Abstract
Mitochondria are essential to providing ATP thereby satisfying the energy demand of the incessant electrical activity and contractile action of cardiac muscle. Emerging evidence indicates that mitochondrial dysfunction can adversely impact cardiac electrical functioning by impairing the intracellular ion homeostasis and membrane excitability through reduced ATP production and excessive reactive oxidative species (ROS) generation, resulting in increased propensity to cardiac arrhythmias. In this review, the molecular mechanisms linking mitochondrial dysfunction to cardiac arrhythmias are discussed with an emphasis on the impact of increased mitochondrial ROS on the cardiac ion channels and transporters that are critical to maintaining normal electromechanical functioning of the cardiomyocytes. The potential of using mitochondria-targeted antioxidants as a novel anti-arrhythmia therapy is highlighted.
Introduction
The normal functioning heart requires coordinated, rhythmic electrical activity and contractile action. At rest, the heart pumps about 280 liters of blood throughout the human body per hour, and the energy demand to meet this unceasing action consumes nearly 10% of the total body O2 uptake [1]. Over 90% of the cellular ATP consumed in the heart is produced by the mitochondria through oxidative phosphorylation (OXPHOS) [2]. As the predominant energy generator in the heart, mitochondria account for ~30% of the volume of cardiac cells, forming a network surrounding sarcoplasmic reticulum (SR), myofilaments and t-tubules [3]. It is estimated that one third of the cardiac ATP generated by mitochondria is used for sarcolemmal and SR ion channels and transporters, which are required for the electrical activity of the cardiac cells [4]. Therefore, mitochondrial dysfunction readily disrupts the cardiac rhythm through depleting energy supply to these channels and transporters [5, 6].
In addition to producing ATP, mitochondria also generate reactive oxygen species (ROS) as a by-product of OXPHOS. It is now widely accepted that in addition to their critical bioenergetic function, mitochondria function as signaling hubs in large part by regulating redox signaling in the cell [7, 8]. Under physiological conditions, trace amount of ROS establish a network of mitochondria-driven signals that integrate metabolism with gene transcription and enzymatic activity [9, 10]. Short term increases in ROS signals trigger adaptive responses and facilitate preconditioning, increasing cellular and tissue resistance against insult [11, 12]. On the other hand, persistently elevated ROS levels can trigger maladaptive responses and persistent abnormalities that compromise function at the molecular, cellular and tissue levels [13–15]; In this regard, excessive production of ROS elicits pathologic changes by altering cellular function and increasing cell death [16]. Emerging evidence has shown that excessive mitochondrial ROS production can impair cardiac excitability by affecting the function of various channels and transporters through direct interaction such as post-translational redox modification of cysteine (S-glutathionylation, sulfhydration and S-nitrosation) or tyrosine (nitration) residues [17–19]. Excessive mitochondrial ROS can also modulate ion channel/transporter function indirectly via associated signaling molecules, such as ROS-sensitive kinases including calcium-calmodulin-dependent protein kinase (CaMKII), cSrc and protein kinase C (PKC), or via redeox-sensitive transcription factors, such as NFκB [20–22].
Mitochondria are also critically involved in the homeostatic regulation of cellular cations such as Ca2+, Na+ and K+, disturbance of which can has important consequences for cardiac contractility, energetics and electrical activity [23–25]. There is a complex interrelationship between sarcolemmal and mitochondrial cation regulation. Mitochondria can uptake and extrude Ca2+, for example, modulating cardiomyocyte function by serving as a dynamic buffer for sarcolemmal Ca2+ [26, 27]. Changes in sarcolemmal cation concentration, on the other hand, can influence mitochondrial structure [28, 29], energetics [30, 31] and mitochondria-dependent cell death [32]. Much of the mitochondria-sarcolemma cation interdependence is mediated by the ion channels or transporters located on the inner membrane of mitochondria (see below).
Many central metabolic systems operate totally or partially within the mitochondria. These systems dynamically regulate cellular energetic status and sarcolemmal ATP-sensitive potassium (sarcKATP) currents through oscillating mitochondrial membrane potential (ΔΨm) in response to the changes in the supply of fuel substrates and O2 [33–35]. In the presence of metabolic stress such as myocardial ischemia, depolarization of ΔΨm diminishes mitochondrial ATP production, resulting in the opening of the sarcKATP channels, which creates a “current sink” in the myocardium, capable of slowing or blocking cardiac electrical propagation, thereby fomenting arrhythmias (see below) [33, 36].
After a brief review on the ionic basis of cardiac excitability, mitochondrial energetics/ROS production, and mitochondrial/sarcolemmal cation homeostasis, the role of mitochondrial dysfunction in influencing myocyte excitability and cardiac arrhythmogenesis will be discussed, with an emphasis on the impact of mitochondrial ROS on sarcolemmal and sarcoplasmic channel/transporter functioning. In addition, the potential antiarrhythmic therapies targeting mitochondrial dysfunction in cardiac diseases will be highlighted.
Ionic basis of cardiac excitability and contractile function
The normal contractile function of the mammalian heart depends on proper myocardial electrical activity, including the sequential activation of cells in specialized conducting system, the normal propagation of electrical activity through the myocardium, and the generation of action potentials in individual cardiomyocytes [37, 38]. The normal cardiac cycle begins with the action potential originating in the sinoatrial node, propagating through the atria to the atrioventriular node. The electrical activity then spreads through the His bundle and Purkinje fibers to the cardiac apex, exciting the working ventricular myocardium [39]. The propagation of myocardial electrical activity depends on electrical coupling mediated by gap junctions, ensuring the coordination of the electromechanical functioning of the working myocardium [40]. Myocardial action potentials are generated by the sequential activation and inactivation of ion channels conducting depolarizing, Na+ and Ca2+, and repolarizing, K+, currents [37, 38]. During the action potential, Ca2+ influx through voltage-gated Ca2+ channels triggers the release of Ca2+ ions into the cytosol from the sarcoplasmic reticulum (SR) via ryanodine receptor 2 (RyR2). Ca2+ binds to the protein troponin-C of the troponin-tropomyosin complex, leading to cardiomyocyte longitudinal shortening. The synchronous shortening of the ventricular myocytes results in the contraction of the heart and the systolic ejection of blood [41]. The subsequent diastolic relaxation of the myocytes depends on the repolarization of membrane potential and the removal of Ca2+ from the sarcomere [41]. Myocardial action potential repolarization is determined by multiple outward K+ currents through voltage-gated K+ (Kv) and inwardly rectifying K+ (Kir) channels, whereas removal of Ca2+ from sarcomere depends on sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), which retrieves cytosolic Ca2+ into SR, as well as Na+/Ca2+ exchanger (NCX), an antiporter membrane protein extruding Ca2+ from the cell. Factors that interfere with the aforementioned channel functioning may impair cardiac excitability and lead to cardiac arrhythmias.
Mitochondrial energetics and ROS production
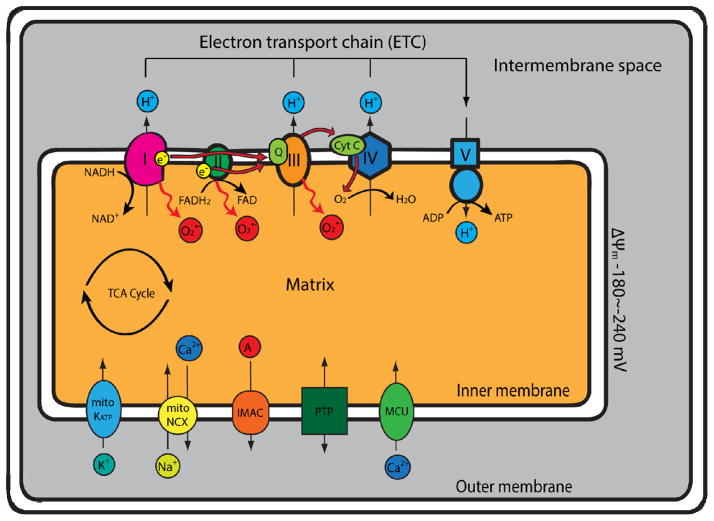
The mitochondria are organelles containing double-membrane structure (inner and outer membranes) that create separate compartments, the intermembrane space and mitochondrial matrix. Mitochondria utilize glucose and fatty acids, the primary metabolic substrates for the myocardium, to generate ATP through OXPHOS. Glucose and fatty acids are sequentially oxidized to produce acetyl-CoA, the metabolic intermediate allowing the production of reducing equivalents nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) through the tricarboxylic acid (TCA) cycle. These reducing equivalents feed electrons to the electron-transport chain (ETC) along the mitochondrial inner membrane, where the electrons flow through the complexes (I, II, III and IV) of ETC, and finally to molecular oxygen to produce H2O. As electrons flow through ETC, redox reaction occurs at complex I, III and IV, which drives proton (H+) across the inner membrane, from mitochondrial matrix into intermembrane space, establishing the proton gradient and the strongly negative mitochondrial membrane potential, ΔΨm (~−180 to −240 mV). The energy stored in ΔΨm and proton gradient drives H+ flow through mitochondrial ATP synthase (complex V), the final complex of the ETC, back into the matrix, converting ADP to ATP (Figure 1).
Figure 1. Schematic overview of oxidative phosphorylation and superoxide production in mitochondria.
Reducing equivalents nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) generated from the tricarboxylic acid (TCA) cycle donate electrons to the electron transport chain (ETC) on the mitochondrial inner membrane. The electrons pass through the ETC (complex I & II → coenzyme Q [Q] → complex III → cytochrome C [Cyt C] → complex IV → molecular O2), where coupled redox reactions drives proton (H+) from the matrix, across the inner membrane, into the intermembrane space, forming the proton gradient and the negative mitochondrial membrane potential, ΔΨm (~−180 to −240 mV). The proton-motive force then drives H+ flow through mitochondrial ATP synthase (complex V) and generates ATP. An estimated 1–5% of the electrons prematurely leak to O2 at complexes I, II or III, leading to the formation of superoxide (O2•−). Several important mitochondrial channels are also located on the inner membrane: the mitochondrial KATP channels (mitoKATP), the mitochondrial Na/Ca exchanger (mitoNCX), the inner membrane anion channel (IMAC), the mitochondrial permeability transition pore (PTP) and the mitochondrial calcium uniporter (MCU).
