Abstract
Although multiple culture assays have been designed to identify “endothelial progenitor cells” (EPCs), the phenotype of cells grown in culture often remains undefined. We sought to define and characterize the pro-angiogenic cell population within human peripheral blood mononuclear cells. Mononuclear cells were isolated from peripheral blood and grown under angiogenic conditions for 7 days. Formed colonies (CFU-As) were identified and analyzed for proliferation, mRNA and surface antigen expression, tube-forming ability and chromosomal content. Colonies were composed of a heterogeneous group of cells expressing the leukocyte antigens CD45, CD14, and CD3, as well as the endothelial proteins vascular endothelial (VE) cadherin, von Willebrand's Factor (vWF), CD31 and endothelial nitric oxide synthase (eNOS). Colony cells expressed increased levels of pro-angiogenic growth factors, and they formed tubes in Matrigel. In comparison with colonies from the CFU-Hill assay, our assay resulted in a greater number of colonies (19±9 vs. 13±7; p<0.0001) with a substantial number of cells expressing an endothelial phenotype (20.2±7.4% vs. 2.2±1.2% expressing eNOS, p=0006). Chromosomal analysis indicated the colony cells were bone marrow-derived. We, therefore, describe a colony forming unit assay that measures bone marrow-derived circulating mononuclear cells with the capacity to proliferate and mature into proangiogenic leukocytic and endothelial-like cells. This assay, therefore, reflects circulating, bone marrow-derived pro-angiogenic activity.
Keywords: stem cells, cardiac disease, cell-based assays
Atherosclerosis is initiated by endothelial injury due to cardiovascular risk factors such as diabetes, hypertension, and hypercholesterolemia that results in arterial intimal inflammation, hyperplasia and thrombosis. 1 It has been proposed that bone marrow-derived cells circulating in peripheral blood participate in both the repair of the injured endothelium and in neovascularization of ischemic tissue through either direct incorporation, or via paracine effects. These cells have been collectively referred to as endothelial progenitor cells (EPC). 2-5
Different methods of identifying EPCs have been developed.2 Colonies of cells with endothelial characteristics grown from human peripheral blood mononuclear cells in vitro under angiogenic conditions were first described by Asahara et al., who regarded the colony forming units (CFUs) to be circulating EPCs.6 Since then, various culture assays and flow cytometry have been developed to detect circulating EPCs, and they have been associated with endothelial function, atherosclerosis burden, and cardiovascular clinical outcomes, suggesting that they play a role in vascular health.7-9
Despite this common association with atherosclerotic disease, both the assays and the cells being measured, are heterogeneous..Different blood cell isolation procedures and culture conditions result in growth varying from independent, isolated cells to colonies with various morphologies. One culture assay, the “late-outgrowth” assay, relies on long incubation periods and gives rise to rare colonies of endothelial cells.10,11 A shorter-term culture assay, the CFU-Hill, gives rise to cells of a monocyte-macrophage lineage with potent pro-angiogenic paracrine effects, but no apparent endothelial cells.10,12 EPCs identified using flow cytometry via the markers CD34, CD133, and vascular endothelial growth factor receptor 2 (VEGFR2) also appear to be of the monocyte-macrophage lineage, and no consensus on cell markers specific for EPCs has been reached.13
We sought to develop an assay to identify cells circulating in the peripheral blood with angiogenic and vascular repair capacity. Herein, we describe the angiogenic colony forming unit (CFU-A) assay, which identifies bone marrow-derived peripheral blood mononuclear cells capable of proliferating into colonies of cells which express endothelial and leukocyte phenotypes and produce angiogenic factors.
Materials and Methods
Study subjects
Healthy volunteers without chronic diseases, hypercholesterolemia (LDL cholesterol level <120 mg/dl), hyperglycemia (glucose <99 mg/dL), hypertension (blood pressure <135 mmHg systolic and <85 mmHg diastolic), or obesity (BMI 19-26), and with at least a 5-year non-smoking status, were recruited. In addition, 4 patients who had sex-mismatched bone marrow transplants were studied. Venous blood was drawn after an overnight fast. The study was approved by the Emory University Institutional Review Board and all subjects gave informed consent.
