Abstract
Early pathology and tissue loss in Alzheimer’s disease (AD) occurs in the hippocampus, a brain region that has recently been implicated in relational processing irrespective of delay. Thus, tasks that involve relational processing will especially tax the hippocampal memory system, and should be sensitive to even mild dysfunction typical of early AD. Here we used a short-lag, short-delay memory task previously shown to be sensitive to hippocampal integrity in an effort to discriminate cognitive changes due to healthy aging from those associated with very mild AD. Young adults, healthy older adults, and individuals with very mild AD (N = 30 for each group) participated in our investigation, which entailed attempting to find an exact match to a previously presented target among a series of stimuli that varied in perceptual similarity to the target stimulus. Older adults with very mild AD were less accurate than healthy older adults, who, in turn, were impaired relative to young adults. Older adults with very mild AD were also particularly susceptible to interference from intervening lure stimuli. A measure based on this finding was able to explain additional variance in differentiating those in the very mild stage of AD from healthy older adults after accounting for episodic memory and global cognition composite scores in logistic regression models. Our findings suggest that cognitive changes in early stage AD reflect aging along with an additional factor potentially centered on sensitivity to interference, thereby supporting multifactorial models of aging.
Keywords: Alzheimer’s disease, relational memory, hippocampus, aging, online representation
1. Introduction
Episodic memory impairments across long retention intervals are generally described as the chief cognitive symptom of those with early Alzheimer’s disease (AD; Weintraub, Wicklund, & Salmon, 2012). This deficit maps on to the first site of tau pathology in early AD, which occurs in the transentorhinal cortex of the medial temporal lobe (MTL) before spreading to the entorhinal cortex and then the hippocampus (Braak & Braak, 1991). This tau pathology occurs in the context of amyloid-beta deposition in cortical regions, potentially occurring as the first manifestation of the disease (Sperling et al., 2011). Areas earliest and most heavily affected by amyloid include regions of the default-mode network, which have dense connections to the MTL (Buckner et al., 2005; Buckner, Andrews-Hanna, & Schacter, 2008; Sperling et al., 2009). Consequently, neuropsychological tests assessing the MTL, such as delayed recall of word lists or narratives, have been primarily relied on for the earliest diagnosis of clinical AD. However, recent studies evaluating the function of the hippocampal memory system have shown that hippocampus is necessary for relational processing irrespective of delay (Hannula, Tranel, & Cohen, 2006; Olson, Page, Moore, Chatterjee, & Verfaellie 2006; Piekema, Kessels, Mars, Petersson, & Fernández, 2006; Warren, Duff, Tranel, & Cohen, 2011; Watson, Voss, Warren, Tranel, & Cohen, 2013), suggesting that tasks requiring on-line relational processing may be helpful in discriminating healthy aging from the earliest stages of AD. Here we report an investigation testing the hypothesis that a task requiring ongoing, rather than long-term, relational memory processing can discriminate healthy aging from very mild AD.
Early neuropsychological research in amnesic patients with hippocampal damage, such as HM, seemed to reveal a focal cognitive deficit in which declarative information could not be recalled after a sufficient delay. In contrast, simple information could be recalled normally after shorter delays (e.g., a few seconds) and non-declarative information could be learned and retained normally (Cohen & Squire, 1980; Corkin, 1968; Graf & Schacter, 1985; Milner, Corkin, & Teuber, 1968; Sidman, Stoddard, & Mohr, 1968; Wickelgren, 1968). Further neuropsychological work and research with animal models of amnesia led to the conclusion that the major contribution of the hippocampus to cognition was supporting the encoding and explicit retrieval of declarative information in a long-term memory system (e.g., Squire, 1992). However, converging evidence from neuropsychological investigations of hippocampal amnesic patients and functional neuroimaging of healthy adults suggests that this description of hippocampal function is incomplete. In the last decade, it has been shown that if the information to be learned is relational (i.e., the relationships among individual elements must be processed: Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001), then the hippocampus is engaged irrespective of delay. For instance, hippocampal patients are impaired at remembering relational information at delays of only a few seconds (Hannula et al., 2006; Watson et al., 2013). These reports complement findings from neuroimaging studies in which hippocampal activity was observed when novel or relational information needed to be maintained over delays of approximately ten seconds (i.e., the timescale of working memory; Hannula & Ranganath, 2008; Ranganath & D’Esposito, 2001).
