Abstract
Background
Heart failure (HF) is the leading cause of hospitalization among older Americans. Subsequent discharge to skilled nursing facilities (SNF) is not well described.
Methods and Results
We performed an observational analysis of Medicare beneficiaries ≥65 years of age, discharged alive to SNF or home after ≥3-day hospitalization for HF in 2005 and 2006 within the Get With The Guidelines–HF Program. Among 15 459 patients from 149 hospitals, 24.1% were discharged to an SNF, 22.3% to home with home health service, and 53.6% to home with self-care. SNF use varied significantly among hospitals (median, 10.2% versus 33.9% in low versus high tertiles), with rates highest in the Northeast. Patient factors associated with discharge to SNF included longer length of stay, advanced age, female sex, hypotension, higher ejection fraction, absence of ischemic heart disease, and a variety of comorbidities. Performance measures were modestly lower for patients discharged to SNF. Unadjusted absolute event rates were higher at 30 days (death, 14.4% versus 4.1%; rehospitalization, 27.0% versus 23.5%) and 1 year (death, 53.5% versus 29.1%; rehospitalization, 76.1% versus 72.2%) after discharge to SNF versus home, respectively (P<0.0001 for all). After adjustment for measured patient characteristics, discharge to SNF remained associated with increased death (hazard ratio, 1.76; 95% confidence interval, 1.66 to 1.87) and rehospitalization (hazard ratio, 1.08; 95% confidence interval, 1.03 to 1.14).
Conclusions
Discharge to SNF is common among Medicare patients hospitalized for HF, and these patients face substantial risk for adverse events, with more than half dead within 1 year. These findings highlight the need to better characterize this unique patient population and understand the SNF care they receive.
Keywords: heart failure, discharge status, disposition, skilled nursing facility, outcomes, risk, mortality, rehospitalization
After acute hospitalization, many older patients in the United States are discharged to skilled nursing facilities (SNF).1–4 Medicare benefits cover up to 100 days of SNF care for patients hospitalized for at least 3 days who also have a skilled need.5 This SNF care accounts for over $20 billion in Medicare costs annually.6 Although heart failure (HF) is the leading cause of hospitalization and rehospitalization for Medicare patients,7 variations in the use of SNF and clinical outcomes of patients discharged to SNF after HF hospitalization are relatively unknown. Consequently, efforts to learn more about the discharge status and related outcomes of patients after HF hospitalization are needed.8
The Get With The Guidelines–Heart Failure (GWTG-HF) registry linked to Centers for Medicare and Medicaid Services (CMS) claims data provides an ideal cohort of hospitalized patients with HF to further examine discharge status and differences in care and outcomes of patients discharged to SNF.9,10 We set out to describe patient and hospital characteristics associated with discharge to SNF, assess hospital performance measures for patients discharged to SNF, and determine mortality and rehospitalization rates for HF patients discharged to SNF as compared with home.
Methods
Settings and Patients
Baseline patient data were obtained from the GWTG-HF Program, an ongoing observational, voluntary, continuous quality-improvement initiative of the American Heart Association.9 Participating institutions submit patient information on consecutive eligible patients admitted to the hospital with a primary diagnosis of HF regardless of left ventricular ejection fraction (LVEF). Trained personnel abstract the data using standardized definitions. Data collection includes demographics, clinical characteristics, use and contraindications for evidence-based therapies, in-hospital outcomes, and admission and discharge location. Clinical data use the point-of-service, interactive, Internet-based Patient Management Tool (Outcome Sciences, Inc, Cambridge, MA). The Internet-based system performs checks to ensure the completeness of the reported data. Additionally, data quality is monitored independently, and reports are generated to confirm the completeness and accuracy of submitted data. All participating institutions are required to comply with local regulatory and privacy guidelines and to submit the program protocols for review and approval by their institutional review boards. Because data are used primarily at the local site for quality improvement, sites are granted a waiver of informed consent under the common rule. Outcome Sciences, Inc, serves as the data collection and coordination center for GWTG-HF. The Duke Clinical Research Institute serves as the data analysis center.
GWTG-HF registry patients were linked with inpatient CMS claims files using indirect identifiers as previously published.10 To be eligible for study inclusion, patients had to be discharged alive between January 1, 2005, and December 31, 2006, from a hospital fully participating in the GWTG-HF program, age 65 years or older, and enrolled in fee-for-service Medicare. Hospitals with >25% of GWTG-HF history panel forms incomplete and hospitals with <25 eligible study records were excluded. Under these criteria, 23 509 patients were initially eligible for the study. If there were multiple hospitalizations for the same patient during the eligibility period, we selected only the first. For the purposes of creating comparable groups for modeling outcomes by discharge to SNF versus home, we excluded patients discharged to a location other than SNF or home under self-care or home health (n=3207; including another short-term hospital for inpatient care, n=403; intermediate care facility, n=611; rehabilitation facility, n=405; long-term care hospital, n=91; hospice at home, n=244; hospice at medical facility, n=197), patients with length of stay less than 3 days who would technically not meet CMS criteria for postacute SNF (n=4593), and patients admitted from a SNF (n=250). The final study population consisted of 15 459 unique older patients hospitalized at 149 sites.
