Abstract
Objective
Endothelial cell activation drives early atherosclerotic plaque formation. Both fibronectin deposition and accumulation of oxidized LDL (oxLDL) occur early during atherogenesis and both are implicated in enhanced endothelial cell activation. However, interplay between these responses has not been established. The objective of our study was to determine whether endothelial matrix composition modulates the inflammatory properties of oxLDL.
Approach and Results
We now show that oxLDL-induced NF-κB activation, proinflammatory gene expression, and monocyte binding is significantly enhanced when endothelial cells are attached to fibronectin compared to basement membrane proteins. This enhanced response does not result from altered oxLDL receptor expression, oxLDL uptake, or reactive oxygen species production, but instead results from oxLDL-induced activation of the fibronectin-binding integrin α5β1. Preventing α5β1 signaling (blocking antibodies, knockout cells) inhibits oxLDL-induced NF-κB activation and VCAM-1 expression. Furthermore, oxLDL-drives α5β1-dependent integrin signaling through the focal adhesion kinase (FAK) pathway and FAK inhibition (PF-573228, siRNA) blunts oxLDL-induced NF-κB activation, VCAM-1 expression, and monocyte adhesion. Lastly, treatment with the α5β1 signaling inhibitor, ATN-161, significantly blunts atherosclerotic plaque development in ApoE deficient mice, characterized by reduced VCAM-1 expression and macrophage accumulation without affecting fibrous cap size.
Conclusions
Our data suggest that α5β1-mediated crosstalk between fibronectin and oxidized LDL regulates inflammation in early atherogenesis and therapeutics that inhibit α5 integrins may reduce inflammation without adversely affecting plaque structure.
Keywords: oxidized LDL, integrin, fibronectin, inflammation, atherosclerosis
INTRODUCTION
Hypercholesterolemia, an elevation in circulating cholesterol, remains the most recognized atherogenic risk factor with genetic and epidemiological studies linking low density lipoproteins (LDL) to atherosclerosis 1. While native LDL acts benignly on vascular pathology, post-translational modifications such as oxidation and glycation enhance the atherogenic nature of LDL. Modified LDL exhibits reduced LDL receptor affinity and enhanced uptake by scavenger receptors (i.e. CD36 and the lectin-like oxidized LDL (oxLDL) receptor (LOX-1)). While the role of oxLDL in atherosclerosis has yet to be definitively proven, multiple lines of evidence suggest that oxLDL contributes to endothelial cell activation, a phenotypic conversion characterized by enhanced permeability and proinflammatory gene expression. Although early reports failed to show oxLDL-induced proinflammatory gene expression in endothelial cells 2, subsequent studies demonstrate that oxidized LDL stimulates vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) expression to facilitate leukocyte adhesion 3, 4. ApoE−/− mice lacking the endothelial oxLDL receptor LOX-1 exhibit reduced plaque size 5, whereas enhancing endothelial LOX-1 expression exacerbates atherosclerotic plaque development 6.
Current data from both cell culture and animal models suggest that extracellular matrix remodeling regulates endothelial cell activation. While the macrovasculature is largely devoid of fibronectin under healthy conditions 7, 8, the subendothelial basement membrane remodels into a fibronectin rich matrix simultaneously with enhanced endothelial cell proinflammatory gene expression during early atherogenesis. Endothelial cells interacting with fibronectin show augmented inflammatory responses to shear stress 7, 9, and limiting fibronectin deposition in atheroprone mice either genetically or with peptide inhibitors blunts both inflammation and early atherosclerotic plaque formation 8, 10.
OxLDL promotes matrix remodeling during multiple pathological conditions 11, 12, however much less is known concerning how matrix composition affects the cellular response to oxLDL. Early studies found that minimally modified LDL could activate endothelial β1 integrins to promote apical fibronectin deposition 13. This apical fibronectin deposition was suggested to facilitate monocyte attachment directly through fibronectin interactions with the leukocyte integrin α4β1. However, it was later shown that α4β1 interactions with fibronectin play a minor role in leukocyte adhesion to the atherosclerotic endothelium compared to the canonical α4β1 ligand VCAM-1 14. We previously found that adhesion to a fibronectin matrix significantly enhances oxLDL-induced endothelial cell permeability 15, suggesting that fibronectin deposition may alter the cellular response to oxLDL. Therefore, we sought to determine whether matrix composition affects oxLDL-induced endothelial cell inflammatory response.
MATERIALS and METHODS
An expanded methods section can be found in the online-only Data Supplement.
RESULTS
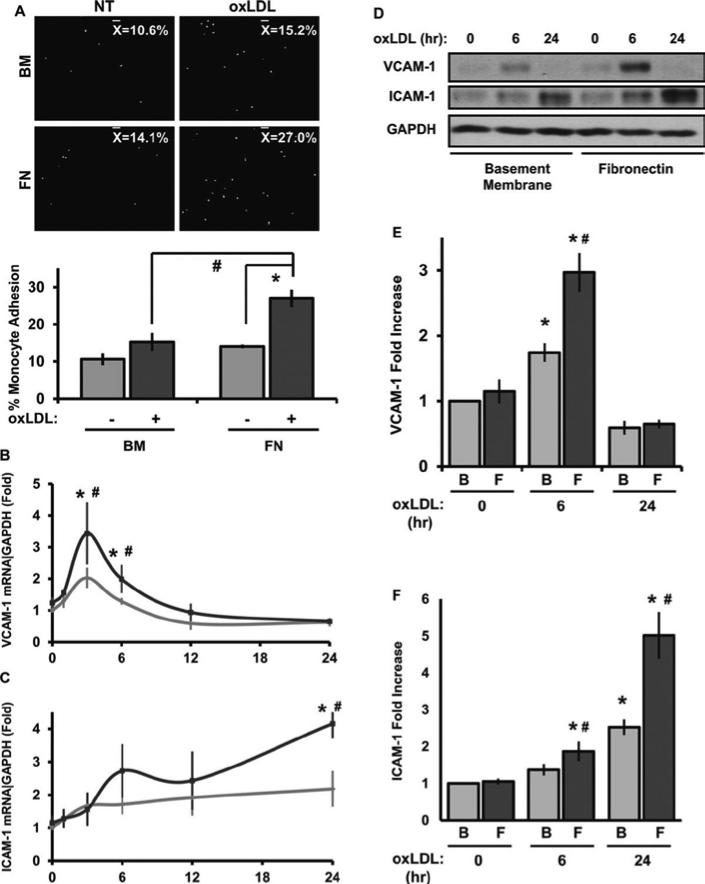
Leukocyte interactions with the endothelium represent a critical and tightly regulated process in the inflammatory response. To assess the role of cell-matrix interactions in oxLDL-induced inflammation, we compared oxLDL-induced monocyte binding in human aortic endothelial cells (HAEC) plated on either basement membrane proteins or fibronectin. While oxLDL treatment did not stimulate attachment of primary human monocytes or THP-1 monocytes in endothelial cells on basement membrane proteins (Figure 1A and Supplemental Figure I), endothelial cells on fibronectin showed a nearly 2-fold increase in monocyte attachment, suggesting that fibronectin augments oxLDL's inflammatory properties.