As an inevitable byproduct of OXPHOS, ROS are produced as a result of incomplete reduction or a surplus of electrons in the ETC. The relationship of OXPHOS to ROS production is not entirely clear and is probably not always an inverse proportion [42], but it has been estimated that 0.1–1% of the electrons flowing through the ETC prematurely leak to O2 at complexes I, II or III, causing the formation of superoxide (O2•−), one of the major ROS in cardiac cells (Figure 1) [43]. The rate of ROS production in mitochondrial matrix depends on the proton motive force, the NADH/NAD+ ratio, the reduced coenzyme Q10 (CoQH2)/coenzyme Q10 (CoQ) ratio, and the local O2 concentration. Under conditions of high proton motive force, high CoQH2/CoQ or increased NADH/NAD+ ratio, mitochondrial ROS production is increased [44]. Manganese superoxide dismutase (MnSOD), the primary antioxidant enzyme locating in mitochondria, catalyzes dismutation of superoxide to hydrogen peroxide (H2O2) and O2 [45]; complementary systems aimed at scavenging resulting H2O2 such as peroxisomal catalase, cytosolic and mitochondrial glutathione peroxidases and mitochondrial and cytosolic peroxiredoxins then convert H2O2 to H2O (and O2 in the case of catalase). Although other cellular origins of ROS, including NADPH oxidase, xanthine oxidase and uncoupled NO synthase may contribute to the production of ROS, mitochondria are viewed as the major source of ROS in cardiomyocytes.
Under pathological conditions such as diabetes mellitus [46, 47], pathological cardiac hypertrophy [48], myocardial ischemia/reperfusion [49, 50] or heart failure [51, 52], the efficiency of respiratory chain is impaired, leading to increased electron leak from the ETC and, hence, increased mitochondrial ROS production. Accumulating ROS levels can trigger the opening of mitochondrial channels, mitochondrial permeability transition pore (PTP) [53] and inner membrane anion channel (IMAC) [35], leading to simultaneous depolarization of ΔΨm in the mitochondrial network and further increases in ROS generation from ETC, a phenomenon known as mitochondrial ROS-induced ROS release [54, 55].
An excessive amount of ROS, including superoxide, H2O2, hydroxyl radicals (•OH), and peroxynitrite (ONOO−/ONOOH), can lead to detrimental reactions with cellular lipids and proteins, resulting in cardiomyocyte damage, dysfunction and death. Impaired mitochondrial function also leads to the accumulation of deleterious metabolites (NADH, ADP, lactate and H+), and the depletion of redox-defense antioxidant scavengers glutathione (GSH) [56], both of which can aggravate cellular damage caused by increased oxidative stress.
Increased mitochondrial ROS also reduce ATP production. It has been shown that superoxide can activate mitochondrial uncoupling proteins, leading to increased proton leak and uncoupling of mitochondrial ATP production from oxygen consumption [57, 58]. In addition, excessive mitochondrial ROS can cause oxidative damage to components of ETC, including complex I, II [59], IV [60], ATP synthase [59], and cytochrome c oxidase [61], leading to impaired ATP production and increased ETC electron leak that favors ROS generation. Under pathological conditions like tissue hypoxia, decreased O2 tension favors ROS generation while limiting ATP production [62]. Increased ROS can further impair ATP synthesis by inhibiting ETC. Increased mitochondrial ROS, therefore, can impair cellular electrical function directly by ROS-mediated signaling and oxidative damage (as discussed below), as well as indirectly by reducing ATP production that is essential for ion channel/transporters to function [5, 6].
Interdependent regulation of mitochondrial and sarcolemmal cation homeostasis
As mentioned above, sarcolemmal cation concentration is tightly controlled in cardiomyocytes by ion channels and transporters located on plasma membrane and SR. Mitochondria also harbor ion channels and transporters. Mitochondrial cation influx and efflux not only contribute to the dynamic regulation of cytoplasmic ionic homeostasis but also play a critical role in modulating mitochondrial function.
Mitochondrial Ca2+ is crucial for the regulation of energy production, mitochondrial morphology and cell death [63–65]. Under physiological conditions, increased mitochondrial Ca2+ activates tricarboxylic acid dehydrogenases [63, 66] and ATP synthase [67], promoting OXPHOS and ATP production. Elevated Ca2+ levels also increase mitochondrial fragmentation by regulating the mitochondrial fission factor DLP1 [65, 68]. Excessive mitochondrial Ca2+, however, can increase ROS production [69–71] and induce apoptotic cell death by activating the mitochondrial permeability transition pore (PTP) [64]. Mitochondrial Ca2+ uptake is mediated mainly by the mitochondrial Ca2+ uniporter (MCU) driven by mitochondrial membrane potential ΔΨm. Recently, a coiled-coil domain-containing protein 109A (CCDC109A) has been identified to encode MCU [72, 73]. In addition to MCU, several other mechanisms have been reported to mediate mitochondrial Ca2+ uptake, including the rapid mode of uptake (RaM) [74, 75], ryanodine receptor 1 (Ryr1) [76, 77] and Ca2+-selective conductance (mCa1 and mCa2) [78]. Tight interactions exist between endoplasmic/sarcoplasmic reticulum (ER/SR) and mitochondria, where Ca2+ is concentrated at the hundred micromolar range in microdomains containing inositol 1,4,5-triphosphate (IP3)/ryanodine (RyR2) receptors that permit Ca2+ transport from ER/SR into mitochondria [79–81]. These functional microdomains allow the cross-talk between mitochondria and SR, modulating Ca2+ handling and matching energy supply and demand by regulating mitochondrial respiration [82, 83]. SR-mitochondria communication has been implicated in ischemia-reperfusion injury [84] and cardiac arrhythmias [85]. Mitochondrial Ca2+ efflux is primarily mediated by the mitochondrial Na+-Ca2+ exchanger (mNCX)[86] [26], although the mitochondrial Ca2+/H+ antiporter [87] and PTP [88, 89] have also been implicated in mitochondrial Ca2+ efflux mechanisms. With the capacity of taking up or extruding Ca2+ through multiple mechanisms, mitochondria have been viewed as an efficient Ca2+ buffer, shaping Ca2+ dynamics in multiple cell types [26, 27, 90, 91]. To what extent mitochondria contribute to cellular Ca2+ dynamics under physiological and pathological conditions remains under debate [92]. A recent review on the available quantitative data suggests that mitochondria do not act as a significant buffer of cytosolic Ca2+ under physiological conditions. With prolonged elevation of cytosolic Ca2+ levels, however, mitochondrial Ca2+ uptake can increase 10- to 1000-fold and begin to impact cellular Ca2+ dynamics significantly [25].
Mitochondrial Na+ is regulated by Na+/H+ exchanger (NHE)-mediated Na+ uptake [93] and mNCX-mediated Na+ extrusion [94]. Under physiological conditions, energized mitochondria extrude protons, and the resulting pH gradient drives the Na+ gradient between mitochondrial matrix (lower [Na+]) and cytosol (higher [Na+])[93, 95]. Sarcolemmal Na+ levels increase significantly in pathological conditions such as heart failure [96, 97]. The rise in cytosolic [Na+] during heart failure widens the Na+ gradient across mitochondria, leading to greater driving force for mNCX to extrude Ca2+ from mitochondria, thereby resulting in decreased mitochondrial [Ca2+] and altered mitochondrial energetics [82].
Potassium transport also plays important roles in mitochondrial structure and function. Mitochondrial matrix volume is controlled by K+ fluxes: K+ influx is mediated by Ca2+-dependent (KCa) [98, 99] and ATP-dependent (mitoKATP) [100] K+ channels, whereas K+ efflux is conducted through a K+/H+ exchanger (KHE) [28]. KHE is activated with the expansion of mitochondrial volume, preventing excess matrix swelling [28, 101]. The activation of KCa and mitoKATP channels, on the other hand, increases mitochondrial matrix volume [28, 29]. Under pathological conditions, sarcolemmal and mitochondrial Ca2+ can become overloaded, triggering the activation of KCa [28, 29]. Mitochondrial Ca2+ overload can also activate mitoKATP and suppress the activity of KHE through depolarizing ΔΨm, loss of proton gradient and decreased ATP synthesis [29]. Mitochondrial Ca2+ overload, therefore, can result in mitochondrial swelling through the activation of KCa/mitoKATP and the inhibition of KHE. In addition, K+ influx mediated by KCa [102] and mitoKATP channels [103, 104] have been implicated in mediating the beneficial effects of ischemic preconditioning, which will be discussed later in this review.
Mitochondrial ROS and cardiac sodium channels
Cardiac voltage-gated Na+ (Nav) channels consist of heteromeric assembly of a pore-forming α subunit and auxiliary β subunits that modulate channel functions. Nav1.5 (SCN5A) is the major Nav α subunit expressed in the mammalian myocardium, whereas multiple Nav β subunits (Navβ1, β2, β3, β4.1 and β4.2) have been described in the cardiomyocytes [39]. Voltage-gated Na+ channels play a critical role in the membrane excitability of cardiomyocytes by generating the rapid upstroke (phase 0) of the action potential. In addition, Nav channels, along with cardiac gap junctions, govern the impulse conduction velocity in the myocardium.
Abnormal cardiac Na+ channel function has been described in hereditary cardiac diseases such as long QT syndrome (LQTS), Brugada syndrome and progressive cardiac conduction defect (PCCD) [105, 106] and in acquired cardiac conditions including myocardial ischemia [107, 108] and heart failure [109, 110]. Upon increased oxidative stress, the slowly inactivating component of sodium current (late INa) is shown to be increased in cardiomyocytes, leading to prolongation of action potential duration (APD), early after depolarizations (EAD), increased Na+/Ca2+ exchange and subsequent cellular Ca2+ overload, all of which are arrhythmogenic [111, 112]. Using a heterologous expression system, we have demonstrated that elevated intracellular NADH level leads to reduction of peak INa through a protein kinase C (PKC)-mediated increase in mitochondrial ROS production; NADH-induced INa reduction can be reversed by a mitochondrial-specific antioxidant, mito-TEMPO, or by inhibiting the mitochondrial respiratory chain [113]. Inhibition of other cellular source of ROS, including the NADPH oxidase, xanthine oxidase or NO synthase [113] did not alter the reduction in INa or the arrhythmic risk resulted from the loss of INa.
