Abstract
Objectives
The aim of this study was to determine the association of SCN5A cardiac sodium (Na+) channel mRNA splice variants in white blood cells (WBCs) with risk of arrhythmias in heart failure (HF).
Background
HF is associated with upregulation of two cardiac SCN5A mRNA splice variants. that encode prematurely truncated, nonfunctional Na+ channels. Since circulating WBCs demonstrate similar SCN5A splicing patterns, we hypothesized that these WBC-derived splice variants might further stratify HF patients at risk for arrhythmias.
Methods
Simultaneously obtained myocardial core samples and WBCs were compared for SCN5A variants C (VC) and D (VD). Circulating variant levels were compared between HF patients divided into three groups: HF without an implantable cardioverter-defibrillator (ICD), HF with an ICD without appropriate intervention, and HF with an ICD with appropriate intervention.
Results
Myocardial tissue-derived SCN5A variant expression levels strongly correlated with circulating WBC samples for both VC and VD variants (r = 0.78 and 0.75, respectively). After controlling for covariates, HF patients who had received an appropriate ICD intervention had higher expression levels of both WBC-derived SCN5A variants compared to HF patients with ICDs who had not (OR= 3.25 (95% CI 1.64–6.45; p=0.001)). Receiver operating characteristics analysis revealed that circulating SCN5A variants levels were highly associated with the risk for appropriate ICD intervention (area under the curve ≥ 0.97).
Conclusions
Circulating expression levels of SCN5A variants were strongly associated with myocardial tissue levels. Furthermore, circulating variant levels were correlative with arrhythmic risk as measured by ICD events in a HF population within one year.
Keywords: sudden death, sodium channel, blood test
Introduction
Heart failure (HF) represents a growing global health care concern. HF is increasing in prevalence, and up to half of all HF patients suffer arrhythmic sudden death (1, 2). Currently, placement of an implanted cardioverter-defibrillator (ICD) is an established interventional therapy to decrease the risk of arrhythmia-related sudden death in HF patients. Both the American College of Cardiology and the American Heart Association endorse the placement of ICDs to reduce total mortality as part of their national guidelines for primary prevention of sudden cardiac death in high-risk HF subjects (3). Based on the criteria for determining “high risk” by these guidelines, however, up to 70% of patients who receive an ICD never suffer from a malignant arrhythmia (4, 5), and somewhere between 15 and 40% of patients who are eligible for an ICD never receive one (6). Moreover, the majority of sudden deaths occur in HF patients that do not meet the current criteria for ICD implantation (7–9). These data suggest that current risk stratification using markers such as left ventricular ejection fraction alone is suboptimal (10). Other methods employed for risk stratification include signal averaged electrocardiogram (sensitivity 62.4% and specificity 77.4% at 2 years) (11), T-wave alternans (sensitivity 74% and specificity 44% at 1 year) (12), and invasive electrophysiological testing (sensitivity 62% and specificity 62% at 1 year) (11), techniques which are not widely employed given poor accuracy, as well as equipment and personnel costs required for implementation. In addition, while risk may change over time, these more demanding techniques are often limited to a single assessment per patient. Therefore, there is an unmet need for a convenient, inexpensive, and non-invasive test to stratify risk for sudden cardiac death and arrhythmias in the HF population.
Alternative mRNA splicing is a post-transcriptional mechanism that can change substantially the pattern of gene expression by creating a variety of gene products from a single DNA message. Up to 95% of multi-exon human genes have alternative spliced forms, suggesting that alternative splicing is one of the most significant components of the functional complexity of the human genome (13, 14). We previously reported that both angiotensin II and hypoxia, signals common to HF, increase two myocardial splicing factors, RBM25 and associated factor LUC7L3 (15, 16). The activated RMB25/LUC7L3 splicing complex increases SCN5A C (VC) and D (VD) variants, decreases the full-length SCN5A transcript and protein, and decreases Na+ current (17). Interestingly, HF results in Na+ current reductions in the range of those seen in Brugada Syndrome, an inherited arrhythmogenic condition at high risk for sudden cardiac death (16, 18). SCN5A variants result from splicing at cryptic splice sequences in the terminal exon of SCN5A (exon 28) (16, 19). SCN5A variants are shorter and encode prematurely truncated, nonfunctional Na+ channel proteins missing part of the C terminus and can represent >50% of the SCN5A transcripts during HF (16, 19). A mouse model of this degree of variant expression showed an 80% reduction in cardiac Na+ current, a significant reduction in myocardial conduction velocity, and an increase in arrhythmic risk (19).
SCN5A transcripts and variants have been noted in circulating white blood cells (WBCs) (16, 19). Circulating molecular biomarkers are attractive given the convenience and comfort of access compared to more traditional methods. Therefore, we sought to determine the association of expression levels of SCN5A cardiac sodium (Na+) channel mRNA splice variants in white blood cells (WBCs) with risk fof arrhythmias in heart failure (HF).
Methods
Correlation of cardiac tissue and circulating levels of VC and VD variants
Simultaneous human blood and heart tissue were obtained with an IRB-approved protocol (2009-0881) at Christ Advocate Hospital from patients undergoing left ventricular assist device implantation. These patients were not included in the clinical trial. The characteristics of these patients are presented in Table 1.
Table 1.