Figure 1. Matrix controls oxLDL-induced inflammation.
(A) HAECS on either BM or FN were treated with oxLDL (100 μg/mL) for 6 hours and human primary monocyte adhesion was analyzed. Results are expressed as percent adherent to the endothelial monolayer. Representative images are shown, n=4. (B and C) HAECs on different matrices were treated with oxLDL for the indicated times. mRNA was analyzed by qRT-PCR for VCAM-1, ICAM-1, and GAPDH, n=4. (D, E, and F) VCAM-1, ICAM-1, and GAPDH protein expression was determined by Western blotting. Representative Western blots are shown, n=7. Values are means ± SE, *p < 0.05 compared with no treatment condition, #p < 0.05 comparing matrices.
Given the importance of endothelial ICAM-1 and VCAM-1 for monocyte adhesion, we next tested whether matrix composition affects their expression following oxLDL treatment. Treatment with 100 μg/mL oxidized LDL induced a rapid and transient increase in VCAM-1 mRNA (Figure 1B) whereas ICAM-1 mRNA expression was delayed but sustained (Figure 1C). Consistent with the monocyte adhesion data, endothelial cells on fibronectin show greater VCAM-1 and ICAM-1 expression at both the mRNA (Figure 1B/C) and protein level (Figure 1DF). Native LDL did not induce VCAM-1 expression, suggesting that these effects are specific to oxLDL (Supplemental Figure II). Importantly, the oxLDL utilized in these studies contained less than 10 pg/ml of endotoxin (Supplemental Figure III), which is 4 orders of magnitude below the threshold for endotoxin-induced ICAM-1/VCAM-1 expression (100 ng/ml; Supplemental Figure III). While high concentrations of oxLDL can stimulate endothelial cell apoptosis, treatment with 100 μg/mL of oxLDL did not induce endothelial cell apoptosis as assessed by annexin V binding and analysis of cleaved caspase 3 and PARP (Supplemental Figure IV).
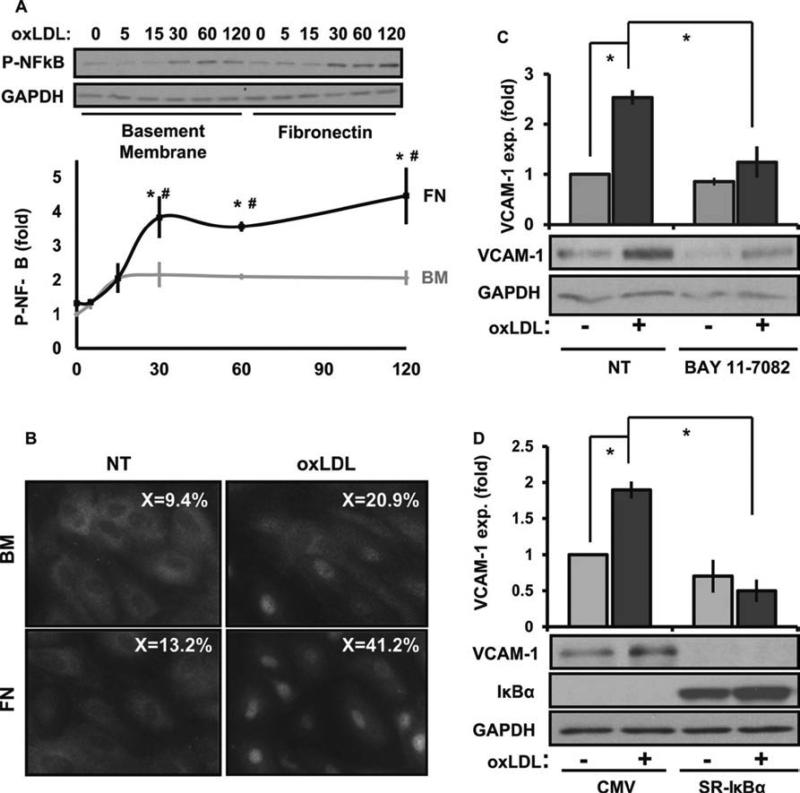
The transcription factor NF-KB regulates the expression of proinflammatory genes, such as ICAM-1 and VCAM-1 16. NF-KB-dependent transcription requires both nuclear translocation of the p65 subunit (hereafter referred to as NF-KB) and NF-KB phosphorylation on serine 536 in the transactivation domain 16. Therefore, we next tested whether matrix composition affects oxLDL-induced proinflammatory signaling by assessing NF-KB phosphorylation and nuclear translocation. Endothelial cells on fibronectin showed enhanced oxLDL-induced NF-KB phosphorylation (Figure 2A) and nuclear translocation (Figure 2B) compared to cells on basement membrane proteins. Consistent with NF-κB driven expression, inhibiting NF-KB either with a pharmacological inhibitor (BAY 11-7082; Figure 2C) or a dominant negative IKB construct (super-repressor IKB (SR-IKB); Figure 2D) significantly blunted oxLDL-induced VCAM-1 expression. Taken together, these results suggest that matrix composition affects the ability of oxLDL to activate NF-KB-dependent transcription of pro-inflammatory genes.
Figure 2. Matrix regulates VCAM-1 expression through NF-KB.
(A) HAECs plated on BM or FN were treated with oxLDL (100 μg/mL) for the indicated times. Immunoblotting was performed for P-NF-κB (p65, Ser536) and GAPDH. Representative Western blots are shown, n=5. (B) HAECs on different matrices were treated with oxLDL for 1 hour and NF-κB nuclear translocation determined by immunocytochemistry. Representative images are shown, n=3. (C) HAECs were treated with Bay11-7082 (10 μM, 1 hr) and oxLDL-induced VCAM-1 expression analyzed. Representative Western blots are shown, n=3. (D). oxLDL-induced VCAM-1 expression was determined in HAECs infected with either a CMV or SR-IκBα expressing adenovirus. Representative Western blots are shown, n=3. Values are means ± SE, *p < 0.05 compared with no treatment condition, #p < 0.05 comparing matrices.
Adhesion to fibronectin could enhance oxLDL's proinflammatory response by altering the expression of oxLDL receptors, affecting oxLDL uptake, or modifying oxLDL-induced signaling, such as reactive oxygen species (ROS) production. However, fluorescence activated cell sorting (FACS) analysis of the endothelial oxLDL receptors LOX-1, TLR4, and CD36 showed no difference in the surface expression in response to matrix composition (Supplemental Figure V). Matrix composition also failed to affect uptake of Dil-labeled oxLDL (Supplemental Figure V). Since oxLDL-induced inflammation requires ROS in multiple cell types 17, we next tested for matrix-dependent ROS production following oxLDL treatment. HAECs on either basement membrane or fibronectin were pretreated with with 2’, 7’-dichlorofluorescein diacetate (DCFDA), a redox-sensitive fluorogenic dye, and then treated with oxidized LDL. Like receptor expression and function, oxidized LDL-induced ROS production did not differ based on matrix composition (Supplemental Figure V).