Cytosolic NADH and mitochondrial ROS are increased in a mouse model of nonischemic cardiomyopathy, resulting in cardiac INa reduction without altering membrane Na+ channel protein expression levels; the reduced INa can be restored by NAD+ or mito-TEMPO treatment, both mitochondrial antioxidants [52]. Consistent with these findings, reduced conduction velocity in human failing myocardium, which is associated with INa reduction, can be improved by NAD+ treatment [52]. Interestingly, the A280V mutation in glycerol-3-phosphate dehydrogenase 1-like (GPD1-L) protein, a mutation known to cause Brugada syndrome [114], reduces INa through increasing intracellular NADH levels and mitochondrial ROS [113, 115]; the reduction in INa by A280V GPD1-L can be reversed also by the treatment of NAD+ or mito-TEMPO [113]. These observations highlight the critical role of mitochondrial ROS as a mediator to transduce altered cardiac metabolism (reflected in NADH/NAD+ ratio) to the modulation of cardiac sodium channel function under pathological conditions such as myocardial ischemia or heart failure.
Cellular redox state, mitochondria, and Ca2+ homeostasis
Calcium ions are important intracellular signaling molecules, responsible for the regulation of numerous cellular processes in cardiomyocytes including excitation-contraction coupling, enzyme activity, transcription regulation and cell death [116]. The intracellular Ca2+ levels ([Ca2+]i) fluctuate markedly between systole and diastole, yet the changes in [Ca2+]i are highly regulated. Abnormal Ca2+ handling has been implicated in the mechanical dysfunction and arrhythmogenesis observed in cardiac diseases such as cardiac hypertrophy [117, 118], heart failure [119, 120] and myocardial ischemia [121, 122].
In cardiomyocytes, the cycling of [Ca2+]i begins with the entry of Ca2+ into the cells through voltage-gated Ca2+ channels, including the L- (low threshold) and T- (transient) type channels. The L-type Ca2+ channel is the predominant Ca2+ channel isoform in ventricular cardiomyocytes, whereas T-type Ca2+ channel is expressed mainly in pacemaker, atrial and Purkinje cells [123]. The Ca2+ entry via Ca2+ channels triggers SR Ca2+ release via RyR2, resulting in the sarcomere contraction. Ca2+ uptake into SR by SERCA and extrusion by NCX lower [Ca2+]i to baseline, allowing sarcomere relaxation. Multiple signaling pathways, including CaMKII, β-adrenergic receptors (β-AR), protein kinase A (PKA) and PKC, are involved in the modulation of the activities of these Ca2+ handling proteins (see reviews by Bers [116]). All these Ca2+ handling proteins contain thiol groups or methionines that are susceptible to the direct regulation by ROS or reducing agents.
Increased cellular ROS are known to cause a net increase in [Ca2+]i in cardiomyocytes [124]. The effects of ROS on L-type Ca2+ channel current (ICaL) in cardiomyocytes, however, are controversial. It has been shown that direct H2O2 application or increased mitochondrial ROS increases ICaL and the sensitivity of ICaL to isoproterenol in guinea pig ventricular myocytes [125] and that oxidized LDL enhances ICaL through lysophosphatidylcholine-induced mitochondrial ROS [126]. In contrast to these observations, others report a decrease in ICaL in ventricular cardiomyocytes with increased ROS [127, 128]. The discrepancies observed among the studies may be attributed to the differences in the animal species and type of ROS. It is critical to note that although grouped under the acronym of “ROS”, different reactive species containing molecular oxygen varying greatly in reactivity, diffusion coefficient, and oxidization potential.
The effects of ROS on other Ca2+ handling proteins, on the contrary, are more consistent. Increased oxidative stress enhances the open probability of RyR2 and increases the efflux of Ca2+ from SR [18]. Photoactivated or antimycin A-induced mitochondrial ROS production triggers a transient increase in Ca2+ sparks from RyR2 [129]. The activities of SERCA, in contrast to RyR2, are inhibited upon increased oxidative stress [130–132]. This effect can be, at least partially, attributed to decreased mitochondrial ATP supply for SERCA pump secondary to mitochondrial dysfunction [17].
CaMKII has been shown to play an important role in modulating RyR2 and SERCA function in response to increased oxidative stress in cardiomyocytes (See reviews by Maier et al [133] and by Rokita et al [134]). The activity of NCX, an antiporter that removes one Ca2+ ion from cytosol in exchange for the import of 3 Na+ ions, has been shown to be enhanced by ROS [135, 136]. The net effects of ROS on these Ca2+ handling proteins result in cytosolic Ca2+ overload and depletion of the SR Ca2+ store, leading to multiple detrimental effects including a prolonged action potential, delayed afterdepolarizations (DADs), contractile dysfunction and the activation of Ca2+-dependent signaling (e.g., CaMKII, calcineurin and NFAT) [17, 137].
As was indicated earlier, mitochondria also play an important role in cytosolic Ca2+ regulation by taking up and releasing Ca2+. The capacity of mitochondria to take up or release Ca2+ can have substantial impact on the spaciotemporal dynamics of Ca2+ signaling in cardiomyocytes [25, 27]. It has been demonstrated recently that impaired mitochondrial function, with depolarized mitochondrial membrane potential and ATP depletion, can lead to calcium transient alternans by affecting the capacity of the mitochondrial network to handle Ca2+ on a beat-to-beat basis. This change predisposes to the development of cardiac arrhythmias [138]. Pharmacological inhibition of MCU with Ru360 has been reported to reduce the incidence of ventricular arrhythmias induced by ischemia-reperfusion in the rat heart [139]. Increased oxidative stress has also been shown to alter mitochondrial [Ca2+] through modulating mitochondrial NCX function or cytosolic Ca2+ levels, contributing to the perturbation of intracellular Ca2+ homeostatsis [129, 140, 141]. For instance, increased oxidative stress during ischemia can depolarize ΔΨm [142] and increase cytosolic Ca2+ levels [121, 122], which can force mitochondrial NCX into reverse transport mode and transport Ca2+ into cytosol [94, 143].
As discussed earlier, mitochondrial Ca2+ is a positive effector of OXPHOS under physiological conditions, yet Ca2+ overload can lead to mitochondrial dysfunction, at least partially, by increasing ROS production [69–71]. Multiple mechanisms have been proposed to explain Ca2+-induced mitochondrial ROS production: (1) Enhanced ROS output from increased respiratory chain activity: Increased Ca2+ stimulates TCA cycle and OXPHOS, and enhanced metabolic rate simply results in more respiratory chain electron leakage and ROS as by-products [144, 145]. (2) Stimulation of nitric oxide synthase (NOS): Ca2+ is known to activate NOS and generate NO•, which has been shown to inhibit complex IV and enhance ROS generation from the Q cycle at complex III [146]. (3)Cytochrome c-mediated ETC inhibition: increased Ca2+ can enhance cytochrome c dislocation from mitochondrial inner membrane, either by competing cardiolipin binding sites or by increasing PTP opening, which can result in complex III inhibition and enhanced ROS generation [147, 148]. (4) Cross-talk with K+ influx: as discussed earlier, mitochondrial Ca2+ overload can activate KCa/mitoKATP, leading to increased K+ influx and enhanced ROS production [102, 149].
Taken together, current evidence suggests a reciprocal interaction between Ca2+-induced ROS production and ROS-induced Ca2+-overload. The cross-talk between Ca2+ and ROS regulatory systems is normally under tight control to maintain normal physiological functions. With pathological stimuli such as myocardial ischemia and pressure/volume overload, multiple signaling and ionic mechanisms can lead to increased cellular and mitochondrial Ca2+ levels, resulting in increased ROS generation. Overt ROS production triggered by increased mitochondrial Ca2+ signals can lead to further increase in mitochondrial Ca2+ and ROS levels. This positive feed-forward loop consisting of Ca2+-induced ROS production, ROS-induced ROS release and ROS-induced Ca2+ overload can exceed cellular capacity of ROS scavenging and Ca2+ clearance, resulting in cellular damage and death [141].
ROS, mitochondria and cardiac potassium channels
Multiple voltage-gated K+ (Kv) channels and non-voltage-gated inwardly rectifying (Kir) channels contribute to myocardial action potential repolarization [38, 39]. Functional K+ channels are integral membrane protein complexes consisting of pore-forming (α) subunits, multiple accessory (β) subunits and regulatory proteins [38, 39]. The α subunits of Kv channels are six-transmembrane-spanning domain (S1-S6) proteins, and functional Kv channels are composed of four α-subunits; In addition, a number of different types of Kv channel accessory subunits have been identified and shown to interact with Kv α subunits to modulate channel biophysical properties and cell surface expression [39, 150].
Based on differences in time- and voltage-dependent properties and pharmacological sensitivities, two main types of Kv channels have been distinguished: transient outward Kv (Ito) and delayed rectifier Kv (IK) currents. Currents classified as Ito activate and inactivate rapidly upon membrane depolarization and underlie early (phase 1) repolarization, whereas IK currents activate on depolarization with variable kinetics and underlie late (phase 2 & 3) repolarization [39]. The heterogeneities in the biophysical properties and expression levels of various K+ currents contribute to the inter-species and inter-regional differences in action potential waveforms [39, 151].