Clinical characteristics of patients used for correlation of SCN5A variants between myocardial tissue and blood
| Subject ID | Age | Gender | RACE | Ischemic cardiomyopathy |
EF | WBC Count |
|---|---|---|---|---|---|---|
| Tissue-1 | 65 | M | Asian | Y | ≤20 | NA |
| Tissue-2 | 58 | M | Caucasian | Y | ≤20 | 8.1 |
| Tissue-3 | 58 | F | AA | Y | ≤20 | NA |
| Tissue-4 | 70 | M | Caucasian | N | >20 | NA |
| Tissue-5 | 47 | M | Caucasian | N | ≤20 | NA |
| Tissue-6 | 72 | M | Caucasian | Y | ≤20 | 7.2 |
| Tissue-7 | 70 | F | Caucasian | Y | >20 | NA |
| Tissue-8 | 34 | M | AA | N | ≤20 | 14.4 |
| Tissue-9 | 72 | M | Hispanic | N | ≤20 | NA |
| Tissue-10 | 78 | M | Caucasian | Y | >20 | 8.7 |
| Tissue-11 | 63 | M | AA | N | ≤20 | 10.3 |
| Tissue-12 | 58 | M | AA | N | ≤20 | 10.0 |
| Tissue-13 | 67 | F | AA | Y | ≤20 | 8.9 |
AA, African American; F, female; M, male; N: No; NA: not available; Y: Yes
The clinical characteristics of study population and recruitment criteria
This was a cross–sectional, cohort, comparison trial entitled “Sodium Channel Splicing in Heart Failure Trial,” (SOCS-HEFT, ClinicalTrials.gov Identifier NCT01185587) and conducted at the University of Illinois at Chicago (UIC) and the Jesse Brown Veterans Administration Medical Center (JBVAMC) in Chicago, Illinois. The study was approved by the Collaborative UIC/Northwestern/JBVAMC Institutional Review Board (IRB). All study subjects signed a written, informed consent prior to enrollment. Subjects were comprised of adult patients (age ≥ 18 years) with systolic HF (defined by echocardiography-derived left ventricular ejection fraction or LVEF ≤ 50%). The subjects were assigned to four groups: those who did not have HF (i.e. control); those with HF without an ICD (HF); those with HF, an ICD, and no evidence of appropriate event-driven therapy [ICD(−)Event]; and finally, those with HF, an ICD, and evidence of appropriate event-driven therapy [ICD(+)Event]. Control patients were defined by normal left ventricular systolic and diastolic function by echocardiographic assessment. The pre-enrollment evaluation for all groups included reviewing the electronic medical records and subject interviews for history, a physical examination, and current medication at the time of enrollment. Demographic data obtained included: age, race, body mass index, and New York Heart Association (NYHA) functional class.
ICD implantation was at least one year prior to enrollment. An appropriate ICD event was adjudicated by an independent, blinded, clinical cardiac electrophysiologist. An “event” was defined as any device therapy delivered to interrupt ventricular fibrillation or ventricular tachycardia excluding anti-tachycardia pacing. ICD programming was at the discretion of the attending physician. The ICD implant indication was predominantly primary prevention (77%). All LVEF determinations were made by echocardiography or cardiac magnetic resonance imaging. LVEF was determined in a 2-year window prior to enrollment.
Exclusion criteria
Any patient with a history of congenital heart disease or use of illicit drugs was excluded. Patients on immunosuppressive medications, or who had evidence of a chronic infection, acute or chronic inflammatory illness, or any illness expected to result in death within 18 months of enrollment were excluded. Control patients had to be free of HF symptoms, diastolic dysfunction, and left ventricular systolic dysfunction documented by any cardiac imaging or diagnosis in the electronic medical record within one year of study enrollment. Other exclusion criteria for the control group included Long-QT Syndrome, Brugada Syndrome, or a history of significant illness (i.e. myocardial infarction, cardiac hospitalization, cardiac arrhythmia, infection, or cancer) within 12 months of study enrollment.
Laboratory methods
Blood samples were collected in PAXgene® Blood RNA tubes (BD, Franklin Lakes, NJ) following the manufacturer’s procedure. Samples were stored for up to three days at room temperature or five days at 2–8°C. Total RNA was isolated using the PAXgene Blood RNA isolation kit and then converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Qiagen, Valencia, CA).
Total RNA was isolated from WBCs and human heart tissue with the RNeasy Mini and RNeasy Lipid Tissue Mini Kits, respectively (Qiagen) and then converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Qiagen). Only samples with an optical density (OD) 260/280 > 1.8, an OD 260/230 > 1.5, and total RNA > 6 µg were used. Both tissue and blood samples were stored in liquid nitrogen. Under these conditions, repeated measures of the same sample varied by less than 2%. Quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) was performed to detect the abundance of SCN5A variants by using iQ™ SYBR® Green Supermix (Bio-Rad) and 7500 Fast Real-Time PCR System (Life Technologies). The primer sequences used were HE27F (5’- CTGCGCCACTACTACTTCACCAACA-3’); HSCN5AE28A/R (5’- GGAAGAGCGTCGGGGAGAAGAAGTA-3’); HSCN5AE28C/R (5’- TCTCTTCTCCCCTCCTGCTGGTCA-3’); HSCN5AE28D/R (5’- GGAAGAGCGTCGGGGAGAAGAAGTA-3’). qRT-PCR thermal cycling conditions were an initial uracil-N-glycosylase incubation at 50°C for two minutes. iTaq™ DNA polymerase was activated with an initial denaturation step at 95°C for five minutes, followed by cycles of denaturation at 95°C for 15 seconds, and annealing and extension at 60°C for one minute. Each sample was measured for the target gene SCN5A, VC, VD, and β-actin. Samples were run in triplicate and averaged. Representative qRT-PCR amplification plots and the sample data are shown in supplemental Figure 1 and supplemental Table 1. To correct for WBC SCN5A expression between subjects, variants levels were expressed as a percentage of the variant with respect to the total Na+ channel mRNA including variants and normalized to the level of β-actin.