Outcome Measures
The main outcome measures were all-cause mortality and all-cause rehospitalization at 30 days and 1 year from the time of discharge, chosen because of ongoing public reporting efforts targeting these measures.11–13
Data Analyses
Continuous variables are presented as medians with differences between the 25th and 75th percentiles and categorical variables are presented as percentages. We compared baseline characteristics for patients discharged to SNF versus those discharged elsewhere using χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables. We also compared characteristics among hospitals divided by high, middle, and low tertiles for rates of SNF discharge. Event rates for mortality and rehospitalization were calculated using the Kaplan-Meier method.
Multivariable logistic regression modeling was used to assess in-hospital patient and institutional correlates of discharge to SNF. A generalized estimating equation method was used to adjust for clustering within hospitals.14 The initial model included demographics (age, sex, race [white versus other]), medical history (chronic or recurrent atrial fibrillation, atrial flutter, cerebrovascular accident or transient ischemic attack, chronic obstructive pulmonary disease or asthma, diabetes, hyperlipidemia, hypertension, peripheral vascular disease, anemia, implantable cardioverter-defibrillator [ICD], pacemaker, chronic dialysis, renal insufficiency defined as serum creatinine chronically >2.0 mg/dL, depression, alcohol abuse, smoking), HF history (prior diagnosis of HF, ischemic etiology defined as prior myocardial infarction or coronary revascularization), clinical data at time of enrollment (systolic blood pressure, heart rate, blood urea nitrogen), and length of stay as determined from the from the case report form. Tests for linearity were assessed among continuous variables, and nonlinear variables were transformed into categorical variables based on commonly used cut-points in the literature. Additionally, interactions between age and sex were tested.
Missing data were <5% for all variables used in modeling, except for admission serum sodium (12.8%), serum creatinine (10.6%), hemoglobin (13.9%), heart rate (9.3%), and ejection fraction (7.8%). For the purposes of modeling predictors of discharge to SNF and for determining adjusted hazards ratios for the outcomes of interest, we excluded those patients with all laboratory data missing (n=860) and used conditional mean imputation for all remaining missing data.
A 2-tailed probability value of <0.05 was considered statistically significant for all tests. All analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC).
Results
Among 15 459 Medicare beneficiaries discharged alive to SNF or home after an index hospitalization for HF of 3 days or more at GWTG-HF hospitals who were not admitted from SNF, the median age was 80 years, just more than half were women, nearly half had ischemic heart disease, median LVEF was 45%, and median length of stay was 5 days (Table 1).
Table 1.
Comparison of Baseline Characteristics for Patients, Stratified by Discharge Status
| Characteristics, n (%) or Median [25th and 75th Percentiles] |
Overall (n=15 459) |
Discharged to SNF (n=3727) |
Discharged to Home (n=11 732) |
P Value* |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 80 [74–86] | 84 [78–88] | 79 [73–84] | <0.001 |
| Female | 8544 (55.3) | 2440 (65.5) | 6104 (52.0) | <0.001 |
| White race | 12 472 (80.7) | 3128 (83.9) | 9344 (79.6) | <0.001 |
| Medicaid payment source | 895 (5.8) | 251 (6.7) | 644 (5.5) | 0.005 |
| Medical history | ||||
| Atrial fibrillation, chronic or recurrent | 5235 (34.8) | 1344 (37.2) | 3891 (34.0) | <0.001 |
| Atrial flutter | 287 (1.9) | 73 (2.0) | 214 (1.9) | 0.558 |
| COPD or asthma | 4320 (28.7) | 1034 (28.6) | 3286 (28.7) | 0.943 |
| Diabetes, insulin–treated | 2352 (15.6) | 592 (16.4) | 1760 (15.4) | 0.139 |
| Diabetes, non–insulin–treated | 3571 (23.7) | 784 (21.7) | 2787 (24.3) | 0.001 |
| Hyperlipidemia | 5848 (38.8) | 1131 (31.3) | 4717 (41.2) | <0.001 |
| Hypertension | 10 970 (72.8) | 2603 (72.1) | 8367 (73.1) | 0.247 |
| Peripheral vascular disease | 1963 (13.0) | 484 (13.4) | 1479 (12.9) | 0.448 |
| CVA/TIA | 2307 (15.3) | 717 (19.9) | 1590 (13.9) | <0.001 |
| Anemia | 2669 (17.7) | 830 (23.0) | 1839 (16.1) | <0.001 |
| Dialysis, chronic | 440 (2.9) | 92 (2.5) | 348 (3.0) | 0.126 |
