Abstract
Human skeletal aging is characterized as a gradual loss of bone mass due to an excess of bone resorption not balanced by new bone formation. Using human marrow cells, we tested the hypothesis that there is an age-dependent increase in osteoclastogenesis due to intrinsic changes in regulatory factors [macrophage-colony stimulating factor (M-CSF), receptor activator of NF-κB ligand (RANKL), and osteoprotegerin (OPG)] and their receptors [c-fms and RANK]. In bone marrow cells (BMCs), c-fms (r=0.61, p=0.006) and RANK expression (r=0.59, p=0.008) were increased with age (27-82 years, n=19). In vitro generation of osteoclasts was increased with age (r=0.89, p=0.007). In enriched marrow stromal cells (MSCs), constitutive expression of RANKL was increased with age (r=0.41, p=0.049) and expression of OPG was inversely correlated with age (r=-0.43, p=0.039). Accordingly, there was an age-related increase in RANKL/OPG (r=0.56, p=0.005). These data indicate an age-related increase in human osteoclastogenesis that is associated with an intrinsic increase in expression of c-fms and RANK in osteoclast progenitors, and, in the supporting MSCs, an increase in pro-osteoclastogenic RANKL expression and a decrease in anti-osteoclastogenic OPG. These findings support the hypothesis that human marrow cells and their products can contribute to skeletal aging by increasing the generation of bone-resorbing osteoclasts. These findings help to explain underlying molecular mechanisms of progressive bone loss with advancing age in humans.
Keywords: Aging, Osteoclast, Marrow Stromal Cell, RANKL, OPG
In humans, peak bone mass is reached during the third decade, with a subsequent decline in bone mass with age [Riggs et al., 1981; Riggs et al., 1982]. Skeletal aging is characterized as an excess of bone resorption that is not balanced by new bone formation. Although there is much information about sex hormone deficiency and development of osteoporosis, more information is needed about the mechanism(s) by which the aging process influences bone loss. A better understanding of intrinsic age-related changes in human bone cells may offer new approaches to mitigate or avoid skeletal aging.
Osteoclasts are large, multinucleated cells derived from the non-adherent hematopoietic stem cells present in bone marrow [Walker, 1973]. Osteoclast differentiation is regulated locally through three major factors: macrophage-colony stimulating factor (M-CSF), receptor activator of NF-κB ligand (RANKL), and osteoprotegerin (OPG). Binding of M-CSF to the c-fms cell surface receptor on osteoclast progenitors upregulates the expression of RANK, the receptor for RANKL [Arai et al., 1991], which promotes osteoclastogenesis [Lacey et al., 1998]. OPG is a soluble decoy receptor of RANKL and inhibits osteoclastogenesis [Yasuda et al., 1998].
Adherent MSCs have potential to differentiate into multiple cell types, including osteoblasts. Many studies [Mueller and Glowacki, 2001; D'Ippolito et al., 1991; Zhou et al. 2008; Zhou et al., 2011; Geng et al., 2011; Zhou et al., 2012] but not all [Bellantuono et al., 2009] show an age-related decline in osteoblastogenic potential of human MSCs. Studies in mice show an age-related increase in stromal/osteoblastic cell-mediated osteoclastogenesis [Perkins et al., 1994; Kahn et al., 1995; Cao et al., 2005]. Expression of RANKL and M-CSF was increased in marrow stromal cells from older animals, OPG was decreased, and osteoclast formation was greater when stromal/osteoblastic cells from old rather than young donors were used to induce osteoclastogenesis [Cao et al., 2003]. Previous studies with human cells showed an age-dependent increase in constitutive secretion of pro-osteoclastogenic IL-6 and IL-11[Cheleuitte et al., 1995]. The RANK/RANKL/OPG signaling pathway maintains the balance between the activity of osteoblasts and osteoclasts in order to prevent bone loss and ensure normal bone turnover [Trouvin et al., 2010]. In a previous study, OPG expression was significantly lower in hMSCs obtained from subjects older than 65 years of age than from those younger than 55 years [Makhluf et al., 2000]. These studies test the hypothesis that there are age-dependent changes in the expression of osteoclast differentiation factors and receptors in the RANKL/RANK/OPG and M-CSF/c-fms pathways in human marrow cells.