Perhaps even more intriguing are the findings detailing impaired performance of hippocampal amnesic patients on what might typically be termed perceptual tasks, in which the participant must make comparisons among several simultaneously-presented stimuli in order to, for example, decide which stimulus does not match the others. Critically, these tasks impose no delay and all of the information needed to correctly respond to a trial is in front of the participant. For example, patients with hippocampal damage were impaired in making discriminations between scenes (Lee, Buckley, et al., 2005; Lee, Bussey, et al., 2005), and research employing functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG) has provided complimentary evidence for a role attributed to the hippocampus in the processing and discrimination of complex visual scenes (Lee, Scahill, & Graham, 2008; Riggs et al., 2009). Similar deficits in simultaneous comparison of multiple single objects have been reported in patients with MTL damage including hippocampus and nearby cortical regions (Barense, Gaffan, & Graham, 2007) and patients with focal hippocampal damage have impairments in the ability to separate or integrate visual information about single objects (Warren, Duff, Jensen, Tranel, & Cohen, 2012).
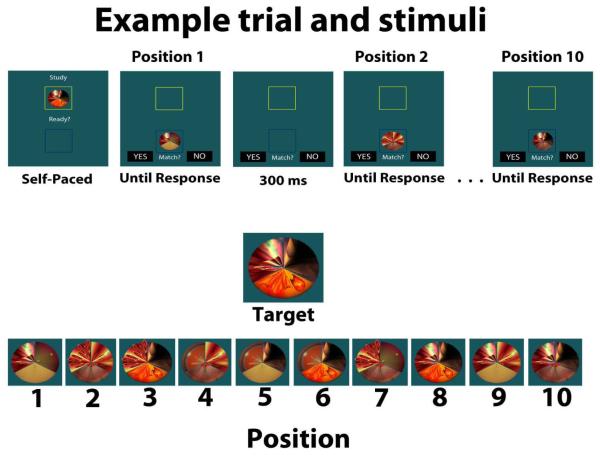
One report particularly germane to the current study found that patients with hippocampal damage were impaired while performing a complex visual search task (Warren et al., 2011). In the task, participants sought a target matching a centrally-positioned sample item. The surrounding search array was composed of objects that had parametrically manipulated levels of similarity to the target. Items were composed of three sections filled with distinctive designs expected to provoke relational processing, and sections were manipulated to create lure items that matched the sample and target items to varying degrees (i.e., zero, one, or two matching features; see Figure 1). In this study, Warren and colleagues (2011) monitored eye movement behavior during visual search by hippocampal amnesic patients and healthy comparison participants. The authors found that in addition to reduced rates of target detection (despite no imposed delay) the patients’ representation of the target stimulus degraded as a function of the number of fixated lures. Hippocampal patients and healthy comparisons alike had longer fixations to items resembling the target. However, whereas this effect was robust throughout search in the comparison participants, for hippocampal patients this effect was seen only for lures viewed shortly after (re)viewing the target stimulus. The duration of their fixations to lures resembling the target decreased the more lures they fixated without having re-sampled the central sample item, which suggests a fading of the internal representation of the target during visual search (Warren et al., 2011).
Figure 1).
Top panel: Example timing and sequence of a trial. Participants study the target item and press a button to initiate the search sequence. At each position they make a decision as to whether the presented item is an exact match to the target item. There are ten stimuli presented sequentially on each trial. Bottom panel: Shows target and the ten stimuli shown during the search sequence of this trial. The stimulus at position eight is the match to the target. Stimuli at positions four, five, and six represent feature overlap levels zero, one, and two, respectively.