Similar to the Kv channels, multiple functionally distinct types of Kir channel pore-forming α-subunits (Kir1-6) have been identified. The Kir α-subunits, like Kv channels, assemble as tetramers to form functional Kir channels [39, 152, 153], although Kir α-subunits have only two, instead of six, transmembrane domains. It has been shown that cardiac IK1 channels reflect the heteromeric assembly of the Kir α-subunits, Kir 2.1 and Kir 2.2 [154–158] and that the predominant form of KATP channels in cardiac sarcolemma reflects the assembly of Kir6.2 and SUR2A [159]. While Kv currents contribute importantly to the repolarization of action potentials in mammalian ventricular myocardium, the Kir currents also contribute to shaping the resting and active membrane properties of cardiomyocytes. Among several types of Kir currents expressed in mammalian heart, IK1 contributes to the terminal phase of repolarization and the maintenance of resting membrane potentials in mammalian ventricular myocytes [39, 152, 153], whereas current conducted by sarcKATP channels (IKATP), gated by intracellular ATP/ADP levels, plays an important role in regulating cellular metabolism and electrophysiological responses to metabolic stresses such as myocardial ischemia [160, 161].
Increased ROS have been shown to reduce the expression of Kv currents, including Ito and multiple delayed rectifier IK (including IKr, IKs and IKur) currents in ventricular cardiomyocytes [162–164], which can be reversed by increasing intracellular redox buffer reduced glutathione (GSH) [165, 166]. Decreased repolarizing Kv currents in cardiac ventricles can lead to delayed repolarization and prolonged action potential duration (APD), predisposing to the development of ventricular arrhythmias [39]. It has been shown that increased oxidative stress can impact the repolarizing Kv currents by modulating the expression levels of the mRNA and protein encoding these K+ channels [164] or by altering the phosphorylation status of these K+ channels through modulating the activities of PKC, PKA or protein tyrosine phosphatases [167–169]. It is not clear whether increased ROS can modulate Kv channel activities through direct oxidation of thiol groups.
Mitochondria also regulate myocyte membrane excitability through modulating sarcKATP channels. The sarcKATP channels, gated by ATP and thereby sensing the cellular energy status, are present at high density in the sarcolemmal membrane. SarcKATP channels are inhibited by ATP and activated by ADP, Mg or low pH, conditions that are associated with inadequate fuel supply, ischemia, and increased oxidative stress [104]. Upon ATP depletion and increased oxidative stress (e.g., during myocardial ischemia), sarcKATP channels are triggered to open, producing an inwardly rectifying repolarizing current. Because of the high cell surface expression density of sarcKATP channels, myocardial APD can be significantly shortened even with the opening of only 1% of the sarcKATP channels [170]. The opening of sarcKATP channels may be intrinsically protective against ATP depletion: shortened APD can reduce inward Ca2+ transient, thereby decreasing Ca2+-mediated cardiac energy consumption and preventing Ca2+ overload-induced cell death. Nevertheless, with adequate numbers of sarcKATP channels in the open state, cardiomyocytes can become hyperpolarized and rendered unexcitable [171]. This creates a current sink capable of slowing or blocking electrical propagation in the myocardium, promoting the development of cardiac arrhythmia [36, 172]. Indeed, it has been demonstrated that pharmacological inhibition of sarcKATP channels can reduce the incidence of ventricular arrhythmias in animal models [173–175] and in human [176–178].
Upon substrate deprivation or increased oxidative stress, sarcKATP currents and APD oscillate [179, 180]. The oscillations are synchronized with the fluctuations in mitochondrial membrane potential, ΔΨm [35]. Under conditions of increased oxidative stress, ΔΨm depolarizes, diminishing the amount of free energy available for ATP production [35]. There is evidence suggesting that ΔΨm depolarization of mitochondrial network is mediated by cell-wide ROS production induced by focal increases in mitochondrial ROS, a process known as “ROS-induced ROS release” [54, 181]. The inner membrane anion channel (IMAC) has been shown to play an important role in influencing ΔΨm. The use of IMAC inhibitors can prevent mitochondrial depolarization of ΔΨm, preventing the oscillation in KATP currents and APDs upon increased oxidative stress [35]. In addition, pharmacological inhibition of IMAC was shown to prevent ventricular arrhythmias in intact mammalian hearts upon increased oxidative stress or ischemia [182, 183].
Another group of KATP channels are located on the inner membrane of the mitochondria (mitoKATP channels), which have been shown to play an important role in mediating the protective effects of ischemic preconditioning [103, 104]. In the resting heart, mitoKATP channel opening increases mitochondrial ROS production, which in turn triggers downstream signaling leading to gene transcription and cell growth [184]. The opening of mitoKATP channels before the onset of ischemia allows K+ influx in mitochondria, partially dissipating ΔΨm (“partial uncoupling”), which results in a compensatory increase in proton pumping and cellular respiration to maintain ΔΨm and oxidative phosphorylation [103]. In addition, as ΔΨm is depolarized during ischemia, mitoKATP opening provides additional K+ influx to complensate for the lower driving force and to maintain mitochondrial volume, which is essential for maintaining a functioning ETC system [184]. Blocking mitoKATP channels abolishes the anti-arrhythmic effects of ischemic preconditioning [185], although some, but not all, pharmacological openers of mitoKATP channels were shown to be protective against ischemia-induced arrhythmias [175, 186, 187]. It is important to point out that many of the reports on mitoKATP channel function were based on results using pharmacological agents like diazoxide (mitoKATP channel opener) or hydroxydeconate (5-HD, a mitoKATP channel inhibitor), which are known to have other effects on mitochondrial function [184]. Recently, ROMK (Kcnj1) has been identified to encode a mitoKATP channel [188]. Targeting the molecular identity of mitoKATP channel may circumvent the caveats of a pharmacological approach and provide direct evidence of mitoKATP function.
Mitochondrial ROS and cardiac gap junction remodeling
Gap junctions, the membrane channels formed by the assembly of a pair of hemichannels consist of six connexin proteins, mediate the cell-to-cell communication of small metabolites and ions and play a critical role in cardiac impulse conduction [189]. There are three major connexin isoforms expressed in the heart: connexin (Cx) 40, Cx43 and Cx45. While Cx43 is extensively expressed in both the atrial and ventricular cardiomyocytes, Cx40 is predominantly expressed in the atria and specialized conduction system. Cx45 expression is restricted to the sinoatrial node, atrioventricular node and adjoining His bundles [190]. Cx43 expression is known to be downregulated in various cardiac diseases, including cardiac hypertrophy [191], heart failure [192, 193], myocardial ischemia [194, 195] and cardiomyopathy [195]. Reduction in Cx43 can lead to slow conduction velocity, increased heterogeneity and exaggerated anisotropic properties of the ventricles [196, 197], all of which are arrhythmogenic and may facilitate the initiation and maintenance of ventricular arrhythmias [198, 199].
The activation of renin-angiotensin system (RAS), a hallmark of cardiomyopathy and heart failure [200, 201], is known to increased myocardial oxidative stress and downregulate ventricular Cx43 [202–204]. Transgenic mouse models with increased cardiac RAS activity [204, 205] have been shown to have high incidence of conduction block, ventricular arrhythmias and sudden death because of reduced cardiac Cx43 and impaired gap junction function [204, 205]. Using a gene targeted, cardiac-specific angiotensin converting enzyme overexpression mouse model (ACE8/8) [204], we have demonstrated that enhanced RAS signaling results in increased expression of the activated form of cSrc (p-cSrc at Tyr416), a redox-sensitive tyrosine kinase, in ventricular myocardium. This activation leads to Cx43 reduction, impaired gap junction function, and subsequent increase in the propensity for ventricular arrhythmias and sudden cardiac death [13, 206]. The downregulation of Cx43 and increased arrhythmia risk in ACE8/8 mice can be ameliorated by pharmacologic inhibition of RAS [207] and cSrc [206]. It is known that increased myocardial p-cSrc results in the downregulation of Cx43 via the competition between p-cSrc and Cx43 for a binding site at zonula occludens-1, an intercalated disk scaffolding protein, leading to Cx43 destabilization and degradation [208]. Increased p-cSrc levels can also impair gap junction function through tyrosine phosphorylation of Cx43 [209]. Using the same ACE8/8 mouse model, we have recently demonstrated that cardiac ROS, specifically mitochondrial ROS, were markedly increased with enhanced RAS signaling [13, 206]. Treatment with mitochondria-targeted antioxidant, MitoTEMPO, but not the other types of antioxidants, reduces cSrc phosphorylation, restores the Cx43 expression, normalizes gap junction conduction, as well as ameliorates ventricular arrhythmias and sudden cardiac death in ACE8/8 mice [13]. These data suggest that mitochondrial oxidative stress plays a critical role in AngII–induced gap junction remodeling and arrhythmia. As mitochondrial ROS are increased in cardiac diseases such as cardiac hypertrophy [48], myocardial ischemia [50, 210] and heart failure [51, 52], conditions that are known to be associated with RAS activation, ventricular Cx43 downregulation and increased risk of arrhythmias, it would be of great interest to test if the treatment with mitochondria-targeted antioxidant can normalize Cx43 expression and prevent life-threatening arrhythmias in these pathological conditions.
Conclusion
In summary, mitochondrial dysfunction is prevalent in arrhythmogenic cardiac diseases including cardiac hypertrophy, heart failure and myocardial ischemia. Reduced ATP synthesis and increased ROS production associated with mitochondrial dysfunction can lead to malfunction of various cellular mechanisms that are required to maintain normal electrical functioning and intracellular ionic homeostasis in cardiomyocytes. As summarized in Figure 2 and Table 1, mitochondrial dysfunction can lead to reduced peak INa and downregulation of Cx43, resulting in abnormal conduction and increased propensity for re-entrant type cardiac arrhythmias. Increased cellular ROS also increases late INa and reduces repolarizing Kv currents, leading to impaired repolarization, prolonged APD, EADs, increased electrical heterogeneity in the myocardium, and increased arrhythmia susceptibility. Mitochondrial dysfunction also leads to disrupted intracellular Ca2+ homeostasis in cardiomyocytes, resulting in cytosolic Ca2+ overload and proarrhythmic DADs. Finally, mitochondrial dysfunction also causes the depolarization of ΔΨm and the opening of sarcoKATP channels, creating a current sink for the propagating depolarization wave, potentiating conduction block and arrhythmia. These observations suggest that mitochondria-targeted antioxidants may prove a more efficacious alternative to traditional ion channel blocking drugs to address arrhythmia in associated with cardiac diseases.