Materials and Methods
Preparation of Low-Density Bone Marrow Cells
Bone marrow samples were obtained with IRB approval and annual review as femoral tissue discarded during total hip replacement for osteoarthritis [Zhou et al., 2008]. Criteria for exclusion were rheumatoid arthritis, cancer, and other comorbid conditions that could affect skeletal metabolism including renal insufficiency, alcoholism, active liver disease, malabsorption, hyperthyroidism, ankylosing spondylitis, aseptic necrosis, hyperparathyroidism, morbid obesity, and diabetes. Also excluded were patients who were taking medications that may influence skeletal metabolism (e.g. thyroid hormone, glucocorticoids, NSAIDs, and bisphosphonates). There were equal numbers of men and women in these studies. Young was defined as < 50 years of age; older was defined as > 55 years of age [Zhou et al., 2008]. Low-density mononuclear bone marrow cells (BMCs) from 9 young (mean 44.2 ± 7.2 years) and 10 older (mean 70.8 ± 9.3) subjects (age range, 27 to 82) were isolated by density centrifugation with Ficoll/Histopaque 1077 (GE Healthcare, Piscataway, NJ) [Zhou et al., 2008]. This procedure sediments dense differentiated cells, such as erythrocytes, granulocytes, and macrophages [Böyum, 1968] and enriches for undifferentiated mononuclear cells. Total RNA from 10×106 freshly isolated BMCs was extracted with Trizol reagent (Invitrogen) and 2 μg was reverse-transcribed into cDNA with M-MLV (Promega). Constitutive expression of c-fms [Kirma et al., 2007], RANK [Atkins et al., 2000], and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was measured by semi-quantitative reverse-transcriptase PCR (RT-PCR). One-twentieth of the cDNA was used in each 50 μl PCR reaction with conditions optimized to reflect the exponential phase of amplification (Table 1). PCR products were separated on 2% agarose gel electrophoresis, quantified by densitometry of captured gel images with KODAK Gel Logic 200 Imaging System and KODAK Molecular Imaging Software (New Haven, CT). Gene expression levels were normalized to the densitometric units of the internal control GAPDH.
Table 1. Primer pairs and amplification conditions.
| Target mRNA | Primer sequences (5′ → 3′) | Annealing temperature (°C) | Product size (bp) | Reference |
|---|---|---|---|---|
| GAPDH | F: ACCACAGTCCATGCCATCAC R: TCCACCACCCTGTTGCTGTA |
60 | 452 | Clontech, BC004109.1 |
| OPG | F: GAACCCCAGAGCGAAATACA R: CGCTGTTTTCACAGAGGTCA |
60 | 441 | Makhluf et al., 2000 |
| c-fms | F: CAGATTGGTATAGTCCCGCTCTCT R: TCCAACTACATTGTCAAGGGCAAT |
60 | 360 | Kirma et al., 2007 |
| RANK | F: CCTACGCACAAGGCGAAGATGC R: CGTAGACCACGATGATGTCGCC |
62 | 702 | Atkins et al., 2000 |
| TRAP | F: CTGGCTGATGGTGCCACCCCTG R: CTCTCAGGCTGCAGGCTGAGG |
61 | 469 | Eslami et al., 2011 |
| RANKL | F: ATCCCATCTGGTTCCCATAA R: CCCTGACCAATACTTGGTGC |
55 | 276 | Eslami et al., 2011 |
| M-CSF | F: ATGACAGACAGGTGGAACTGCCAGTGTAGAGG R: TCACACAACTTCAGTAGGTTCAGGTGATGGGC |
60 | 495 | Clonetech, XM_002150.2 |
Culture of Low-Density Bone Marrow Cells for Osteoclast Differentiation
Low-density mononuclear BMCs from 8 young (mean 44.5 ± 4.4 years) and 7 older (mean 67.6 ± 9.7 years) subjects (range, 36 to 84 years) were cultured for in vitro generation of osteoclasts. BMCs were cultured in 60-mm dishes (seeding density 1.43 × 106 cells/cm2) with phenol red-free α-MEM medium (Gibco), 10% Fetal Bovine Serum-heat inactivated (FBS-HI) (Invitrogen), 100 U/ml penicillin, 0.1 μM streptomycin (Gibco), and 0.5 μM fungizone. Half of the medium was changed twice each week with care to preserve the cell-cell interaction between adherent and non-adherent cells. With some samples, total RNA was isolated for expression of the osteoclast-specific marker, tartrate-resistant acid phosphatase (TRAP) [Atkins et al., 2000; Eslami et al., 2011]. Other cultures were cytochemically stained for TRAP [Eslami et al., 2011]; TRAP-positive multinucleated cells with 3 or more nuclei were counted without knowledge of sample identity. In a validation study, TRAP gene expression was shown to be correlated with expression of cathepsin-K and with number of osteoclasts generated in vitro [Eslami et al., 2011].
In other experiments, to test for responsiveness of in vitro osteoclastogenesis to exogenous regulators, recombinant human soluble RANKL (PreproTech, Rocky Hill, NJ) was added with each change of medium in BMC cultures from 3 younger subjects (mean age 46.7 ± 4.2 years). Recombinant human OPG (PreproTech) was added to cultures from 8 older subjects (mean 67.0 ± 7.0). Expression of TRAP is presented as mean ± S.D.
Preparation of Human Marrow Stromal Cells (MSCs)
Aliquots of 30×106 mononuclear cells isolated from 13 young (mean 44.6 ± 4.5 years) and 10 older (mean 68.6 ± 10.4 years) subjects were seeded in 100-mm tissue culture dishes. Non-adherent cells were removed at 18 hrs and adherent MSCs were expanded to passage 2. Upon near-confluence, RNA was isolated and assayed for constitutive expression of RANKL [Eslami et al., 2011], M-CSF [Eslami et al., 2011], OPG [Makhluf et al., 2000] and GAPDH (Table 1).