| Renal insufficiency, chronic | 2801 (18.6) | 726 (20.1) | 2075 (18.1) | 0.008 |
| Depression | 1423 (9.4) | 536 (14.8) | 887 (7.7) | <0.001 |
| Smoking | 1426 (9.2) | 242 (6.5) | 1184 (10.1) | <0.001 |
| Cardiac history | ||||
| Ischemic etiology for heart failure | 6732 (43.5) | 1363 (36.6) | 5369 (45.8) | <0.001 |
| Valvular heart disease | 1113 (7.4) | 281 (7.8) | 832 (7.3) | 0.301 |
| LVEF, % | 45 [30–55] | 46 [30–60] | 43 [30–55] | <0.001 |
| LVEF <40% | 5673 (36.7) | 1143 (30.7) | 4530 (38.6) | <0.001 |
| ICD | 1069 (7.1) | 151 (4.2) | 918 (8.0) | <0.001 |
| Pacemaker | 1573 (10.4) | 371 (10.3) | 1202 (10.5) | 0.703 |
| Vital sign-admission | ||||
| BMI, kg/m2 | 26.3 [22.5–31.3] | 25.6 [21.8–30.6] | 26.6 [22.7–31.6] | <0.001 |
| Heart rate, bpm | 81 [70–96] | 81 [70–96] | 80 [70–95] | 0.078 |
| Systolic blood pressure, mm Hg | 138 [119–158] | 134 [116–155] | 139 [120–159] | <0.001 |
| Labs at admission | ||||
| Serum sodium, mEq/L | 138 [135–141] | 138 [135–141] | 138 [135–141] | 0.207 |
| Hemoglobin, g/dL | 11.8 [10.5–13.2] | 11.4 [10.2–12.8] | 12.0 [10.6–13.3] | <0.001 |
| BNP, pg/mL | 767 [385–1520] | 836 [436–1690] | 741 [370–1470] | <0.001 |
| Serum creatinine, mg/dL | 1.3 [1.0–1.8] | 1.3 [1.0–1.8] | 1.3 [1.0–1.9] | 0.008 |
| BUN, mg/dL | 26 [18–38] | 28 [20–41] | 26 [18–37] | <0.001 |
| Troponin, ng/mL | 0.05 [0.03–0.11] | 0.06 [0.03–0.14] | 0.05 [0.03–0.10] | <0.001 |
| Hospital characteristics | ||||
| No. of beds | 355 [217–575] | 357 [211–575] | 353 [217–571] | 0.020 |
| Academic medical center designation | 7989 (51.7) | 2086 (56.0) | 5903 (50.3) | <0.001 |
| Region | <0.001 | |||
| Northeast | 4501 (29.1) | 1350 (36.2) | 3151 (26.9) | |
| Midwest | 3668 (23.7) | 934 (25.1) | 2734 (23.3) | |
| South | 5604 (36.3) | 1039 (27.9) | 4565 (38.9) | |
| West | 1586 (10.3) | 374 (10.0) | 1212 (10.3) | |
| Discharge data | ||||
| Length of stay, d | 5 [4–7] | 6 [4–9] | 5 [3–7] | <0.001 |
| Comfort measures only at discharge | 124 (1.3) | 75 (3.4) | 49 (0.7) | <0.001 |
| Discharge care performance measures | 124 (0.8) | 75 (2.0) | 49 (0.4) | <0.001 |
| Documentation of LV function (percentage of those eligible) | ||||
| Patients with LVSD and no documented contraindications to ACEI/ARB† |
14 292 (94.4) | 3322 (92.5) | 10 970 (95.0) | <0.001 |
| Prescribed ACEI/ARB at discharge among patients eligible for measure (percent of those eligible) |
4710 (30.5) | 912 (24.5) | 3798 (32.4) | <0.001 |
| Patients with LVSD and no documented contraindications to β–blockers |
4020 (85.4) | 722 (79.2) | 3298 (86.8) | <0.001 |
| Prescribed β–blockers at discharge among patients eligible for measure (percent of those eligible) |
5131 (33.2) | 1004 (26.9) | 4127 (35.2) | <0.001 |
| Patients with smoking history discharged with smoking cessation counseling (percent of those eligible) |
4638 (90.4) | 858 (85.5) | 3780 (91.6) | <0.001 |
SNF indicates skilled nursing facility; COPD, chronic obstructive pulmonary disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; LVEF, left ventricular ejection fraction; ICD, implantable cardioverter-defibrillator; BMI, body mass index; BNP, b-type natriuretic peptide; BUN, blood urea nitrogen; and SD, systolic dysfunction.
P values were calculated by comparing only nonmissing row values.
CMS performance measures otherwise exclude patients discharged to SNF.
Frequencies of Discharge Status
From the final cohort, 3727 (24.1%) study patients were discharged to an SNF. Among the original population considered for the study (before exclusions for shorter length of stay, admission location, or nonhome non-SNF discharge status), SNF accounted for 18.1% of discharges.
Patient Characteristics Associated With Discharge to an SNF
A wide variety of patient factors were associated with discharge to a SNF versus home (Table 1). In multivariable analysis, patient characteristics associated with discharge to a SNF included longer length of stay, advanced age, a variety of comorbidities (history of depression, stroke, anemia, diabetes, peripheral vascular disease, chronic obstructive pulmonary disease or asthma), female sex, hypotension, uremia, higher ejection fraction, hypernatremia, and absence of hyperlipidemia, valvular heart disease, myocardial infarction, coronary revascularization, or ICD (Table 2). Addition of an age-by-sex interaction term was not significant in the model (P=0.91).