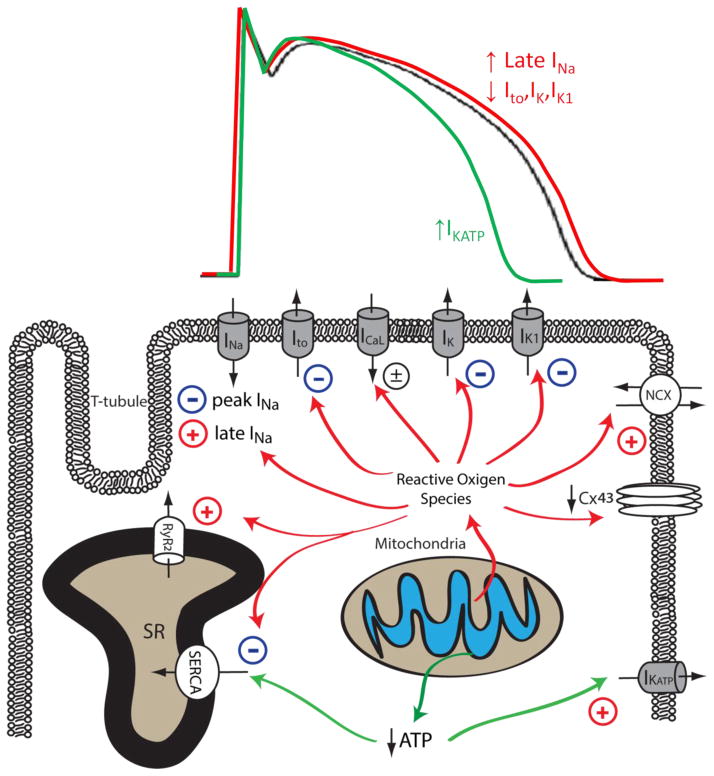
Figure 2. Effects of mitochondrial dysfunction on myocardial ion channels and calcium handling proteins.
Mitochondrial dysfunction upon increased pathological or metabolic stress can lead to increased ROS production and reduced ATP synthesis. Increased cellular ROS can lead to the inhibition of peak INa, Ito, IK, IK1, SERCA and the downregulation of Cx43, whereas the activity of late INa, NCX and RyR are enhanced by increased oxidative stress. Reduced ATP production secondary to mitochondrial dysfunction can inhibit the activity of SERCA and increase the activity of sarcolemmal KATP channels. The impact of mitochondrial dysfunction on AP waveforms is illustrated on top: increased mitochondrial ROS production can prolong AP duration by increasing late INa and reducing repolarizing K+ currents (Red). Under conditions such as acute ischemia, mitochondrial ATP production is reduced, leading to opening of sarcolemmal KATP channel and AP shortening (Green). The Baseline AP waveform is shown in black.
Table 1.
Impact of mitochondrial dysfunction on cardiac arrhythmogenicity
| Target Channel/Transporter | Impact of Mitochondrial Dysfunction | Impact on electrical/ionic homeostasis | Pro-arrhythmic mechanism |
|---|---|---|---|
| Peak INa | ↓ | ↓ Na+ influx | Slow conduction |
| Late INa | ↑ | ↑ Na+ influx, prolonged APD | EAD |
| Ito | ↓ | ↓ K+ influx, prolonged APD | EAD |
| ICaL | ± | ||
| IK | ↓ | ↓ K+ influx, prolonged APD | EAD |
| IK1 | ↓ | ↓ K+ influx, prolonged APD | EAD |
| IKATP | ↑ | ↑ K+ influx, shortened APD | Current sink, slow conduction |
| NCX | ↑ | Cytosolic Ca2+ overload | DAD |
| Cx43 | ↓ | Impaired gap junction function | Slow conduction |
| SERCA | ↓ | Cytosolic Ca2+ overload | DAD |
| RyR2 | ↑ | Cytosolic Ca2+ overload | DAD |
| mitoNCX | Reverse mode | Cytosolic Ca2+ overload | DAD |
| mitoKATP/mitoKCa | ↑ | ↑Mitochondrial K+ influx | Protective, mechanism of ischemic-preconditioning |
Peak INa: peak Na+ current; Late INa: late Na+ current; Ito: transient outward K+ current; ICaL: L-type Ca2+ current; IK: delayed rectifier K+ current; IK1: inwardly rectifying K+ current; IKATP: ATP-sensitive K+ current; NCX: Na+/Ca2+ exchanger; Cx43: connexin 43; SERCA: sarco/endoplasmic reticulum Ca2+-ATPase; RyR2: ryanodine receptor 2; mitoNCX: mitochondrial Na+/Ca2+ exchanger; mitoKATP: mitochondrial ATP-sensitive K+ current; mitoKCa: mitochondrial Ca2+-sensitive K+ current; APD: action potential duration; EAD: early after-depolarization; DAD: delayed after-depolarization.
Highlights.
We review the mechanisms by which mitochondrial dysfunction causes arrhythmias
Mitochondrial dysfunction leads to increased reactive oxidative species production
Mitochondrial dysfunction impairs sarcolemmal and sarcoplasmic channel functions
Mitochondrial dysfunction results in impaired intracellular cation homeostasis
The use of mitochondria-targeted antioxidants might be a novel antiarrhythmic therapy
Acknowledgments
This work was funded by National Institutes of Health (NIH) Grants RO1 HL104025 (SCD), HL106592 (SCD), a Veterans Affairs MERIT grants BX000859 (SCD) and American Heart Association Midwest Affiliation Postdoctoral Fellowship AHA13POST14380029 (KCY); Dr. Bonini is funded by American Heart Association (13GRNT16400010; 09SDG2250933) and the U.S. Department of Defense W911NF-12-1-0493).
Footnotes
Conflict of Interest:
Dr. Dudley is an inventor of 13/551,790 A Method for Ameliorating or Preventing Arrhythmic Risk Associated with Cardiomyopathy by Improving Conduction Velocity and 13/507,319 A Method for Modulating or Controlling Connexin43 (Cx43) Level of a Cell and Reducing Arrhythmic Risk
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris DA, Das AM. Control of mitochondrial ATP synthesis in the heart. Biochem J. 1991;280(Pt 3):561–573. doi: 10.1042/bj2800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaper J, Meiser E, Stammler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res. 1985;56:377–391. doi: 10.1161/01.res.56.3.377. [DOI] [PubMed] [Google Scholar]
- 4.Schramm M, Klieber HG, Daut J. The energy expenditure of actomyosin-ATPase, Ca2+-ATPase and Na+, K+-ATPase in guinea-pig cardiac ventricular muscle. J Physiol. 1994;481(Pt 3):647–662. doi: 10.1113/jphysiol.1994.sp020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overend CL, Eisner DA, O’Neill SC. Altered cardiac sarcoplasmic reticulum function of intact myocytes of rat ventricle during metabolic inhibition. Circ Res. 2001;88:181–187. doi: 10.1161/01.res.88.2.181. [DOI] [PubMed] [Google Scholar]
- 6.Silverman HS, Stern MD. Ionic basis of ischaemic cardiac injury: insights from cellular studies. Cardiovasc Res. 1994;28:581–597. doi: 10.1093/cvr/28.5.581. [DOI] [PubMed] [Google Scholar]
- 7.Broadley SA, Hartl FU. Mitochondrial stress signaling: a pathway unfolds. Trends Cell Biol. 2008;18:1–4. doi: 10.1016/j.tcb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol. 2011;301:H647–653. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pung YF, Sam WJ, Stevanov K, Enrick M, Chen CL, Kolz C, Thakker P, Hardwick JP, Chen YR, Dyck JR, Yin L, Chilian WM. Mitochondrial oxidative stress corrupts coronary collateral growth by activating adenosine monophosphate activated kinase-alpha signaling. Arterioscler Thromb Vasc Biol. 2013;33:1911–1919. doi: 10.1161/ATVBAHA.113.301591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sripathi SR, He W, Atkinson CL, Smith JJ, Liu Z, Elledge BM, Jahng WJ. Mitochondrial-nuclear communication by prohibitin shuttling under oxidative stress. Biochemistry. 2011;50:8342–8351. doi: 10.1021/bi2008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun JZ, Tang XL, Park SW, Qiu Y, Turrens JF, Bolli R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. The Journal of clinical investigation. 1996;97:562–576. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. The Journal of biological chemistry. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 13.Sovari AA, Rutledge CA, Jeong EM, Dolmatova E, Arasu D, Liu H, Vahdani N, Gu L, Zandieh S, Xiao L, Bonini MG, Duffy HS, Dudley SC., Jr Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circulation. Arrhythmia and electrophysiology. 2013;6:623–631. doi: 10.1161/CIRCEP.112.976787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders PN, Koval OM, Jaffer OA, Prasad AM, Businga TR, Scott JA, Hayden PJ, Luczak ED, Dickey DD, Allamargot C, Olivier AK, Meyerholz DK, Robison AJ, Winder DG, Blackwell TS, Dworski R, Sammut D, Wagner BA, Buettner GR, Pope RM, Miller FJ, Jr, Dibbern ME, Haitchi HM, Mohler PJ, Howarth PH, Zabner J, Kline JN, Grumbach IM, Anderson ME. CaMKII is essential for the proasthmatic effects of oxidation. Science translational medicine. 2013;5:195ra197. doi: 10.1126/scitranslmed.3006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zafari AM, Harrison DG. Free radicals in heart failure: therapeutic targets for old and new drugs. Congest Heart Fail. 2002;8:129–130. doi: 10.1111/j.1527-5299.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- 16.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal NT, Makielski JC. Redox control of cardiac excitability. Antioxid Redox Signal. 2013;18:432–468. doi: 10.1089/ars.2011.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami M, Okabe E. Superoxide anion radical-triggered Ca2+ release from cardiac sarcoplasmic reticulum through ryanodine receptor Ca2+ channel. Mol Pharmacol. 1998;53:497–503. doi: 10.1124/mol.53.3.497. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 20.Clerk A, Michael A, Sugden PH. Stimulation of multiple mitogen-activated protein kinase sub-families by oxidative stress and phosphorylation of the small heat shock protein, HSP25/27, in neonatal ventricular myocytes. Biochem J. 1998;333(Pt 3):581–589. doi: 10.1042/bj3330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Windt LJ, Lim HW, Haq S, Force T, Molkentin JD. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J Biol Chem. 2000;275:13571–13579. doi: 10.1074/jbc.275.18.13571. [DOI] [PubMed] [Google Scholar]
- 22.Shang LL, Sanyal S, Pfahnl AE, Jiao Z, Allen J, Liu H, Dudley SC., Jr NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. 2008;294:C372–379. doi: 10.1152/ajpcell.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy E, Eisner DA. Regulation of intracellular and mitochondrial sodium in health and disease. Circ Res. 2009;104:292–303. doi: 10.1161/CIRCRESAHA.108.189050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.JOU, Pan S, Sheu SS. Perspectives on: SGP symposium on mitochondrial physiology and medicine: molecular identities of mitochondrial Ca2+ influx mechanism: updated passwords for accessing mitochondrial Ca2+-linked health and disease. J Gen Physiol. 2012;139:435–443. doi: 10.1085/jgp.201210795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochondrial calcium uptake. Proc Natl Acad Sci U S A. 2013;110:10479–10486. doi: 10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drago I, De Stefani D, Rizzuto R, Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci U S A. 2012;109:12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garlid KD, Paucek P. Mitochondrial potassium transport: the K+ cycle. Biochim Biophys Acta. 2003;1606:23–41. doi: 10.1016/s0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 29.Kaasik A, Safiulina D, Zharkovsky A, Veksler V. Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol. 2007;292:C157–163. doi: 10.1152/ajpcell.00272.2006. [DOI] [PubMed] [Google Scholar]
- 30.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J Biol Chem. 2001;276:2586–2599. doi: 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
