Abstract
Bone erosion is a central feature of rheumatoid arthritis and is associated with disease severity and poor functional outcome. Erosion of periarticular cortical bone, the typical feature observed on plain radiographs in patients with rheumatoid arthritis, results from excessive local bone resorption and inadequate bone formation. The main triggers of articular bone erosion are synovitis, including the production of proinflammatory cytokines and receptor activator of nuclear factor κB ligand (RANKL), as well as antibodies directed against citrullinated proteins. Indeed, both cytokines and autoantibodies stimulate the differentiation of bone-resorbing osteoclasts, thereby stimulating local bone resorption. Although current antirheumatic therapy inhibits both bone erosion and inflammation, repair of existing bone lesions, albeit physiologically feasible, occurs rarely. Lack of repair is due, at least in part, to active suppression of bone formation by proinflammatory cytokines. This Review summarizes the substantial progress that has been made in understanding the pathophysiology of bone erosions and discusses the improvements in the diagnosis, monitoring and treatment of such lesions.
Introduction
The skeleton is composed of trabecular bone, the fine bony network hosting the bone marrow, and cortical bone, the dense bony shell that provides structural support in weight-bearing regions. Both types of bone are targeted for erosion in rheumatoid arthritis (RA). Moreover, RA is an independent risk factor for generalized osteopenia and osteoporosis, involving trabecular and cortical bone in the axial and appendicular skeleton. Articular bone erosion represents localized bone loss (osteolysis), initially involving cortical bone, and destruction of the natural barrier between the extraskeletal tissue and the intertrabecular spaces of the bone marrow cavity. Osteolysis results from an imbalance in which bone resorption by osteoclasts is favoured over bone formation by osteoblasts. Understanding the mechanisms that define the formation of bone erosions requires insight into the biology of bone homeostasis and the molecular regulation of the differentiation and function of osteoclasts and osteoblasts.1–3
Although several pathological processes can lead to bone erosion, including malignancy, metabolic processes such as hyperparathyroidism, and chronic inflammatory diseases such as histiocytosis and sarcoidosis, the most common cause is RA. Initially described more than 100 years ago,4,5 articular bone erosions have now become a central element in the diagnosis, treatment and monitoring of RA. Moreover, these lesions are an expected consequence of seropositive RA if the disease is not treated in a timely and effective fashion. Erosions reflect the clinical consequence of the tight interaction between immune activation and skeletal modelling and remodelling. Indeed, research into the interface between the immune system and bone has now led to a new field, termed osteoimmunology.6–9
Definition of bone erosion
Bone erosion is a radiological term and reflects the fact that imaging is used for detection.10 Erosions are visible on plain radiographs as breaks in the cortical bone surface, and are often accompanied by loss of the adjacent trabecular bone. By contrast, bone cysts are areas of osteolysis inside the trabecular bone compartment, without any signs of cortical bone destruction. Although erosions can also be observed in forms of arthritis other than RA, such as gout, psoriatic arthritis, spondyloarthritis and even osteoarthritis, they are included in the diagnostic criteria for RA.11 Owing to the severity and typical distribution pattern along multiple peripheral skeletal sites, as well as absence of concomitant new bone formation, the appearance of bone erosions is unique in RA and is substantially different from other types of arthritis.
Bone erosions constitute a key outcome measure in RA and are predictive of a more severe course of disease with a higher degree of disability and increased mortality. 12–14 Clinical trials with all major antirheumatic agents approved for disease-modification of RA have been validated for their ability to retard, or even arrest, structural damage, which is a composite of bone erosion and cartilage degradation. Furthermore, radiography is widely used to assess structural damage in clinical practice and to monitor the efficacy of therapy in retarding structural damage. Thus, at present, detection and quantification of bone erosion constitutes a major instrument for disease diagnosis, as well as for monitoring and measurement of efficacy of drug therapy in patients with RA. This Review focuses on bone erosion in RA, and does not discuss cartilage damage. Nonetheless, cartilage damage is an equally important feature of structural damage in RA that occurs through fundamentally different mechanisms to bone erosions.15
Anatomic factors and microstructure
Bone erosions do not emerge at random locations, but show a predilection for certain anatomical sites. Detailed analysis of the distribution of bone erosions has been conducted by high-resolution CT, high-resolution ultrasonography and MRI. The radial aspects of the finger joints were revealed to be ‘hot spots’ for bone erosions, whereas the ulnar aspects were less frequently affected, and the palmar and volar surfaces of the joints were virtually spared from such lesions.16–18 This distinct localization pattern of bone erosion in RA is intriguing and could be linked with certain anatomical features, as described later. Moreover, bone erosions typically emerge at the site at which the synovium comes into direct contact with bone (known as bare areas), suggesting that anatomical factors render these areas of juxta-articular bone susceptible to erosion.19,20 This concept is also supported by studies in healthy individuals using high-resolution CT, which have shown that bone erosions of less than 2 mm in diameter can be found in healthy individuals, with a distribution pattern that exactly reflects that of the erosive lesions seen in patients with RA.15,21 Anatomical factors that predispose skeletal sites for erosions include: the presence of mineralized cartilage, a tissue particularly prone to destruction by bone-resorbing cells; the insertion sites of ligaments to the bone surface, which transduce mechanical forces to the bone and could induce microdamage;22 and inflamed tendon sheaths (termed tenosynovitis), which pass by the bone surface, and enable the spread of inflammation from the tendon to the articular synovium.23
The small bone channels that penetrate cortical bone carry microvessels and bridge the outer synovial membrane and the inner bone marrow space; these channels are also prone to erosive change early in the course of RA. The microvessels located within these channels facilitate homing of osteoclast precursor cells to the bone, which, upon contact with bone and receipt of the appropriate molecular signals, differentiate into osteoclasts. Widening of cortical bone channels, as a result of osteoclast-mediated bone resorption, is a typical early change in animal models of arthritis.24,25 Moreover, cortical fenestrations have been described in high-resolution CT scans of joints from patients with RA, which probably reflect such widening of cortical bone channels.15 These changes are in accordance with the known reduction in cortical bone mass in RA, which has been documented in several studies in patients with RA and seems to be closely related to disease activity and the development of bone erosions.26–30
Evolution of bone erosions