Taken together, these data suggest a critical role for the hippocampus in relational binding and comparison irrespective of delay (Voss et al., 2011; Warren et al., 2012). Thus, if the essence of hippocampal function is not necessarily memory for declarative information at long delays, but rather relational binding and memory at any delay (even a delay of just several saccades), then tests assessing these processes may be able to detect dysfunction of the hippocampus, thereby providing tools sensitive to the earliest stages of AD. In the current study, we used a behavioral paradigm inspired by Warren et al. (2011) that proved sensitive to hippocampal dysfunction. Data were collected from young adults, healthy older adults, and participants with very mild AD in an effort to isolate effects related to aging versus those due to the earliest stages of AD. Given the effects found in Warren et al., (2011) with hippocampal amnesic patients, we hypothesized this task would be sensitive to the earliest changes that occur within the hippocampus due to very mild AD.
2. Materials and Methods
2.1. Participants
Sixty-four older adults, age range 62-94, were recruited to participate from the Charles F. and Joanne Knight Alzheimer’s Disease Research Center (Knight ADRC). Of the 64 participants recruited, 30 were healthy older adults and 34 had very mild AD. The presence and severity of Alzheimer’s disease was assessed using the Washington University Clinical Dementia Rating (CDR) Scale (Morris, McKeel, Fulling, Torack, & Berg, 1988; Morris, 1993). The CDR is a 90-minute interview assessment conducted by a trained clinician that assesses the patient and collects information from family members to determine changes in cognition and function. The CDR employs a scale with the values 0, 0.5, 1, 2, and 3, mapping on to no AD, very mild AD, mild AD, moderate AD, and severe AD, respectively. The AD participants in the present study only included individuals with the earliest stage of AD dementia and had a CDR rating of 0.5. The CDR has been shown to be particularly sensitive to detecting the earliest stages of AD, is highly reliable (Burke et al., 1988), and has a very high concordance with a neuropathological diagnosis of AD confirmed at autopsy, even in individuals with a CDR of 0.5 (Berg et al., 1998; Storandt, Grant, Miller, & Morris, 2006).
Several participants with AD (N = 7) had difficulty completing the task and those sessions were terminated before completion. Participants were included in the study if they completed more than half of the experiment’s main phase test trials (≥ 22 of 42 total trials); this criterion excluded an additional four participants with very mild AD. Three individuals with very mild AD completed more than half the experiment and thus their data were retained for analysis; all other data presented here reflect complete test sessions. Thus, the final numbers of participants in the older adult groups were as follows: healthy older adults (CDR 0) = 30, very mild AD patients (CDR 0.5) = 30. To further dissociate the effects of aging from very mild AD, 31 young adults from the Urbana-Champaign community, aged 19-28, completed the experiment. One participant’s data were discarded due to a very low accuracy level (d’ value more than three standard deviations less than the group mean), leaving the final number of participants in this sample at 30. All participants signed informed consent documents and this experiment was approved by the institutional review boards of Washington University and the University of Illinois at Urbana-Champaign. Participants were monetarily compensated for their participation.
The AD group was significantly older than the healthy older adult group (see Table 1 for demographic information). Therefore, all comparisons involving the healthy older adult group and AD patients either included age as a covariate, or when analyses also involved young adults, a separate confirmatory analysis was conducted on the older adult samples including age as a covariate to partial out the effect of age on any findings of AD status.
Table 1.
Participant Demographics
| Variable | Young | CDR 0 | CDR 0.5 |
|---|---|---|---|
| N | 30 | 30 | 30 |
| % female | 50% | 53% | 47% |
| Age M (SD) | 21.7 (2.1) | 71.3 (6.7) | 76.1 (7.6) |
| MMSE M (SD) | - | 28.7 (1.2) | 27.1 (2.3) |
MMSE = Mini Mental Status Exam
2.2. Procedure
We employed a task that required participants to maintain an internal representation of a target stimulus while attempting to find an exact match to that target when viewing a series of highly similar objects presented on a computer display. The experiment was conducted using Presentation software (Neurobehavioral Systems, http: http://www.neurobs.com/). Figure 1 displays an example of the stimuli and an example trial. The stimuli used here were novel, computer-generated objects originally used by Warren et al. (2011). Each stimulus was composed of three distinct sections that together formed a circular object, with each section containing a unique, novel design (i.e., a “feature”). Three different, distinctive features were available for each section, yielding a total of 27 unique stimuli that were used throughout the experiment.