- 31.Territo PR, Mootha VK, French SA, Balaban RS. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol. 2000;278:C423–435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 32.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 33.Brown DA, O’Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc Res. 2010;88:241–249. doi: 10.1093/cvr/cvq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 36.Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fozzard HA. Excitation-contraction coupling in the heart. Adv Exp Med Biol. 1991;308:135–142. doi: 10.1007/978-1-4684-6015-5_11. [DOI] [PubMed] [Google Scholar]
- 38.Roden DM, Balser JR, George AL, Jr, Anderson ME. Cardiac ion channels. Annu Rev Physiol. 2002;64:431–475. doi: 10.1146/annurev.physiol.64.083101.145105. [DOI] [PubMed] [Google Scholar]
- 39.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 40.Kanno S, Saffitz JE. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001;10:169–177. doi: 10.1016/s1054-8807(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 41.ter Keurs HE. Electromechanical coupling in the cardiac myocyte; stretch-arrhythmia feedback. Pflugers Arch. 2011;462:165–175. doi: 10.1007/s00424-011-0944-3. [DOI] [PubMed] [Google Scholar]
- 42.Jafri MS, Dudycha SJ, O’Rourke B. Cardiac energy metabolism: models of cellular respiration. Annu Rev Biomed Eng. 2001;3:57–81. doi: 10.1146/annurev.bioeng.3.1.57. [DOI] [PubMed] [Google Scholar]
- 43.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisiger RA, Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]
- 46.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 47.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54:1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Novalija E, Kevin LG, Eells JT, Henry MM, Stowe DF. Anesthetic preconditioning improves adenosine triphosphate synthesis and reduces reactive oxygen species formation in mitochondria after ischemia by a redox dependent mechanism. Anesthesiology. 2003;98:1155–1163. doi: 10.1097/00000542-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 50.Tompkins AJ, Burwell LS, Digerness SB, Zaragoza C, Holman WL, Brookes PS. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta. 2006;1762:223–231. doi: 10.1016/j.bbadis.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 52.Liu M, Gu L, Sulkin MS, Liu H, Jeong EM, Greener I, Xie A, Efimov IR, Dudley SC., Jr Mitochondrial dysfunction causing cardiac sodium channel downregulation in cardiomyopathy. J Mol Cell Cardiol. 2013;54:25–34. doi: 10.1016/j.yjmcc.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenner C, Moulin M. Physiological roles of the permeability transition pore. Circ Res. 2012;111:1237–1247. doi: 10.1161/CIRCRESAHA.112.265942. [DOI] [PubMed] [Google Scholar]
- 54.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 55.Brady NR, Hamacher-Brady A, Westerhoff HV, Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- 56.Werns SW, Fantone JC, Ventura A, Lucchesi BR. Myocardial glutathione depletion impairs recovery of isolated blood-perfused hearts after global ischaemia. J Mol Cell Cardiol. 1992;24:1215–1220. doi: 10.1016/0022-2828(92)93088-2. [DOI] [PubMed] [Google Scholar]
- 57.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 58.Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J Biol Chem. 2003;278:37223–37230. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- 60.Tatarkova Z, Kuka S, Racay P, Lehotsky J, Dobrota D, Mistuna D, Kaplan P. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2011;60:281–289. doi: 10.33549/physiolres.932019. [DOI] [PubMed] [Google Scholar]
- 61.Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS Lett. 2000;466:323–326. doi: 10.1016/s0014-5793(00)01082-6. [DOI] [PubMed] [Google Scholar]
- 62.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972;128:161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 65.Hom JR, Gewandter JS, Michael L, Sheu SS, Yoon Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol. 2007;212:498–508. doi: 10.1002/jcp.21051. [DOI] [PubMed] [Google Scholar]
- 66.Denton RM, Richards DA, Chin JG. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978;176:899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szabadkai G, Simoni AM, Bianchi K, De Stefani D, Leo S, Wieckowski MR, Rizzuto R. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Przygodzki T, Sokal A, Bryszewska M. Calcium ionophore A23187 action on cardiac myocytes is accompanied by enhanced production of reactive oxygen species. Biochim Biophys Acta. 2005;1740:481–488. doi: 10.1016/j.bbadis.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Duan Y, Gross RA, Sheu SS. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol. 2007;585:741–758. doi: 10.1113/jphysiol.2007.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kowaltowski AJ, Naia-da-Silva ES, Castilho RF, Vercesi AE. Ca2+-stimulated mitochondrial reactive oxygen species generation and permeability transition are inhibited by dibucaine or Mg2+ Arch Biochem Biophys. 1998;359:77–81. doi: 10.1006/abbi.1998.0870. [DOI] [PubMed] [Google Scholar]
- 72.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bazil JN, Dash RK. A minimal model for the mitochondrial rapid mode of Ca2+ uptake mechanism. PLoS One. 2011;6:e21324. doi: 10.1371/journal.pone.0021324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim Biophys Acta. 2001;1504:248–261. doi: 10.1016/s0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 76.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 77.Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH. The mitochondrial ryanodine receptor in rat heart: a pharmaco-kinetic profile. Biochim Biophys Acta. 2007;1768:1784–1795. doi: 10.1016/j.bbamem.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 78.Michels G, Khan IF, Endres-Becker J, Rottlaender D, Herzig S, Ruhparwar A, Wahlers T, Hoppe UC. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 2009;119:2435–2443. doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- 79.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 80.Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 82.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103:279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seppet EK, Kaambre T, Sikk P, Tiivel T, Vija H, Tonkonogi M, Sahlin K, Kay L, Appaix F, Braun U, Eimre M, Saks VA. Functional complexes of mitochondria with Ca, MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim Biophys Acta. 2001;1504:379–395. doi: 10.1016/s0005-2728(00)00269-3. [DOI] [PubMed] [Google Scholar]
- 84.Ruiz-Meana M, Abellan A, Miro-Casas E, Agullo E, Garcia-Dorado D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. Am J Physiol Heart Circ Physiol. 2009;297:H1281–1289. doi: 10.1152/ajpheart.00435.2009. [DOI] [PubMed] [Google Scholar]
- 85.Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D. The SR-mitochondria interaction: a new player in cardiac pathophysiology. Cardiovasc Res. 2010;88:30–39. doi: 10.1093/cvr/cvq225. [DOI] [PubMed] [Google Scholar]
- 86.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bernardi P, von Stockum S. The permeability transition pore as a Ca2+ release channel: new answers to an old question. Cell Calcium. 2012;52:22–27. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 90.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 91.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Rourke B, Blatter LA. Mitochondrial Ca2+ uptake: tortoise or hare? J Mol Cell Cardiol. 2009;46:767–774. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jung DW, Apel LM, Brierley GP. Transmembrane gradients of free Na+ in isolated heart mitochondria estimated using a fluorescent probe. Am J Physiol. 1992;262:C1047–1055. doi: 10.1152/ajpcell.1992.262.4.C1047. [DOI] [PubMed] [Google Scholar]
- 94.Kim B, Matsuoka S. Cytoplasmic Na+-dependent modulation of mitochondrial Ca2+ via electrogenic mitochondrial Na+-Ca2+ exchange. J Physiol. 2008;586:1683–1697. doi: 10.1113/jphysiol.2007.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donoso P, Mill JG, O’Neill SC, Eisner DA. Fluorescence measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. J Physiol. 1992;448:493–509. doi: 10.1113/jphysiol.1992.sp019053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jelicks LA, Siri FM. Effects of hypertrophy and heart failure on [Na+]i in pressure-overloaded guinea pig heart. Am J Hypertens. 1995;8:934–943. doi: 10.1016/0895-7061(95)00219-F. [DOI] [PubMed] [Google Scholar]
- 97.Pogwizd SM, Sipido KR, Verdonck F, Bers DM. Intracellular Na in animal models of hypertrophy and heart failure: contractile function and arrhythmogenesis. Cardiovasc Res. 2003;57:887–896. doi: 10.1016/s0008-6363(02)00735-6. [DOI] [PubMed] [Google Scholar]
- 98.Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 99.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 100.Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–26069. [PubMed] [Google Scholar]
- 101.Brierley GP, Jurkowitz MS, Farooqui T, Jung DW. K+/H+ antiport in heart mitochondria. J Biol Chem. 1984;259:14672–14678. [PubMed] [Google Scholar]
- 102.Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am J Physiol Cell Physiol. 2007;292:C148–156. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- 103.O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Rourke B. Myocardial KATP channels in preconditioning. Circ Res. 2000;87:845–855. doi: 10.1161/01.res.87.10.845. [DOI] [PubMed] [Google Scholar]
- 105.Bezzina CR, Rook MB, Wilde AA. Cardiac sodium channel and inherited arrhythmia syndromes. Cardiovasc Res. 2001;49:257–271. doi: 10.1016/s0008-6363(00)00272-8. [DOI] [PubMed] [Google Scholar]
- 106.Schott JJ, Alshinawi C, Kyndt F, Probst V, Hoorntje TM, Hulsbeek M, Wilde AA, Escande D, Mannens MM, Le Marec H. Cardiac conduction defects associate with mutations in SCN5A. Nat Genet. 1999;23:20–21. doi: 10.1038/12618. [DOI] [PubMed] [Google Scholar]