Table 2.
Multivariable Model of Patient Correlates of Discharge to SNF
| Covariate | Odds Ratio | 95% CI Lower Limit for OR |
95% CI Upper Limit for OR |
χ2 | P Value |
|---|---|---|---|---|---|
| Length of stay, per day | 1.12 | 1.11 | 1.13 | 386 | <0.001 |
| Age, per 10 –y increase | 1.98 | 1.82 | 2.15 | 260 | <0.001 |
| Depression, history of | 2.11 | 1.84 | 2.41 | 120 | <0.001 |
| Female | 1.53 | 1.42 | 1.66 | 116 | <0.001 |
| Systolic blood pressure, per 10 increase | 0.94 | 0.93 | 0.96 | 58.4 | <0.001 |
| CVA/TIA, history of | 1.55 | 1.36 | 1.77 | 44.3 | <0.001 |
| Hyperlipidemia, history of | 0.74 | 0.68 | 0.81 | 44.4 | <0.001 |
| Anemia, history of | 1.31 | 1.18 | 1.45 | 25.5 | <0.001 |
| Diabetes, history of | 1.19 | 1.08 | 1.30 | 13.1 | <0.001 |
| Valvular heart disease | 0.72 | 0.60 | 0.86 | 12.9 | <0.001 |
| CABG/PCI, history of | 0.80 | 0.70 | 0.92 | 9.40 | 0.002 |
| ICD | 0.76 | 0.63 | 0.91 | 8.50 | 0.004 |
| BUN, per 5 mg/dL increase | 1.03 | 1.01 | 1.05 | 7.89 | 0.005 |
| COPD or asthma, history of | 1.11 | 1.02 | 1.21 | 5.47 | 0.019 |
| LVEF <40% | 0.91 | 0.83 | 0.99 | 5.13 | 0.023 |
| Prior myocardial infarction | 0.86 | 0.75 | 0.98 | 5.11 | 0.024 |
| Peripheral vascular disease, history of | 1.13 | 1.00 | 1.27 | 4.03 | 0.045 |
| Sodium, per 10 mEq/L increase | 1.05 | 1.00 | 1.11 | 3.87 | 0.049 |
| White race | 1.15 | 1.00 | 1.32 | 3.74 | 0.053 |
| Heart rate, per 10 bpm increase | 1.02 | 1.00 | 1.04 | 3.60 | 0.058 |
| Dialysis, chronic | 1.27 | 0.84 | 1.91 | 1.27 | 0.26 |
| Pacemaker | 1.07 | 0.94 | 1.21 | 1.02 | 0.31 |
| HF history: ischemic/CAD | 0.95 | 0.84 | 1.08 | 0.62 | 0.43 |
| Serum creatinine, mg/dL | 0.97 | 0.88 | 1.07 | 0.41 | 0.52 |
| Smoking | 0.97 | 0.84 | 1.12 | 0.20 | 0.66 |
| Hypertension, history of | 1.00 | 0.91 | 1.09 | 0.01 | 0.93 |
| Atrial flutter or atrial fibrillation, history of | 1.00 | 0.92 | 1.09 | 0.00 | 0.95 |
SNF indicates skilled nursing facility; CVA/TIA, cerebrovascular accident/transient ischemic attack; LVEF, left ventricular ejection fraction; ICD, implantable cardioverter-defibrillator; CABG/PCI, coronary artery bypass graft surgery/percutaneous coronary intervention; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; and CAD, coronary artery disease.
Full model of all patient covariates considered. n=14 599 (excludes 860 patients with all laboratory data missing).
Hospital Characteristics Associated With Rates of Discharge to SNF
At the hospital level, rates of discharge to SNF ranged widely (Table 3). There was significant regional variation in the use of SNF with use highest in the Northeast (30.0%) and lowest in the West (23.6%, P<0.0001, Table 1). Hospitals with the lowest rates of discharge to SNF were more likely to care for racial minorities and slightly younger patients (Table 3).
Table 3.
Comparison of Hospital Characteristics Stratified by Tertiles of Rates of SNF Discharge
| Variable, n (%) or Median [25th and 75th Percentiles] |
Hospitals With Lowest Tertile of Percent Discharges to SNF |
Hospitals With Middle Tertile of Percent Discharges to SNF |
Hospitals With Highest Tertile of Percent Discharges to SNF |
P Value* |
|---|---|---|---|---|
| Hospital characteristics | (n=50) | (n=50) | (n=50) | |
| Percentage of discharges to SNF | 10.2 [5.3–14.5] | 23.4 [20.5–25.3] | 33.9 [30.0–42.1] | <0.001 |
| No. of beds | 295 [116–406] | 263 [121–442] | 220 [94–324] | 0.100 |
| Academic medical center designation | 19 (38.0) | 19 (38.0) | 20 (40.0) | 0.838 |
| Region | <0.001 | |||
| Northeast | 5 (10.0) | 13 (26.0) | 15 (30.0) | |
| Midwest | 12 (24.0) | 16 (32.0) | 13 (26.0) | |
| South | 27 (54.0) | 17 (34.0) | 14 (28.0) | |
| West | 6 (12.0) | 4 (8.0) | 7 (14.0) | |
| Patient demographics | (n=3782) | (n=6281) | (n = 5396) | |
| Age | 79 [73–85] | 80 [74–86] | 81 [75–86] | <0.001 |
| Female | 2037 (53.9) | 3451 (54.9) | 3056 (56.6) | 0.007 |
| White race | 2785 (73.6) | 5228 (83.2) | 4459 (82.6) | <0.001 |
SNF indicated skilled nursing facility.