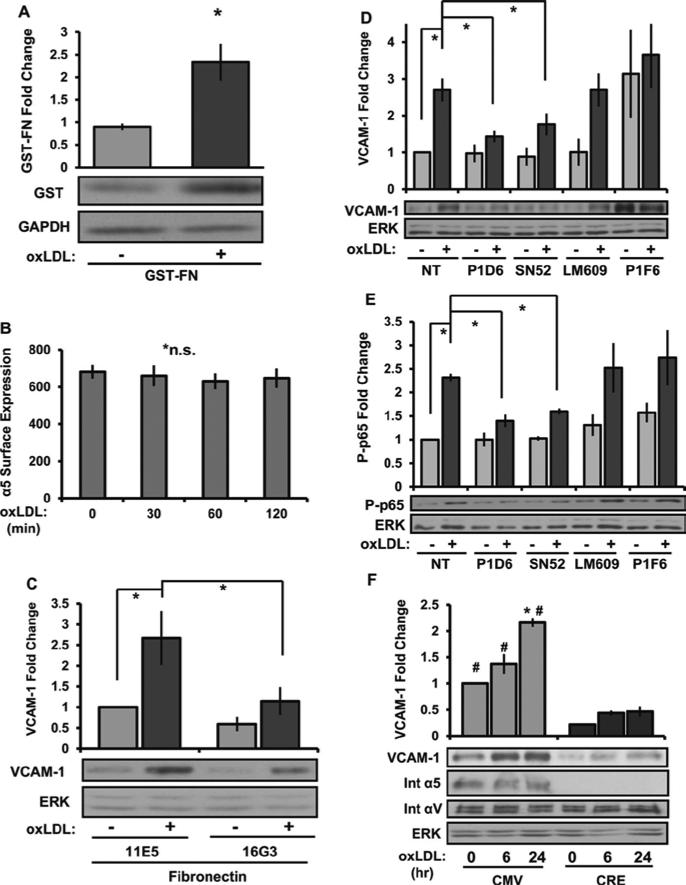
Previous reports suggest that oxLDL can activate endothelial β1 integrins 13, presumably α5β1, the main fibronectin-binding integrin in endothelial cells. Since oxLDL-induced α5β1 integrin signaling could contribute to the altered NF-κB activation observed, we next tested whether oxLDL could stimulate α5β1 activation and α5β1-dependent signaling. To measure α5β1 activation, we utilized an α5β1-specific ligand mimetic consisting of a glutathione S-transferase (GST) fusion protein containing the 9th to 11th FN type III repeats (GST-FNIII9-11) as previously described 18. Treatment with oxLDL enhanced GST-FNIII9-11 retention (Figure 3A) without affecting α5 surface expression (Figure 3B), consistent with enhanced α5β1 integrin activation. To test whether subsequent α5β1 ligation is required for oxLDL-induced VCAM-1 expression, we utilized blocking antibodies either to the integrin binding site in fibronectin (16G3) or to the α5β1 integrin (P1D6, SNAKA52). Blocking either fibronectin with 16G3 (Figure 3C) or the α5β1 integrin with P1D6 or SNAKA52 (Figure 3D) significantly blunted oxLDL-induced VCAM-1 expression. Control antibodies that bind to fibronectin but do not inhibit integrin binding (11E5) or that bind to other fibronectin binding integrins such as αvβ3 (LM609) and αvβ5 (P1F6) did not affect oxLDL-induced VCAM-1 expression. Consistent with these findings, the α5 blocking antibodies also prevented oxLDL-induced NF-KB activation whereas αVβ3 and αVβ5 blocking antibodies did not (Figure 3E). To verify the significance of integrin α5 in oxLDL-induced inflammation, we isolated mouse aortic endothelial cells (MAECs) from mice expressing a floxed α5 integrin allele (gift of Richard Hynes, MIT). Following Cre-mediated excision of the α5 gene, oxLDL-induced VCAM-1 expression was significantly reduced (Figure 3F). Together, these data suggest that α5β1 integrin activation plays a critical role in oxLDL-induced inflammation.
Figure 3. Integrin α5 regulates oxLDL-induced VCAM-1 and NF-KB activation.
(A) HAECs were treated with oxLDL (100 μg/mL) for 30 min, and α5β1 activation was determined by measuring GST-FNIII9-11 retention by Western blotting. Representative images are shown. n=4. (B) α5 surface expression in HAECs treated with oxLDL was determined by FACS analysis and is expressed as average mean fluorescence intensity, n=3. (C) HAECs pretreated with the nonblocking (11E5, 40 μg/ml) or fibronectin-blocking antibody (16G3, 40 μg/ml) for 1 hour were treated with oxLDL for 6 hours and VCAM-1 expression was determined by Western blotting, n=4. (D and E) HAECs plated on FN were treated with blocking antibodies for α5 (P1D6, SNAKA52), αvα3 (LM609), or αvα5 (P1F6) at 10 μg/mL for 1 hour, and oxLDL-induced VCAM-1 expression (6 hr) and NF-κB activation (1 hr) were determined by Western blotting, n=4. (F) oxLDL-induced VCAM-1 expression was determined in conditionally immortalized MAE cells either wildtype (CMV) or deficient for α5 integrins (CRE). Representative Western blots for VCAM-1, integrin α5, integrin αV, and ERK are shown, n=3. Values are means ± SE, *p < 0.05 compared with no treatment condition. #p < 0.05 compared with α5 deficient (Cre) cells.
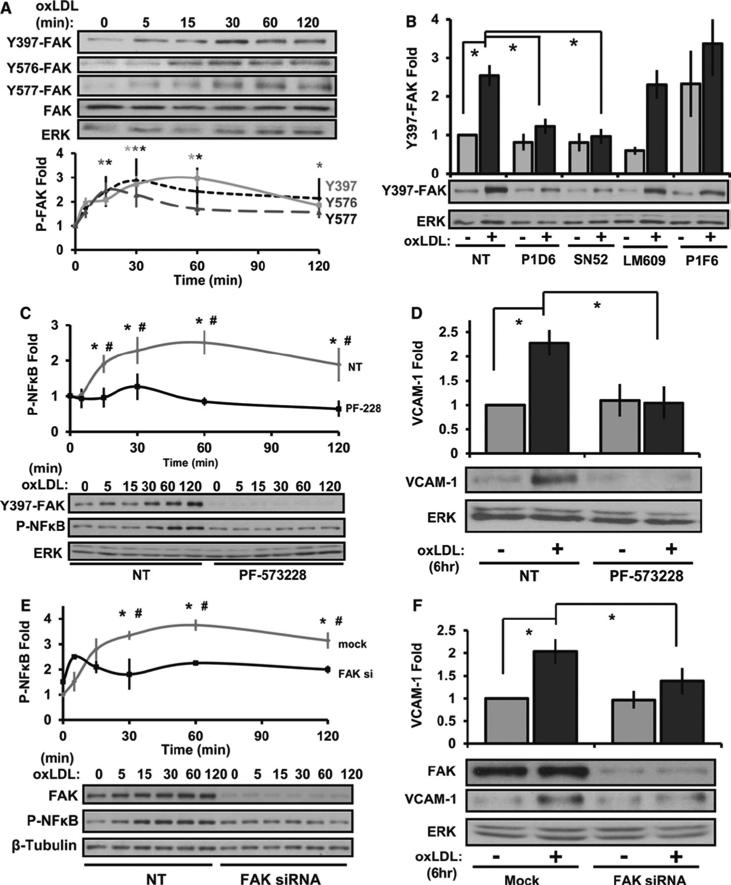
Since our data demonstrates a crucial role for α5 in oxLDL-induced inflammation, we next investigated the role of focal adhesion kinase (FAK), a common integrin signaling partner 19. OxLDL stimulated a rapid increase in FAK phosphorylation at the autophosphorylation site (Tyr397) as well as in the kinase domain (Tyr576, Tyr577) required for full FAK kinase activity. (Figure 4A). Consistent with the role of α5β1 activation, FAK stimulation by oxLDL was completely inhibited by the α5β1-blocking antibodies P1D6 and SNAKA52, whereas the αvβ3-blocking antibody LM609 and the αvβ5-blocking antibody P1F6 were without effect (Figure 4B). The ATP-competitive FAK inhibitor, PF-573228, significantly repressed oxLDL-induced NF-κB activation (Figure 4C), VCAM-1 expression (Figure 4D), and monocyte adhesion (Supplemental Figure VI). Additionally, FAK-targeted siRNA (~90% knockdown) similarly blunted oxLDL-induced proinflammatory responses (Figure 4E/F). Together, these data demonstrate that FAK signaling couples α5β1 to the NF-κB pathway following oxLDL stimulation thereby supporting the hypothesis that integrin signaling mediates oxLDL-induced endothelial cell activation.