Prior to participating in the actual experiment, participants completed four practice trials with different stimuli that were structured identically to those in the actual experiment. The full experiment contained 42 trials. During a trial, participants were presented with a sample target stimulus at the top of the display and instructed to study the stimulus. After studying the item, the participant pressed a button that initiated a search sequence. During this phase of the trial, the target stimulus disappeared from the top of the display and the first potentially matching stimulus appeared at the bottom of the display. Participants were instructed to indicate whether the stimulus at the bottom of the display was an exact match with the studied sample by pressing one of two keyboard keys indicating “yes” or “no.” During a trial, participants saw ten serially presented stimuli; each stimulus was presented 300 ms after the previous match/mismatch response. Nine of the stimuli presented during each trial were lures, and their similarity to the target (“feature overlap”) was parametrically varied such that a lure could share zero, one, or two identical features with the target. One of the ten stimuli shown was the target, that is, an exact match to the sample item. To maximize interference and engage the MTL memory system, on most trials (36 of 42) the target was the sixth, eighth, or tenth object presented. These 36 critical trials were split evenly between the different target position conditions (i.e., target at ordinal positions six, eight, and ten). Additionally, an equal number of stimuli presented throughout the critical trials shared zero, one, or two features with the target stimulus. In the six remaining trials, the target was the second item and all subsequently presented items on these trials shared no features with the target; these catch trials were introduced in order to keep participants from learning to reject all items shown early in a trial without evaluating them.
2.3. Data analysis
Both accuracy and response time (RT) measures were analyzed to evaluate performance on this task. A signal detection approach was utilized to assess accuracy, and d’ values were calculated for each participant at each of the three possible target positions. The d’ measure was derived by calculating the hit rate and false alarm (FA) rate at (ordinal) positions six, eight, and ten; these d’ values are heretofore referred to respectively as d’6, d’8, and d’10. In the case of a hit rate of 1 or an FA rate of 0, d’ values were calculated by using 1-(1/2N) and 1/2N respectively, with N equaling the number of trials contributing to the analysis (Green & Swets, 1966). When only assessing the hit rate or FA rate individually, the raw values were used. In addition to the d’ measure, a FA rate was calculated across the trials for each participant at each level of feature overlap. To assess how the FA rate changed as the trial unfolded, levels representing the stages of the trial were formed by combining positions 2-4, 5-7, and 8-10 into “early,” “middle,” and “late” in the trial, respectively, forming the factor “trial stage.” Responses to items at position one were discarded, as response times on these trials were much longer than responses to items at the other nine positions across all populations, likely reflecting a task-switching cost between the study phase and the test phase.
Prior to statistical analysis of the RT measures, the RT data were trimmed in the following manner. Only correct responses were considered for the RT analysis, and RTs shorter than 250 ms or longer than 6000 ms were discarded; this resulted in the removal of 2.6% of all data. Following this initial pruning of the data, values that were three standard deviations greater than an individual’s mean were discarded, resulting in the additional removal of 1.8% of the remaining data set. To account for overall age- and dementia-related changes in RT, which can mislead the interpretation of group × condition interactions (Faust, Balota, Spieler, & Ferraro, 1999) the trimmed RTs for each participant were standardized by transforming them into z scores using each participant’s overall trimmed mean and standard deviation; all statistical analyses were performed on the standardized RT data which are referred to as zRT. The main zRT measure focused on correct rejections so as to analyze zRT across the groups as a function of feature overlap and trial stage. zRT to the target was conducted in a separate analysis in order to avoid confounding “yes” vs “no” responses.