Longitudinal radiographic studies have shown that bone erosions emerge early in the pathogenesis of RA, affecting approximately half of untreated patients by 6 months after disease onset.31 Radiographic studies in patients with RA of less than 3 months’ duration suggest that bone erosions can be detected as early as a few weeks after disease onset in some patients.32 Moreover, several studies in patients with RA have shown that relationships exist between bone erosions and the development of osteopenia and osteoporosis.33–35
Detection of bone erosions has improved owing to marked technological advances in musculoskeletal imaging. CT, high-resolution ultrasonography and MRI can reliably detect even small bone erosions in patients with RA.36,37 Imaging has also highlighted and substantiated the role of inflammation in triggering bone erosions, showing that not only synovitis, but also inflammation of the adjacent intertrabecular space (osteitis), correlates with the development of radiographic bone erosions.38,39 Whether bone erosions lead to osteitis, or osteitis is a result of bone erosion, remains unclear.40 Bone erosions are usually considered as irreversible damage, although detailed longitudinal assessment of these lesions with respect to repair is still scarce. Spontaneous repair, however, is rare, which contrasts with the substantial periosteal bone-formation response noted in conjunction with bone erosions in patients with psoriatic arthritis or osteoarthritis.41
Osteoclasts mediate bone erosion
Osteoclasts, giant multinucleated cells derived from the monocyte lineage, are the only cells capable of resorbing bone in the body.2,3 Osteoclasts are designed to resorb bone by adhering tightly to the bone surface through interactions with both integrins and extracellular matrix proteins, as well as by assembling junctions, which seal the bone surface and the osteoclast and thereby separate bone from the surrounding extracellular space. Proton pumps along the osteoclasts’ ruffled border then create an acidic milieu, enabling solubilization of calcium from bone. Matrix enzymes synthesized by the osteoclast, including cathepsin K, matrix metalloproteinase 9 and tartrate-resistant acid phosphatase type 5 (TRAP), degrade the bone matrix.2
Osteoclasts populate the interface between inflammatory synovial tissue and the periarticular bone surface. The first indirect description of bone-resorbing cells in RA dates back to the 19th century,5 and was revisited by Bromley and Woolley42 and by Leisen et al.43 in the 1980s. Osteoclasts in RA were definitively identified and characterized in detail by use of modern immunohistochemical and molecular techniques by Gravallese et al.44 in 1998. The multinucleated cells at the pannus–bone interface demonstrate all of the phenotypic features of bona fide osteoclasts, including formation of a ruffled border and expression of specific markers including TRAP, cathepsin K and the calcitonin receptor. Osteoclast precursor cells accumulate inside the so-called synovial pannus —the dense inflammatory synovially derived tissue located both at the interface with bone and inside the bone erosions themselves. Similar changes are also found in all relevant animal models of inflammatory arthritis, and studies involving these animal models demonstrated the crucial role of osteoclasts in the pathogenesis of articular bone erosion in arthritis induced by adjuvant,45 collagen,46,47 serum transfer48 and TNF.49,50 By inducing arthritis in osteoclast-free mice, it was shown that these mice are completely protected from bone erosions.48,49 Osteoclasts have, therefore, emerged as an essential cell type in the erosive process.
Pathways for osteoclast differentiation
Development of osteoclasts occurs locally in the synovial tissue as a result of expression of the two essential osteoclastogenic mediators, macrophage colony-stimulating factor 1 (M-CSF)51,52 and receptor activator of nuclear factor κB ligand (RANKL; also known as TNF ligand superfamily member 11).53–58 This process involves the migration of monocyte-lineage cells from the bone marrow into the secondary lymphatic organs and finally into the joints. The differentiation step at which monocytes enter the joint is unclear. Indeed, monocytes could be already committed to a certain monocyte lineage, such as M1 or M2 macrophages, dendritic cells or osteoclast precursor cells when they enter the joint. Some data suggest that TNF, a key proinflammatory cytokine expressed in RA synovial tissue, stimulates the migration of osteoclast precursor cells from the bone marrow into the periphery.59,60 In addition, TNF stimulates expression of surface receptors such as osteoclast-associated immunoglobulin-like receptor before the precursor cells enter the joint, and these receptors facilitate differentiation. 61 Once within the microenvironment of the joint, these cells are exposed to M-CSF and RANKL, and differentiate toward osteoclasts. Final differentiation into bone-resorbing osteoclasts is then achieved following contact with the bone surface.
Blockade of osteoclast differentiation by inhibiting RANKL, or M-CSF plus RANKL, has been shown to arrest bone erosion in virtually all animal models of inflammatory arthritis.45–50,62 One clinical trial in patients with RA has also shown that blockade of RANKL using a neutralizing antibody (denosumab) slows the progression of bone erosion, whereas it does not retard inflammation.63 Thus, direct targeting of osteoclasts in RA protects bone from the consequences of inflammation even in the absence of inhibition of inflammation itself. This observation also largely excludes the possibility that bone resorption exerts positive feedback loops on synovial inflammation.
Triggers of bone erosion
Preclinical autoimmunity
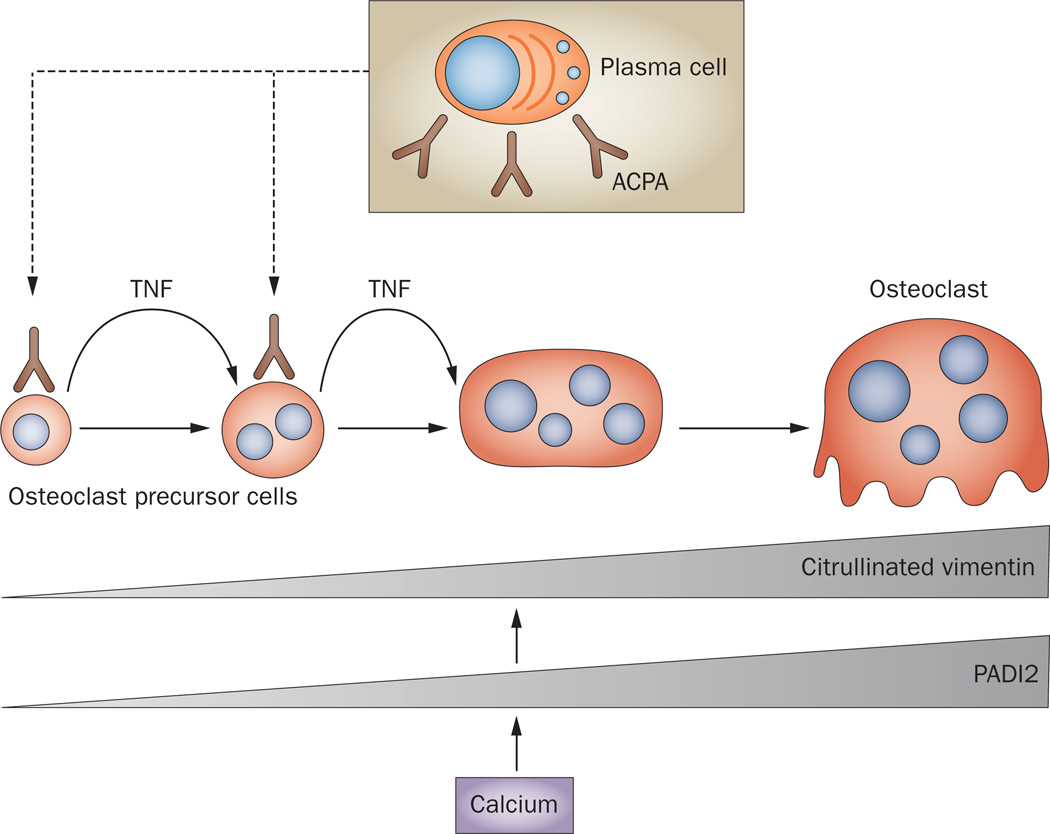
Autoimmunity is among the strongest prognostic indicators of structural damage in RA. Compelling evidence for the link between autoimmunity and articular erosion was revealed by the presence of anti-citrullinated protein antibodies (ACPA) and anti-carbamylated protein antibodies in the serum of patients with RA, which can emerge long before the onset of synovitis and independently predict bone erosion in these patients.64–67 This observation is consistent among several cohorts of patients with RA and is independent of measures of disease activity, such as 28-joint disease activity score, or inflammation, as measured by levels of C-reactive protein.64–67 A study in 2012 revealed that ACPA recognize citrullinated vimentin expressed on the surface of osteoclast precursor cells.68 Binding of ACPA to the cell surface increases cellular differentiation to bone-resorbing osteoclasts via autocrine stimulation of TNF production. Osteoclasts express high levels of the enzyme peptidyl-arginine deiminase type 2 (PADI2), which is induced by calcium flux and is responsible for protein citrullination (Figure 1). In osteoclast-lineage cells, PADI2 activation leads to preferential citrullination of vimentin, inducing a change in its subcellular localization and rendering it accessible for ACPA binding. Induction of osteoclastogenesis by ACPA binding is also highlighted by high levels of markers of bone resorption in the serum of patients with RA,9 reflecting osteoclast-mediated bone resorption. It will be of interest to determine if this effect is specific to citrullinated vimentin or if antibodies specific to other citrullinated proteins have similar effects. These findings shed new light on the interplay between inflammation and bone damage in RA, and support the concept that structural changes to bone occur at disease onset, or even before disease onset, in patients with RA (Figure 2).