Figure 4. FAK regulates oxLDL-induced VCAM-1 and NF-KB activation.
(A) HAECs were treated with oxLDL (100 μg/mL) for the indicated times. Immunoblotting was performed for phospho-FAK Y397, Y576, Y577, total FAK, and ERK. Representative images are shown, n=4. (B) HAECs pretreated with blocking antibodies to α5 (P1D6, SNAKA52), αvβ3 (LM609), or αvα5 (P1F6) were treated with oxLDL for 1 hour. Immunoblotting was performed for phospho-FAK Y397 and ERK. Representative images are shown, n=3. (C) HAECs were pretreated with PF-573228 (10 μM; 1 hour) and treated with oxLDL for indicated times. Immunoblotting was performed for phospho-FAK Y397, P-NF-κB, and ERK, n=4. (D) HAECs pretreated with PF-573228 were treated with oxLDL for 6 hours, and VCAM-1 expression was determined by Western blotting. Representative images are shown, n=3. (E) HAECs were transfected with anti-FAK siRNA and treated for the indicated times. Immunoblotting was performed for FAK, P-NF-κB, and β-tubulin. Representative images are shown, n=4. (F) HAECs were transfected with anti-FAK siRNA then treated with oxLDL for 6 hours. Immunoblotting was performed for FAK, VCAM-1, and ERK. Representative images are shown, n=4. Values are means ± SE, *p < 0.05 compared with no treatment condition. #p < 0.05 comparing treatment conditions.
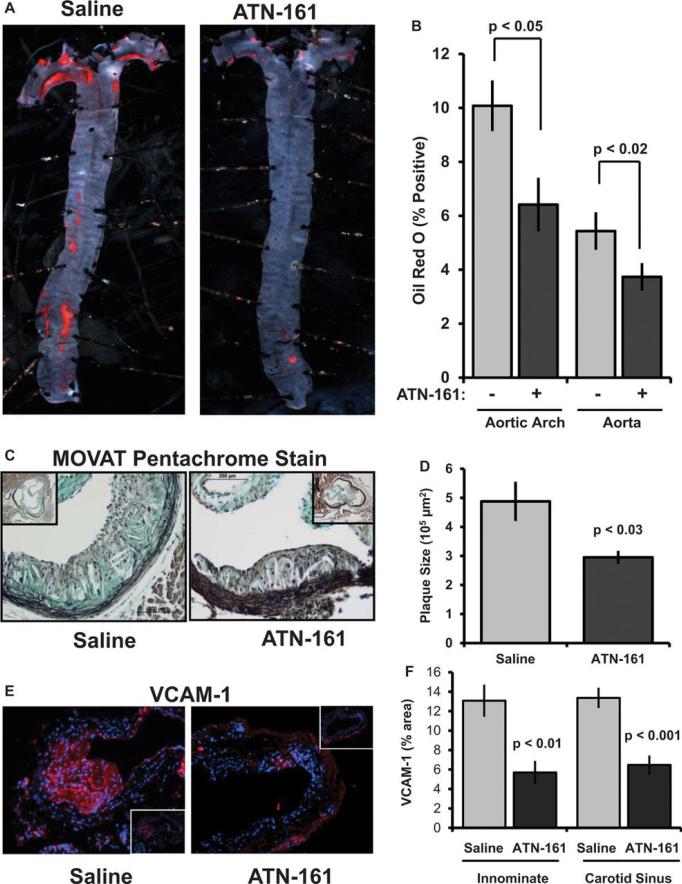
Immunohistochemical analysis of plaques from human patients and atherosclerosis-prone mice demonstrated enhanced integrin α5 expression in both the endothelial cell layer and the invading macrophage foam cells (Supplemental Figure VII). Similarly, quantitative real-time PCR showed enhanced α5 expression in the aortic arch of Western diet-fed ApoE knockout mice compared to chow-fed ApoE mice and C57Bl/6J mice (Supplemental Figure VII). Since α5 signaling contributes to oxLDL-induced inflammation, we next tested if inhibiting α5 signaling in vivo was sufficient to reduce atherosclerosis in hypercholesterolemic mice. Male, 8-10 week old ApoE−/− mice were fed a high-fat Western Diet for 8 weeks to induce atherosclerosis. Upon initiation of Western diet feeding, mice were treated with either saline or the α5 signaling inhibitor ATN-161, a peptide mimetic of the PHSRN sequence of fibronectin 20, for the entire 8 week feeding regimen. ATN-161 was administered intraperitoneally at 5 mg/kg three times a week consistent with previously published efficacy reports in murine cancer models 21, 22. Treatment with the ATN-161 peptide did not affect mouse weight, blood glucose levels, total cholesterol, HDL cholesterol, LDL cholesterol, or triglyceride levels (Supplemental Figure VIII). However, ATN-161 treatment significantly limited diet-induced atherosclerosis as demonstrated by Oil Red O staining of the aorta (Figure 5A/B). Analysis of plaque cross sections using MOVAT staining showed a ~40% reduction in plaque size in the aortic root (Figure 5C/D) and a ~60% reduction in the carotid sinus (Supplemental Figure IX). Consistent with a role for α5β1 in endothelial activation, ATN-161 treatment reduced plaque-associated VCAM-1 expression in both the carotid sinus and innominate arteries (Figure 5E/F; Supplemental Figure X).
Figure 5. Inhibiting integrin α5 signaling in vivo is sufficient to delay atherosclerosis.