After these initial analyses, we also tested whether task performance could successfully discriminate healthy aging (CDR 0) from very mild AD (CDR 0.5), compared to the classification results achieved by using standard neuropsychological measures. Three hierarchical logistic regression analyses were conducted that used data from this task along with measures derived from the extensive neuropsychological testing administered to the participants of this study. First, we wished to directly compare our task and delayed recall of Logical Memory Story A to see if data from our task explained variance in CDR status after accounting for delayed recall performance. Also, using a similar factor structure previously reported in this population (Johnson, Storandt, Morris, Langford, & Galvin, 2008), we evaluated our task with two composite scores from the neuropsychological data: an episodic memory score and a global cognition score. The episodic memory composite score was composed of an average of the following scores: the sum of the three free recall trials on the Selective Reminding Task (Grober, Buschke, Crystal, Bang, & Dresner, 1988), Paired Associate Learning from the Wechsler Memory Scale (WMS; Wechsler & Stone, 1973), and immediate recall from WMS Logical Memory Story A. The global cognition score was composed of the averaged scores on the above tests making up the episodic memory score, plus averaged scores from tests which formed three other composites: semantic memory, working memory, and visual spatial function. The semantic memory composite score included scores from the Information subtest on the Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1955), Boston Naming Test (Goodglass, 1983), and Animal Naming (Goodglass, 1983). The working memory composite score was composed of WMS Mental Control, Digit Span Forward and Digit Span Backward, and Letter Fluency for S and P (Thurstone & Thurstone, 1949). The visual spatial composite included the WAIS Block Design and Digit Symbol subtests, and Trail Making A and B (Armitage, 1946).
The episodic memory composite and delayed recall measure were included in the logistic regression analysis because of the voluminous literature on declines in episodic memory and early AD (Salmon, 2000). The inclusion of a global cognition composite was due to the impairments on multiple cognitive domains that have been observed in early AD, deficits that can powerfully discriminate between healthy aging and AD (Johnson et al., 2008; Johnson, Storandt, Morris, & Galvin, 2009). With the exception of the delayed-recall measure we focused on composite scores rather than any individual neuropsychological sub-task. Composite scores of multiple tests generated an amount of data more comparable to that in the experimental task used here, which produced more data than that collected on any individual standard neuropsychological task. Consequently, direct comparisons between this task and an individual neuropsychological task may be unfair, as the latter measure may be noisier. The one exception to this policy was with delayed recall, a test which has so many times in the literature yielded large deficits in early AD, Including delayed recall in the episodic memory composite did not change the results. The variable chosen (post hoc) for inclusion from the new task described in this report was d’ 10, based on the finding to be reported here of declining accuracy as a function of trial stage associated with very mild AD (see Results). In all logistic regression analyses, age was entered as a factor due to age differences between the two groups. Neuropsychological data was available for 51 of the 60 CDR 0 and CDR 0.5 participants (CDR 0, N = 24; CDR 0.5, N = 27), and thus the logistic regression analyses only included these participants.
In the logistic regression analyses, age and one of the neuropsychological scores were entered in step one to establish the variance associated with these measures, and then d’10 was entered to determine if this carried any unique explanatory power; three separate models were run for delayed recall, episodic memory, and global cognition. Finally, the reverse analysis was completed where age and d’10 were entered, followed by delayed recall, episodic memory, or global cognition.
Further, an anonymous reviewer suggested we perform a receiver operating characteristic (ROC) analysis to determine an optimal score on our task that best dissociated CDR 0 participants from CDR 0.5 participants. Thus, for both of the older adult groups we conducted the ROC analysis on their scores for d’10 using the pROC package with R (Robin et al., 2011) to determine a cutoff score that best separates the groups, as well as measures of sensitivity and specificity.
3. Results
3.1. Study time analysis
Since the study phase on each trial was self-paced, it is possible that study time may have differed between groups, and that those who spent longer observing the object would do better on the task. To assess this, we compared the mean study time for the three groups in a one-way ANOVA; study time data for one young adult was unavailable due to a computer error. This analysis indicated a main effect of group, F (2, 86) = 29.00, p < .001, which was driven by the young adult group studying the sample item for shorter periods of time (M = 6.4 s, SD = 1.9 s) than the CDR 0 (M = 14.65 s, SD = 6.3 s), t (57) = 6.76, p < .001, and CDR 0.5 groups (M = 13.75 s, SD = 4.3 s), t (57) = 8.45, p < .001. There was no difference in study time between the two older adult groups, t (58) = .65, p = .52. Further, within the two older adult groups we analyzed whether individual differences in study time modulated accuracy by correlating study time with the d’ measures at each position and d’ overall; no significant effects were observed, (all r’s < .2, all p’s > .13). Finally, given that the young adults studied the sample item for the shortest duration, but performed most accurately in the task (see below), it is unlikely that the results here are due to speed-accuracy tradeoffs during the study phase.