- 107.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 108.Fozzard HA, Makielski JC. The electrophysiology of acute myocardial ischemia. Annu Rev Med. 1985;36:275–284. doi: 10.1146/annurev.me.36.020185.001423. [DOI] [PubMed] [Google Scholar]
- 109.Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res. 2004;61:208–217. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 110.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 111.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 112.Ma JH, Luo AT, Zhang PH. Effect of hydrogen peroxide on persistent sodium current in guinea pig ventricular myocytes. Acta Pharmacol Sin. 2005;26:828–834. doi: 10.1111/j.1745-7254.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- 113.Liu M, Liu H, Dudley SC., Jr Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ Res. 2010;107:967–974. doi: 10.1161/CIRCRESAHA.110.220673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC., Jr Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu M, Sanyal S, Gao G, Gurung IS, Zhu X, Gaconnet G, Kerchner LJ, Shang LL, Huang CL, Grace A, London B, Dudley SC., Jr Cardiac Na+ current regulation by pyridine nucleotides. Circ Res. 2009;105:737–745. doi: 10.1161/CIRCRESAHA.109.197277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 117.Moore RL, Yelamarty RV, Misawa H, Scaduto RC, Jr, Pawlush DG, Elensky M, Cheung JY. Altered Ca2+ dynamics in single cardiac myocytes from renovascular hypertensive rats. Am J Physiol. 1991;260:C327–337. doi: 10.1152/ajpcell.1991.260.2.C327. [DOI] [PubMed] [Google Scholar]
- 118.Bentivegna LA, Ablin LW, Kihara Y, Morgan JP. Altered calcium handling in left ventricular pressure-overload hypertrophy as detected with aequorin in the isolated, perfused ferret heart. Circ Res. 1991;69:1538–1545. doi: 10.1161/01.res.69.6.1538. [DOI] [PubMed] [Google Scholar]
- 119.O’Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 120.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 121.Varadarajan SG, An J, Novalija E, Smart SC, Stowe DF. Changes in [Na+](i), compartmental [Ca2+], and NADH with dysfunction after global ischemia in intact hearts. Am J Physiol Heart Circ Physiol. 2001;280:H280–293. doi: 10.1152/ajpheart.2001.280.1.H280. [DOI] [PubMed] [Google Scholar]
- 122.An J, Varadarajan SG, Novalija E, Stowe DF. Ischemic and anesthetic preconditioning reduces cytosolic [Ca2+] and improves Ca2+ responses in intact hearts. Am J Physiol Heart Circ Physiol. 2001;281:H1508–1523. doi: 10.1152/ajpheart.2001.281.4.H1508. [DOI] [PubMed] [Google Scholar]
- 123.Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 124.Goldhaber JI, Ji S, Lamp ST, Weiss JN. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea pig ventricle. Implications for ischemia and reperfusion injury. J Clin Invest. 1989;83:1800–1809. doi: 10.1172/JCI114085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Viola HM, Arthur PG, Hool LC. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ Res. 2007;100:1036–1044. doi: 10.1161/01.RES.0000263010.19273.48. [DOI] [PubMed] [Google Scholar]
- 126.Fearon IM. OxLDL enhances L-type Ca2+ currents via lysophosphatidylcholine-induced mitochondrial reactive oxygen species (ROS) production. Cardiovasc Res. 2006;69:855–864. doi: 10.1016/j.cardiores.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 127.Coetzee WA, Opie LH. Effects of oxygen free radicals on isolated cardiac myocytes from guinea-pig ventricle: electrophysiological studies. J Mol Cell Cardiol. 1992;24:651–663. doi: 10.1016/0022-2828(92)91049-b. [DOI] [PubMed] [Google Scholar]
- 128.Hammerschmidt S, Wahn H. The effect of the oxidant hypochlorous acid on the L-type calcium current in isolated ventricular cardiomyocytes. J Mol Cell Cardiol. 1998;30:1855–1867. doi: 10.1006/jmcc.1998.0749. [DOI] [PubMed] [Google Scholar]
- 129.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 130.Scherer NM, Deamer DW. Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2+-ATPase. Arch Biochem Biophys. 1986;246:589–601. doi: 10.1016/0003-9861(86)90314-0. [DOI] [PubMed] [Google Scholar]
- 131.Kukreja RC, Kearns AA, Zweier JL, Kuppusamy P, Hess ML. Singlet oxygen interaction with Ca2+-ATPase of cardiac sarcoplasmic reticulum. Circ Res. 1991;69:1003–1014. doi: 10.1161/01.res.69.4.1003. [DOI] [PubMed] [Google Scholar]
- 132.Xu KY, Zweier JL, Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca2+-ATPase function by direct attack on the ATP binding site. Circ Res. 1997;80:76–81. doi: 10.1161/01.res.80.1.76. [DOI] [PubMed] [Google Scholar]
- 133.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 134.Rokita AG, Anderson ME. New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII) Circulation. 2012;126:2125–2139. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eigel BN, Gursahani H, Hadley RW. ROS are required for rapid reactivation of Na+/Ca2+ exchanger in hypoxic reoxygenated guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;286:H955–963. doi: 10.1152/ajpheart.00721.2003. [DOI] [PubMed] [Google Scholar]
- 136.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271:H823–833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 137.Choudhary G, Dudley SC., Jr Heart failure, oxidative stress, and ion channel modulation. Congest Heart Fail. 2002;8:148–155. doi: 10.1111/j.1527-5299.2002.00716.x. [DOI] [PubMed] [Google Scholar]
- 138.Florea SM, Blatter LA. The role of mitochondria for the regulation of cardiac alternans. Front Physiol. 2010;1:141. doi: 10.3389/fphys.2010.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Garcia-Rivas GJ, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deng J, Wang G, Huang Q, Yan Y, Li K, Tan W, Jin C, Wang Y, Liu J. Oxidative stress-induced leaky sarcoplasmic reticulum underlying acute heart failure in severe burn trauma. Free Radic Biol Med. 2008;44:375–385. doi: 10.1016/j.freeradbiomed.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 141.Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed) 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Griffiths EJ, Ocampo CJ, Savage JS, Rutter GA, Hansford RG, Stern MD, Silverman HS. Mitochondrial calcium transporting pathways during hypoxia and reoxygenation in single rat cardiomyocytes. Cardiovasc Res. 1998;39:423–433. doi: 10.1016/s0008-6363(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 144.Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G. The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol B. 1998;168:149–158. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- 145.Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 1985;35:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- 146.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 147.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grijalba MT, Vercesi AE, Schreier S. Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+-stimulated generation of reactive oxygen species by the respiratory chain. Biochemistry. 1999;38:13279–13287. doi: 10.1021/bi9828674. [DOI] [PubMed] [Google Scholar]
- 149.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067–2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 150.Abbott GW, Xu X, Roepke TK. Impact of ancillary subunits on ventricular repolarization. J Electrocardiol. 2007;40:S42–46. doi: 10.1016/j.jelectrocard.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002;90:939–950. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- 152.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 153.Lopatin AN, Nichols CG. Inward rectifiers in the heart: an update on IK1. J Mol Cell Cardiol. 2001;33:625–638. doi: 10.1006/jmcc.2001.1344. [DOI] [PubMed] [Google Scholar]
- 154.McLerie M, Lopatin AN. Dominant-negative suppression of IK1 in the mouse heart leads to altered cardiac excitability. J Mol Cell Cardiol. 2003;35:367–378. doi: 10.1016/s0022-2828(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 155.Zobel C, Cho HC, Nguyen TT, Pekhletski R, Diaz RJ, Wilson GJ, Backx PH. Molecular dissection of the inward rectifier potassium current IK1 in rabbit cardiomyocytes: evidence for heteromeric co-assembly of Kir2.1 and Kir2.2. J Physiol. 2003;550:365–372. doi: 10.1113/jphysiol.2002.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K+ current IK1 as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 158.Nichols CG, Makhina EN, Pearson WL, Sha Q, Lopatin AN. Inward rectification and implications for cardiac excitability. Circ Res. 1996;78:1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- 159.Akrouh A, Halcomb SE, Nichols CG, Sala-Rabanal M. Molecular biology of KATP channels and implications for health and disease. IUBMB Life. 2009;61:971–978. doi: 10.1002/iub.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhuo ML, Huang Y, Liu DP, Liang CC. KATP channel: relation with cell metabolism and role in the cardiovascular system. Int J Biochem Cell Biol. 2005;37:751–764. doi: 10.1016/j.biocel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 161.Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- 162.Cerbai E, Ambrosio G, Porciatti F, Chiariello M, Giotti A, Mugelli A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991;84:1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- 163.Liang H, Li X, Li S, Zheng MQ, Rozanski GJ. Oxidoreductase regulation of Kv currents in rat ventricle. J Mol Cell Cardiol. 2008;44:1062–1071. doi: 10.1016/j.yjmcc.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X, Tang K, Xie B, Li S, Rozanski GJ. Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system. Am J Physiol Heart Circ Physiol. 2008;295:H416–424. doi: 10.1152/ajpheart.91446.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li S, Li X, Li YL, Shao CH, Bidasee KR, Rozanski GJ. Insulin regulation of glutathione and contractile phenotype in diabetic rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H1619–1629. doi: 10.1152/ajpheart.00140.2006. [DOI] [PubMed] [Google Scholar]