8 week old ApoE−/− mice were fed a high fat, Western Diet for 8 weeks during which mice were treated with either saline or ATN-161 (5 mg/kg) by intraperitoneal injection. (A, B) Oil Red O staining of aortas was performed, and plaque area was analyzed as the percent Oil Red O positive area. (C, D) Plaque size in the aortic root was quantified following Russell-MOVAT pentachrome staining. Plaque area was calculated as the neointimal area inside the internal elastic lamina. (E, F) VCAM-1 expression in the carotid sinus and innominate artery was determined by immunofluorescence immunohistochemistry and expressed as the positive area in the vessel wall. Images taken at 20X with insets at 10X. Analysis was performed using NIS elements software. n=8-10 mice per group. Values are means ± SE.
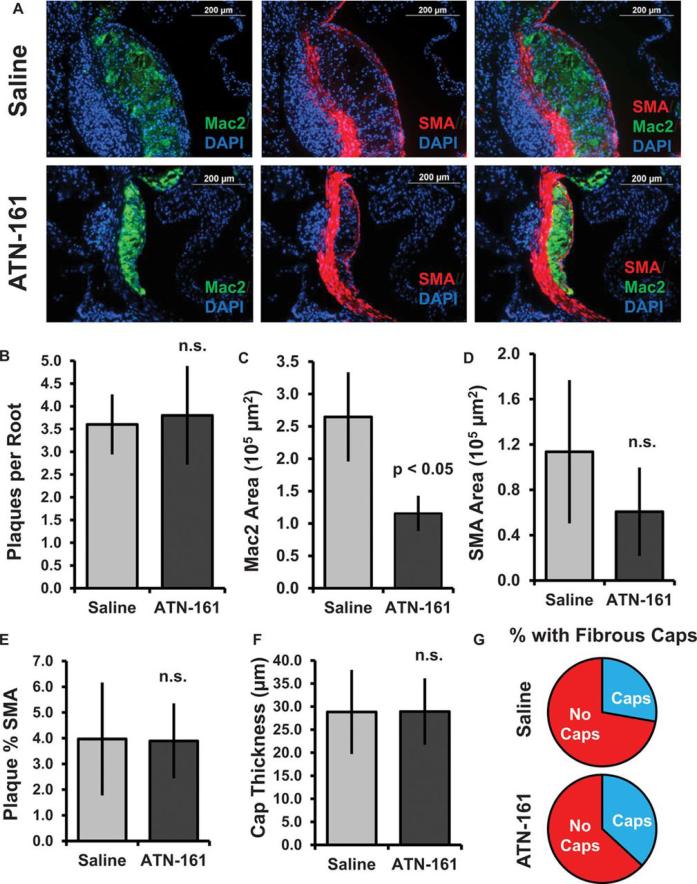
While deletion of plasma fibronectin was previously shown to reduce plaque size and macrophage content 8, the smooth muscle rich fibrous cap was similarly ablated suggesting that targeting this pathway could lead to the formation of vulnerable plaques prone to rupture. To assess whether α5 inhibitors similarly affect plaque composition, we analyzed macrophage (Mac2-positive) and smooth muscle (SMA-positive) content of these plaques in the aortic root (Figure 6A). The number of plaques per section of the aortic root was similar between saline and ATN-161 treated groups (Figure 6B), suggesting that ATN-161 reduces plaque area by limiting plaque size. ATN-161 treatment reduced the Mac2-positive regions in the aortic root and carotid sinus by 65% and 75%, respectively, indicative of diminished macrophage levels (Figure 6C; Supplemental Figure IX). While SMA-positive smooth muscle staining showed a trend toward lower levels (Figure 6D), this effect was not statistically significant. Furthermore, the percent area of the plaque positive for smooth muscle actin (Figure 6E), the thickness of the fibrous caps when present (Figure 6F), and the percentage of plaques scored positive for SMA-rich fibrous caps (Figure 6G) were all unaltered in the ATN-161 treated mice. These data demonstrate that integrin α5 signaling contributes to the development of atherosclerosis and blocking integrin α5 function significantly reduces plaque size by diminishing macrophage levels without affecting early smooth muscle recruitment.
Figure 6. Inhibiting integrin α5 signaling in vivo reduces macrophage content without altering fibrous cap formation.
(A) Aortic roots from saline and ATN-161 treated mice were stained by immunohistochemistry for Mac2 (macrophage marker, green), smooth muscle actin (smooth muscle cell marker, red) and DAPI (blue). Representative 40X images are shown. (B) The number of individual plaques per aortic root were quantified for each mouse. (C) Macrophage area was analyzed by quantifying the Mac2-positive area for each aortic root. (D) Smooth muscle area was analyzed by quantifying the SMA-positive area for each aortic root. (E) The percentage of the plaque that was SMA positive was calculated by dividing the SMA-positive area by the total plaque area and averaged for each group. (F) Thickness of the individual fibrous caps (averaged from more than four regions per cap) was calculated and expressed as the average cap thickness within each group. (G) Plaques were scored for SMA-positive fibrous caps, and the percentage of fibrous cap-positive plaques were calculated by dividing by the total number of plaques in each group. All analysis was performed using NIS elements software, n=10 mice per group. Values are means ± SE.
DISCUSSION
Transition to a fibronectin-rich matrix occurs prior to inflammatory cell recruitment, and limiting fibronectin deposition in vivo inhibits atherosclerosis and neointimal hyperplasia 7, 8, 10. Oxidized LDL accumulates early in atherosclerosis and promotes endothelial cell activation 15, 23. We demonstrate here that composition of the endothelial matrix modulates oxLDL-induced NF-κB activation, NF-κB-dependent proinflammatory gene expression (ICAM-1, VCAM-1), and monocyte attachment. This matrix-dependent response to oxLDL does not result from altered surface expression of oxLDL receptors, oxLDL uptake, or changes in ROS production. Instead, oxLDL treatment activates the fibronectin-binding integrin α5β1, and preventing α5β1 signaling (blocking antibodies, knockout cells) abolishes oxLDL-induced NF-κB activation and VCAM-1 expression. Both mouse and human atherosclerotic plaques show α5 expression in the endothelium and macrophages, and treating Western Diet-fed ApoE knockout mice with the α5β1 signaling inhibitor, ATN-161, significantly reduces VCAM-1 expression, macrophage content, and atherosclerotic plaque size. Together, these data demonstrate that α5β1 integrin signaling critically regulates oxLDL-induced proinflammatory responses in early atherogenesis.
Despite nearly 30 years of study, several controversies still remain concerning the role of oxLDL in endothelial activation, including the presentation of oxLDL to endothelial cells, the degree of LDL oxidation, and the mechanism of oxLDL-induced inflammation. The predominant theory on LDL oxidation suggests that LDL becomes oxidized after accumulation in the intima 24. While this model might limit the potential for endothelial interaction with oxLDL, oxidation of the apoB100 protein on LDL reduces its affinity for matrix suggesting that it could diffuse to the endothelial surface 25. Additionally, several recent studies have found a pool of electronegative LDL similar to highly oxidized LDL, termed L5, present in human plasma 26, 27. Early studies identified minimally modified LDL, but not highly oxidized LDL, as the predominant inflammatory species 28 and showed an important role for the oxidized phospholipid oxPAPC in mediating this effect 28, 29. These studies did not observe ICAM-1 or VCAM-1 expression by oxLDL, MM-LDL or oxPAPC. However, other groups have shown oxLDL-induced ICAM-1 and VCAM-1 expression in arterial endothelial cells 3, 4. Consistent with this, highly electronegative L5 LDL (similar to highly oxidized LDL) from human plasma induces VCAM-1 expression in arterial endothelial cells 26. Therefore, the endothelial cell inflammatory response to oxLDL likely differs based on the degree of LDL oxidation.