Statistical analysis
Age, sex, race, ischemia (defined as a chart review revealing a diagnosis of coronary artery disease, ischemic cardiomyopathy, previous coronary bypass surgery, previous percutaneous coronary intervention, or test results indicating obstructive coronary artery disease), LVEF, medications, New York Heart Association (NYHA) Class, and QRS duration measurements were recorded. Clinical characteristics were reported as means ± standard deviations for continuous variables and frequencies for categorical variables. Differences between the groups were examined by t-tests and chi-square tests for continuous and categorical variables, respectively. Results with p<0.05 were considered statistically significant in all analyses.
Linear regression, based on ordinary least squares (OLS), was used to determine the degree of correlation between normalized variant levels in the ventricle and blood. A probability value p < 0.05 was taken to indicate statistical correlation. The diagnostic odds ratio (DOR) is an overall measure of diagnostic accuracy that combines both sensitivity and specificity: [sensitivity/(1-sensitivity)]/[(1-specificity)/specificity]. We compared the summary DORs and their corresponding 95% confidence intervals (CIs) across different diagnostic predictors: normalized variants VC and VD in the blood, New York Heart Association (NYHA) class III/IV, ACE inhibitors, antiarrhythmic drugs, LVEF ≤ 20%, and QRS duration ≥ 120 ms. Univariate analysis was performed to calculate DORs and their corresponding 95% CIs.
Receiver Operating Characteristic (ROC) curves were generated for both splicing variants and LVEF ≤ 20%. Sensitivity (the proportion of true positive ICD patients with an event) and the specificity (the proportion of ICD patients without an event) were evaluated. A commonly used measure of overall diagnostic effectiveness is the Youden index, which is defined as (sensitivity + specificity) -1. We determined the optimal cutoff value by maximizing the Youden index. The sensitivities and specificities were calculated from the data across all possible cutoff values within the range of the test results, and we selected the cutoff value leading to the highest Youden index.
Results
Correlation of cardiac tissue and circulating levels of VC and VD variants
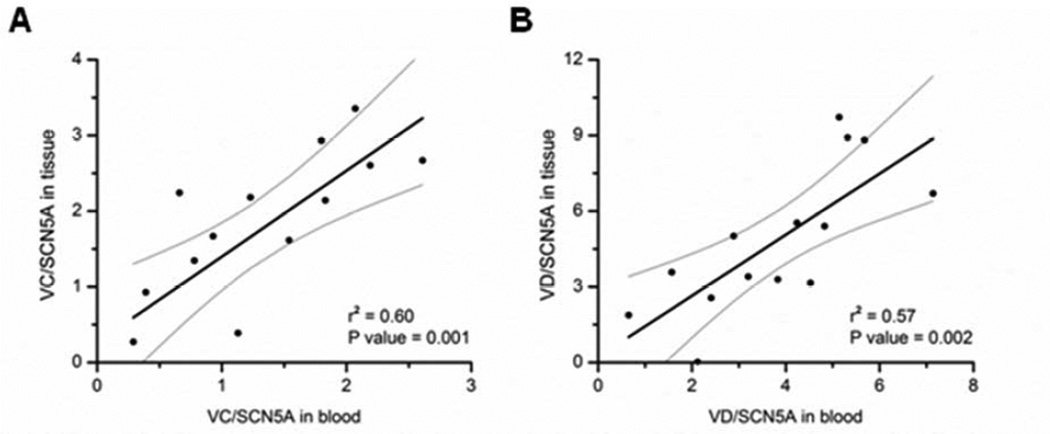
Paired analysis of SCN5A variants from circulating WBC and ventricular tissue demonstrated strong correlation (Figure 1, r= 0.78 and 0.75, respectively for variants VC and VD), demonstrating that WBC-derived expression of SCN5A variants are correlative of levels in myocardial tissue. Supplemental Figure 2 shows that the variant levels were independent of the WBC count.
Figure 1. Correlation of cardiac tissue and WBC mRNA abundances of SCN5A variants VC and VD.
Panel A shows tissue levels of the variant VC as a function of WBC levels measured in the same patient. Panel B shows tissue levels of the variant VD as a function of WBC levels measured in the same patient. The best-fit linear regression is displayed as a solid black line. Grey lines represent the 95% confidence intervals.