Statistical Analyses
Group data are presented as mean values ± standard deviation. Quantitative data were analyzed with Mann-Whitney test, Spearman correlation test, or paired t-test when appropriate. Because subanalyses did not detect gender differences in any of the studies, data for cells from men and women were pooled. A value of p<0.05 was considered significant. If the p value was higher than 0.05 but less than 0.10, it was described as a trend.
Results
Effects of Subject Age on Receptors c-fms and Rank in Bone Marrow Cells
Constitutive expression of c-fms in freshly isolated BMCs was correlated with age (Fig. 1A, r=0.61, p=0.006); the amount of c-fms mRNA was 1.7-fold greater in BMCs from the older subjects (0.67 ± 0.23) than for the younger subjects (0.40 ± 0.16, p=0.017). Constitutive RANK expression was also correlated with age (r=0.59, p=0.008); the amount of RANK mRNA was 1.9-fold greater in BMCs from the older subjects (0.61 ± 0.32) than for the younger subjects (0.32 ± 0.36, p=0.028). Further, there was a strong positive correlation between RANK and c-fms expression (r=0.85, p<0.0001; Fig. 1B).
Fig. 1.
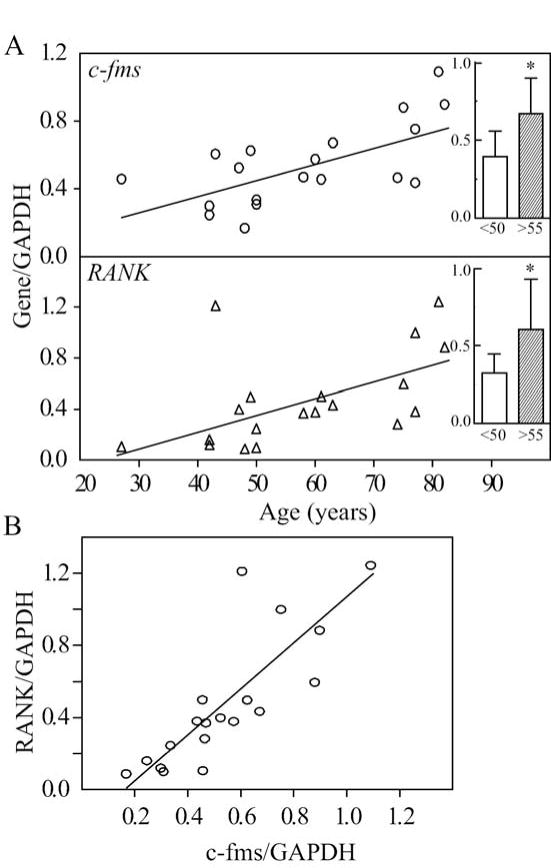
Effects of subject age on constitutive expression of c-fms and RANK in low-density mononuclear bone marrow cells (BMCs) isolated from 19 subjects (age range, 27 to 82 years). (A) There were age-dependent increases in expression of c-fms (Spearman r=0.61, p=0.006) and RANK (r=0.59, p=0.008) in BMCs. Expression of c-fms and RANK was 1.7-fold (*p=0.017) and 1.9-fold (*p=0.028) higher, respectively, in BMCs from the older subjects (inset, shaded bars, >55 years, n=10) than for the younger subjects (open bars, <50 years, n=9). (B) There was a correlation between RANK and c-fms gene expression (r=0.85, p<0.0001).
Effects of Subject Age on In Vitro Differentiation of Osteoclasts
In vitro osteoclastogenesis was assessed in a series of BMC cultures by enumeration of TRAP-positive multinucleated cells (Fig. 2A). Osteoclastogenesis was correlated with age (Spearman r=0.89, p=0.007, Fig. 2B); the number of osteoclasts generated in cultures of cells from the older subjects (939 ± 124) was 1.9-fold higher than for the younger group (489 ± 145, p=0.029, Fig. 2B, inset). Osteoclastogenesis was also monitored by expression of TRAP and was correlated with age (r=0.75, p=0.037) (Fig. 2C); expression of TRAP was 1.3-fold higher in BMC cultures from older subjects (1.08 ± 0.06) than for younger subjects (0.84 ± 0.15, p=0.029, Fig. 2C, inset). The number of in vitro-generated osteoclasts was correlated with expression of TRAP (r=0.74, p=0.046).
Fig. 2.