Colony forming unit (CFU) assay
Our CFU-A assay was modified from previous assay descriptions.7,14 Mononuclear cells were isolated from 16 ml of whole blood by density-gradient centrifugation using CPT® tubes (Becton Dickenson), washed and resuspended in growth medium (Dulbecco's Modified Eagle Medium (DMEM) supplemented with 20% fetal bovine serum and 6.5% endothelial cell growth supplement (ECGS, Becton Dickenson). To eliminate potential contamination by mature circulating endothelial cells,the cells were plated in 6-well culture dishes coated with human fibronectin (Biocoat, Becton Dickinson) and, after 24 hours, non-adherent cells were replated onto new fibronectin-coated 24-well dishes (1 million cells/well). Growth medium was changed every two days and colonies/well were counted seven days after plating. A colony was identified as multiple thin, flat cells emanating from a central cluster of rounded cells.6,7 Reproducibility was tested by in 15 blood samples drawn 1 week apart from the same individuals. The overall correlation between the repeated assays was 0.84 (p<0.001).
The commercially-available CFU-Hill assay (Endocult, Stem Cell Technologies, Vancouver) was performed per manufacturer's directions for comparison.7,15 Both assay colonies were counted by a single, blinded observer in a minimum of 4 wells. Average number of colonies per 1 million mononuclear cell are reported.
Proliferation Assay
To determine whether the colonies were derived from mononuclear precursor cells via proliferation, 5-bromo-2’-deoxyuridine (BrdU, Sigma) (1 μg/ml) was used to label cells for 24 hours on day 3 or 6 of growth. Subsequently, immunostaining with anti-BrdU (DAKO, USA) was used to detect proliferating cells within the colonies (see below).
Reverse transcriptase polymerase chain reaction (RT-PCR)
RNA was isolated from freshly-isolated mononuclear cells and from 7-day colonies using Qiagen RNeasy Mini Kit. cDNA was prepared from RNA samples (Super Array). RT- PCR was performed in ABI 7900 (Applied Biosystem) instrument using pathway focused gene expression PCR arrays from Super Array (human endothelial biology, angiogenesis). mRNA expression levels were determined using SYBR Green-based real-time PCR. Results were analyzed using PCR Array data analysis web portal to convert threshold cycles into fold-changes.
Immunocytochemistry
We performed immunocytochemistry for leukocyte and endothelial antigens.7 Colonies were fixed in 2% para-formaldehyde for 1 hour, washed in phosphate buffered saline (PBS) and blocked with 5% normal serum in 2% bovine serum albumin (BSA) in PBS for 30 min. Cells were incubated with the primary antibody (mouse anti-CD31 1:300 (eBioscience), mouse anti-CD45 1:5000 (eBioscience), mouse anti-CD3 1:300 (eBioscience), mouse anti-CD14 1:200 (Santa Cruz), mouse anti-vascular endothelial (VE) cadherin 1:50 (Abcam), rabbit polyclonal anti-endothelial nitric oxide synthase (eNOS) 1:1000 (Santa Cruz), rabbit polyclonal anti-von Willibrand's Factor (vWF) 1:1000 (Chemicon)) in 2% BSA for 1 hour, then with biotinylated horse anti-mouse IgG for monoclonal primary antibodies and biotinylated goat anti-rabbit IgG for polyclonal antibodies (1:200) (Vector Lab) for 30 min. After another PBS wash, cells were stained with streptavidin-conjugated quantum dots (QDot 605; Invitrogen), 1:100, for 1 hour and counterstained with Hoechst nuclear stain. Images were acquired on a Zeiss LSM 510 confocal microscope.
Flow cytometry
To detect the surface expression of endothelial and hematopoietic lineage marker proteins, harvested colonies from 24-well plates were suspended in PBS after washing and incubated for 1 hour in the presence of the anti-CD14, -CD3, -CD45, -eNOS, -vWF, or -VE cadherin then conjugated with biotinylated secondary antibodies. After washing with PBS, cells were stained with streptavidin-conjugated quantum dots (QDot 605; Invitrogen), 1:100, and analyzed by flow cytometry (Facs Calibur, Becton-Dickinson).