SCN5A variants expression in HF
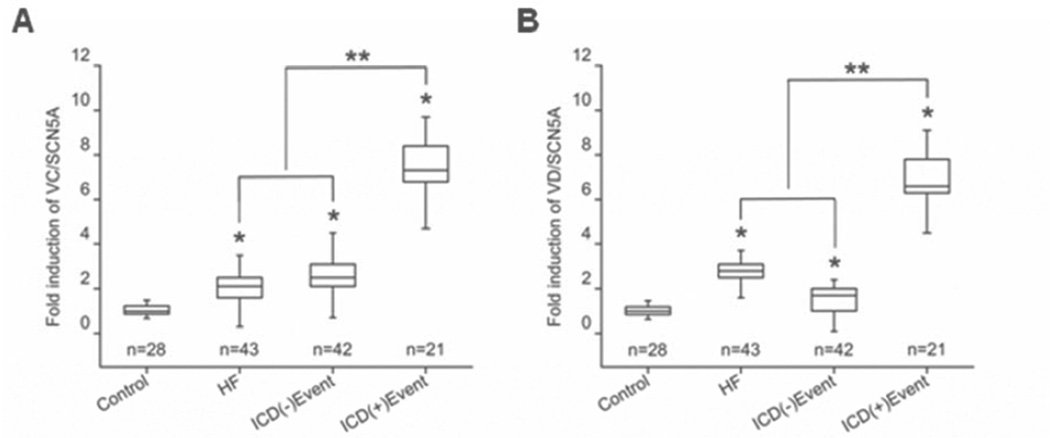
Table 2 shows the clinical characteristics of patients enrolled in the study. By omnibus F test in the one-way ANOVA, both VC and VD varied with NYHA class, β blocker use, and antiarrhythmic drug use (amiodarone in all cases except three patients in the ICD(+)Event group whose records indicated use of digoxin, sotalol, and an unspecified drug, respectively; supplemental Table 2). VC but not VD was influenced by QRS duration. Figure 2 displays median and interquartile ranges (IQR) for VC and VD across the three groups. Patients in the HF and ICD(−)Event group exhibited significantly lower median and IQR for both WBC-derived VC and VD variants compared to patients in the ICD(+)Event group [VC: HF 2.1 (IQR 1.7 – 2.5), ICD(−)Event 2.5 (IQR 2.1 – 3.1), ICD(+)Event 7.3 (IQR 6.8 – 8.4); VD: HF 2.8 (IQR 2.5 – 3.1), ICD(−)Event 1.7 (IQR 1.1 – 2.0), ICD(+)Event 6.6 (IQR 6.3 – 7.8). The expression of VC and VD variants were also significantly increased in all three HF groups (HF, ICD(−)Event, ICD(+)Event) compared to the control group (p<0.05).
Table 2.
The clinical characteristics of the study population
| Control (n=28) |
HF (n=43) |
ICD Without Event (n=42) |
ICD With Event (n=21) |
P Value (VC) |
P Value (VD) |
|
|---|---|---|---|---|---|---|
| Age—yr | 68.1 ± 12.1 | 59.9 ± 15.1 | 63.5 ± 11.1 | 62.1 ± 12.0 | 0.317 (Age≤60 yrs) | 0.485 |
| Male Sex—no. (%) | 26 (92.9) | 28 (65.1) | 29 (69.0) | 15 (71.4) | 0.777 | 0.722 |
| Race—no. (%) | ||||||
| African American | 23 (82.1) | 29 (67.4) | 26 (61.9) | 16 (76.2) | 0.705 | 0.575 |
| Caucasian | 5 (17.9) | 3 (7.0) | 5 (11.9) | 1 (4.8) | 0.470 | 0.422 |
| Hispanic | 0 (0) | 9 (20.9) | 9 (21.4) | 4 (19.0) | 0.493 | 0.432 |
| Asian | 0 (0) | 1 (2.3) | 1 (2.4) | 0 (0) | 0.839 | 0.079 |
| Other | 0 (0) | 1 (2.3) | 1 (2.4) | 0 (0) | 0.532 | 0.646 |
| NYHA Class—no. (%) | 0.002 | |||||
| 0.006 (I/II vs. III/IV) | ||||||
| I | 0 (0) | 2 (4.7) | 0 (0) | 0 (0) | ||
| II | 0 (0) | 9 (20.9) | 9 (21.4) | 0 (0) | ||
| III | 0 (0) | 11 (25.6) | 18 (42.9) | 4 (19.0) | ||
| IV | 0 (0) | 21 (48.8) | 15 (35.7) | 17 (81.0) | ||
| Ischemic Cardio-myopathy—no. (%) | 0.462 | |||||
| 7 (25.0) | 19 (44.2) | 31 (73.8) | 12 (57.1) | 0.814 | ||
| Medications—no.(%) | ||||||
| β Blocker | 8 (28.6) | 35 (81.4) | 37 (88.1) | 21 (100) | 0.005 | 0.046 |
| ACE Inhibitor | 12 (42.9) | 29 (67.4) | 29 (69.0) | 15 (71.4) | 0.661 | 0.607 |
| ARB | 3 (10.7) | 3 (7.0) | 7 (16.7) | 4 (19.0) | 0.450 | 0.709 |
| Aldosterone | ||||||
| Antagonist | 0 (0) | 3 (7.0) | 14 (33.3) | 8 (38.1) | 0.301 | 0.904 |
| Antiarrhythmic | 0.052 | |||||
| Drug | 0 (0) | 2 (4.7) | 4 (9.5) | 8 (38.1) | 0.021 | |
| QRS Duration >120 ms—no. (%) | 2 (7.1) | 8 (20.0) | 18 (43.9) | 15 (71.4) | 0.014 | 0.150 |
| LVEF % | 54.6 ± 1.9 | 26.0 ± 7.7 | 27.4 ± 7.0 | 26.1 ± 5.5 | 0.414 (LVEF≤20%) | 0.382 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection factor;; NYHA Class, New York Heart Association class
Figure 2. The WBC expression of SCN5A variants in the test groups.