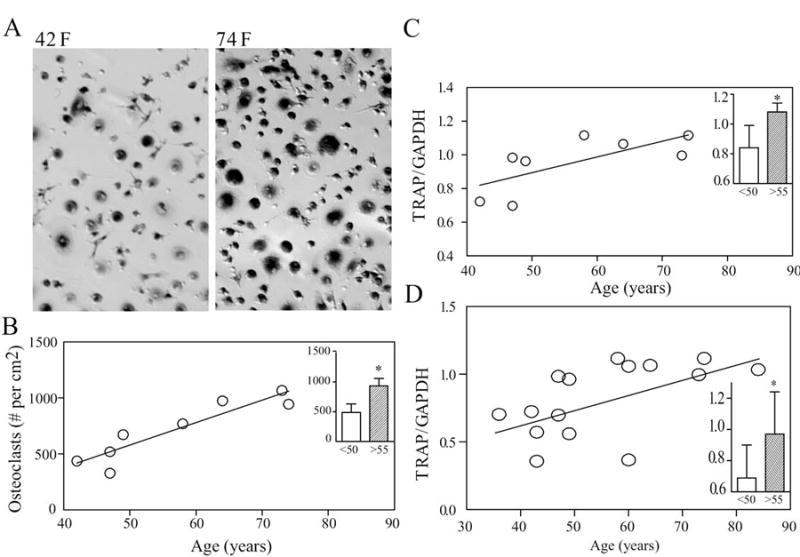
Effect of subject age on in vitro osteoclastogenesis in cultures of BMCs after 14 days. (A) Photomicrographs show TRAP-positive multinucleated cells in BMC cultures from a 74-year-old female subject (right), compared with a 42-year-old female subject (left). (B) The number of generated osteoclasts was positively correlated with age of subject (r=0.89, p=0.007, n=8). The number of osteoclasts was 1.9-fold (p=0.029) higher in BMC cultures from the older subjects (inset, shaded bar, n=4) than for the younger subjects (open bar, n=4). (C) Expression of TRAP in a series of samples was correlated with age (r=0.75, p=0.037, n=8). TRAP gene expression was 1.3-fold (p=0.029) higher in BMC cultures from the older subjects (inset, shaded bar, n=4) than for the younger subjects (open bar, n=4). (D) For a series of 15 different samples, TRAP mRNA was correlated with the age of subjects (r=0.60, p=0.019). TRAP gene expression was 1.4-fold (p=0.014) higher in older subjects (inset, shaded bar, n=8) than for the younger subjects (inset, open bar, n=7).
Expression of TRAP in a second series of 15 samples was also correlated with age (r=0.60, p=0.019, Fig. 2D); expression of TRAP was 1.4-fold higher in BMC cultures from older subjects (0.97 ± 0.27) than for younger subjects (0.69 ± 0.21, p=0.014, Fig. 2D, inset).
In Vitro Effects of Recombinant OPG and RANKL
To assess the responsiveness of in vitro osteoclastogenesis to exogenous regulatory factors, we tested for the expected effects of a range of doses of RANKL on osteoclastogenesis with BMCs from young subjects and the effects of a range of doses of OPG on cells from older subjects. With BMCs from 3 subjects between 42 to 50 years of age, addition of 10 ng/mL RANKL increased TRAP expression by 20.2 ± 12.7% (Fig. 3A, p=0.044), and 25 ng/mL RANKL increased TRAP expression by 29.4 ± 12.3 % (p=0.041). With cells from 8 subjects between 58 and 79 years of age, addition of 1 ng/mL OPG inhibited TRAP expression to an average 93.2% (Fig. 3B, p=0.035) and addition of 10 ng/mL OPG inhibited TRAP expression to an average 87.4% relative to each control (p=0.004).
Fig. 3.
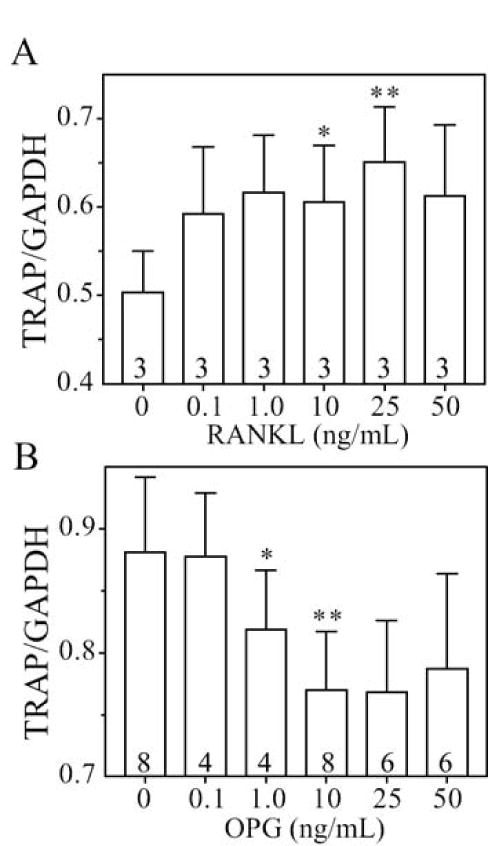
Effects of exogenous factors on osteoclast differentiation in BMC cultures from young or old subjects. (A) Recombinant human RANKL stimulated osteoclast differentiation in BMC cultures from 3 subjects younger than 50 years. Bars represent the mean values ± S.D. *p=0.044; **p=0.041 (B) Recombinant human OPG inhibited osteoclast differentiation in BMC cultures from 4 to 8 subjects older than 55 years. Bars represent the mean values of 4-8 specimens. *p=0.035; **p=0.004
Effects of Subject Age on RANKL and OPG in Human Marrow Stromal Cells
We tested whether subject age influences constitutive expression of osteoclastogenic regulatory factors in stromal cells. Constitutive expression of pro-osteoclastogenic RANKL in hMSCs was correlated with age (Fig. 4A, r=0.41, p=0.049); there was a trend of 2.3-fold higher expression of RANKL in MSCs from older subjects (0.35 ± 0.27) than for younger subjects (0.15 ± 0.11, p=0.072). Constitutive expression of anti-osteoclastogenic OPG in hMSCs was inversely correlated with age (r=-0.43, p=0.039); there was a trend for 1.4-fold higher expression of OPG in younger subjects (0.73 ± 0.28) than that in older group (0.55 ± 0.19, p=0.088). There was an age-related increase in the ratio of RANKL/OPG (r=0.56, p=0.005) (Fig. 4B). M-CSF expression did not appear to vary with age in this series.