Matrigel tube-formation assay
In 24-well fibronectin-coated culture dishes, 250 l of the 1:1 Matrigel:PBS solution was applied per well and allowed to polymerize at 37C for 30min-2hours. After polymerization, 106 pre-plated, non-adherent mononuclear cells or cells from 4 day-old colonies were plated in each well with 1 ml DMEM and 20% FBS, 6.5% ECGS, and observed for tube-formation for up to 5 days.
Cell migration assay
CFU cell migration from 16 subjects was assayed using the BD Biocoat Angiogenesis Endothelial Cell Invasion System (Becton Dickenson). CFU cells (50,000/well) in culture medium were distributed into 24-well multiwell insert/receiver plate containing a fluorescence-blocking microporous polyethylene terephthalate membrane (3.0 μm pore size) evenly coated with human fibronectin,VEGF (50 ng/ml) and stromal cell derived factor 1 alpha (SDF-1α) (50 ng/well) were used as chemoattractants. To quantitate the number of cells that migrated through the pores and attached to the underside of the insert membrane, cells were labeled with a fluorescent dye and were measured using a bottom-reading fluorescent plate reader.
FISH (fluorescent in situ hybridization)
Peripheral blood mononuclear cells from 4 sex-mismatched bone marrow transplant recipients were cultured via the CFU-A colony assay protocol above. At 7 days, fluorescent in situ hybridization was performed on the colonies, and on interphase cells from a concurrent peripheral blood chromosome culture. The Vysis CEP X Spectrum Orange/Y Spectrum Green Direct Labeled Fluorescent DNA Probe Kit (Abbott#30-161059) was used to detect the presence of X and/or Y chromosomes in the colonies and interphase cells. A G-banded metaphase karyotype was also produced as a confirmation of FISH results.
Statistical analysis
To test for differences among groups with respect to continuous variables, we used the paired or unpaired t-test, as appropriate. Correlations between cell counts were made using linear regression analysis. P values ≤ 0.05 were considered to be statistically significant. Data analysis was performed with SPSS software, version 14.0 (SPSS Inc, Chicago, IL).
Results
CFU assay comparison
We compared the CFU-A assay with the commercial CFU-Hill assay, which has been previously studied.10,16 Mononuclear cells were isolated from peripheral blood from 59 subjects (mean 45±11 years) and grown in vitro using both assays. Colonies were identified as clusters of rounded cells surrounded by spindle-shaped cells at the periphery under the microscope and were similar in morphology in both assays (Figure 1A). Colony counts ranged from 3 to 48 for the CFU-A assay, and from 1 to 34 for the CFU-Hill assay. Significantly more colonies grew from the CFU-A assay than the CFU-Hill assay (19±9 vs. 13±7; p<0.0001). Correlation was poor (r2 = 0.08, p=0.03), suggesting potentially important differences between the two assays.
Figure 1.