P values were calculated by comparing only nonmissing row values.
Discharge Quality Measures and Care Stratified by Discharge Status
To assess the extent to which SNF patients were treated differently than their counterparts, eligibility for and completion of HF performance measures at the time of hospital discharge were assessed according to discharge status. Patients discharged to SNF were slightly less likely to be eligible for angiotensin-converting enzyme inhibitor/angiotensin receptor blocker and β-blocker therapies at the time of hospital discharge, associated with the higher LVEF among patients discharged to SNF (Table 1). The frequency of application of GWTG care performance measures in eligible patients was modestly less in patients discharged to SNF, with absolute differences in rates <10% (measures listed Table 1). Procedures during the index hospitalization were rare in all patients regardless of discharge status; ICD placement, cardioversion, percutaneous coronary intervention, coronary artery bypass graft surgery, and intra-aortic balloon pump placement all occurred at rates <1%.
Outcomes Stratified by Discharge Status
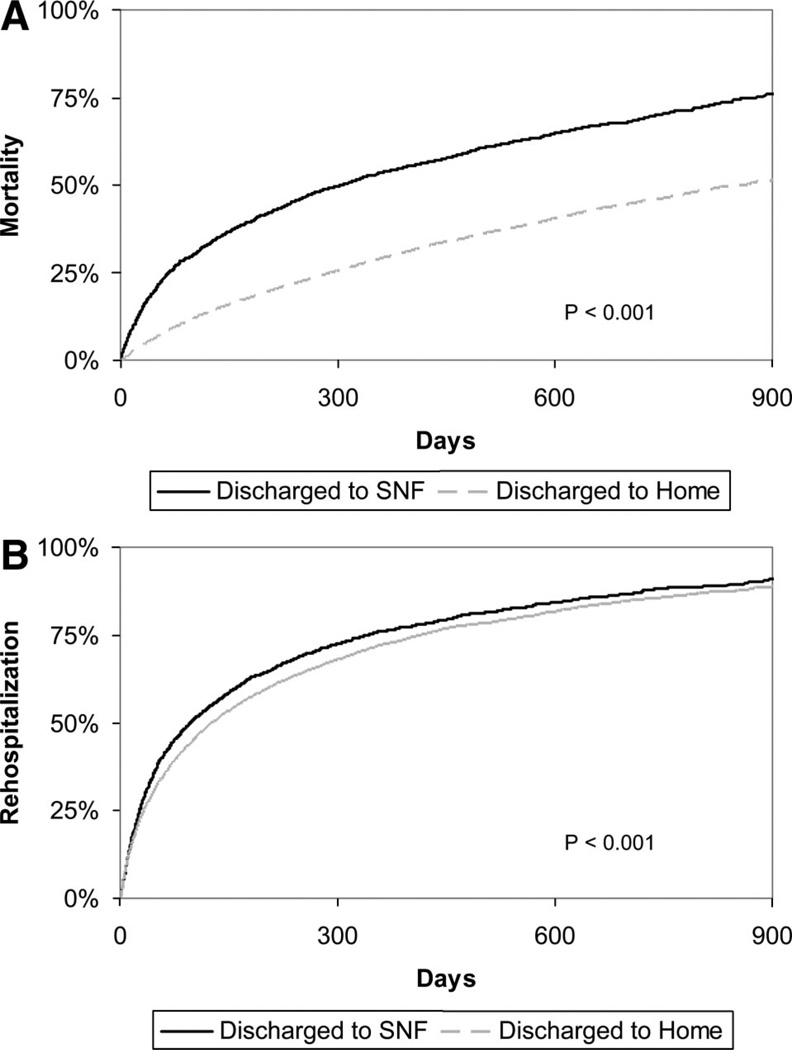
Patients discharged to an SNF had high rates of adverse events. Unadjusted postdischarge all-cause mortality was markedly higher for patients discharged to SNF compared with patients discharged elsewhere: 30-day mortality, 14.4% versus 4.1%, and 1-year mortality, 53.5% versus 29.1%, respectively; P<0.0001 (Figure, A). All-cause rehospitalization rates were also higher in the patients discharged to SNF in comparison to their non-SNF counterparts: 30-day rehospitalization, 27.0% versus 23.5%, and 1-year rehospitalization, 76.1% versus 72.2%, respectively; P<0.0001 (Figure, B). For the combined end point of death or rehospitalization, rates were 35.2% versus 25.5% at 30 days and 83.6% versus 74.8% for SNF versus non-SNF discharge status, respectively; P<0.0001. Adjustment for in-hospital patient factors partially attenuated the association between discharge status and outcomes (Table 4).