Fig. 4.
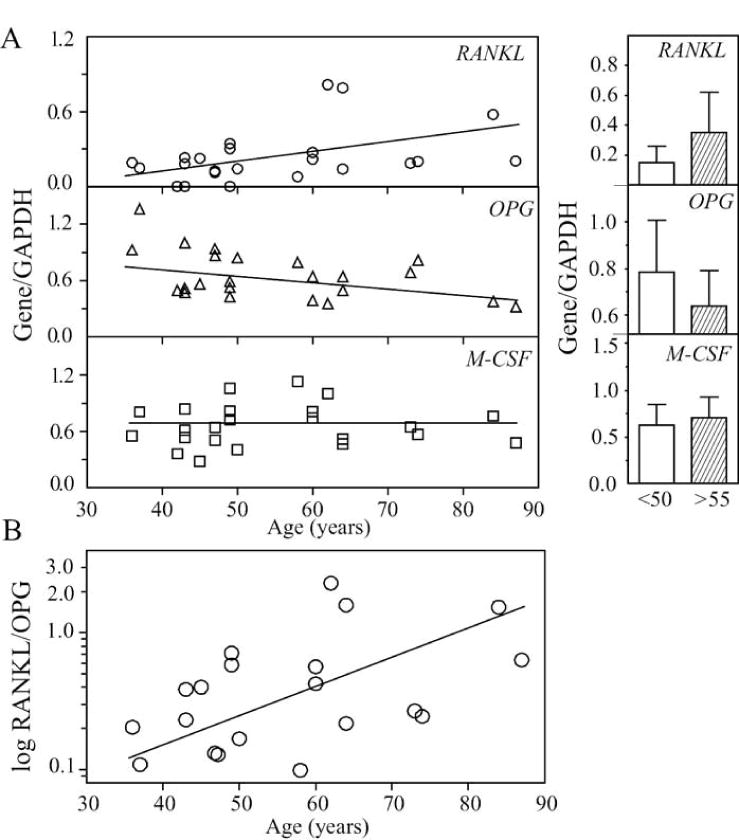
Effects of subject age on constitutive expression of osteoclast differentiation factors in hMSCs. (A) With the age of subjects (n=23), there was a positive correlation for RANKL expression (r=0.41, p=0.049) and an inverse correlation for OPG (r=-0.43, p=0.039). There was a trend of 2.3-fold (p=0.072) higher expression of RANKL in hMSCs from the older subjects (shaded bar, n=10) than for the younger subjects (open bar, n=13) and a trend of 1.4-fold (p=0.088) higher expression of OPG in hMSCs from the younger subjects (open bar, n=13) than that for the older subjects (shaded bar, n=10). M-CSF expression did not appear to vary with age. (B) The ratio of RANKL/OPG was significantly correlated with the age of subject (r=0.56, p=0.005).
Discussion
These studies show an age-related increase in in vitro osteoclastogenesis with human marrow cells. Further, they show that constitutive expression of multiple osteoclast regulatory factors and receptors are influenced by age (age range 27 to 87 years). The addition of exogenous osteoclastogenic factors was not required for osteoclast formation in this study because high seeding density and presence of supporting stromal cells and their products facilitate in vitro osteoclast differentiation, compared with other human or murine cell methods [Koshihar et al., 2002; MacDonald et al., 1987; Thavarajah et al., 1991]. Koshihara et al. studied marrow cells from elderly women with hip fracture (age range 64-96), using 20% selected horse serum and adding 10-8 M 1α,25(OH)2D3 [Koshihar et al., 2002]; those stimuli may have been needed because of the lower seeding density in their experiments (0.5 × 106/cm2) than in ours (1.43 × 106/cm2). Many studies with murine marrow require exogenous growth factors, such as RANKL or M-CSF, to stimulate osteoclast formation [Kahn et al., 1995; Udagawa et al., 1999]. Using supplements and selected sera may confound assessment of constitutive osteoclastogenesis, a goal of this investigation. Phenol red-free growth medium was used to avoid the indicator's estrogenic effects [Berthois et al., 1986]. Osteoclast differentiation in BMC cultures was the result of interactions between osteoclast progenitors in the non-adherent fractions and regulatory factors produced by the adherent stromal cells. As evidence of clinical relevance of studies with human marrow cultures, Koshihara et al. found an inverse correlation between bone mineral density of the spine and in vitro osteoclastogensis [Koshihara et al., 2002].