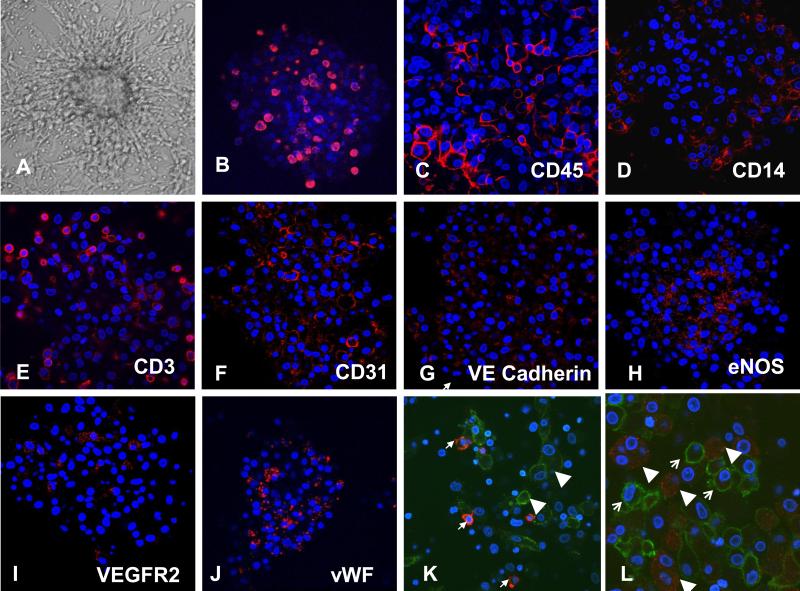
CFU-A. (A) Phase-contrast microscopy (10× magnification). (B) Proliferating cells (red) seen using BrdU incorporation on day 6. (C-L) Immunocytochemistry demonstrating leukocyte (C-E) and endothelial (F-J) antigens (red) as labeled. (K, L) Double-immunostaining of CFU-A cells for monocyte antigen CD14 (green) and endothelial antigens (red) vWF (K) and eNOS (L), demonstrating no co-expression. Nuclear counterstaining (blue) with Hoechst. (40× magnification). Typical examples from n = 20 subjects.
Colony cell proliferation
Proliferative potential is considered one criterion for progenitor cells. To determine whether CFU-A cells proliferate, Brd-U incorporation was studied on culture day 3 and 6. Brd-U immunostaining on both day 4 and 7 revealed proliferating cells interspersed throughout the colony (Figure 1B). Levels of proliferating cells appeared similar at both time points (data not shown).
RT-PCR
To analyze the pro-angiogenic potential and endothelial lineage of the developing CFU-A colonies, we compared RNA expression of angiogenesis- and endothelium-related proteins in the isolated mononuclear cells to the mature CFU-A colonies. Expression of 30 different pro-angiogenic or endothelial genes was up-regulated up to 210-fold in the CFU-A cells compared to mononuclear cells (Table 1). In particular, we found a 5-, 7- and 11-fold increase in expression of the endothelial-specific proteins CD31, eNOS and vWF RNA, respectively, in the mature colony as compared to mononuclear cells. This data supports the notion that CFU-A colonies contain cells with endothelial and pro-angiogenic characteristics.
Table 1.
RNA expression of select proteins in CFU-A colonies as compared to circulating mononuclear cells in volunteer subjects.
| Relative increase | p value | |
|---|---|---|
| Anaioaenesis-related | ||
| Matrix metalloproteinase-2 | 90.7 | 0.029 |
| Matrix metalloproteinase-9 | 27.4 | 0.0007 |
| Interferon gamma | 11.6 | 0.003 |
| Chemokine (C-X-C motif) ligand 9 (CXCL9) | 6.4 | 0.02 |
| Midkine (neurite growth promoting factor 2) | 3.2 | 0.04 |
| Vascular endothelial growth factor D | 3.0 | 0.008 |
| Endothelial cell-related | ||
| Angiotensin I converting enzyme | 211.0 | 0.0007 |
| Colony stimulating factor 2 | 147.0 | 0.003 |
| Tumor necrosis factor receptor superfamily 10c | 28.1 | 0.001 |
| Plasminogin activator urokinase | 14.9 | 0.0009 |
| Von Willibrand's Factor | 10.9 | <0.0001 |
| Prostacyclin synthase ^ | 10.6 | <0.0001 |
| Arachidonate 5-lipoxygenase | 7.0 | 0.032 |
| Endothelial nitric oxide synthase (NOS 3) | 7.0 | 0.0001 |
| Vascular endothelial growth factor receptor (FLT-1) | 6.3 | 0.05 |
| Platelet/endothelial cell adhesion molecule-1 (CD31) | 5.4 | 0.009 |