Panel A shows fold induction compared to control of SCN5A variant VC in the heart failure (HF), ICD(−)event, and ICD(+)event groups. Panel B shows fold induction compared to control of SCN5A variant VD in the heart failure (HF), ICD(−)event, and ICD(+)event groups. The fold induction values are displayed as median, interquartile ranges, minimum, and maximum. * p<0.05 as compared to control. ** p<0.05 comparing the ICD(+)Event group to the combined HF and ICD(−)Event groups using an independent-samples Mann-Whitney U test.
Effect of population characteristics on SCN5A variant expression
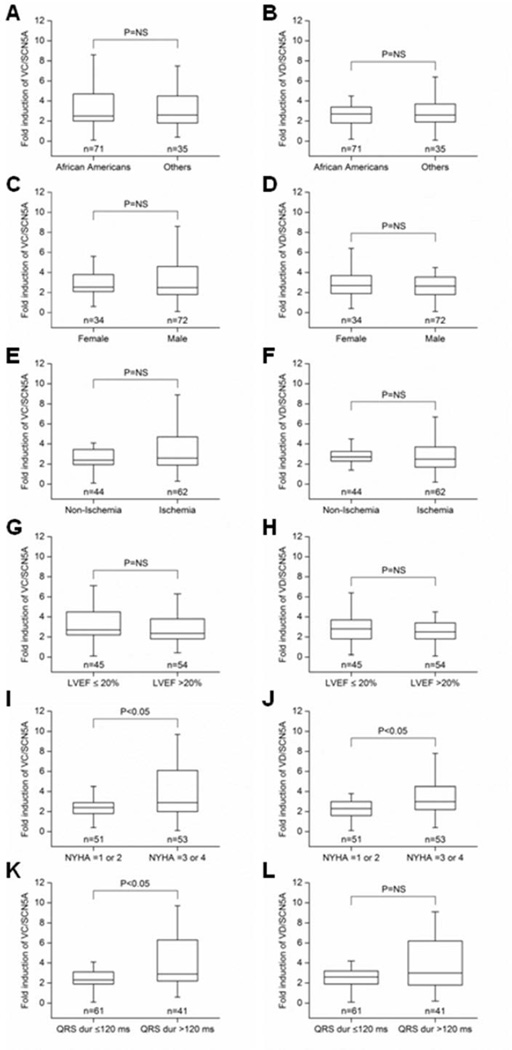
There was no difference in the expression of VC and VD across races (p = NS; Figures 3A, 3B), between sexes (p = NS; Figures 3C, 3D), between subjects with an ischemia history and those without an ischemia history (p = NS; Figures 3E, 3F), or between subjects with an LVEF ≤ 20% and those with LVEF > 20% (p = NS; Figures 3G, 3H). Worsening NYHA class, however, was associated with an induction of both WBC-derived SCN5A variants (NYHA Class I-II versus NYHA Class III-IV: 2.8 ± 1.7 versus 4.1 ± 2.7 and 2.5 ± 1.6 versus 3.8 ± 2.4 for VC and VD, respectively (p < 0.05 for each; Figures 3I–J). VC expression also demonstrated significant changes between QRS duration ≤ 120 ms versus > 120 ms (3.0 ± 2.1 versus 4.2 ± 2.6, respectively, p < 0.05; Figure 3K). A similar trend toward significance was evident in VD expression (2.9 ± 1.9 versus 3.6 ± 2.4, p > 0.05; Figure 3L).
Figure 3. The effect of clinical characteristics on the WBC expression of SCN5A VC and VD.
Panels A, C, E, G, I, and K as well as B, D, F, H, J, and L compare the effects of race, sex, origin of the cardiomyopathy, left ventricular ejection fraction (LVEF), New York Heart Association heart failure stage (NYHA), and QRS duration on WBC variant VC and VD levels, respectively.
Predictors of ICD events
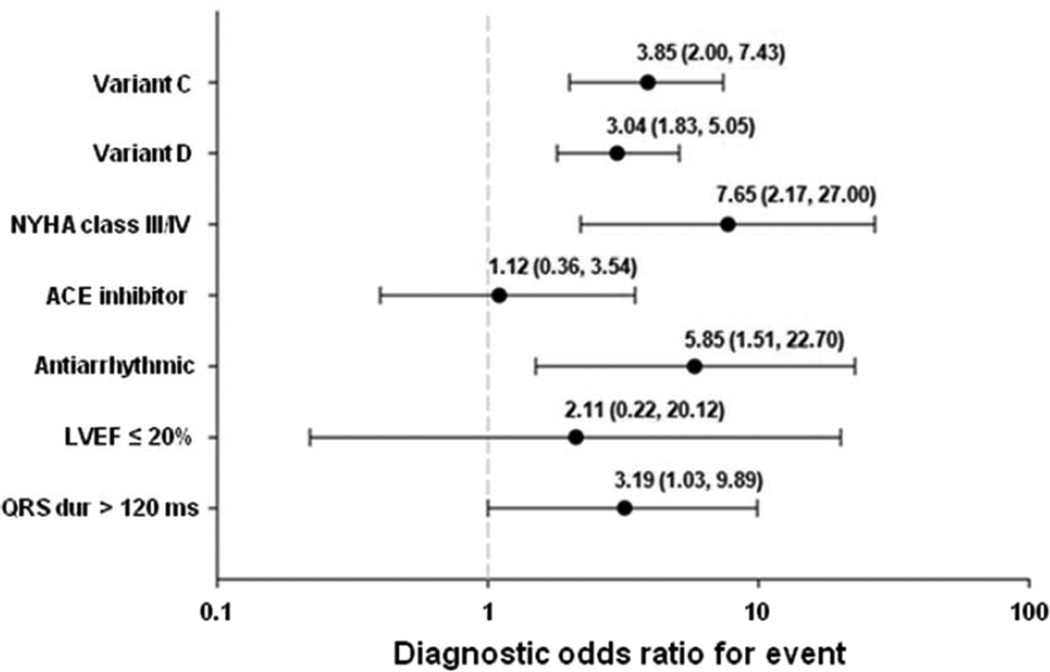
By univariate analysis (Figure 4), NYHA class III/IV (DOR 7.65; 95% CI 2.17, 27.00), antiarrhythmic drug use (DOR 5.85; 95% CI 1.51, 22.70), WBC-derived VC expression (DOR 3.85; 95% CI 2.00, 7.43), WBC-derived VD expression (DOR 3.04; 95% CI 1.83, 5.05), and QRS duration ≥ 120 ms (DOR 3.19; 95% CI 1.03, 9.89) demonstrated association with increased risk of an ICD event. In contrast, an LVEF ≤ 20% (DOR 2.11; 95% CI 0.22, 20.12) and ACE inhibitors (DOR 1.12; 95% CI 0.36, 3.54) were not associated with ICD events.