Vascular fibronectin deposition affects leukocyte recruitment in multiple ways. Published work and data presented herein show that fibronectin deposition into the subendothelial matrix affects the endothelial cell activation response to multiple stimuli 7, 8, 10. However, apical deposition of fibronectin containing the alternatively spliced connecting segment (CS-1) domain provides direct interaction sites for α4β1 integrins on leukocytes 13. Although MM-LDL stimulates apical fibronectin deposition, subsequent ex vivo analysis demonstrate that only a minority (<25%) of monocyte interactions with atherosclerotic endothelial occurs through apical CS-1 fibronectin 14. Interestingly, MM-LDL-induced apical fibronectin deposition is postulated to occur through α5β1 integrin activation, as MM-LDL enhances β1 integrin ligation and blocking α5β1 prevents monocyte-binding to MM-LDL stimulated endothelial cells 13. However, maximal induction of monocyte binding with MM-LDL takes 4 hours 13, 28 whereas α5β1 activation occurs rapidly (Figure 3A). Furthermore, cycloheximide treatment inhibits MM-LDL induced monocyte binding suggesting new gene expression is required 28, and MM-LDL does not affect α5β1 expression 13. While data presented herein provides direct proof that oxLDL-induces α5β1 activation, our work suggests that α5β1-dependent signaling following interaction with subendothelial fibronectin supports oxLDL-induced proinflammatory gene expression.
While the redox-sensitive transcription factor NF-κB classically mediates proinflammatory ICAM-1 and VCAM-1 expression, there are conflicting reports as to NF-κB's role in oxLDL-induced endothelial inflammatory gene expression 30, 31. Although macrophages stimulated with MM-LDL show enhanced NF-κB signaling, MM-LDL fails to activate NF-κB in endothelial cells 31, 32. However, highly oxidized LDL promotes NF-κB activation in endothelial cells, smooth muscle cells, and fibroblasts 30, and our data support an important role for NF-κB activation in matrix-specific VCAM-1 expression by oxLDL. Interestingly, oxLDL stimulates ROS production independent of matrix composition, suggesting that ROS production is insufficient to activate NF-κB. In our system, oxLDL activates NF-κB through an α5β1 integrin-dependent pathway requiring the tyrosine kinase FAK. While multiple groups implicate FAK signaling in NF-κB activation and proinflammatory gene expression 33, 34, the mechanisms by which oxLDL and α5β1-dependent FAK signaling converge to activate NF-κB remain unknown.
While the integrin αvβ3 has received considerable attention in cardiovascular disease models 35, much less is known concerning α5β1 integrins. Analysis of mRNA isolated from human plaques and abdominal aortic aneurysms shows enhanced α5 expression 36, 37 while protein levels were shown to be enhanced following carotid injury and induced arteriogenesis 38, 39. Immunohistochemistry from mouse and human plaques suggest that α5β1 expression occurs predominantly in the endothelial layer and plaque macrophages (Supplemental Figure VII). Our data suggest that endothelial α5 integrins contribute to proinflammatory gene expression and monocyte recruitment. The α5 integrin inhibitor ATN-161, a derivative of the fibronectin synergy sequence known to bind specifically to α5 40, significantly reduces VCAM-1 expression, macrophage content, and atherosclerotic plaque size in hypercholesterolemic mice. Although ATN-161 can interact with multiple integrin β subunits 41, 42, ATN-161 has only been shown to inhibit α5β1 integrin signaling 43-45. While these data are consistent with a role for α5 in endothelial cell activation, ATN-161 treatment may limit plaque formation through effects on other cell types as well. Inhibiting α5β1 in plaque macrophages could influence macrophage migration 46, phagocytosis 47, and gene expression 48. Future studies examining cell type-specific deletion of α5 integrins should shed further light into the cellular mechanisms underlying this therapeutic effect.
Matrix-specific integrin signaling may contribute to multiple processes during atherosclerotic plaque formation, including endothelial activation, fibrous cap formation, and plaque angiogenesis 35. Several published studies suggest that limiting fibronectin deposition blunts early inflammation in the atherosclerotic plaque 8, 10, and work from our group has shown that fibronectin enhances endothelial activation in response to multiple atherogenic stimuli 7, 15, 49. While genetic deletion of plasma fibronectin reduces endothelial activation and plaque inflammation 8, fibronectin deletion similarly reduced fibrous cap formation suggesting that depleting fibronectin could destabilize the plaque promoting plaque rupture 8. However, we show that inhibiting the fibronectin-binding integrin α5β1 similarly reduces plaque inflammation without affecting early smooth muscle recruitment (Figure 5, 6), potentially due to an important role for other fibronectin-binding integrins in smooth muscle growth and migration. While other groups have shown an important role for α5β1 in smooth muscle growth and migration in cell culture models 50, our current data suggest that α5β1 may show limited expression in the smooth muscle, at least at early stages of plaque development. Taken together, our data suggests that α5β1 integrin inhibitors, currently in clinical trials targeting cancer, could be redirected for use in atherosclerosis.
Supplementary Material
SIGNIFICANCE.
Endothelial cell activation facilitates monocyte recruitment into forming atherosclerotic plaques, and fibronectin deposition into the endothelial matrix enhances endothelial activation. We now show that oxidized LDL, a classic proatherogenic factor, stimulates the fibronectin binding receptor, integrin α5β1. We further demonstrate that inhibiting α5β1 reduces oxLDL-induced proinflammatory adhesion molecule (VCAM-1) expression in cell culture models. Furthermore, treating atherosclerosis-prone mice with an integrin α5 inhibitor reduces VCAM-1 expression, macrophage content, and plaque burden without perturbing the protective fibrous cap. These findings are of high scientific interest because integrin inhibitors targeting α5 currently in clinical trials for other pathologies should be considered as potential therapeutics for atherosclerosis.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Richard Hynes (Massachusetts Institute of Technology) for providing the integrin α5flox/flox mice, Dr. Kenneth Yamada (National Institute of Dental and Craniofacial Research, NIH) and Dr. Martin Schwartz (Yale University) for providing the 11E5 and 16G3 antibodies, Dr. Martin Humphries (University of Manchester) for providing the SNAKA52 antibody, Dr. Luca Gusella (Mount Sinai Hospital) for providing the retroviral temperature-sensitive large T antigen construct, Dr. Andrew Yurochko (LSUHSC-Shreveport) for providing primary human monocytes, and Dr. Sushil Jain (LSUHSC-Shreveport) for providing THP-1 monocytes.