| Vascular cell adhesion molecule-1 (VCAM1) | 4.7 | <0.0001 |
| Superoxide dismutase 1 (soluble) | 3.4 | 0.01 |
| Housekeeping | ||
| GAPDH | 1.3 | 0.17 |
| ACTB | 1.0 | 0.37 |
| 18scRNA | 1.0 | 0.99 |
Subject n = 3; includes all RNA with ≥ 3 fold increase
Immunocytochemistry
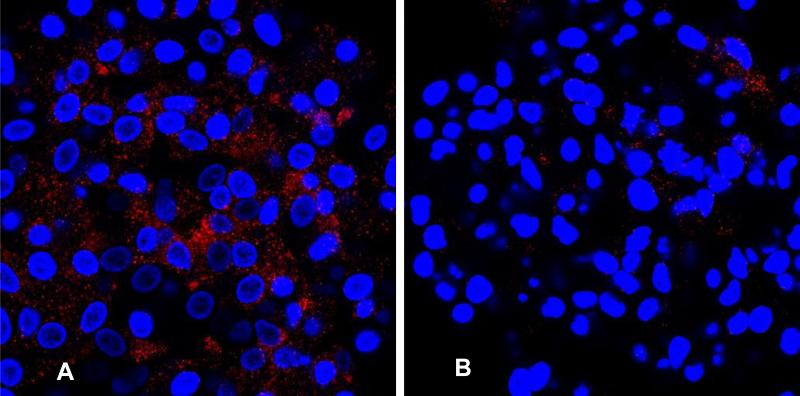
Immunocytochemistry for leukocyte and endothelial proteins was performed on the colonies. Many CFU-A colony cells stained for the leukocyte antigen CD45, while subgroups of cells, primarily located at the periphery, stained for the monocyte-antigen CD14 and lymphocyte-antigen CD3. Staining for endothelial antigens CD31, VEGF–receptor 2, VE-cadherin, and vWF also occurred throughout the colony, while eNOS staining occurred primarily centrally (Figure 1C-J). Double-staining demonstrated that cells that stained for vWF or eNOS did not stain for CD14 (Figure 1K, L). These observations suggest that CFU-A colonies are comprised of leukocytes of monocytic and lymphocytic lineage, as well as of endothelial-like cells. In comparison, the CFU-Hill colonies subjectively appeared to stain primarily for monocyte and lymphocyte markers and only sparsely for eNOS (Figure 2).
Figure 2.
Immunostaining for eNOS demonstrating increased staining in the CFU-A (A) as compared to the CFU-Hill (B). Nuclear counterstaining with Hoechst (blue). 40x magnification. Typical example from n = 5 subjects.
Flow cytometry
Quantification of cell types in the CFU-A colonies at day 7 using flow cytometry demonstrated that the majority of cells expressed CD45 (80%) and CD3 (60%) antigens. However, a sizable minority (20%) also expressed the endothelial markers eNOS and vWF. In contrast, only 2% of cells in the CFU-Hill colonies expressed the endothelial specific proteins eNOS and VWF (p<0.001; Table 2).
Table 2.
Comparison of cell frequency in CFU-A vs CFU-Hill colonies from volunteer subjects measured by flow cytometry.
| Cell antigen | CFU-A | CFU-Hill | P value |
|---|---|---|---|
| Cd45 (%) | 83.4±14 | 77.5±18 | 0.56 |
| Cd3 (%) | 59.5±19 | 60.1±28 | 0.97 |
| vWf (%) | 19.6±3.9 | 2.2±1.4 | <0.00001 |
| eNOS (%) | 20.2±7.4 | 2.2±1.2 | 0.0006 |
Subject n =7; % of total cells positive for antigen.
Tube-forming assay
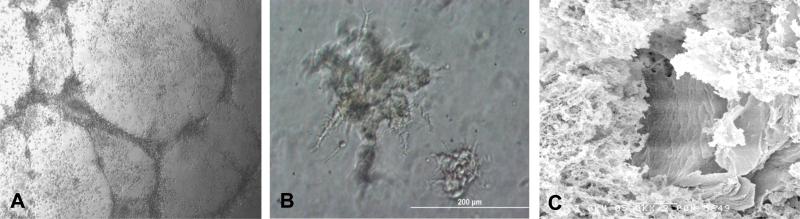
To assess the angiogenic capacity of the circulating mononuclear cells and the resultant CFU-A colony-derived cells, we performed Matrigel tube-forming assays on non-adherent mononuclear cells as well as maturing colonies. The mononuclear cells formed networks of tubes which contained distinct lumens as assessed by electron microscopy. Furthermore, when individual maturing colonies were transferred to Matrigel, tube-like structures emerged from their centers at 24 hours (Figure 3).