Figure 1.
Autoantibodies against citrullinated proteins and osteoclastogenesis. Plasma cells produce ACPA with specificity for citrullinated vimentin, which bind to osteoclast precursor cells and stimulate the release of TNF, which in turn enhances the differentiation of these cells into mature osteoclasts. During the osteoclast differentiation process, production of the PADI2 enzyme is induced by calcium. The activity of PADI2 leads to citrullination of vimentin, which is abundantly expressed on the surface of osteoclast-lineage cells. Abbreviations: ACPA, anti-citrullinated protein antibodies; PADI2, peptidyl-arginine deiminase type 2.
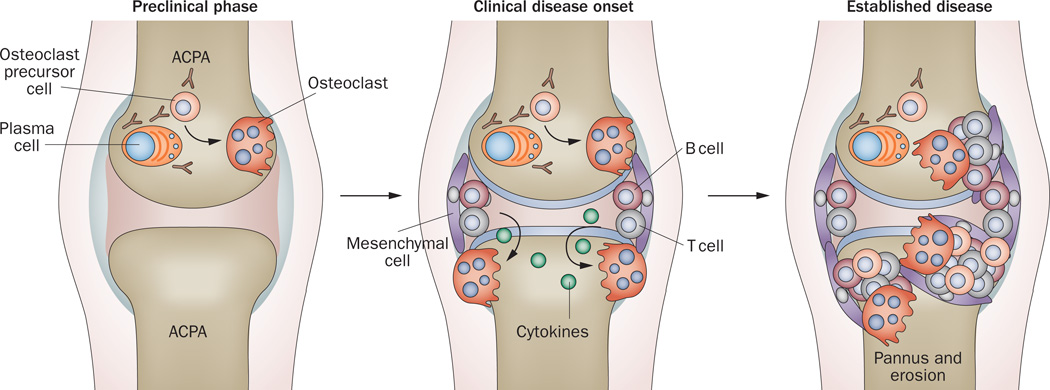
Figure 2.
Evolution of bone erosion in the course of RA. During the preclinical phase of RA, ACPA are produced early on by plasma cells. ACPA can stimulate osteoclast differentiation and lead to initial bone loss. These early changes may initiate in the bone marrow adjacent to the joint. Synovitis at the onset of clinical disease leads to production of cytokines, which stimulate osteoclastogenesis by inducing expression of RANKL, and synergize with RANKL to enhance bone erosion. Established RA is characterized by the presence of large bone erosions filled with inflamed, synovially derived pannus tissue. Abbreviations: ACPA, anti-citrullinated protein antibodies; RA, rheumatoid arthritis; RANKL, receptor activator of nuclear factor κB ligand.
Innate immunity
Amassing evidence is indicating a role for the innate immune system in bone erosion in RA. Osteoclasts are viewed as key innate immune cells in bone, as they express innate immune receptors, analogous to macrophages and dendritic cells, which regulate their ability to respond to inflammation in the joint, and thus direct their fate and function. These receptors include immunoreceptor tyrosine-based activation motif (ITAM)-bearing receptors, which have an important co-stimulatory role with RANKL and M-CSF in osteoclastogenesis. 69 A role for Toll-like receptors (TLRs) in the pathogenesis of inflammation and bone erosion in RA has also been proposed, as TLR stimulation of synoviocytes induces expression of RANKL, thereby favouring osteoclastogenesis. TLR ligands can also activate pathways that suppress osteoclastogenesis, by inhibiting expression of receptor activator of NFκB (RANK; also known as TNF receptor superfamily member 11A), thus limiting pathologic bone loss associated with inflammation.70 The role of the innate immune system in bone erosion in RA thus remains a fertile area for further research.
Transition from autoimmunity to inflammation
Changes in immune balance could be capable of retarding or even preventing the transition from ACPA-positive healthy individuals to patients with inflammatory arthritis. Regulatory mechanisms involving a balance of T-cell subsets might be crucial in blocking the clinical manifestations of RA. Although T cells, and in particular type 17 T-helper (TH17) cells, have always been considered to be the cell types that trigger osteoclastogenesis,62,71 T cells may also facilitate maintenance of bone homeostasis. For example, expression of cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) on T cells, in particular regulatory T (TREG) cells, is a potent signal for blockade of osteoclast differentiation. 72–75 CTLA4 disrupts the co-stimulation of T cells and thereby acts as an immunomodulatory signal. Binding of CTLA4 to the cell surface receptors CD80 and CD86 on osteoclast precursors arrests further differentiation of these cells into osteoclasts, even in the presence of the stimulatory factors M-CSF and RANKL.75 This concept has been translated into therapeutic use in patients with the introduction of CTLA4–Ig (abatacept), which blocks T-cell co-stimulation and thus blocks inflammation and bone erosion in patients with RA. Moreover, the fact that an immunoregulatory molecule protects bone extends the spectrum of currently described immune–bone interactions and demonstrates dual roles for cell types and factors in both the immune system and the skeleton. Thus, immune activation facilitates bone resorption, whereas immune modulation has protective effects on the skeleton.
Synovitis
Once synovial inflammation develops from autoimmunity in RA, additional triggers augment the process of bone erosion. It follows, then, that tight control of synovial inflammation would protect from bone erosion progression; this prediction has held true in patients with RA receiving tightly controlled anti-inflammatory treatment with DMARDs and glucocorticoids.76,77 Synovitis is a rich source of proinflammatory cytokines, which drive the process of osteoclast differentiation.15 TNF, IL-1 and IL-6 enhance osteoclastogenesis through permissive action on RANKL expression in mesenchymal cells, as well as through direct effects on osteoclast precursor cells, and in so doing generate an appropriate microenvironment for osteoclastogenesis. Moreover, the expression of receptors for osteoclast differentiation, such as RANK, is stimulated by synovially derived cytokines (Table 1).78–83 It should be noted that the greater the severity of synovitis, the more extensive the erosive process. In accordance, both ultrasonography and MRI studies have shown that the extent of synovitis and osteitis is related to the likelihood of later bone damage.38,39 Furthermore, it seems that levels of acute phase reactants, such as C-reactive protein, are also positively correlated with the likelihood of the development of bone erosions in RA.84 As synovitis develops shortly before clinical disease onset in patients with RA, it is not surprising that the risk of bone erosions increases after the clinical onset of disease.85,86
Table 1.