SOURCES OF FUNDING
This work was supported by the National Institute of Health [R01 HL098435 to A.W.O]; the Louisiana Board of Regents Superior Toxicology Fellowship [LEQSF (2008-13)-FG-20 to A.Y.J.] and the American Heart Association Predoctoral Fellowship [14PRE18660003 to A.Y.J.].
ABBREVIATIONS LIST
- ANOVA
analysis of variance
- apoE
apolipoprotein E
- BM
basement membrane
- CS-1
connecting segment 1
- ERK
extracellular signal-regulated kinase
- FN
fibronectin
- HAEC
human aortic endothelial cells
- HDL
high density lipoprotein
- ICAM-1
intercellular adhesion molecule-1
- IκBα
inhibitor of kappa B alpha
- LDL
low density lipoprotein
- LOX-1
oxidized low density lipoprotein receptor 1
- MAEC
mouse aortic endothelial cells
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- oxLDL
oxidized low density lipoprotein
- oxPAPC
oxidized 1-palmitoyl-2-arachidonyl-sn- glycero-3-phosphorylcholine
- qRT-PCR
quantitative real time PCR
- ROS
reactive oxygen species
- SMA
smooth muscle actin
- SR-IκB
super repressor-inhibitor of kappa B
- TLR4
toll-like receptor 4
- VCAM-1
vascular cell adhesion molecule-1
- vWF
von Willebrand Factor
Footnotes
DISCLOSURES
Dr. Andrew Mazar owns patents on the ATN-161 compound.
REFERENCES
- 1.Goldstein J, Brown M. The LDL receptor. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JA, Territo MC, Wayner E, Carlos TM, Parhami F, Smith CW, Haberland ME, Fogelman AM, Berliner JA. Partial characterization of leukocyte binding molecules on endothelial cells induced by minimally oxidized LDL. Arterioscler Thromb. 1994;14:427–433. doi: 10.1161/01.atv.14.3.427. [DOI] [PubMed] [Google Scholar]
- 3.Xu S, Ogura S, Chen J, Little P, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cellular and molecular life sciences : CMLS. 2012 doi: 10.1007/s00018-012-1194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amberger A, Maczek C, Jurgens G, Michaelis D, Schett G, Trieb K, Eberl T, Jindal S, Xu Q, Wick G. Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones. 1997;2:94–103. doi: 10.1379/1466-1268(1997)002<0094:ceoive>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta J, Sanada N, Hu C, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K-i, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat P, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circulation research. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 6.White S, Sala-Newby G, Newby A. Overexpression of scavenger receptor LOX-1 in endothelial cells promotes atherogenesis in the ApoE(−/−) mouse model. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2011;20:369–373. doi: 10.1016/j.carpath.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orr A, Sanders J, Bevard M, Coleman E, Sarembock I, Schwartz M. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. The Journal of cell biology. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohwedder I, Montanez E, Beckmann K, Bengtsson E, Dunér P, Nilsson J, Soehnlein O, Fässler R. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO molecular medicine. 2012;4:564–576. doi: 10.1002/emmm.201200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurdagul A, Chen J, Funk S, Albert P, Kevil C, Orr A. Altered nitric oxide production mediates matrix-specific PAK2 and NF-κB activation by flow. Molecular biology of the cell. 2013;24:398–408. doi: 10.1091/mbc.E12-07-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang H-Y, Korshunov V, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1074–1079. doi: 10.1161/ATVBAHA.108.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneiderhan W, Schmid-Kotsas A, Zhao J, Grünert A, Nüssler A, Weidenbach H, Menke A, Schmid R, Adler G, Bachem M. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis. Hepatology (Baltimore, Md.) 2001;34:729–737. doi: 10.1053/jhep.2001.27828. [DOI] [PubMed] [Google Scholar]
- 12.Hu C, Dandapat A, Sun L, Chen J, Marwali M, Romeo F, Sawamura T, Mehta J. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovascular research. 2008;79:287–293. doi: 10.1093/cvr/cvn110. [DOI] [PubMed] [Google Scholar]
- 13.Shih P, Elices M, Fang Z, Ugarova T, Strahl D, Territo M, Frank J, Kovach N, Cabanas C, Berliner J, Vora D. Minimally modified low-density lipoprotein induces monocyte adhesion to endothelial connecting segment-1 by activating beta1 integrin. The Journal of clinical investigation. 1999;103:613–625. doi: 10.1172/JCI5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circulation research. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 15.Orr A, Stockton R, Simmers M, Sanders J, Sarembock I, Blackman B, Schwartz M. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. The Journal of cell biology. 2007;176:719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitra S, Deshmukh A, Sachdeva R, Lu J, Mehta JL. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci. 2011;342:135–142. doi: 10.1097/MAJ.0b013e318224a147. [DOI] [PubMed] [Google Scholar]
- 18.Orr A, Ginsberg M, Shattil S, Deckmyn H, Schwartz M. Matrix-specific suppression of integrin activation in shear stress signaling. Molecular biology of the cell. 2006;17:4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nature reviews. Molecular cell biology. 2004;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 20.Khalili P, Arakelian A, Chen G, Plunkett M, Beck I, Parry G, Doñate F, Shaw D, Mazar A, Rabbani S. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Molecular cancer therapeutics. 2006;5:2271–2280. doi: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 21.Cianfrocca M, Kimmel K, Gallo J, Cardoso T, Brown M, Hudes G, Lewis N, Weiner L, Lam G, Brown S, Shaw D, Mazar A, Cohen R. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. British journal of cancer. 2006;94:1621–1626. doi: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livant D, Brabec R, Pienta K, Allen D, Kurachi K, Markwart S, Upadhyaya A. Anti-invasive, antitumorigenic, and antimetastatic activities of the PHSCN sequence in prostate carcinoma. Cancer research. 2000;60:309–320. [PubMed] [Google Scholar]
- 23.Rosenfeld M, Palinski W, Ylä-Herttuala S, Butler S, Witztum J. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis (Dallas, Tex.) 1990;10:336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 24.Goyal T, Mitra S, Khaidakov M, Wang X, Singla S, Ding Z, Liu S, Mehta J. Current Concepts of the Role of Oxidized LDL Receptors in Atherosclerosis. Current atherosclerosis reports. 2012 doi: 10.1007/s11883-012-0228-1. [DOI] [PubMed] [Google Scholar]
- 25.Oorni K, Pentikainen MO, Annila A, Kovanen PT. Oxidation of low density lipoprotein particles decreases their ability to bind to human aortic proteoglycans. Dependence on oxidative modification of the lysine residues. J Biol Chem. 1997;272:21303–21311. doi: 10.1074/jbc.272.34.21303. [DOI] [PubMed] [Google Scholar]