Figure 3.
Matrigel tube-forming assay. (A) Phase contrast micrograph of tube-formation by non-adherent mononuclear cells. (B) Tubes sprouting from CFU-A cells transplanted on day 4 to Matrigel. (C) Electron microscopy demonstrating lumen of tube. Typical example from n = 4 subjects.
Cell migration assay
To assess the response to specific pro-angiogenic stimuli, migration of CFU cells across a porous membrane were measured.. Cell migration was significantly stimulated by VEGF and SDF-1alpha as compared to control (21 (p < 0.00001) and 41 (p=0.04) vs 15 cells/well),
Sex-mismatched bone marrow transplant (BMT) subjects
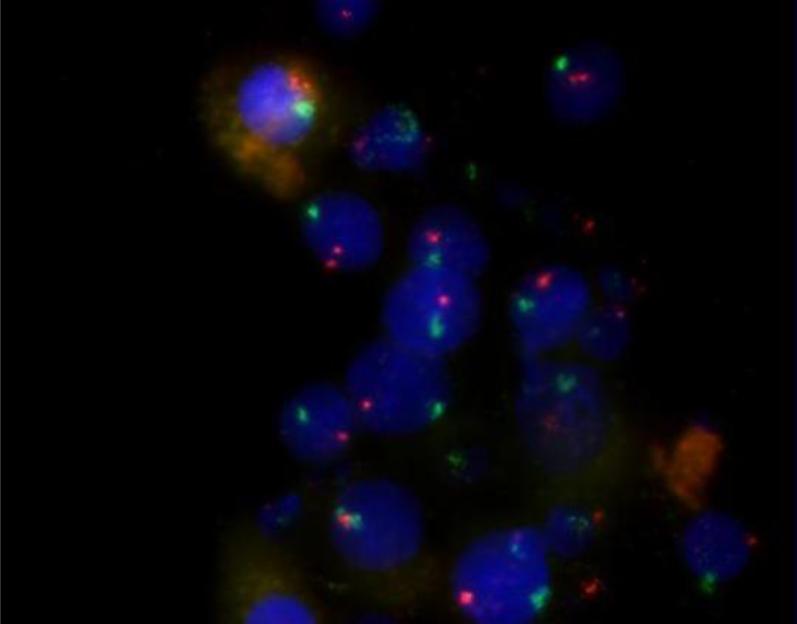
In order to determine the origin of the CFU-A cells, 3 female BMT recipients who had male donors were studied. All cells in the colonies as well as the interphase and metaphase cells were male, having an X and a Y chromosome (Figure 4). A fourth male BMT recipient who had a female donor was studied. All of this patient's cells in colonies, interphase cells and metaphase cells were female, having two X chromosomes. These results confirm the bone marrow origin of the CFU-A colonies.
Figure 4.
Fluorescent in situ hybridization demonstrating the bone marrow origin of CFU-A colony cells. Colony cells staining for Y chromosomes (green) indicate they are from the bone marrow of a female subject who received a male donor bone marrow transplant. X chromosome = orange. Nuclear counterstaining with Hoechst (blue). Typical examples from n = 4 volunteer subjects. Magnification 63×.
Discussion
Peripheral blood mononuclear cells contribute to angiogenesis and vascular repair, processes important in the pathogenesis of atherosclerosis and ischemic heart disease, and have been collectively called EPCs. Culture assays have been used to identify these cells, however culture conditions and outgrowth morphology have varied considerably between the assays, and not all assays have been optimally characterized. We sought to characterize a distinct culture assay, the CFU-A assay. We demonstrate that CFU-A are peripheral blood mononuclear cells which (i) are bone marrow-derived, (ii) are proliferative, and (iii) give rise to heterogeneous cell colonies containing both endothelial- and hematopoietic-like cells, which have (iv) significantly upregulated pro-angiogenic protein expression and (iv) the ability to form capillary-like tubes. Thus, the CFU-A assay reflects circulating, bone marrow-derived, pro-angiogenic cell activity.