The clinical effects of cytokines during RA
| Cytokine | Proinflammatory effect | Skeletal effect |
|---|---|---|
| TNF | Key inhibitor of bone formation in RA | Pro-osteoclastogenic by inducing RANKL and by direct stimulation of osteoclast precursors through TNF receptor 1; also inhibits bone formation |
| IL-6 | Major proinflammatory cytokine in RA | Pro-osteoclastogenic by inducing RANKL and by direct stimulation of osteoclast precursors through induction of gp130 signalling |
| M-CSF | Growth factor with potential proinflammatory effects | Essential factor for osteoclast differentiation |
| RANKL | No effect on inflammation | Essential factor for osteoclast differentiation |
| IL-1 | Moderate proinflammatory effects in RA | Pro-osteoclastogenic by inducing RANKL and RANK expression |
| IL-17 | Potential proinflammatory effects in RA | Pro-osteoclastogenic by inducing RANKL and by blocking anti-osteoclastogenic factors, such as IL-4, IFN-γ and IL-12 |
| IL-15 | Cytokine involved in innate and adaptive immune responses with potential proinflammatory effects in RA | Pro-osteoclastogenic by inducing RANKL |
| IL-33 | Proinflammatory and antiinflammatory effects; no definite role in inflammation in RA | Potent inhibitor of osteoclastogenesis and bone resorption |
| Dkk-1 | Wnt inhibitor with no effect on inflammation in RA | Key inhibitor of bone formation in RA |
Abbreviations: Dkk-1, Dickkopf-related protein 1; IFN-γ, interferon-γ; M-CSF, macrophage colony-stimulating factor; RA, rheumatoid arthritis; RANK, receptor activated of nuclear factor κB; RANKL, receptor activated of nuclear factor κB ligand.
Early intervention with antirheumatic therapy is the most efficacious strategy for the prevention of bone erosions. Standard small-molecule antirheumatic drugs for RA, such as glucocorticoids, methotrexate and leflunomide, seem to have bone-sparing effects simply based on their ability to effectively reduce synovitis.87,88 It is noteworthy that even glucocorticoids, which definitely show negative effects on bone balance, can enable protection from bone erosions by effectively reducing the burden of synovitis. By contrast, incomplete control of synovitis enables osteoclast-mediated bone erosions to advance, resulting in progression of structural damage.89–91 Thus, patients with a low level of disease activity and even those in clinical remission can demonstrate progression of bone erosion, particularly if treated with conventional antirheumatic drugs.89–91 It is likely that progression of bone erosion in these patients is a result of residual, subclinical synovitis and osteitis, which is sufficient to trigger continued osteoclast differentiation and bone erosions. However, one can not exclude the possibility that, in some patients with RA, the processes of inflammation and bone erosion become dissociated and bone erosion may continue after inflammation has ceased. Thus, for instance, altered mechanical load and other mechanical factors might precipitate further bone damage in RA.
Proinflammatory cytokines in bone erosion
Cytokine inhibition, including with TNF blockers (infliximab, etanercept, adalimumab, certolizumabpegol and golimumab) and IL-6 receptor (IL-6R) blockade (tocilizumab), is one of the most effective approaches to slow or arrest the bone erosive process in RA (Figure 3) and may prevent the progression of systemic bone loss.88 The explanation for this effect is twofold. First, cytokine blockade is typically more effective than traditional antirheumatic drugs in reducing synovitis, including subclinical synovitis. However, at this time, no evidence has shown that the burden of synovitis is different amongst patients with RA in remission, treated with either conventional drugs or by cytokine blockers. Second, blockade of TNF and IL-6R exerts direct effects to limit the process of osteoclastogenesis. This concept is supported by observations from clinical trials92 as well as by pathophysiological studies showing that TNF and IL-6–IL-6R complexes directly induce differentiation of osteoclast precursor cells to become bone-resorbing osteoclasts.78,82
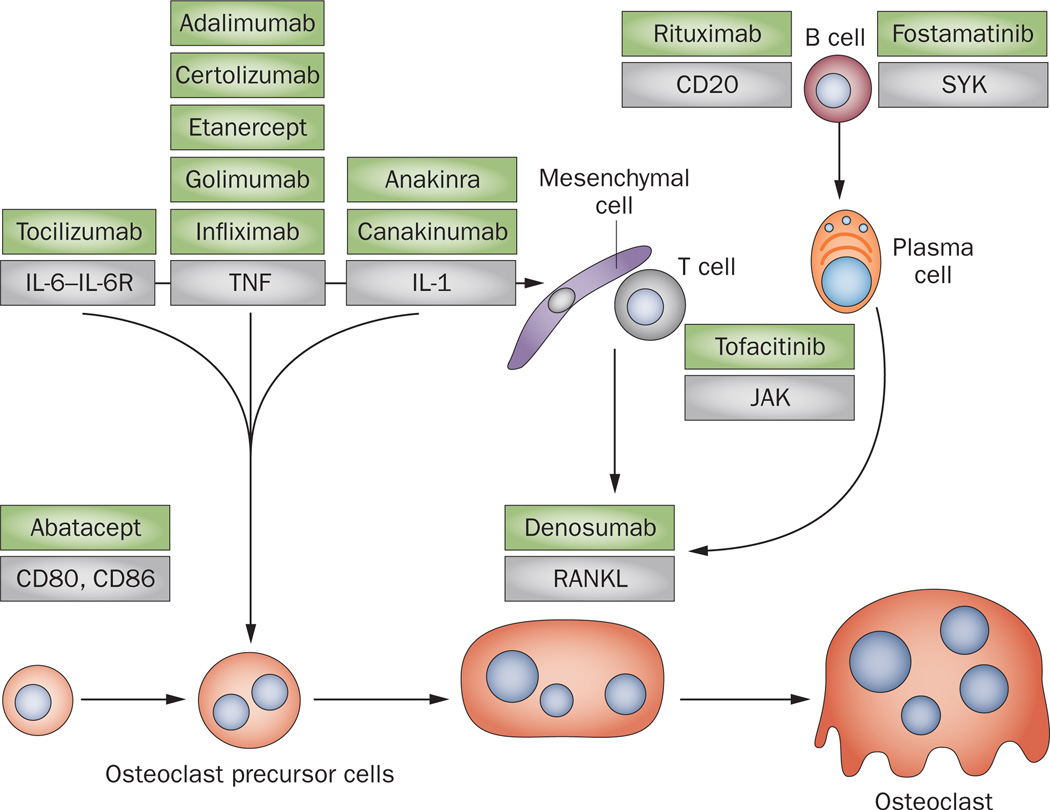
Figure 3.