3.2. Accuracy measures
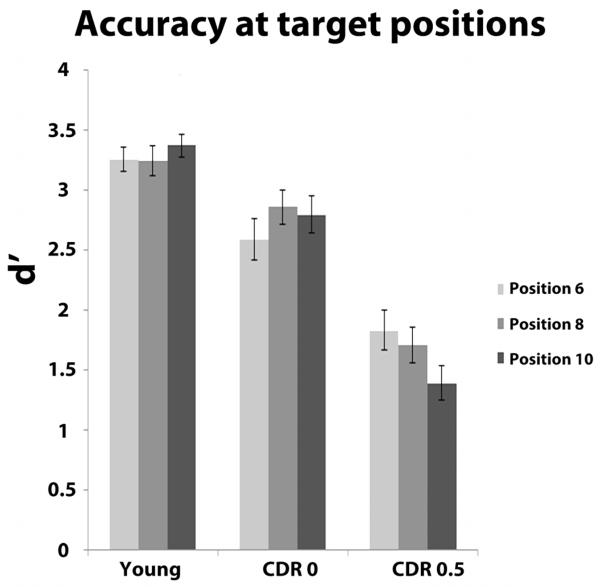
Figure 2 displays the d’ values as a function of group and position. Overall, young adults were more accurate than healthy older adults, who were in turn more accurate than those with very mild AD; also, the very mild AD group showed declining accuracy as position increased. The mixed effect ANOVA revealed a main effect of group, F (2, 87) = 44.86, p < .001, as well as a significant group × position interaction, F (4,174) = 5.95, p < .001. Across all positions, young adults (M = 3.29, SD = .48) were more accurate than the CDR 0 participants (M = 2.75, SD = .79), t (58) = 3.21, p = .002, who in turn had better signal detection than the CDR 0.5 group (M = 1.64, SD = .75), t (58) = 5.58, p < .001). The interaction reflected differential effects of the position factor between the two older adult groups. Specifically, the CDR 0 group showed an increase in accuracy from d’6 (M = 2.59, SD = .95) to d’8 (M = 2.86, SD = .76), t (29) = 2.39, p = .02, whereas the CDR 0.5 group displayed significant decreases in accuracy when comparing d’6 (M = 1.83, SD = .91) or d’8 (M = 1.71, SD = .80) to d’10 (M = 1.39, SD = .79), t (29) = 4.32, p < .001, and, t (29) = 2.64, p = .01, respectively. Furthermore, the differences in signal detection across position were attributable to changes in the FA rate for the CDR 0 group, but changes in the hit rate for the CDR 0.5 group. The CDR 0 group’s FA rate decreased significantly from position six (M = .07, SD = .07) to position eight (M = .03, SD = .05), t (29) = 2.9, p = .01. For the CDR 0.5 group, a declining hit rate largely contributed to the decrease in d’, as there was a reliable difference in the hit rate from position six (M = .67, SD = .22) to position ten (M = .56, SD = .22), t (29) = 2.8, p = .002., whereas the FA rate from position six (M = .11, SD = .09) to position ten (M = .13, SD = .09) only increased modestly, t = 1.6, p = .12. Table 2 provides detailed descriptive statistics for all groups. Visual inspection of the data suggested that in contrast to the other two groups, the CDR 0.5 group may have a linear decrease in d’ across the three levels of position; this was confirmed by a group × position interaction for the linear term, F (2,87) = 11.54, p < .001. This interaction was not an effect of differing age between the CDR 0 and CDR 0.5 group, because a separate 3 × 2 ANCOVA with the two older groups (and age as a covariate) yielded the same result, F (1,57) = 14.01, p < .001.
Figure 2).
Accuracy as assessed by d’ values. Error bars represent ± one s.e.m.
Table 2.