The CFU-A assay appears to be different from other popular EPC culture assays. The “late outgrowth” assay identifies circulating proliferative cells which form homogeneous cobblestone colonies of endothelial cells.10,11 However, these circulating cells are extremely rare, if present at all, in the adult human circulation and may originate from the vessel wall instead of the bone marrow.17,18 Hill et al. described a shorter duration CFU assay based on the work of Asahara et al, and a commercial variant of this assay, called the CFU-Hill, was developed.7 This assay was originally believed to reflect true EPCs and was associated with endothelial function and atherosclerotic disease in patients.9 However, since then, investigators demonstrated that the CFU-Hill assay yields almost exclusively hematopoietic-like cell colonies, with almost no eNOS expression and no in vitro capillary-tube formation capability.10,16 Instead, these bone marrow-derived progenitor cells facilitate angiogenesis through paracrine mechanisms.12 While the CFU-A assay grows colonies morphologically similar to the CFU-Hill assay, the CFU-A colonies contain both hematopoietic-like cells as well as endothelial-like cells, Importantly, almost 20% of the cells express endothelial-specific antigens vWF and eNOS (compared to 2% of the CFU-Hill colony cells), and the cells migrate in response to the angiogenic stimulant VEFG and form capillary-like tubes in Matrigel, indicating their vasculogenic potential. Our immunocytochemical doublestaining experiments further supported endothelial-lineage of some colony cells by showing that they were not monocyte/macrophage lineage cells expressing an endothelial phenotype. In addition, almost 60% more colonies formed using the CFU-A than the CFU-Hill assay, with little correlation in CFU number between these assays. We have also shown a robust and distinct relationship between the CFU-A assay and clinical atherosclerosis (“Circulating Pro-angiogenic Cell Activity Is Associated with Cardiovascular Disease Risk”, Journal of Biomolecular Screening). Finally, unlike the late outgrowth colony assay cells, all the CFU-A colony cells are bone-marrow derived.
The differences between the CFU-A and the other EPC assays are likely due to different isolation and culture conditions resulting in different circulating cell selection, growth pattern and phenotype manifestation. Important differences include 1) the culture duration (7 vs. 5 days for the CFU-Hill assay vs.>2 weeks for the late outgrowth assay), 2) the elimination of early adherent cells (potential mature endothelial cells) (24 hour vs 48 hours for the CFU-Hill assay vs. no elimination for the late outgrowth assay), and 3) the culture media used (DMEM with 20% FBS and 6.5% EGCS vs. Endocult media for the CFU-Hill with a proprietary formulation). These differences have likely resulted in the substantially different colony characteristics we observed.
The different cell phenotypes found in CFU-A colonies supports the CFU-A's potential pro-angiogenic role. An effective bone marrow-derived reparative response to vascular injury involves multiple cell types, including both hematopoietic and endothelial-like cells, with some cells offering only paracrine stimulation while others incorporating directly into the injured vessel.19,20,16,21 The appearance of both hematopoietic and endothelial-like cells in a common colony may reflect their common precursor, the hemangioblast, which can differentiate into both lineages.22 Alternatively, the CFU-A colonies may be derived cooperatively from more differentiated precursor cells , potentially including monocytes which develop a vascular phenotype and have been shown to be involved in angiogenesis and vascular repair.23 Either way, the culture technique and the resultant biphenotypic CFU-A colony growth may reflect the bone marrow-derived vascular repair activity more comprehensively than other more selective techniques.