Site of action of antirheumatic drugs on osteoclast differentiation and bone erosion. Inhibitors (green boxes) of TNF, IL-1 and IL-6R block the expression of RANKL by mesenchymal cells and T cells; they also directly interfere with osteoclastogenesis. Abatacept inhibits osteoclast differentiation by directly engaging CD80 and CD86 on the surface of osteoclast precursor cells. T-cell activation is targeted by small-molecule tyrosine kinase inhibitors such as tofacitinib, an inhibitor of JAK. B cells differentiate into plasma cells, which are a source of RANKL. B cells are depleted by an antibody against CD20 (rituximab) and are inhibited by small-molecule tyrosine kinase inhibitors such as fostamatinib, an inhibitor of SYK. Abbreviations: IL-6R, IL-6 receptor; JAK, Janus kinase; RANKL, receptor activator of nuclear factor κB ligand; SYK, spleen tyrosine kinase.
The direct effect on osteoclastogenesis may not be limited to cytokine-blocking agents, but may also apply to inhibitors of intercellular protein kinases.93 Novel compounds in clinical testing for their disease-modifying effect are tofacitinib, an inhibitor of Janus-associated kinase (JAK),94,95 and fostamatinib, a tyrosine-protein kinase inhibitor that targets spleen tyrosine kinase (SYK).96 These tyrosine kinases are expressed by immune cells, including osteoclasts, and function to integrate cytokine signalling and cellular responses during inflammation.96 As a consequence, their inhibition may simultaneously result in anti-inflammatory as well as anti-erosive effects in patients with RA. Direct inhibition of osteoclasts and bone erosion is particularly probable when kinases, such as SYK97 and Bruton tyrosine kinase (BTK), are targeted by small-molecule drugs as these kinases are preferentially expressed by antigen-presenting cells such as B cells and monocytes.98 As previously discussed, IL-1 might participate in triggering bone erosions in patients with RA.99 This cytokine has been shown to be a pivotal trigger for cartilage and bone loss in animal models of inflammatory arthritis.79,80 In RA, however, the role of IL-1 blockade in improving clinical signs of disease is limited. Nevertheless, IL-1 blockade yields protection from bone erosion in RA.99 Even when taking into account its modest anti-inflammatory effect in RA, the role of IL-1 blockade in protecting patients from bone erosion in diseases that are triggered by IL-1, such as gout, may be substantial.
Candidate cytokines that combine proinflammatory and pro-osteoclastogenic properties include IL-17 and IL-15. IL-17 is a proinflammatory cytokine that induces production of prostaglandins, nitric oxide, cytokines and chemokines. IL-17 induces the production of IL-1 and TNF in macrophages and fibroblasts and is synergistic with IL-1 in the upregulation of inflammatory mediators released by synovial fibroblasts.100–102 Thus, a role for IL-17 in RA pathogenesis has been highlighted.103 IL-17 is produced by several cell lineages including TH17 cells and mast cells, and is a potent inducer of RANKL expression on the surface of osteoblasts and synovial fibroblasts.104,105 At the same time, IL-17 blocks the function of compensatory anti-osteoclastogenic factors such as TREG cells and IL-4, and thereby inhibits local bone erosion as well as systemic bone loss in a mouse model of TNF-mediated arthritis.106 IL-15 is another proinflammatory cytokine that regulates innate and adaptive immune responses, as well as mediating osteoclastogenesis and bone erosion.107 Single-nucleotide polymorphisms in the gene encoding IL-15 were shown to be correlated with joint destruction in RA in a multicohort study, supporting a direct role for this cytokine in articular bone erosion.108
Not all proinflammatory cytokines trigger bone loss. For instance, the alarmin IL-33 and the dendritic cell-derived cytokines IL-23 and IL-12, as well as the type I and type II interferons, are all potent suppressors of osteoclastogenesis.6,109,110 Although these factors are also expressed in synovial tissue, their actions cannot outweigh the bone-resorptive effects of the aforementioned proinflammatory signals within RA synovium, which result in net bone loss.
New advances to promote erosion repair
Once established, bone erosions rarely repair.111,112 Indeed, spontaneous repair of erosions is virtually absent and, even with the use of potent anti-inflammatory therapeutic strategies such as blockade of TNF or IL-6R, only limited signs of repair of bone erosions are noted.113,114 If present, repair manifests as new bone apposition (that is, sclerosis) at the base of the erosion and seems to involve the juxta-articular bone marrow.113 Indeed, histopathology of synovial tissue of patients with RA has revealed an abundance of osteoclasts in bone erosions, but a paucity or even absence of mature osteoblasts, 115 suggesting that certain molecules in the synovium effectively block differentiation of bone-forming osteoblasts. Histomorphometric studies in a mouse model of RA demonstrated that bone formation at sites of erosion is markedly limited, with bone formation rates similar to those in healthy controls.115,116 Synovitis in RA thereby seems to foster focal articular bone loss by blocking local bone formation, which leads to a fundamental imbalance in bone homeostasis (Figure 4). By contrast, data in an animal model has shown that, once synovitis resolves, osteoblasts do populate the surfaces of eroded bone and form new bone to repair erosions.117 The implication of this finding is that in patients in whom repair is not seen, inflammation might not be fully controlled. Indeed, when sensitive radiographic techniques are used to study the joints of patients thought to be in clinical remission, residual inflammation is often seen.91 The relevance of erosion repair to functional status will be important to determine, as will understanding the possible detrimental effects of residual inflammation on the cardiovascular system and on bone in the axial and appendicular skeleton.
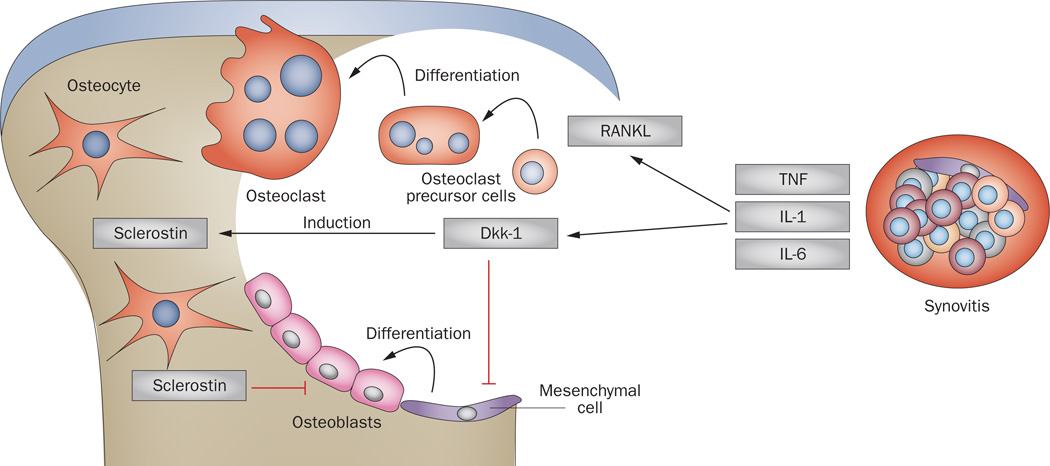
Figure 4.