Figure 4. Univariate analysis of clinical characteristics on discrimination of ICD events.
The data are presented as the odds ratio and 95% confidence intervals.
Sensitivity and specificity of SCN5A variants for determination of ICD events
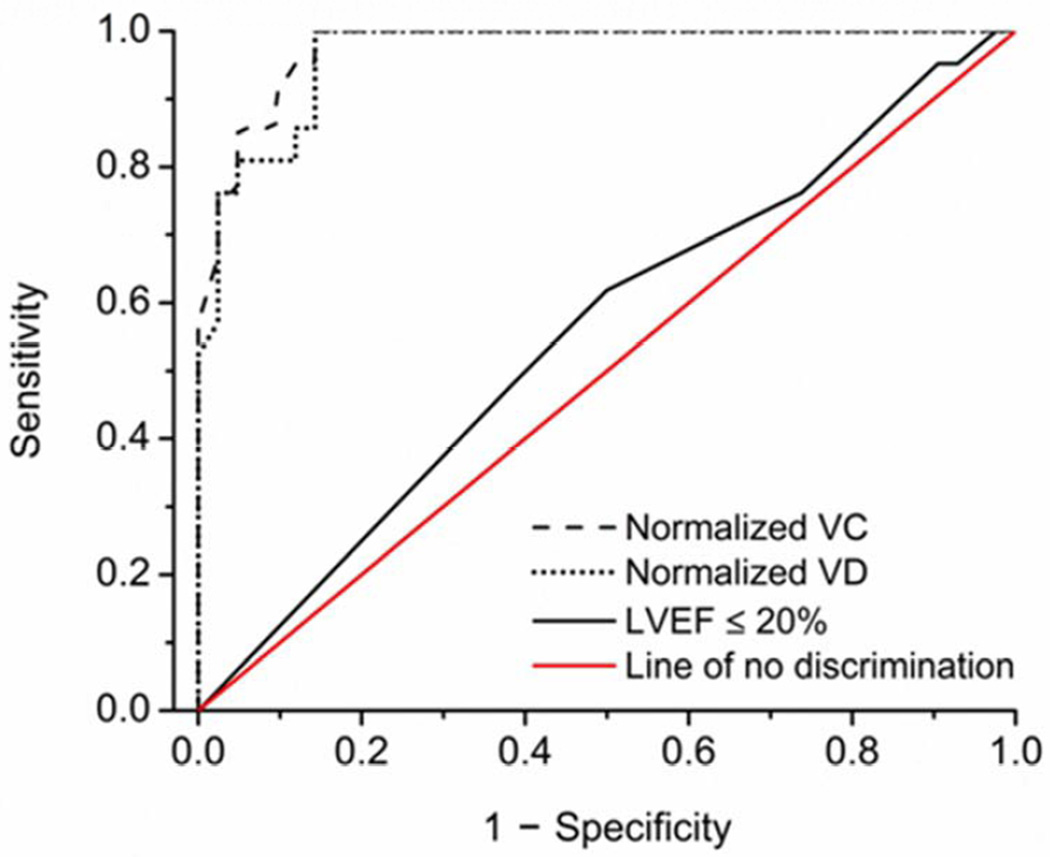
ROC curves were generated to evaluate the performance of the expression of the WBC-derived variants in distinguishing between the ICD patients with and without the events and then compared to those generated for an LVEF ≤ 20%. The area under the ROC curve was 0.98 (95% CI 0.95, 1.00), 0.97 (95% CI 0.93, 1.00) and 0.56 (95% CI 0.41, 0.71) for VC, VD and LVEF ≤ 20%, respectively (Figure 5). The values for the optimal Youden index and cutoff as well as the corresponding maximum sensitivities and specificities are shown in Table 3. To address any model overfitting, we performed a 7-fold cross validation of each logistic regression associated with ICD intervention (from VC, VD, and LVEF < 20%), and plotted the distribution of sensitivity and specificity values for cutoffs based on the regressions on the ROC curves. The plots (Supplemental Figure 3) illustrate that both VC and VD variants provided superior performance to LVEF in our data set.
Figure 5. Receiver operation characteristics curves for WBC SCN5A variant VC and VD discrimination of ICD events.