To validate the responsiveness of the culture system to exogenous factors, we added recombinant OPG and soluble RANKL to BMC cultures from older and younger subjects, respectively. The inhibition of osteoclastogenesis by exogenous recombinant OPG was expected [Dunn et al., 2007] and suggests in vitro rejuvenation of old marrow cells. Stimulation of osteoclast differentiation by RANKL with cells from younger subjects is consistent with other studies [Matsuzaki et al., 1998].
Availability of a wide range of age of subjects undergoing orthopedic surgery with discarded marrow made possible these analyses of the effects of age on osteoclastogenesis regulatory factors. Age had multiple effects on constitutive expression of receptors and endogenous regulatory factors. Age-related increases in the expression of both c-fms and RANK may indicate an age-dependent increase in the number osteoclast progenitors and/or the number of receptors per cell, which would increase their responsiveness to M-CSF and RANKL, as suggested in mice [Cao et al., 2005]. A correlation between c-fms and RANK expression is consistent with mouse data that binding of M-CSF to c-fms up-regulates the expression RANK receptors on the cell surface and in turn increases the response of osteoclast progenitors to RANKL [Arai et al., 1991].
In addition to the intrinsic differences in expression of the receptors c-fms and RANK, the age-related increase in in vitro osteoclastogenesis may be explained by changes in MSCs and their regulation of osteoclast differentiation. Human MSCs showed an age-related increase in pro-osteoclastogenic RANKL and a decrease in anti-osteoclastogenic OPG. These findings are consistent with those in mice [Cao et al., 2005], but, unlike in mouse cells, age-related effects on M-CSF expression were not observed in these human MSCs. Jiang et al. detected elevated RANKL expression in fresh whole marrow aspirates from older subjects, but did not provide data on OPG or on the receptors [Jiang et al., 2008]. Our data indicate a correlation between age and the ratio of RANKL to OPG. The ratio of RANKL to OPG may be a key determinant for the levels of osteoclastogenesis and bone resorption. Abdallah et al. showed that the ratio of RANKL/OPG was significantly higher in iliac bone biopsies from women with hip fractures than for controls and was associated with increased fracture susceptibility [Abdallah et al., 2005]. In a comparison of marrow cells from postmenopausal women, we reported that the RANKL/OPG ratio in cells from women taking the anti-osteoporosis drug alendronate at the time of marrow procurement was 52% of that for age-matched control women [Eslami et al., 2011]. That difference in the RANKL/OPG ratios indicates that the marrow cells retain effects of in vivo alendronate therapy and that alendronate acts to suppress osteoclast differentiation. Gori et al. reported that support of human osteoclastogenesis by stromal-osteoblast lineage cells is developmentally regulated; undifferentiated human MSCs expressed a high RANKL/OPG ratio to initiate and support osteoclast formation, but as in vitro differentiation to mature osteoblasts occurred, the RANKL/OPG ratio decreased and osteoclast formation was not supported [Gori et al., 2000]. Giner et al. found the same decline in RANKL/OPG as osteoblast cultures matured [Giner et al., 2008].
Two recent studies demonstrated that for mice, osteocytes, rather than osteoblasts or MSCs, are the major source of RANKL for osteoclast formation [Nakashima et al., 2011; Xiong et al., 2011]. There is as yet no information about relative effects of human osteocytes in regulating osteoclast differentiation. An important question related to skeletal aging is whether and how osteocytes could contribute to age-related oseoclastogenesis [Matsuo, 2012] in view of the fact that aging is associated with fewer osteocytes in bone for both humans [Busse et al., 2010] and mice [Manolagas and Parfitt, 2010]. There is some uncertainty regarding humans and mice and age-related changes in bone remodeling. Studies in mice indicate that cancellous bone remodeling either declines [Almeida et al., 2007; Shahnazari et al., 2012] or increases [Cao et al., 2005]. Whereas the effects of age on bone remodeling have been relatively well studied in women, there have been few studies in men. In women, advancing age is associated with a decline in periosteal bone formation, a decline in the volume of bone formed by each basic multicellular unit (BMU), continued resorption by each BMU, and high remodeling after menopause [Seeman, 2008]. Available data indicate that loss of trabecular bone is predominantly due to decreased formation at the level of individual bone remodeling units and that an increase in remodeling rate does not play a major role in men [Compston, 2011].
Age-related intrinsic changes in human MSCs described here for expression of RANKL and OPG add to previously reported age-related increases in other pro-osteoclastogenic cytokines interleukin 6 [Cheleuitte et al., 1995; Koshihara et al., 2002], interleukin 11[Cheleuitte et al., 1995], and prostaglandin E2 [Koshihara et al., 2002]. Human osteoblasts also show effects of age; osteoblast from elders expressed more IL-6 and less OPG than cells from young subjects [Eriksen et al., 2010]. Taken together with the finding of an age-related decrease in proliferation and osteoblast differentiation in hMSCs [Zhou et al., 2008], these findings support the hypothesis that human marrow cells and their products can contribute to skeletal aging by increasing the generation of bone-resorbing osteoclasts and by decreasing the renewal of bone-forming osteoblasts. These findings help to explain underlying molecular mechanisms of progressive bone loss with advancing age in humans.