Disruption of bone homeostasis by synovitis. Inflammation within synovial tissue induces osteoclastogenesis through increased expression of RANKL, and by the production of proinflammatory cytokines that drive osteoclastogenesis and synergize with RANKL. In addition, expression of Dkk-1 by synovial fibroblasts leads to inhibition of osteoblast differentiation and consequently of bone formation. Dkk-1 itself induces expression of another anti-anabolic molecule, sclerostin, by osteocytes. Abbreviations: Dkk-1; Dickkopf-related protein 1; RANKL, receptor activator of nuclear factor κB ligand.
Limited repair of bone erosions in RA seems to involve the induction of signals that block new bone formation. Administration of parathyroid hormone has been shown to achieve repair of bone erosions in a mouse model of arthritis, when combined with TNF inhibitors.118 Other candidate molecules that could limit bone formation and repair include antagonists of the Wnt signalling pathway, one of the strongest bone anabolic pathways. Production of Dickkopf-related protein 1 (Dkk-1), for instance, is induced by TNF in synovial fibroblasts and is expressed in the synovium of patients with RA.116 Dkk-1 potently interferes with Wnt signalling and blocked new bone formation in a mouse model of RA.116 Moreover, expression of other Wnt antagonists such as secreted Frizzled-related protein-1 and sclerostin can be induced during inflammation and may also inhibit repair of bone erosion by suppressing bone formation.117,119,120 Whether blockade of Dkk-1 or other Wnt antagonists such as sclerostin will trigger repair or even healing of bone erosion is, however, unclear at present, and despite some evidence from animal models of arthritis, appropriate clinical studies have to be undertaken to address this question.
Conclusions
In summary, articular bone erosion is a hallmark of RA and is relevant for diagnosis, treatment and monitoring of the disease. Knowledge of the mechanisms that induce bone erosion has increased substantially owing to refinements in imaging technologies as well as new insights into pathophysiology. Data obtained over the past few years have further revised the concepts of pathogenesis, shifting us from a perception of erosions as irreversible, end-stage lesions to a dynamic view of erosion as an active process. Most importantly, bone erosion begins early in the course of RA and is integrally entwined with innate immune mechanisms, autoimmunity and synovitis. Further insights into the molecular pathways of bone loss and bone formation during inflammation should facilitate the development of new therapeutic strategies for repair of bone erosions.
Key points.
-
▪
Articular bone erosions are a central clinical feature of rheumatoid arthritis
-
▪
Imaging techniques enable early detection of bone erosions and provide insights into disease pathogenesis
-
▪
Bone erosion is a result of enhanced osteoclast differentiation and inhibition of osteoblast-mediated bone repair
-
▪
Autoantibodies and cytokines, including proinflammatory cytokines and receptor activator of nuclear factor κB ligand, are the major precipitating factors in bone erosion in rheumatoid arthritis
-
▪
Antirheumatic therapies block progression of bone erosion by mitigating synovial inflammation and restoring bone balance
Review criteria.
A search for original articles published between 1970 and 2012 was performed using the PubMed database with the search terms “rheumatoid arthritis”, “bone” and “erosion”, both alone and in combination. Only full-text papers published in the English language were considered. The reference lists of identified papers were also used to identify further relevant articles.
Acknowledgements
The work of G. Schett is supported by the Deutsche Forschungsgemeinschaft (SPP1468-IMMUNOBONE), the Bundesministerium für Bildung und Forschung (BMBF; project ANCYLOSS) and the MASTERSWITCH project of the European Union and the IMI funded project BTCure. The work of E. Gravallese is supported by the NIH (R01 AR055952) and by the American College of Rheumatology Research and Education Foundation (Within our Reach: Finding a Cure for Rheumatoid Arthritis campaign).
Footnotes
Competing interests
G. Schett declares no competing interests. E. Gravallese declares an association with the following companies: Abbott Laboratories, Lilly. See the article online for full details of the relationships.
Contributor Information
Georg Schett, Department of Internal Medicine 3, University of Erlangen-Nuremberg, Krankenhausstrasse 12, 91054 Erlangen, Germany.
Ellen Gravallese, Department of Medicine, University of Massachusetts Memorial Medical Center and University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655, USA.
References
- 1.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu. Rev. Cell. Dev. Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 3.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 4.Baker WM. The formation of abnormal synovial cysts in the connection with the joints. St Bartolomews Hospital Reports. 1855;21:177–190. [Google Scholar]
- 5.Weichselbaum A. Die feineren Veränderungen des Gelenkknorpels bei fungöser Synovitis und Karies der Gelenkenden [German] Archiv. Pathol. Anat. Physiol. Klin. Med. 1878;73:461–475. [Google Scholar]
- 6.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 7.Arron JR, Choi Y. Osteoimmunology: bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 8.Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat. Rev. Endocrinol. 2010;6:698–706. doi: 10.1038/nrendo.2010.190. [DOI] [PubMed] [Google Scholar]
- 9.Schett G, Saag KG, Bijlsma JW. From bone biology to clinical outcome: state of the art and future perspectives. Ann. Rheum. Dis. 2010;69:1415–1419. doi: 10.1136/ard.2010.135061. [DOI] [PubMed] [Google Scholar]
- 10.Sharp JT, Lidsky MD, Collins LC, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum. 1971;14:706–720. doi: 10.1002/art.1780140605. [DOI] [PubMed] [Google Scholar]
- 11.Aletaha D, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/ European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 12.Ødegård S, et al. Association of early radiographic damage with impaired physical function in rheumatoid arthritis: a ten-year, longitudinal observational study in 238 patients. Arthritis Rheum. 2006;54:68–75. doi: 10.1002/art.21548. [DOI] [PubMed] [Google Scholar]
- 13.Scott DL, et al. The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford) 2000;39:122–132. doi: 10.1093/rheumatology/39.2.122. [DOI] [PubMed] [Google Scholar]
- 14.Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–2017. doi: 10.1002/1529-0131(200109)44:9<2009::AID-ART349>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.McInnes I, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 16.Stach CM, et al. Periarticular bone structure in rheumatoid arthritis patients and healthy individuals assessed by high-resolution computed tomography. Arthritis Rheum. 2010;62:330–339. doi: 10.1002/art.27252. [DOI] [PubMed] [Google Scholar]
- 17.Døhn UM, et al. Rheumatoid arthritis bone erosion volumes on CT and MRI: reliability and correlations with erosion scores on CT, MRI and radiography. Ann. Rheum. Dis. 2007;66:1388–1392. doi: 10.1136/ard.2007.072520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakefield RJ, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000;43:2762–2770. doi: 10.1002/1529-0131(200012)43:12<2762::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.McGonagle D, Tan AL, Møller Døhn U, Ostergaard M, Benjamin M. Microanatomic studies to define predictive factors for the topography of periarticular erosion formation in inflammatory arthritis. Arthritis Rheum. 2009;60:1042–1051. doi: 10.1002/art.24417. [DOI] [PubMed] [Google Scholar]
- 20.Martel W, Hayes JT, Duff IF. The pattern of bone erosion in the hand and wrist in rheumatoid arthritis. Radiology. 1965;84:204–214. doi: 10.1148/84.2.204. [DOI] [PubMed] [Google Scholar]
- 21.Ejbjerg B, et al. Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum. 2004;50:1097–1106. doi: 10.1002/art.20135. [DOI] [PubMed] [Google Scholar]