Receiver operation characteristics (ROC) curves for normalized VC and VD are compared to LVEF ≤ 20%. The area under the ROC curve (95% CI) are 0.98 (0.95, 1.00), 0.97 (0.93, 1.00), and 0.56 (0.41, 0.71) for VC, VD and LVEF ≤ 20%, respectively. The line of no discrimination is also shown.
Table 3.
Optimal discrimination values for VC and VD variants
| Index test | Optimal cutoff value |
Optimal Youden index |
Corresponding sensitivity (95% CI) |
Corresponding specificity (95% CI) |
|---|---|---|---|---|
| Normalized VC | 4.2 | 0.9 | 1.0 (0.85, 1.00) | 0.9 (0.78, 0.96) |
| Normalized VD | 2.9 | 0.9 | 1.0 (0.85, 1.00) | 0.9 (0.78, 0.96) |
| LVEF ≤ 20% | 4.5 | 0.1 | 0.6 (0.41, 0.79) | 0.5 (0.35, 0.64) |
LVEF, Left ventricular ejection fraction; VC, SCN5A variant C; VD, SCN5A variant D
Discussion
Alterations in sodium current, the main current for cardiac conduction, are associated with arrhythmogenesis (20). Since the cloning of SCN5A, encoding the α-subunit of the Na+ channel (21), hundreds of mutations have been reported to cause inherited sudden death syndromes such as Brugada syndrome, the third variant of Long QT syndrome (LQT3), and sudden infant death (20, 22). Moreover, we have shown previously that abnormal mRNA splicing results in SCN5A variants that can contribute to arrhythmic risk and that these variants are increased in HF (16, 17, 19).
Using LVAD core samples and blood samples from the same patient, we now show a significant correlation between normalized variant expression levels in the heart and blood. The results also indicate a graded association between levels of circulating SCN5A variants and an increasing risk of ICD events in patients from control to HF to HF with ICD events. HF patients who had received appropriate ICD intervention had significantly higher levels of SCN5A splice variants compared to controls and to subjects who had not received an intervention. As expected, HF subjects with and without an ICD but with no intervention had similar variant levels. Moreover, the separation between groups allowed for further discriminatory power to risk stratify patients with HF.
The discriminatory capacity of the variant expression to identify groups with and without ICD events was independent of gender, race, etiology of myopathy, or severity of LVEF. SCN5A variants expression was increased with higher NYHA class, consistent with the notion that these patients exhibit worsening HF symptoms and are at higher risk from HF complications such as arrhythmias. As longer QRS duration is a manifestation of cardiac conduction disease and associated with reduced functional sodium channels (23–26), observations of higher variant levels in patients with longer QRS duration were also consistent. Interestingly, variant levels were not associated with severity of LVEF. Since LVEF severity beyond the initial threshold of 30 or 35% has not been a reliable indicator for further risk stratification for ICD implantation (27–29), the current data indicate that measures of circulating variant expression levels may give added information to risk reflected by left ventricular function. Given the cardiac specific nature of the pathophysiological role of SCN5A variants and the high degree of correlation between WBC-derived SCN5A variants levels and ICD interventions, we speculate that circulating SCN5A variants may supplement current methods to improve discrimination of patients who will most likely benefit from ICD implantation.
There are a number of limitations to this study. The small sample size may limit the applicability of the findings to larger population-level cohorts. For example, sample size may have masked weak associations of variants with other potential covariates or identification of other predictors of arrhythmic risk. Given the retrospective imaging and ICD data, unforeseen biases may have been introduced that might reduce the power of WBC-derived variants. Analysis of patients with ICD device interventions for nonlethal arrhythmias, however, revealed that device intervention alone did not alter SCN5A variants levels (data not shown). Additionally, ICD programming was not controlled in this study design. Therefore, potential nonsustained events may have been counted, altering the calculated power of the variants levels. Exclusion of anti-tachycardia pacing as an event mitigates some of this concern. While this study suggested the association of SCN5A mRNA splice variants was independent of race, the total number of patients and limited numbers of Caucasians, Hispanic, or Asian patients make it difficult to be certain the findings apply similarly to all racial groups. We did not evaluate the correlation of variants with multiple ICD interventions in the ICD(+)Event group or over longer than the defined one year period. Despite the correlation between elevated levels of VC and VD with ICD events, it is possible that not all arrhythmic conditions will be similarly associated. Finally, the cost/benefit ratio of any combination of predictive parameters remains to be determined.
In conclusion, we have shown that levels of circulating WBC-derived SCN5A mRNA variants are representative of levels in the myocardium. Moreover, the SCN5A variants levels increased with risk for SCD, and variants levels were significantly elevated in subjects having received an ICD intervention. The degree of separation of variants levels between HF subjects with and without an ICD intervention suggested variant levels had a strong power to discriminate between these two groups. If true in prospective validation trials, WBC SCN5A variant level determinations may help identify which patients with HF might benefit most from device implantation.
Supplementary Material
Acknowledgments
Funding sources: National Institutes of Health grants P01 HL058000 (SCD), R01 HL1024025 (SCD), R01 HL106592 (SCD), Veterans Administration Merit Award (SCD), R41 HL112355 (SCD), and National Center for Research Resources/National Center for Advancing Translational Sciences UL1RR029879 (AAD).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: SCD is the inventor on patent applications: 1) SCN5A Splice Variants for Use in Methods Relating to Sudden Cardiac Death and Need for Implanted Cardiac Defibrillators, PCT/US2012/20564 and 2) SCN5A Splicing Factors and Splice Variants for Use in Diagnostic and Prognostic Methods, 13/291,826.