Figure 1.
Kaplan-Meier curves for freedom from A, all-cause mortality, and B, all-cause rehospitalization stratified by discharge status.
Table 4.
Unadjusted and Adjusted Hazards Ratios for Mortality, Rehospitalization, and Combined Mortality/Rehospitalization for Patients Discharged to SNF Compared With Home Location
| Unadjusted† | Adjusted†* | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Hazard Ratio |
95% CI Lower Limit for Hazard Ratio |
95% CI Upper Limit for Hazard Ratio |
Pr > χ2 | Hazard Ratio |
95% CI Lower Limit for Hazard Ratio |
95% CI Upper Limit for Hazard Ratio |
Pr > χ2 |
| Mortality, all– cause |
2.19 | 2.07 | 2.31 | <0.001 | 1.76 | 1.66 | 1.87 | <0.001 |
| Rehospitalization, all– cause |
1.14 | 1.08 | 1.19 | <0.001 | 1.08 | 1.03 | 1.14 | 0.001 |
| Mortality or rehospitalization |
1.35 | 1.30 | 1.41 | <0.001 | 1.25 | 1.19 | 1.31 | <0.001 |
CI indicates confidence interval.
Adjusted for all covariates listed in Table 2.
n=14 599 (excludes 860 patients with all laboratory data missing).
Discussion
Using a large contemporary prospective observational database, we found that discharge to SNF occurs in approximately 1 in 5 Medicare beneficiaries after hospitalization for HF. The vast majority of patients discharged to a SNF were previously residing at home. Discharge to SNF is associated with patient factors, region of care, and modestly lower performance on existing inpatient quality measures. The subsequent risk for mortality is substantial, with more than half of these patients dead at 1 year. Compared with similar patients discharged to home, discharge to SNF is associated with a 76% increased risk of death after adjustment for a wide-range of patient characteristics known to be associated with adverse outcomes. However, discharge to SNF is by its very nature determined by criteria such as poor mobility, cognitive impairment, frailty, and poor in-home support, which are also important determinants of outcome but are not directly captured in the analyses provided here. Irrespective of the underlying causes of this disproportionate rate of mortality, there are potential implications in terms of prognostic communication, medical decision-making, and targeted assessment of care provided at SNFs.
Given the financial and policy implications, the relative paucity of evidence regarding the use of postacute SNF care in patients with hospitalization for HF represents an important gap in knowledge. Based on available National Health Expenditure data, the CMS Prospective Payment System spent $19.1 billion on SNF care in 2005 and is expected to spend $29.3 billion in 2011.6 As the leading cause of hospitalization and rehospitalization for Medicare patients,7 the care for patients with HF accounts for a significant portion of these costs. A number of high-profile publications have begun to address the utilization and subsequent outcomes of various discharge dispositions. For Medicare hospitalizations involving admission to an intensive care unit, 2 studies have looked at outcomes stratified by discharge location, including skilled care facilities and long-term acute care facilities.15,16 Trends in length of stay and short-term outcomes among Medicare fee-for-service beneficiaries hospitalized with HF were recently published, including the finding that rates of discharge to “SNF or intermediate care facility” rose from 13.2% to 19.6% between 1993 and 2006 at the same time that length of stay has been shortened and 30-day rehospitalizations have increased.17 Among the general SNF population, high 30-day rates of rehospitalization have also recently been recognized.18 Our results complement these purely administrative data analyses by providing prospective collection of clinical data to better characterize those patients discharged to SNF and inform adjusted analysis associating discharge status with outcome. To our knowledge, the findings presented here are the first to systematically characterize subsequent outcomes for patients discharged to SNF and non-SNF locations after acute hospitalization for HF.
The high absolute rates of death after discharge to SNF for patients hospitalized with HF have potential implications for communication, discharge planning, and goals of care. The Institute of Medicine19 and national HF guidelines recommend that patients and their families be “provided with prognostic information to help appropriately plan for their futures.”20 We postulate that the overall process of transitioning a patient with HF from home to hospital to SNF is likely to require significant discussions and planning between the patient, family, and care providers. Thus, this peridischarge period may also represent an opportune time to consider: (1) transmission of tailored information regarding ranges of expectations for survival and rehospitalization in this high risk group of patients; (2) deliberation of alternatives to SNF for certain patients, including hospice; and (3) formal consideration of overall goals of care including code status and plans regarding potential rehospitalization for worsening health status. Data suggest that patients who have prepared advance directives receive care that is better associated with their preferences.21 Formalized processes for assessing and recording patient and family wishes regarding certain therapies including rehospitalization may be particularly useful in the management of patients with HF transitioned to a SNF.22 Further studies that provide longitudinal data regarding the range of patient experiences after discharge to SNF would further help to guide these types of discussions.