- 22.Tan AL, et al. Role of metacarpophalangeal joint anatomic factors in the distribution of synovitis and bone erosion in early rheumatoid arthritis. Arthritis Rheum. 2003;48:1214–1222. doi: 10.1002/art.10963. [DOI] [PubMed] [Google Scholar]
- 23.Hayer S, et al. Tenosynovitis and osteoclast formation as the initial preclinical changes in a murine model of inflammatory arthritis. Arthritis Rheum. 2007;56:79–88. doi: 10.1002/art.22313. [DOI] [PubMed] [Google Scholar]
- 24.Marinova-Mutafchieva L, Williams RO, Funa K, Maini RN, Zvaifler NJ. Inflammation is preceded by tumor necrosis factor-dependent infiltration of mesenchymal cells in experimental arthritis. Arthritis Rheum. 2002;46:507–513. doi: 10.1002/art.10126. [DOI] [PubMed] [Google Scholar]
- 25.Schett G, et al. Analysis of the kinetics of osteoclastogenesis in arthritic rats. Arthritis Rheum. 2005;52:3192–3201. doi: 10.1002/art.21343. [DOI] [PubMed] [Google Scholar]
- 26.Tournis S, et al. Effect of rheumatoid arthritis on volumetric bone mineral density and bone geometry, assessed by peripheral quantitative computed tomography in postmenopausal women treated with bisphosphonates. J. Rheumatol. 2012;39:1215–1220. doi: 10.3899/jrheum.110579. [DOI] [PubMed] [Google Scholar]
- 27.Aeberli D, et al. Reduced trabecular bone mineral density and cortical thickness accompanied by increased outer bone circumference in metacarpal bone of rheumatoid arthritis patients: a cross-sectional study. Arthritis Res. Ther. 2010;12:R119. doi: 10.1186/ar3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp JT, et al. Denosumab prevents metacarpal shaft cortical bone loss in patients with erosive rheumatoid arthritis. Arthritis Care Res. (Hoboken) 2010;62:537–544. doi: 10.1002/acr.20172. [DOI] [PubMed] [Google Scholar]
- 29.Hoff M, et al. Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann. Rheum. Dis. 2009;68:324–329. doi: 10.1136/ard.2007.085985. [DOI] [PubMed] [Google Scholar]
- 30.Haugeberg G, et al. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Ann. Rheum. Dis. 2004;63:1331–1334. doi: 10.1136/ard.2003.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Heijde DM. Joint erosions and patients with early rheumatoid arthritis. Br. J. Rheumatol. 1995;34:74–78. [PubMed] [Google Scholar]
- 32.Machold KP, et al. Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology (Oxford) 2007;46:342–349. doi: 10.1093/rheumatology/kel237. [DOI] [PubMed] [Google Scholar]
- 33.Güler-Yüksel M, et al. Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann. Rheum. Dis. 2009;68:330–336. doi: 10.1136/ard.2007.086348. [DOI] [PubMed] [Google Scholar]
- 34.Solomon DH, et al. The relationship between focal erosions and generalized osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum. 2009;60:1624–1631. doi: 10.1002/art.24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pye SR, et al. Disease activity and severity in early inflammatory arthritis predict hand cortical bone loss. Rheumatology (Oxford) 2010;49:1943–1948. doi: 10.1093/rheumatology/keq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Møller Døhn U, et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann. Rheum. Dis. 2011;70:252–258. doi: 10.1136/ard.2009.123729. [DOI] [PubMed] [Google Scholar]
- 37.Finzel S, et al. A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum. 2011;63:1231–1236. doi: 10.1002/art.30285. [DOI] [PubMed] [Google Scholar]
- 38.Haavardsholm EA, Bøyesen P, Østergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann. Rheum. Dis. 2008;67:794–800. doi: 10.1136/ard.2007.071977. [DOI] [PubMed] [Google Scholar]
- 39.McQueen FM, et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1814–1827. doi: 10.1002/art.11162. [DOI] [PubMed] [Google Scholar]
- 40.Geusens P, Lems WF. Osteoimmunology and osteoporosis. Arthritis Res. Ther. 2011;13:242. doi: 10.1186/ar3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finzel S, Englbrecht M, Engelke K, Stach C, Schett G. A comparative study of periarticular bone lesions in rheumatoid arthritis and psoriatic arthritis. Ann. Rheum. Dis. 2011;70:122–127. doi: 10.1136/ard.2010.132423. [DOI] [PubMed] [Google Scholar]
- 42.Bromley M, Woolley DE. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984;27:968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- 43.Leisen JCDH, Riddle JM, Pitchford WC. The erosive front: a topographic study of the junction between the pannus and the subchondral plate in the macerated rheumatoid metacarpal head. J. Rheumatol. 1988;15:17–22. [PubMed] [Google Scholar]
- 44.Gravallese EM, et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am. J. Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- 45.Kong YY, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 46.Romas E, et al. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am. J. Pathol. 2002;161:1419–1427. doi: 10.1016/S0002-9440(10)64417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lubberts E, et al. Increase in expression of receptor activator of nuclear factor κB at sites of bone erosion correlates with progression of inflammation in evolving collagen-induced arthritis. Arthritis Rheum. 2002;46:3055–3064. doi: 10.1002/art.10607. [DOI] [PubMed] [Google Scholar]
- 48.Pettit AR, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Redlich K, et al. Osteoclasts are essential for TNF-α-mediated joint destruction. J. Clin. Invest. 2002;110:1419–1427. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Redlich K, et al. Tumor necrosis factor α-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 2002;46:785–792. doi: 10.1002/art.10097. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 52.Firestein GS, et al. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J. Exp. Med. 1988;168:1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong BR, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/ osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 56.Kong Y-Y, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 57.Gravallese EM, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 58.Shigeyama Y, et al. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000;43:2523–2530. doi: 10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 59.Li P, et al. RANK signaling is not required for TNFα- mediated increase in CD11hi osteoclast precursors but is essential for mature osteoclast formation in TNFα-mediated inflammatory arthritis. J. Bone Miner. Res. 2004;19:207–213. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- 60.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-α- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J. Clin. Invest. 2003;111:821–831. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hermann S, et al. OSCAR—a key co-stimulation molecule for osteoclasts is induced in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:3041–3050. doi: 10.1002/art.23943. [DOI] [PubMed] [Google Scholar]
- 62.Ohno H, et al. The orally-active and selective c-Fms tyrosine kinase inhibitor Ki20227 inhibits disease progression in a collagen-induced arthritis mouse model. Eur. J. Immunol. 2008;38:283–291. doi: 10.1002/eji.200737199. [DOI] [PubMed] [Google Scholar]
- 63.Cohen SB, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelvemonth, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 64.Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002;46:357–365. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- 65.Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project) Ann. Rheum. Dis. 2004;63:1085–1089. doi: 10.1136/ard.2003.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer O, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann. Rheum. Dis. 2003;62:120–126. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi J, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl Acad. Sci. USA. 2011;108:17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harre U, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 2012;122:1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y, Humphrey MB, Nakamura MC. Osteoclasts—the innate immune cells of the bone. Autoimmunity. 2008;41:183–194. doi: 10.1080/08916930701693180. [DOI] [PubMed] [Google Scholar]