There are some limitations of this study. Endothelial-like cells were identified based on some, but not all, relevant endothelial markers. Double-immunostaining showed that cells expressing endothelial antigens did not co-express monocyte-antigen CD14,however we do not definitively distinguish between endothelial-like cells derived directly from their primitive endothelial progenitors and those derived from more differentiated myeloid cells.10,16,24 We have shown some differences between the CFU-A and the CFU-Hill assays, however, further comparison of molecular expression profiling could provide additional insight. We have not quantified whether the amount of protein being expressed in this in vitro assay correlates with in vivo angiogenesis, nor have we demonstrated any in vivo evidence that CFU-A are directly involved in angiogenesis or vascular repair, both subjects that need further study. However, we do provide compelling evidence of the association between the CFU-A and the development and outcomes of clinical atherosclerotic disease (“Circulating Pro-angiogenic Cell Activity Is Associated with Cardiovascular Disease Risk”, Journal of Biomolecular Screening).
In conclusion, we describe a culture-based assay that is able to reproducibly measure circulating, bone marrow-derived, pro-angiogenic cells and is distinct from the other EPC assays. We believe it will be useful in measuring vascular health, the risk of developing cardiovascular disease, and the testing of pro-angiogenic compounds.
Acknowledgements
Funding: This work was supported by Eli Lilly and Company, Indianapolis, Indiana; a gift from the Marcus Foundation, Atlanta, Georgia; the Georgia Tech/Emory Center for the Engineering of Living Tissues (GTEC) National Science Foundation (NSF) Grant EEC-9731643; PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources; and the Woodruff Fund.
Footnotes
Disclosures: None of the authors have any conflicts of interest.
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993. 362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Rafii DC, Psaila B, Butler J, Jin DK, Lyden D. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:217–222. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- 3.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells.[comment]. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 4.Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition.[see comment]. Arteriosclerosis, Thrombosis & Vascular Biology. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 5.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk.[see comment]. New England Journal of Medicine. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 8.Guven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease.[see comment]. Journal of the American College of Cardiology. 2006;48:1579–1587. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 9.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes.[see comment]. New England Journal of Medicine. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 10.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse Origin and Function of Cells With Endothelial Phenotype Obtained From Adult Human Blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 12.Sieveking DP, Buckle A, Celermajer DS, Ng MKC. Strikingly Different Angiogenic Properties of Endothelial Progenitor Cell Subpopulations: Insights From a Novel Human Angiogenesis Assay. Journal of the American College of Cardiology. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 13.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Experimental Hematology. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Rovira, Bloom ML, Takeda K, Ferrans VJ, Quyyumi AA, Finkel T. Endothelial progenitor cells as putative targets for angiostatin. Cancer Research. 1999;59:5875–5877. [PubMed] [Google Scholar]
- 15.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–1149. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 16.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune Cells Mimic the Morphology of Endothelial Progenitor Colonies In Vitro. Stem Cells. 2007;25:1746–1752. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschi KK, Ingram DA, Yoder MC. Assessing Identity, Phenotype, and Fate of Endothelial Progenitor Cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deindl E, Schaper W. The art of arteriogenesis. Cell Biochemistry and Biophysics. 2005;43:1–15. doi: 10.1385/CBB:43:1:001. [DOI] [PubMed] [Google Scholar]
- 20.Monaco C, Andreakos E, Kiriakidis S, Feldmann M, Paleolog E. T-Cell-Mediated Signalling in Immune, Inflammatory and Angiogenic Processes: The Cascade of Events Leading to Inflammatory Diseases. Current Drug Targets - Inflammation & Allergy. 2004;3:35–42. doi: 10.2174/1568010043483881. [DOI] [PubMed] [Google Scholar]
- 21.Hur J, Yang H-M, Yoon C-H, Lee C-S, Park K-W, Kim J-H, Kim T-Y, Kim J-Y, Kang H-J, Chae I-H, Oh B-H, Park Y-B, Kim H-S. Identification of a Novel Role of T Cells in Postnatal Vasculogenesis: Characterization of Endothelial Progenitor Cell Colonies. Circulation. 2007;116:1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 22.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 23.Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H. Bone Marrow Monocyte Lineage Cells Adhere on Injured Endothelium in a Monocyte Chemoattractant Protein-1-Dependent Manner and Accelerate Reendothelialization as Endothelial Progenitor Cells. Circ Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 24.Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, Zhou JY, Hu SS. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 16:577–584. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]