Although unmeasured confounding almost certainly accounts for at least some of the residual association between discharge status and increased mortality, the persistent increases in mortality after restriction of the cohort and subsequent adjustment for measured covariates raises the possibility that differences in care provided at SNF versus other discharge locations after HF hospitalization may play a role in these adverse outcomes. We saw some differences in the use of discharge quality of-care measures when stratified by discharge status, but the absolute differences in these quality measures for eligible patients were smaller than the absolute difference in adjusted mortality. However, the data analyzed in the present study only characterize care quality up to the time of discharge and provide no insight into the processes of care provided at SNFs. Although no cause-and-effect relationship can or should be drawn from these observational results, the differences seen here emphasize the need for future studies that evaluate postdischarge care of patients with HF, with particular focus of SNF care. Published data on long-term care hospitals suggest that serious violations of Medicare rules are approximately double those of regular hospitals,23 whereas systematic assessment of SNF care is currently lacking.
The GWTG-HF registry participation is largely motivated by a desire to improve key performance measures related to processes of care. Until now, there has been no basis for different processes of care for patients discharged to SNF versus those discharged to home. Even for the frailest of patients without obvious contraindications, application of the performance measures among eligible patients may have the benefit of improving symptoms and quality of life. Despite this, most existing analyses of discharge performance for HF quality measures have excluded patients discharged to SNF. Patients discharged to SNF represent a significant fraction of the more than 1 million HF hospitalizations each year1 and thus should not be merely ignored. In addition to considering how well the existing quality measures apply to patients discharged to SNF, we must consider other processes of care that may be distinctly relevant to this unique population, potentially focusing on frailty, symptom relief, and end-of-life issues for those at the highest risk.
Certain factors and limitations should be considered in the interpretation of the results of this study. Participation in the GWTG-HF registry is voluntary and thus may select for hospitals with certain characteristics, thereby resulting in selection bias. However, patient characteristics and outcomes for hospitals in the precursor program to GWTG-HF were similar to the nation as a whole for fee for service Medicare patients.24 In merging GWTG data with CMS data to obtain longitudinal follow-up, the patient population was restricted to only those patients age 65 years or older. However, 80% of patients hospitalized with HF are age 65 or older,1 and approximately 80% of those are covered by the Medicare fee-for-service program,25 making the findings and conclusions of this study applicable to the majority of HF patients. The data on discharge status are reported by personnel who may not be entirely familiar with the formal differences between various types of discharge destinations, although clear definitions were provided during training and in the operating manual to supplement the case report form and the fields for capturing discharge status were designed to parallel CMS discharge codes. Comparison of discharge codes entered into GWTG-HF with those from the MEDPAR database demonstrated 94% agreement. We cannot exclude the possibility that SNF was being used in a hospice capacity for some patients, although the low rate of “comfort measures only” status among patients discharged to SNF (3.6%) compared with comfort measures only status among those discharged to hospice (25.7%) suggests that this is likely to be a rare occurrence. Missing data were minimal for most variables; in particular, discharge status was missing on only 0.2% of patients initially considered for this analysis. From the data, we cannot conclude whether discharge to SNF has any direct impact on patient outcomes or whether these differences in outcomes are related to unmeasured patient characteristics which also drive discharge disposition decisions. We had only limited data on the living situation of patients after their discharge to SNF, thus limiting the ability to characterize the average length of stay in SNF, the frequency with which patients were transitioned to long-term care, or the rate at which patients were able to leave a SNF and return to home. Finally, as a result of the large sample size, some small differences in absolute terms are still highly statistically significant.
Conclusions
Discharge to SNF occurs in approximately than 1 in 5 patients admitted to the hospital for HF. Patients discharged to SNF face a very high risk for death or rehospitalization. These results highlight the need to evaluate care processes and outcomes in the SNF setting. Additionally, we must consider whether a different set of quality measures are needed for this unique group of patients.
CLINICAL PERSPECTIVE.
After acute hospitalization, many older patients in the United States are discharged to skilled nursing facilities (SNF). Medicare benefits cover up to 100 days of SNF care for patients hospitalized for at least 3 days who also have a skilled need. Although heart failure (HF) is the leading cause of hospitalization and rehospitalization for Medicare patients, subsequent discharge to SNF is not well described. Therefore, we evaluated 15 459 Medicare beneficiaries also enrolled in the Get With The Guidelines Program who were ≥65 years of age and discharged to home or SNF after ≥3-day hospitalization for HF. We found that 24% were discharged to an SNF, 22% to home with home health service, and 54% to home with self-care. SNF use varied widely among hospitals (more than 3-fold difference from 10th to 90th percentile), with rates highest in the Northeast. Patient factors associated with discharge to SNF included longer length of stay, advanced age, female sex, higher ejection fraction, absence of ischemic heart disease, and a variety of comorbidities. Performance measures were modestly lower for patients discharged to SNF. Discharge to SNF was associated with substantial risk for adverse events, with more than half of these patients dead within 1 year. These findings highlight the need to better characterize this unique population, understand the SNF care they receive, and consider whether a different set of quality measures should be applied to these patients.