- 70.Ji D, et al. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-γ in human osteoclast precursors. J. Immunol. 2009;183:7223–7233. doi: 10.4049/jimmunol.0900072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato K, et al. TH17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zaiss MM, et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J. Immunol. 2010;184:7238–7246. doi: 10.4049/jimmunol.0903841. [DOI] [PubMed] [Google Scholar]
- 73.Zaiss MM, et al. Increased bone density and resistance to ovariectomy-induced bone loss in FoxP3-transgenic mice based on impaired osteoclast differentiation. Arthritis Rheum. 2010;62:2328–2338. doi: 10.1002/art.27535. [DOI] [PubMed] [Google Scholar]
- 74.Axmann R, et al. CTLA-4 directly inhibits osteoclast formation. Ann. Rheum. Dis. 2008;67:1603–1609. doi: 10.1136/ard.2007.080713. [DOI] [PubMed] [Google Scholar]
- 75.Zaiss MM, et al. TREG cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–4112. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 76.Bakker MF, et al. Utrecht Rheumatoid Arthritis Cohort Study Group. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 2012;156:329–339. doi: 10.7326/0003-4819-156-5-201203060-00004. [DOI] [PubMed] [Google Scholar]
- 77.Grigor C, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–269. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 78.Lam J, et al. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyce BF, et al. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989;125:1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- 80.Zwerina J, et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl Acad. Sci. USA. 2007;104:11742–11747. doi: 10.1073/pnas.0610812104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishimi YMC, et al. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 82.Axmann R, et al. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo . Arthritis Rheum. 2009;60:2747–2756. doi: 10.1002/art.24781. [DOI] [PubMed] [Google Scholar]
- 83.Poli V, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13:1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jansen LM, van der Horst-Bruinsma IE, van Schaardenburg D, Bezemer PD, Dijkmans BA. Predictors of radiographic joint damage in patients with early rheumatoid arthritis. Ann. Rheum. Dis. 2001;60:924–927. doi: 10.1136/ard.60.10.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kraan MC, et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 1998;41:1481–1488. doi: 10.1002/1529-0131(199808)41:8<1481::AID-ART19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 86.Van de Sande MG, et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann. Rheum. Dis. 2011;70:772–777. doi: 10.1136/ard.2010.139527. [DOI] [PubMed] [Google Scholar]
- 87.Rich E, Moreland LW, Alarcón GS. Paucity of radiographic progression in rheumatoid arthritis treated with methotrexate as the first disease modifying antirheumatic drug. J. Rheumatol. 1999;26:259–261. [PubMed] [Google Scholar]
- 88.Schett G, Stach C, Zwerina J, Voll R, Manger B. How antirheumatic drugs protect joints from damage in rheumatoid arthritis. Arthritis Rheum. 2008;58:2936–2948. doi: 10.1002/art.23951. [DOI] [PubMed] [Google Scholar]
- 89.Cohen G, et al. Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann. Rheum. Dis. 2007;66:358–363. doi: 10.1136/ard.2006.057497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molenaar ET, et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42. doi: 10.1002/art.11481. [DOI] [PubMed] [Google Scholar]
- 91.Brown AK, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–2967. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 92.Vis M, et al. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFκB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006;65:1495–1499. doi: 10.1136/ard.2005.044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pesu M, et al. Therapeutic targeting of Janus kinases. Immunol. Rev. 2008;223:132–142. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Vollenhoven RF, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 95.Fleischmann R, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 96.Weinblatt ME, et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 2010;363:1303–1312. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- 97.Mócsai A, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl Acad. Sci. USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 99.Jiang Y, et al. A multicenter, double-blind, doseranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000;43:1001–1009. doi: 10.1002/1529-0131(200005)43:5<1001::AID-ANR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 100.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jovanovic DV, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 102.Chabaud M, et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 103.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009;361:888–889. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 104.Kotake S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sato K, et al. TH17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zwerina K, et al. Anti IL-17A therapy inhibits bone loss in TNF-α-mediated murine arthritis by modulation of the T-cell balance. Eur. J. Immunol. 2012;42:413–423. doi: 10.1002/eji.201141871. [DOI] [PubMed] [Google Scholar]
- 107.Ogata Y, et al. A novel role of IL-15 in the development of osteoclasts: inability to replace its activity with IL-2. J. Immunol. 1999;162:2754–2760. [PubMed] [Google Scholar]
- 108.Knevel R, et al. Genetic variants in IL15 associate with progression of joint destruction in rheumatoid arthritis: a multicohort study. Ann. Rheum. Dis. 2012;71(Suppl. 1):A56–A57. doi: 10.1136/annrheumdis-2011-200724. [DOI] [PubMed] [Google Scholar]
- 109.Zaiss MM, et al. IL-33 shifts the balance from osteoclast to alternatively activated macrophage differentiation and protects from TNFα-mediated bone loss. J. Immunol. 2011;186:6097–6105. doi: 10.4049/jimmunol.1003487. [DOI] [PubMed] [Google Scholar]
- 110.Quinn JM, et al. IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J. Immunol. 2008;181:5720–5729. doi: 10.4049/jimmunol.181.8.5720. [DOI] [PubMed] [Google Scholar]
- 111.Lukas C, van der Heijde D, Fatenejad S, Landewé R. Repair of erosions occurs almost exclusively in damaged joints without swelling. Ann. Rheum. Dis. 2010;69:851–855. doi: 10.1136/ard.2009.119156. [DOI] [PubMed] [Google Scholar]
- 112.Møller Døhn U, et al. Erosive progression is minimal, but erosion healing rare, in patients with rheumatoid arthritis treated with adalimumab. A 1-year investigator-initiated follow-up study using high-resolution computed tomography as the primary outcome measure. Ann. Rheum. Dis. 2009;68:1585–1590. doi: 10.1136/ard.2008.097048. [DOI] [PubMed] [Google Scholar]
- 113.Finzel S, et al. Interleukin-6 receptor blockade induces limited repair of bone erosions in rheumatoid arthritis: a micro CT study. Ann. Rheum. Dis. doi: 10.1136/annrheumdis-2011-201075. http://dx.doi.org/10.1136/ annrheumdis-2011-201075. [DOI] [PubMed] [Google Scholar]
- 114.Finzel S, et al. Repair of bone erosions in rheumatoid arthritis treated with tumour necrosis factor inhibitors is based on bone apposition at the base of the erosion. Ann. Rheum. Dis. 2011;70:1587–1593. doi: 10.1136/ard.2010.148395. [DOI] [PubMed] [Google Scholar]
- 115.Walsh NC, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J. Bone Miner. Res. 2009;24:1572–1585. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- 116.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 117.Matzelle MM, et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 2012;64:1540–1550. doi: 10.1002/art.33504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Redlich K, et al. Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. Am. J. Pathol. 2004;164:543–555. doi: 10.1016/S0002-9440(10)63144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poole KE, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 120.Semënov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]