- 166.Xu Z, Patel KP, Lou MF, Rozanski GJ. Up-regulation of K+ channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc Res. 2002;53:80–88. doi: 10.1016/s0008-6363(01)00446-1. [DOI] [PubMed] [Google Scholar]
- 167.Chiarugi P. PTPs versus PTKs: the redox side of the coin. Free Radic Res. 2005;39:353–364. doi: 10.1080/10715760400027987. [DOI] [PubMed] [Google Scholar]
- 168.Nakamura TY, Coetzee WA, Vega-Saenz De Miera E, Artman M, Rudy B. Modulation of Kv4 channels, key components of rat ventricular transient outward K+ current, by PKC. Am J Physiol. 1997;273:H1775–1786. doi: 10.1152/ajpheart.1997.273.4.H1775. [DOI] [PubMed] [Google Scholar]
- 169.Li GR, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ Res. 1996;78:903–915. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]
- 170.Faivre JF, Findlay I. Action potential duration and activation of ATP-sensitive potassium current in isolated guinea-pig ventricular myocytes. Biochim Biophys Acta. 1990;1029:167–172. doi: 10.1016/0005-2736(90)90450-3. [DOI] [PubMed] [Google Scholar]
- 171.Bhatnagar A. Contribution of ATP to oxidative stress-induced changes in action potential of isolated cardiac myocytes. Am J Physiol. 1997;272:H1598–1608. doi: 10.1152/ajpheart.1997.272.4.H1598. [DOI] [PubMed] [Google Scholar]
- 172.Aon MA, Cortassa S, Akar FG, Brown DA, Zhou L, O’Rourke B. From mitochondrial dynamics to arrhythmias. Int J Biochem Cell Biol. 2009;41:1940–1948. doi: 10.1016/j.biocel.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vajda S, Baczko I, Lepran I. Selective cardiac plasma-membrane KATP channel inhibition is defibrillatory and improves survival during acute myocardial ischemia and reperfusion. Eur J Pharmacol. 2007;577:115–123. doi: 10.1016/j.ejphar.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 174.Fischbach PS, White A, Barrett TD, Lucchesi BR. Risk of ventricular proarrhythmia with selective opening of the myocardial sarcolemmal versus mitochondrial ATP-gated potassium channel. J Pharmacol Exp Ther. 2004;309:554–559. doi: 10.1124/jpet.103.060780. [DOI] [PubMed] [Google Scholar]
- 175.Wirth KJ, Rosenstein B, Uhde J, Englert HC, Busch AE, Scholkens BA. ATP-sensitive potassium channel blocker HMR 1883 reduces mortality and ischemia-associated electrocardiographic changes in pigs with coronary occlusion. J Pharmacol Exp Ther. 1999;291:474–481. [PubMed] [Google Scholar]
- 176.Cacciapuoti F, Spiezia R, Bianchi U, Lama D, D’Avino M, Varricchio M. Effectiveness of glibenclamide on myocardial ischemic ventricular arrhythmias in non-insulin-dependent diabetes mellitus. Am J Cardiol. 1991;67:843–847. doi: 10.1016/0002-9149(91)90617-t. [DOI] [PubMed] [Google Scholar]
- 177.Aronson D, Mittleman MA, Burger AJ. Effects of sulfonylurea hypoglycemic agents and adenosine triphosphate dependent potassium channel antagonists on ventricular arrhythmias in patients with decompensated heart failure. Pacing Clin Electrophysiol. 2003;26:1254–1261. doi: 10.1046/j.1460-9592.2003.t01-1-00177.x. [DOI] [PubMed] [Google Scholar]
- 178.Lomuscio A, Vergani D, Marano L, Castagnone M, Fiorentini C. Effects of glibenclamide on ventricular fibrillation in non-insulin-dependent diabetics with acute myocardial infarction. Coron Artery Dis. 1994;5:767–771. [PubMed] [Google Scholar]
- 179.Aon MA, Cortassa S, O’Rourke B. Mitochondrial oscillations in physiology and pathophysiology. Adv Exp Med Biol. 2008;641:98–117. doi: 10.1007/978-0-387-09794-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ryu SY, Lee SH, Ho WK. Generation of metabolic oscillations by mitoKATP and ATP synthase during simulated ischemia in ventricular myocytes. J Mol Cell Cardiol. 2005;39:874–881. doi: 10.1016/j.yjmcc.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 181.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O’Rourke B. Effects of 4’-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res. 2008;79:141–149. doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O’Rourke B. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol. 2010;48:673–679. doi: 10.1016/j.yjmcc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K+ channel in cardiac function and cardioprotection. Biochim Biophys Acta. 2003;1606:1–21. doi: 10.1016/s0005-2728(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 185.Vegh A, Parratt JR. The role of mitochondrial KATP channels in antiarrhythmic effects of ischaemic preconditioning in dogs. Br J Pharmacol. 2002;137:1107–1115. doi: 10.1038/sj.bjp.0704966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Headrick JP, Willems L, Ashton KJ, Holmgren K, Peart J, Matherne GP. Ischaemic tolerance in aged mouse myocardium: the role of adenosine and effects of A1 adenosine receptor overexpression. J Physiol. 2003;549:823–833. doi: 10.1113/jphysiol.2003.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Das B, Sarkar C. Is the sarcolemmal or mitochondrial KATP channel activation important in the antiarrhythmic and cardioprotective effects during acute ischemia/reperfusion in the intact anesthetized rabbit model? Life Sci. 2005;77:1226–1248. doi: 10.1016/j.lfs.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 188.Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, O’Rourke B. Mitochondrial ROMK channel is a molecular component of mitoKATP. Circ Res. 2012;111:446–454. doi: 10.1161/CIRCRESAHA.112.266445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lo CW. Role of gap junctions in cardiac conduction and development: insights from the connexin knockout mice. Circ Res. 2000;87:346–348. doi: 10.1161/01.res.87.5.346. [DOI] [PubMed] [Google Scholar]
- 190.Litchenberg WH, Norman LW, Holwell AK, Martin KL, Hewett KW, Gourdie RG. The rate and anisotropy of impulse propagation in the postnatal terminal crest are correlated with remodeling of Cx43 gap junction pattern. Cardiovasc Res. 2000;45:379–387. doi: 10.1016/s0008-6363(99)00363-6. [DOI] [PubMed] [Google Scholar]
- 191.Kostin S, Dammer S, Hein S, Klovekorn WP, Bauer EP, Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res. 2004;62:426–436. doi: 10.1016/j.cardiores.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 192.Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, Kaprielian R, Yacoub MH, Severs NJ. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 193.Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, Bauer EP, Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- 194.Kaprielian RR, Gunning M, Dupont E, Sheppard MN, Rothery SM, Underwood R, Pennell DJ, Fox K, Pepper J, Poole-Wilson PA, Severs NJ. Downregulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation. 1998;97:651–660. doi: 10.1161/01.cir.97.7.651. [DOI] [PubMed] [Google Scholar]
- 195.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 196.Jongsma HJ, Wilders R. Gap junctions in cardiovascular disease. Circ Res. 2000;86:1193–1197. doi: 10.1161/01.res.86.12.1193. [DOI] [PubMed] [Google Scholar]
- 197.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Efimov IR. Connections, connections, connexins: towards systems biology paradigm of cardiac arrhythmia. J Mol Cell Cardiol. 2006;41:949–951. doi: 10.1016/j.yjmcc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 199.van Rijen HV, van Veen TA, Gros D, Wilders R, de Bakker JM. Connexins and cardiac arrhythmias. Adv Cardiol. 2006;42:150–160. doi: 10.1159/000092567. [DOI] [PubMed] [Google Scholar]
- 200.Lonn EM, Yusuf S, Jha P, Montague TJ, Teo KK, Benedict CR, Pitt B. Emerging role of angiotensin-converting enzyme inhibitors in cardiac and vascular protection. Circulation. 1994;90:2056–2069. doi: 10.1161/01.cir.90.4.2056. [DOI] [PubMed] [Google Scholar]
- 201.Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst. 2004;5(Suppl 1):S7–10. doi: 10.3317/jraas.2004.024. [DOI] [PubMed] [Google Scholar]
- 202.De Mello WC. Renin-angiotensin system and cell communication in the failing heart. Hypertension. 1996;27:1267–1272. doi: 10.1161/01.hyp.27.6.1267. [DOI] [PubMed] [Google Scholar]
- 203.De Mello WC. Is an intracellular renin-angiotensin system involved in control of cell communication in heart? J Cardiovasc Pharmacol. 1994;23:640–646. doi: 10.1097/00005344-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 204.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr, Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, Bernstein KE. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, Berul CI, Breitbart RE. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35:1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 206.Sovari AA, Iravanian S, Dolmatova E, Jiao Z, Liu H, Zandieh S, Kumar V, Wang K, Bernstein KE, Bonini MG, Duffy HS, Dudley SC. Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death. J Am Coll Cardiol. 2011;58:2332–2339. doi: 10.1016/j.jacc.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Iravanian S, Sovari AA, Lardin HA, Liu H, Xiao HD, Dolmatova E, Jiao Z, Harris BS, Witham EA, Gourdie RG, Duffy HS, Bernstein KE, Dudley SC., Jr Inhibition of renin-angiotensin system (RAS) reduces ventricular tachycardia risk by altering connexin43. J Mol Med (Berl) 2011;89:677–687. doi: 10.1007/s00109-011-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–1112. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Tada M, Hori M. Functional role of c-Src in gap junctions of the cardiomyopathic heart. Circ Res. 1999;85:672–681. doi: 10.1161/01.res.85.8.672. [DOI] [PubMed] [Google Scholar]
- 210.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]