Acknowledgments
Disclosures
Dr Hernandez received research support from Johnson and Johnson, Medtronic, and Merck and Co; served on the speakers’ bureau for Novartis; and received honoraria from AstraZeneca and Medtronic. Dr Peterson received research support from Bristol-Myers Squibb, Sanofi-Aventis, Merck and Co, and Eli Lilly and served as the principal investigator of the analytic center for the American Heart Association Get With the Guidelines Program. Dr Curtis received research support from Allergan, GlaxoSmithKline, Merck and Co, and Johnson and Johnson (Ortho Biotech). Dr Masoudi served on an advisory board for Amgen and contracted with the American College of Cardiology, Axio Research, and the Oklahoma Foundation for Medical Quality. Dr Bhatt received research grants from AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Heartscape, Sanofi- Aventis, and The Medicines Company. Dr Fonarow received research grants or other research support from GlaxoSmithKline, Pfizer, and the National Institutes of Health and honoraria from Amgen, AstraZeneca, Boston Scientific/Guidant, GlaxoSmithKline, Medtronic, Merck and Co, Novartis, Pfizer, and St Jude Medical and served as a consultant for Boston Scientific/Guidant, GlaxoSmithKline, Medtronic, Merck and Co, Novartis, Pfizer, Relypsa, Scios/ Johnson & Johnson, and St Jude Medical and as chair of the American Heart Association Get With The Guidelines Steering Committee. Dr Fonarow holds the Eliot Corday Chair of Cardiovascular Medicine at UCLA and was supported by the Ahmanson Foundation (Los Angeles, CA). Drs Hernandez, Peterson, and Curtis have made available online detailed listings of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp).
Sources of Funding
Get With The Guidelines–Heart Failure is a program of the American Heart Association and is supported by unrestricted educational grants from GlaxoSmithKline and Medtronic. This study was also supported by grant 1U18HS016964-01 from the Agency for Healthcare Research and Quality.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics: 2010 Update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Hutt E, Ecord M, Eilertsen TB, Frederickson E, Kramer AM. Precipitants of emergency room visits and acute hospitalization in short-stay Medicare nursing home residents. J Am Geriatr Soc. 2002;50:223–229. doi: 10.1046/j.1532-5415.2002.50052.x. [DOI] [PubMed] [Google Scholar]
- 3.Hutt E, Frederickson E, Ecord M, Kramer AM. Associations among processes and outcomes of care for Medicare nursing home residents with acute heart failure. J Am Med Dir Assoc. 2003;4:195–199. doi: 10.1097/01.JAM.0000073964.19754.C0. [DOI] [PubMed] [Google Scholar]
- 4.Fried TR, Mor V. Frailty and hospitalization of long-term stay nursing home residents. J Am Geriatr Soc. 1997;45:265–269. doi: 10.1111/j.1532-5415.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed June 8, 2010];Medicare Coverage of Skilled Nursing Facility Care. http://www.medicare.gov/publications/pubs/pdf/10153.pdf.
- 6. [Accessed June 8, 2010];National Health Expenditure Database. http://www.cms.hhs.gov/NationalHealthExpendData/downloads/tables.pdf.
- 7.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 8.Bonow RO, Bennett S, Casey DE, Jr, Ganiats TG, Hlatky MA, Konstam MA, Lambrew CT, Normand SL, Pina IL, Radford MJ, Smith AL, Stevenson LW, Burke G, Eagle KA, Krumholz HM, Linderbaum J, Masoudi FA, Ritchie JL, Rumsfeld JS, Spertus JA. ACC/AHA Clinical Performance Measures for Adults with Chronic Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures): endorsed by the Heart Failure Society of America. Circulation. 2005;112:1853–1887. doi: 10.1161/CIRCULATIONAHA.105.170072. [DOI] [PubMed] [Google Scholar]
- 9.Hong Y, LaBresh KA. Overview of the American Heart Association “Get With The Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–186. doi: 10.1097/01.hpc.0000243588.00012.79. [DOI] [PubMed] [Google Scholar]
- 10.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krumholz HM, Normand SL, Spertus JA, Shahian DM, Bradley EH. Measuring performance for treating heart attacks and heart failure: the case for outcomes measurement. Health Aff (Millwood) 2007;26:75–85. doi: 10.1377/hlthaff.26.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 13. [Accessed June 8, 2010];Hospital Compare. www.hospitalcompare.hhs.gov.
- 14.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 15.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-Year Outcomes for Medicare Beneficiaries Who Survive Intensive Care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 16.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood) 2010;29:57–64. doi: 10.1377/hlthaff.2009.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Medicine Committee on Quality of Health Care in America: Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 20.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 21.Silveira MJ, Kim STH, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362:1211–1218. doi: 10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed November 28, 2010]. POLST.org. [Google Scholar]
- 23.Berenson A. Long-Term Care Hospitals Face Little Scrutiny. New York Times. 2010 Feb 2; [Google Scholar]
- 24.Curtis LH, Greiner MA, Hannill BG, DiMartino LD, Shea AM, Hernandez AF, Fonarow GC. Representativeness of a national heart failure quality-of-care registry: comparison of OPTIMIZE-HF and non OPTIMIZE-HF Medicare patients. Circ Cardiovasc Qual Outcomes. 2009;2:277–384. doi: 10.1161/CIRCOUTCOMES.108.822692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Congressional Budget Office. Medicare Baseline. 2009 Mar