL, Billington J, Pettigrew D, Cota E, Simpson P, Roversi P, Chen HA, Urvil P, du ML, Barlow PN, Medof ME, Smith RA, Nowicki B, Le BC, Lea SM, Matthews S. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol Cell. 2004;15:647–657. doi: 10.1016/j.molcel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Cota E, Jones C, Simpson P, Altroff H, Anderson KL, du ML, Guignot J, Servin A, Le BC, Mardon H, Matthews S. The solution structure of the invasive tip complex from Afa/Dr fibrils. Mol Microbiol. 2006;62:356–366. doi: 10.1111/j.1365-2958.2006.05375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piatek R, Bruzdziak P, Zalewska-Piatek B, Kur J, Stangret J. Preclusion of irreversible destruction of Dr adhesin structures by a high activation barrier for the unfolding stage of the fimbrial DraE subunit. Biochemistry. 2009;48:11807–11816. doi: 10.1021/bi900920k. [DOI] [PubMed] [Google Scholar]
- 15.Piatek RJ, Bruzdziak P, Zalewska-Piatek BM, Wojciechowski MA, Namiesnik JM, Kur JW. Analysis of the unique structural and physicochemical properties of the DraD/AfaD invasin in the context of its belonging to the family of chaperone/usher type fimbrial subunits. BMC Struct Biol. 2011;11:25. doi: 10.1186/1472-6807-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang L, Nowicki BJ, Urvil P, Goluszko P, Nowicki S, Young SL, Yallampalli C. Epithelial invasion by Escherichia coli bearing Dr fimbriae is controlled by nitric oxide-regulated expression of CD55. Infect Immun. 2004;72:2907–2914. doi: 10.1128/IAI.72.5.2907-2914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas DJ, Lublin DM. Identification of 5'-flanking regions affecting the expression of the human decay accelerating factor gene and their role in tissue-specific expression. J Immunol. 1993;150:151–160. [PubMed] [Google Scholar]
- 18.Fenichel P, Cervoni F, Donzeau M, Hsi BL. Expression and role of complement regulatory proteins on human gametes and pre-implantation embryos. Contracept Fertil Sex. 1995;23:576–580. [PubMed] [Google Scholar]
- 19.Kaul AK, Kumar D, Nagamani M, Goluszko P, Nowicki S, Nowicki BJ. Rapid cyclic changes in density and accessibility of endometrial ligands for Escherichia coli Dr fimbriae. Infect Immun. 1996;64:611–615. doi: 10.1128/iai.64.2.611-615.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venneker GT, Asghar SS. CD59: a molecule involved in antigen presentation as well as downregulation of membrane attack complex. Exp Clin Immunogenet. 1992;9:33–47. [PubMed] [Google Scholar]
- 21.Parker CJ. Molecular basis of paroxysmal nocturnal hemoglobinuria. Stem Cells. 1996;14:396–411. doi: 10.1002/stem.140396. [DOI] [PubMed] [Google Scholar]
- 22.Kaul A, Nagamani M, Nowicki B. Decreased expression of endometrial decay accelerating factor (DAF), a complement regulatory protein, in patients with luteal phase defect. Am J Reprod Immunol. 1995;34:236–240. doi: 10.1111/j.1600-0897.1995.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 23.Stoute JA. Complement-regulatory proteins in severe malaria: too little or too much of a good thing? Trends Parasitol. 2005;21:218–223. doi: 10.1016/j.pt.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Jones J, Morgan BP. Apoptosis is associated with reduced expression of complement regulatory molecules, adhesion molecules and other receptors on polymorphonuclear leucocytes: functional relevance and role in inflammation. Immunology. 1995;86:651–660. [PMC free article] [PubMed] [Google Scholar]
- 25.Murray KP, Mathure S, Kaul R, Khan S, Carson LF, Twiggs LB, Martens MG, Kaul A. Expression of complement regulatory proteins-CD 35, CD 46, CD 55, and CD 59-in benign and malignant endometrial tissue. Gynecol Oncol. 2000;76:176–182. doi: 10.1006/gyno.1999.5614. [DOI] [PubMed] [Google Scholar]
- 26.Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin) Am J Pathol. 1996;149:129–142. [PMC free article] [PubMed] [Google Scholar]
- 27.Varsano S, Rashkovsky L, Shapiro H, Ophir D, Mark-Bentankur T. Human lung cancer cell lines express cell membrane complement inhibitory proteins and are extremely resistant to complement-mediated lysis; a comparison with normal human respiratory epithelium in vitro, and an insight into mechanism(s) of resistance. Clin Exp Immunol. 1998;113:173–182. doi: 10.1046/j.1365-2249.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewulonu UK, Ravi L, Medof ME. Characterization of the decay-accelerating factor gene promoter region. Proc Natl Acad Sci U S A. 1991;88:4675–4679. doi: 10.1073/pnas.88.11.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa J, Ohmichi M, Tasaka K, Kanda Y, Adachi K, Nishio Y, Hisamoto K, Mabuchi S, Hinuma S, Murata Y. Regulation of the PRL promoter by Akt through cAMP response element binding protein. Endocrinology. 2002;143:13–22. doi: 10.1210/endo.143.1.8586. [DOI] [PubMed] [Google Scholar]
- 31.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 32.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 33.Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nippon Sanka Fujinka Gakkai Zasshi. 1985;37:1103–1111. [PubMed] [Google Scholar]
- 34.Nowicki B, Hart A, Coyne KE, Lublin DM, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goluszko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki BJ. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J Infect Dis. 1997;176:158–167. doi: 10.1086/514018. [DOI] [PubMed] [Google Scholar]