In conclusion, these studies show an age-related increase in human osteoclast differentiation, an increase in expression of c-fms and RANK in osteoclast progenitor cells, as well as an increase in constitutive expression of pro-osteoclastogenic RANKL and decrease in anti-osteoclastogenic OPG by human MSCs. Age-related changes in these multiple ligands and receptors provide compounding mechanisms for increased bone resorption. The RANKL/OPG ratio in hMSCs may be an important age-related regulator of osteoclast differentiation. Taken together with the finding of an age-related decrease in proliferation and osteoblast differentiation in hMSCs, these findings support the hypothesis that human marrow cells and their products can contribute to skeletal aging by increasing the generation of bone-resorbing osteoclasts and by decreasing the renewal of bone-forming osteoblasts. These findings help to explain underlying molecular mechanisms of progressive bone loss with advancing age in humans.
Acknowledgments
The authors have no conflicts of interest regarding this research. This work was presented at the 2012 Meeting of the Orthopedic Research Society. This work was conducted in partial fulfillment for the D.M.Sc. in Oral Biology and for Certification in Orthodontics from the Harvard School of Dental Medicine. The authors appreciate the assistance of Sara Anderson and Dr. Shuichi Mizuno.
Grant sponsor: NIH grants AG025015 and AG028114.
References
- Abdallah BM, Stilgren LS, Nissen N, Kassem M, Jørgensen HR, Abrahamsen B. Increased RANKL/OPG mRNA ratio in iliac bone biopsies from women with hip fractures. Calcif Tissue Int. 2005;76:90–97. doi: 10.1007/s00223-004-0074-4. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins GJ, Haynes DR, Graves SE, Evdokiou A, Hay S, Bouralexis S, Findlay DM. Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res. 2000;15:640–649. doi: 10.1359/jbmr.2000.15.4.640. [DOI] [PubMed] [Google Scholar]
- Bellantuono I, Aldahmash A, Kassem M. Aging of marrow stromal (skeletal) stem cells and their contribution to age-related bone loss. Biochim Biophys Acta. 2009;1792:364–370. doi: 10.1016/j.bbadis.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red is tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Busse B, Djonic D, Milovanovic P, Hahn M, Püschel K, Ritchie RO, Djuric M, Amling M. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9:1065–1075. doi: 10.1111/j.1474-9726.2010.00633.x. [DOI] [PubMed] [Google Scholar]
- Cao J, Venton L, Sakata T, Halloran BP. Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J Bone Miner Res. 2003;18:270–277. doi: 10.1359/jbmr.2003.18.2.270. [DOI] [PubMed] [Google Scholar]
- Cao J, Wronski TJ, Iwaniec U, Phleger L, Kurimoto P, Boudignon B, Halloran BP. Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J Bone Miner Res. 2005;20:1659–1668. doi: 10.1359/JBMR.050503. [DOI] [PubMed] [Google Scholar]
- Cheleuitte D, Mizuno S, Glowacki J. In vitro secretion of cytokines by human bone marrow: effects of age and estrogen status. J Clin Endocrinol Metab. 1995;83:2043–2051. doi: 10.1210/jcem.83.6.4848. [DOI] [PubMed] [Google Scholar]
- Compston J. Age-related changes in bone remodelling and structure in men: histomorphometric studies. J Osteoporos. 2011;2011:108324. doi: 10.4061/2011/108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- Dunn MD, Park CH, Kostenuik PJ, Kapila S, Giannobile WV. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone. 2007;41:446–455. doi: 10.1016/j.bone.2007.04.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen CG, Olsen H, Husted LB, Sørensen L, Carstens M, Søballe K, Langdahl BL. The expression of IL-6 by osteoblasts is increased in healthy elderly individuals: Stimulated proliferation and differentiation are unaffected by age. Calcif Tissue Int. 2010;87:414–423. doi: 10.1007/s00223-010-9412-x. [DOI] [PubMed] [Google Scholar]
- Eslami B, Zhou S, Van Eekeren I, LeBoff MS, Glowacki J. Reduced osteoclastogenesis and RANKL expression in marrow from women taking alendronate. Calcified Tissue Intl. 2011;88:272–280. doi: 10.1007/s00223-011-9473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S, Zhou S, Glowacki J. Age-related decline in osteoblastogenesis and1α-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone. Aging Cell. 2011;10:962–971. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner M, Montoya MJ, Vázquez MA, Rios MJ, Moruno R, Miranda MJ, Pérez-Cano R. Modifying RANKL/OPG mRNA expression in differentiating and growing human primary osteoblasts. Horm Metab Res. 2008;40:869–874. doi: 10.1055/s-0028-1082083. [DOI] [PubMed] [Google Scholar]
- Glowacki J. Influence of age on human marrow. Calcif Tissue Int. 1995;56:S50–51. [Google Scholar]
- Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology. 2000;141:4768–4776. doi: 10.1210/endo.141.12.7840. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Mishima H, Sakai S, Liu YK, Ohyabu Y, Uemura T. Gene expression analysis of major lineage-defining factors in human bone marrow cells: effect of aging, gender, and age-related disorders. J Orthop Res. 2008;26:910–917. doi: 10.1002/jor.20623. [DOI] [PubMed] [Google Scholar]
- Kahn A, Gibbons R, Perkins S, Gazit D. Age-related bone loss. A hypothesis and initial assessment in mice. Clin Orthop Relat Res. 1995;313:69–75. [PubMed] [Google Scholar]
- Kirma N, Hammes LS, Liu YG, Nair HB, Valente PT, Kumar S, Flowers LC, Tekmal RR. Elevated expression of the oncogene c-fms and its ligand, the macrophage colony-stimulating factor-1, in cervical cancer and the role of transforming growth factor-beta1 in inducing c-fms expression. Cancer Res. 2007;67:1918–1926. doi: 10.1158/0008-5472.CAN-06-1991. [DOI] [PubMed] [Google Scholar]
- Koshihara Y, Suematsu A, Feng D, Okawara R, Ishibashi H, Yamamoto S. Osteoclastogenic potential of bone marrow cells increases with age in elderly women with fracture. Mech Ageing Dev. 2002;123:1321–1331. doi: 10.1016/s0047-6374(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- MacDonald BR, Takahashi N, McManus LM, Holahan J, Mundy GR, Roodman GD. Formation of multinucleated cells that respond to osteotropic hormones in long term human bone marrow cultures. Endocrinology. 1987;120:2326–2333. doi: 10.1210/endo-120-6-2326. [DOI] [PubMed] [Google Scholar]
- Makhluf HA, Mueller SM, Mizuno S, Glowacki J. Age-related decline in osteoprotegerin expression by human bone marrow cells cultured in three-dimensional collagen sponges. Biochem Biophys Res Commun. 2000;268:669–672. doi: 10.1006/bbrc.2000.2182. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K, Udagawa N, Takahashi N, Yamaguchi K, Yasuda H, Shima N, Morinaga T, Toyama Y, Yabe Y, Higashio K, Suda T. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998;246:199–204. doi: 10.1006/bbrc.1998.8586. [DOI] [PubMed] [Google Scholar]
- Matsuo K. Osteocytes communicate with osteoclast lineage cells via RANKL. IBMS BoneKEy. 2012;9 Article number: 39. [Google Scholar]
- Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- Perkins SL, Gibbons R, Kling S, Kahn AJ. Age-related bone loss in mice is associated with an increased osteoclast progenitor pool. Bone. 1994;15:65–72. doi: 10.1016/8756-3282(94)90893-1. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton LJ., 3rd Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest. 1981;67:328–335. doi: 10.1172/JCI110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Wahner HW, Seeman E, Offord KP, Dunn WL, Mazess RB, Johnson KA, Melton LJ., 3rd Changes in bone mineral density of the proximal femur and spine with aging. Differences between the postmenopausal and senile osteoporosis syndromes. J Clin Invest. 1982;70:716–723. doi: 10.1172/JCI110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E. Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology (Oxford) 2008;47(Suppl 4):iv2–8. doi: 10.1093/rheumatology/ken177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnazari M, Dwyer D, Chu V, Asuncion F, Stolina M, Ominsky M, Kostenuik P, Halloran B. Bone turnover markers in peripheral blood and marrow plasma reflect trabecular bone loss but not endocortical expansion in aging mice. Bone. 2012;50:628–637. doi: 10.1016/j.bone.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Thavarajah M, Evans D, Kanis JA. 1,25(OH)2D3 induces differentiation of osteoclast-like cells from human bone marrow cultures. Biochem Biophys Res Commun. 1991;176:1189–1195. doi: 10.1016/0006-291x(91)90411-y. [DOI] [PubMed] [Google Scholar]
- Trouvin AP, Goëb V. Receptor activator of nuclear factor-κB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging. 2010;5:345–354. doi: 10.2147/CIA.S10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E, Higashio K, Gillespie MT, Martin TJ, Suda T. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone. 1999;25:517–523. doi: 10.1016/s8756-3282(99)00210-0. [DOI] [PubMed] [Google Scholar]
- Walker DG. Osteopetrosis cured by temporary parabiosis. Science. 1973;180:875. doi: 10.1126/science.180.4088.875. [DOI] [PubMed] [Google Scholar]
- Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Greeberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Bueno EM, Kim SW, Amato I, Shen L, Hahne J, Bleiberg I, Morley P, Glowacki J. Effects of age on parathyroid hormone signaling in human marrow stromal cells. Aging Cell. 2011;10:780–788. doi: 10.1111/j.1474-9726.2011.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Glowacki J, Kim SW, Hahne J, Geng S, Mueller SM, Shen L, Bleiberg I, LeBoff MS. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D3 in human marrow stromal cells. J Bone Miner Res. 2012;27:1992–2000. doi: 10.1002/jbmr.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]