References
- 1.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J. 1988;115:869–875. doi: 10.1016/0002-8703(88)90891-5. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Verma A, Sarak B, Kaplan AJ, et al. Predictors of appropriate implantable cardioverter defibrillator (ICD) therapy in primary prevention patients with ischemic and nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2010;33:320–329. doi: 10.1111/j.1540-8159.2009.02566.x. [DOI] [PubMed] [Google Scholar]
- 6.LaPointe NM, Al-Khatib SM, Piccini JP, et al. Extent of and reasons for nonuse of implantable cardioverter defibrillator devices in clinical practice among eligible patients with left ventricular systolic dysfunction. Circ Cardiovasc Qual Outcomes. 2011;4:146–151. doi: 10.1161/CIRCOUTCOMES.110.958603. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–1655. doi: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huikuri HV, Makikallio TH, Raatikainen MJ, Perkiomaki J, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death: appraisal of the studies and methods assessing the risk of sudden arrhythmic death. Circulation. 2003;108:110–115. doi: 10.1161/01.CIR.0000077519.18416.43. [DOI] [PubMed] [Google Scholar]
- 11.Bailey JJ, Berson AS, Handelsman H, Hodges M. Utility of current risk stratification tests for predicting major arrhythmic events after myocardial infarction. J Am Coll Cardiol. 2001;38:1902–1911. doi: 10.1016/s0735-1097(01)01667-9. [DOI] [PubMed] [Google Scholar]
- 12.Costantini O, Hohnloser SH, Kirk MM, et al. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009;53:471–479. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 13.Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet. 2002;30:13–19. doi: 10.1038/ng0102-13. [DOI] [PubMed] [Google Scholar]
- 14.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 15.Zhou A, Ou AC, Cho A, Benz EJ, Jr, Huang SC. Novel splicing factor RBM25 modulates Bcl-x pre-mRNA 5' splice site selection. Mol Cell Biol. 2008;28:5924–5936. doi: 10.1128/MCB.00560-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao G, Xie A, Huang SC, et al. Role of RBM25/LUC7L3 in abnormal cardiac sodium channel splicing regulation in human heart failure. Circulation. 2011;124:1124–1131. doi: 10.1161/CIRCULATIONAHA.111.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao G, Xie A, Zhang J, et al. Unfolded protein response regulates cardiac sodium current in systolic human heart failure. Circ Arrhythm Electrophysiol. 2013;6:1018–1024. doi: 10.1161/CIRCEP.113.000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu D, Barajas-Martinez H, Medeiros-Domingo A, et al. A novel rare variant in SCN1Bb linked to Brugada syndrome and SIDS by combined modulation of Nav1.5 and Kv4.3 channel currents. Heart Rhythm. 2012;9:760–769. doi: 10.1016/j.hrthm.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang LL, Pfahnl AE, Sanyal S, et al. Human heart failure is associated with abnormal C-terminal splicing variants in the cardiac sodium channel. Circ Res. 2007;101:1146–1154. doi: 10.1161/CIRCRESAHA.107.152918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remme CA, Bezzina CR. Sodium channel (dys)function and cardiac arrhythmias. Cardiovasc Ther. 2010;28:287–294. doi: 10.1111/j.1755-5922.2010.00210.x. [DOI] [PubMed] [Google Scholar]
- 21.Gellens ME, George AL, Jr, Chen LQ, et al. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci USA. 1992;89:554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkel BG, Larsen MK, Berge KE, et al. The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J Cardiovasc Electrophysiol. 2012;23:1092–1098. doi: 10.1111/j.1540-8167.2012.02371.x. [DOI] [PubMed] [Google Scholar]
- 23.Santos LF, Rodrigues B, Moreira D, et al. Criteria to predict carriers of a novel SCN5A mutation in a large Portuguese family affected by the Brugada syndrome. Europace. 2012;14:882–888. doi: 10.1093/europace/eur421. [DOI] [PubMed] [Google Scholar]
- 24.Kanter RJ, Pfeiffer R, Hu D, Barajas-Martinez H, Carboni MP, Antzelevitch C. Brugada-like syndrome in infancy presenting with rapid ventricular tachycardia and intraventricular conduction delay. Circulation. 2012;125:14–22. doi: 10.1161/CIRCULATIONAHA.111.054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotoodehnia N, Isaacs A, de Bakker PI, et al. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet. 2010;42:1068–1076. doi: 10.1038/ng.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royer A, van Veen TA, Le Bouter S, et al. Mouse model of SCN5A-linked hereditary Lenegre's disease: age-related conduction slowing and myocardial fibrosis. Circulation. 2005;111:1738–1746. doi: 10.1161/01.CIR.0000160853.19867.61. [DOI] [PubMed] [Google Scholar]
- 27.Buxton AE, Ellison KE, Lorvidhaya P, Ziv O. Left ventricular ejection fraction for sudden death risk stratification and guiding implantable cardioverter-defibrillators implantation. J Cardiovasc Pharmacol. 2010;55:450–455. [PubMed] [Google Scholar]
- 28.Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 29.Saba S. Sudden cardiac death risk stratification and assessment: primary prevention based on ejection fraction criteria. Heart Fail Clin. 2011;7:175–183. doi: 10.1016/j.hfc.2010.12.004. vii. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.