- 36.Goluszko P, Moseley SL, Truong LD, Kaul A, Williford JR, Selvarangan R, Nowicki S, Nowicki B. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J Clin Invest. 1997;99:1662–1672. doi: 10.1172/JCI119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan X, Yokoyama Y, Shinohara A, Takahashi Y, Tamaya T. PTEN augments staurosporine-induced apoptosis in PTEN-null Ishikawa cells by downregulating PI3K/Akt signaling pathway. Cell Death Differ. 2002;9:414–420. doi: 10.1038/sj.cdd.4400982. [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 39.Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003;23:5726–5737. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowicki B, Fang L, Singhal J, Nowicki S, Yallampalli C. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased death rate with inhibition of nitric oxide. Am J Reprod Immunol. 1997;38:309–312. doi: 10.1111/j.1600-0897.1997.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 41.Banadakoppa M, Liebenthal D, Nowak DE, Urvil P, Yallampalli U, Wilson GM, Kishor A, Yallampalli C. Role of transcription factor Sp1 and RNA binding protein HuR in the downregulation of Dr+ Escherichia coli receptor protein decay accelerating factor (DAF or CD55) by nitric oxide. FEBS J. 2013;280:840–854. doi: 10.1111/febs.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beachey EH. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 43.Nowicki B, Selvarangan R, Nowicki S. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J Infect Dis. 2001;183(Suppl 1):S24–S27. doi: 10.1086/318846. [DOI] [PubMed] [Google Scholar]
- 44.Guignot J, Peiffer I, Bernet-Camard MF, Lublin DM, Carnoy C, Moseley SL, Servin AL. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect Immun. 2000;68:3554–3563. doi: 10.1128/iai.68.6.3554-3563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowicki B, Labigne A, Moseley S, Hull R, Hull S, Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990;58:279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lalioui L, Jouve M, Gounon P, Le BC. Molecular cloning and characterization of the afa-7 and afa-8 gene clusters encoding afimbrial adhesins in Escherichia coli strains associated with diarrhea or septicemia in calves. Infect Immun. 1999;67:5048–5059. doi: 10.1128/iai.67.10.5048-5059.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalioui L, Le BC. afa-8 Gene cluster is carried by a pathogenicity island inserted into the tRNA(Phe) of human and bovine pathogenic Escherichia coli isolates. Infect Immun. 2001;69:937–948. doi: 10.1128/IAI.69.2.937-948.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nowicki B, Moulds J, Hull R, Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988;56:1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berger CN, Billker O, Meyer TF, Servin AL, Kansau I. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC) Mol Microbiol. 2004;52:963–983. doi: 10.1111/j.1365-2958.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 50.Servin AL. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin Microbiol Rev. 2005;18:264–292. doi: 10.1128/CMR.18.2.264-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nowicki B, Truong L, Moulds J, Hull R. Presence of the Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. Am J Pathol. 1988;133:1–4. [PMC free article] [PubMed] [Google Scholar]
- 52.O'Brien DP, Israel DA, Krishna U, Romero-Gallo J, Nedrud J, Medof ME, Lin F, Redline R, Lublin DM, Nowicki BJ, Franco AT, Ogden S, Williams AD, Polk DB, Peek RM., Jr The role of decay-accelerating factor as a receptor for Helicobacter pylori and a mediator of gastric inflammation. J Biol Chem. 2006;281:13317–13323. doi: 10.1074/jbc.M601805200. [DOI] [PubMed] [Google Scholar]
- 53.O'Brien DP, Romero-Gallo J, Schneider BG, Chaturvedi R, Delgado A, Harris EJ, Krishna U, Ogden SR, Israel DA, Wilson KT, Peek RM., Jr Regulation of the Helicobacter pylori cellular receptor decay-accelerating factor. J Biol Chem. 2008;283:23922–23930. doi: 10.1074/jbc.M801144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergelson JM, Chan M, Solomon KR, St John NF, Lin H, Finberg RW. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci U S A. 1994;91:6245–6248. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergelson JM, Mohanty JG, Crowell RL, St John NF, Lublin DM, Finberg RW. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55) J Virol. 1995;69:1903–1906. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karnauchow TM, Tolson DL, Harrison BA, Altman E, Lublin DM, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55) J Virol. 1996;70:5143–5152. doi: 10.1128/jvi.70.8.5143-5152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krachler AM, Woolery AR, Orth K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol. 2011;195:1083–1092. doi: 10.1083/jcb.201107132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Black DS, Bliska JB. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peiffer I, Servin AL, Bernet-Camard MF. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect Immun. 1998;66:4036–4042. doi: 10.1128/iai.66.9.4036-4042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