- 26.Abe Y, Fornage M, Yang CY, Bui-Thanh NA, Wise V, Chen HH, Rangaraj G, Ballantyne CM. L5, the most electronegative subfraction of plasma LDL, induces endothelial vascular cell adhesion molecule 1 and CXC chemokines, which mediate mononuclear leukocyte adhesion. Atherosclerosis. 2007;192:56–66. doi: 10.1016/j.atherosclerosis.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Yang JH, Burns AR, Chen HH, Tang D, Walterscheid JP, Suzuki S, Yang CY, Sawamura T, Chen CH. Mediation of electronegative low-density lipoprotein signaling by LOX-1: a possible mechanism of endothelial apoptosis. Circ Res. 2009;104:619–627. doi: 10.1161/CIRCRESAHA.108.190116. [DOI] [PubMed] [Google Scholar]
- 28.Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, Esterson M, Fogelman AM. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85:1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leitinger N, Watson AD, Faull KF, Fogelman AM, Berliner JA. Monocyte binding to endothelial cells induced by oxidized phospholipids present in minimally oxidized low density lipoprotein is inhibited by a platelet activating factor receptor antagonist. Adv Exp Med Biol. 1997;433:379–382. doi: 10.1007/978-1-4899-1810-9_82. [DOI] [PubMed] [Google Scholar]
- 30.Maziere C, Auclair M, Djavaheri-Mergny M, Packer L, Maziere JC. Oxidized low density lipoprotein induces activation of the transcription factor NF kappa B in fibroblasts, endothelial and smooth muscle cells. Biochem Mol Biol Int. 1996;39:1201–1207. doi: 10.1080/15216549600201392. [DOI] [PubMed] [Google Scholar]
- 31.Dwivedi A, Anggard EE, Carrier MJ. Oxidized LDL-mediated monocyte adhesion to endothelial cells does not involve NFkappaB. Biochem Biophys Res Commun. 2001;284:239–244. doi: 10.1006/bbrc.2001.4955. [DOI] [PubMed] [Google Scholar]
- 32.Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99:199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Z, DiMichele LA, Hakim ZS, Rojas M, Mack CP, Taylor JM. Targeted focal adhesion kinase activation in cardiomyocytes protects the heart from ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2012;32:924–933. doi: 10.1161/ATVBAHA.112.245134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petzold T, Orr A, Hahn C, Jhaveri K, Parsons J, Schwartz M. Focal adhesion kinase modulates activation of NF-kappaB by flow in endothelial cells. American journal of physiology. Cell physiology. 2009;297:22. doi: 10.1152/ajpcell.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 36.Al-Fakhri N, Wilhelm J, Hahn M, Heidt M, Hehrlein FW, Endisch AM, Hupp T, Cherian SM, Bobryshev YV, Lord RS, Katz N. Increased expression of disintegrinmetalloproteinases ADAM-15 and ADAM-9 following upregulation of integrins alpha5beta1 and alphavbeta3 in atherosclerosis. J Cell Biochem. 2003;89:808–823. doi: 10.1002/jcb.10550. [DOI] [PubMed] [Google Scholar]
- 37.Cheuk BL, Cheng SW. Differential expression of integrin alpha5beta1 in human abdominal aortic aneurysm and healthy aortic tissues and its significance in pathogenesis. J Surg Res. 2004;118:176–182. doi: 10.1016/S0022-4804(03)00351-2. [DOI] [PubMed] [Google Scholar]
- 38.Cai WJ, Li MB, Wu X, Wu S, Zhu W, Chen D, Luo M, Eitenmuller I, Kampmann A, Schaper J, Schaper W. Activation of the integrins alpha 5beta 1 and alpha v beta 3 and focal adhesion kinase (FAK) during arteriogenesis. Mol Cell Biochem. 2009;322:161–169. doi: 10.1007/s11010-008-9953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickering JG, Chow LH, Li S, Rogers KA, Rocnik EF, Zhong R, Chan BM. alpha5beta1 integrin expression and luminal edge fibronectin matrix assembly by smooth muscle cells after arterial injury. Am J Pathol. 2000;156:453–465. doi: 10.1016/s0002-9440(10)64750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livant DL, Brabec RK, Pienta KJ, Allen DL, Kurachi K, Markwart S, Upadhyaya A. Anti-invasive, antitumorigenic, and antimetastatic activities of the PHSCN sequence in prostate carcinoma. Cancer Res. 2000;60:309–320. [PubMed] [Google Scholar]
- 41.Donate F, Parry GC, Shaked Y, Hensley H, Guan X, Beck I, Tel-Tsur Z, Plunkett ML, Manuia M, Shaw DE, Kerbel RS, Mazar AP. Pharmacology of the novel antiangiogenic peptide ATN-161 (Ac-PHSCN-NH2): observation of a U-shaped dose-response curve in several preclinical models of angiogenesis and tumor growth. Clin Cancer Res. 2008;14:2137–2144. doi: 10.1158/1078-0432.CCR-07-4530. [DOI] [PubMed] [Google Scholar]
- 42.Khalili P, Arakelian A, Chen G, Plunkett ML, Beck I, Parry GC, Donate F, Shaw DE, Mazar AP, Rabbani SA. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Mol Cancer Ther. 2006;5:2271–2280. doi: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 43.Zeng ZZ, Yao H, Staszewski ED, Rockwood KF, Markwart SM, Fay KS, Spalding AC, Livant DL. alpha(5)beta(1) Integrin Ligand PHSRN Induces Invasion and alpha(5) mRNA in Endothelial Cells to Stimulate Angiogenesis. Transl Oncol. 2009;2:8–20. doi: 10.1593/tlo.08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao H, Veine DM, Zeng ZZ, Fay KS, Staszewski ED, Livant DL. Increased potency of the PHSCN dendrimer as an inhibitor of human prostate cancer cell invasion, extravasation, and lung colony formation. Clin Exp Metastasis. 2010;27:173–184. doi: 10.1007/s10585-010-9316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veine DM, Yao H, Stafford DR, Fay KS, Livant DL. A D-amino acid containing peptide as a potent, noncovalent inhibitor of alpha5beta1 integrin in human prostate cancer invasion and lung colonization. Clin Exp Metastasis. 2014 doi: 10.1007/s10585-013-9634-1. [DOI] [PubMed] [Google Scholar]
- 46.Abshire MY, Thomas KS, Owen KA, Bouton AH. Macrophage motility requires distinct alpha5beta1/FAK and alpha4beta1/paxillin signaling events. J Leukoc Biol. 2011;89:251–257. doi: 10.1189/jlb.0710395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vernon-Wilson EF, Aurade F, Brown SB. CD31 promotes beta1 integrin-dependent engulfment of apoptotic Jurkat T lymphocytes opsonized for phagocytosis by fibronectin. J Leukoc Biol. 2006;79:1260–1267. doi: 10.1189/jlb.1005571. [DOI] [PubMed] [Google Scholar]
- 48.Jun HK, Lee SH, Lee HR, Choi BK. Integrin alpha5beta1 activates the NLRP3 inflammasome by direct interaction with a bacterial surface protein. Immunity. 2012;36:755–768. doi: 10.1016/j.immuni.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Orr AW, Hahn C, Blackman BR, Schwartz MA. p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ Res. 2008;103:671–679. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barillari G, Albonici L, Incerpi S, Bogetto L, Pistritto G, Volpi A, Ensoli B, Manzari V. Inflammatory cytokines stimulate vascular smooth muscle cells locomotion and growth by enhancing alpha5beta1 integrin expression and function. Atherosclerosis. 2001;154:377–385. doi: 10.1016/s0021-9150(00)00506-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.