Accuracy at target locations
| Young | CDR 0 | CDR 0.5 | |
|---|---|---|---|
| Position Variable |
6 8 10 | 6 8 10 | 6 8 10 |
|
|
|||
| Hit rate | |||
| M | .92 .91 .92 | .81 .83 .82 | .67 .66 .56 |
| SD | .09 .12 .11 | .20 .17 .21 | .22 .21 .22 |
| False alarm rate | |||
| M | .03 .02 .01 | .07 .03 .04 | .11 .13 .13 |
| SD | .04 .04 .02 | .07 .05 .04 | .09 .10 .09 |
| d ’ | |||
| M | 3.3 3.2 3.4 | 2.6 2.9 2.8 | 1.8 1.7 1.4 |
| SD | .54 .69 .53 | .95 .76 .84 | .91 .80 .79 |
Accuracy data at each of the three possible target positions. CDR = Clinical Dementia Rating CDR 0 = no dementia; CDR 0.5 = very mild Alzheimer’s disease
In an additional analysis suggested by an anonymous reviewer, we evaluated whether the hit rate differed between the groups on the catch trials, where the target appeared early in the trial (position two). Though there were fewer trials where the target appeared in position two, this analysis would be informative as to the nature and degree of impairment in the CDR 0.5 group. A one-way ANOVA comparing the hit rate at position two across the three groups was significant, F (2, 87) = 5.23, p = .007. Post-hoc t-tests revealed this effect was due to the CDR 0.5 group having a lower hit rate (M = .71, SD = .27) compared to the CDR 0 group (M = .86, SD = .22), t (58) = 2.28, p = .03. This effect remained after regressing the influence of age on the hit rate at position two, and conducting a t-test on the residuals, t (58) = 2.14, p = .04. There was no difference in the hit rate at position two between the CDR 0 group and the young adults (M = .88, SD = .15), t (58) = .46, p = .65.
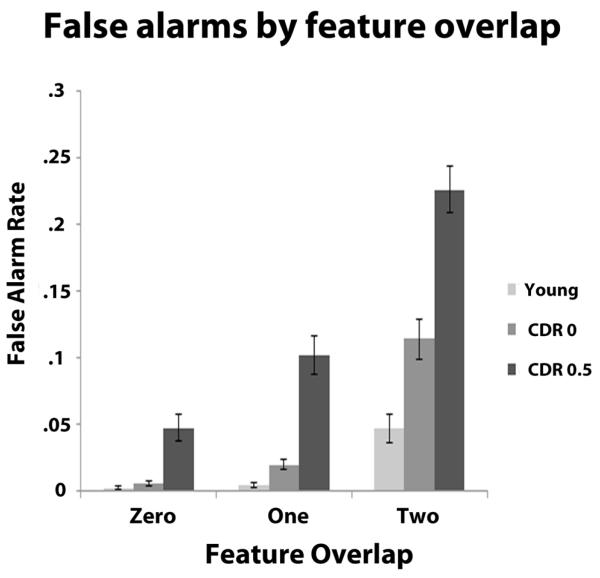
Analysis of the FA rate data from positions 2-10 indicated that young adults had the lowest FA rate followed by the CDR 0 group and the CDR 0.5 group. While all groups had increases in their FA rate as feature overlap increased (see Table 3 for detailed descriptive statistics), the effect of increasing feature overlap on FA rates was larger for the CDR 0 participants compared to young adults, and this pattern was exacerbated when comparing the CDR 0.5 group to the CDR 0 group. FA rates were entered into a 3 × 3 × 3 mixed-measures ANOVA with group as a between-subjects factor and feature overlap (zero, one, or two) and trial stage (early, middle, and late) as within-subject factors. The effects of feature similarity, age, and CDR status were evident in main effects of feature overlap, F (2,174) = 213.22, p < .001 and group, F (2, 87) = 42.05, p < .001, and these were qualified by a feature overlap × group interaction, F (4,174) = 25.53, p < .001. In order to further pursue the nature of this interaction, we conducted a 3 × 2 mixed-measures ANOVA with the factor of feature overlap and group (young adult and CDR 0), which produced a significant interaction, F (2, 116) = 13.55, p < .001 due to the two groups showing no difference in FA rate at feature overlap level zero (mean difference in FA rate = .004), t (58) = 1.78, p = .08, but more FAs for the CDR 0 group at feature overlap level one (mean difference in FA rate = .02), t (58) = 3.45, p = .001, and this difference became larger at feature overlap two (mean difference in FA rate = .07), t (58) = 3.75, p < .001 (Table 3). This pattern of disproportionate increases in the FA rate per group when feature overlap increased was also apparent in a similar 3 × 2 ANCOVA comparing the CDR 0 and CDR 0.5 groups with age as a covariate, F (2, 114) = 6.17, p = .003. Here, the CDR 0.5 group had a significantly higher FA rate compared to the CDR 0 group at each level of feature overlap, but this difference became larger as feature overlap increased (mean difference in FA rates between CDR 0 and CDR 0.5 after adjusting for age: feature overlap level zero = .03; feature overlap level one = .06; feature overlap level two = .08; all t values > 3, all p values < .003; Figure 3). Finally, a main effect of trial position was observed, F (2,174) = 7.77, p = .001 due to a general increase in FAs during the middle portion of a trial (M = .07, SD = .07) compared to the early (M = .06; SD = .06), t (89) = 4.15, p < .001, and the late portions of trials (M = .06; SD = .07), t (89) = 2.21, p = .03. Follow-up analyses indicated this effect occurred only in the two older adult groups and largely as a result of an increase from early to middle, as the CDR 0.5 group had a significant increase from early (M = .12, SD = .07) to middle (M = .14, SD = .07), t (29) = 2.58, p = .02 with no increase from middle to late (M = .13, SD = .08), t (29) = 1.44, p = .16. The CDR 0 group also displayed an increase from early (M = .04, SD = .04) to middle (M = .06, SD = .05), t (29) = 3.44, p = .002, with a small increase from middle to late (M = .04, SD = .04), t = 2.18, p = .04.
Table 3.
False alarm rate by feature overlap and trial stage M (SD)
| Feature Overlap | |||
|---|---|---|---|
| Zero | One | Two | |
| Trial Stage |
|
||
| Young | |||
| Early | .00 (.00) | .00 (.01) | .04 (.06) |
| Middle | .00 (.00) | .00 (.02) | .05 (.06) |
| Late | .00 (.01) | .01 (.02) | .05 (.05) |
| CDR 0 | |||
| Early | .00 (.01) | .02 (.03) | .10 (.09) |
| Middle | .00 (.02) | .02 (.04) | .14 (.11) |
| Late | .01 (.03) | .02 (.04) | .10 (.08) |
| CDR 0.5 | |||
| Early | .03 (.04) | .10 (.10) | .22 (.10) |
| Middle | .06 (.06) | .11 (.08) | .25 (.12) |
| Late | .05 (.06) | .11 (.09) | .22 (.14) |
Note: False alarm rates listed as .00 are due to rounding, as each group had some false alarms at each level of feature overlap and trial stage, accounting for cases where the mean is listed as zero but the standard deviation is greater than zero. Early refers to positions 2-4, Middle 5-7, Late 8-10. CDR = Clinical Dementia Rating CDR 0 = no dementia; CDR 0.5 = very mild Alzheimer’s disease
Figure 3).
False alarm (FA) rate as a function of feature overlap with the target and trial stage Error bars represent ± one s.e.m.
3.3. Standardized RT data
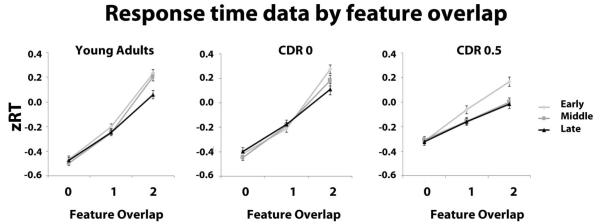
Standardized RT data (zRT) of correct rejections were analyzed in 3 × 3 × 3 mixed-measure ANOVA as described for the analysis of FA rates. There were main effects of group, F (2, 87) = 15.12, p < .001, and feature overlap, F (2, 174) = 583.38, p < .001. More importantly, there was a reliable feature overlap × group interaction, F (4, 174) = 17.34, p < .001. For both the young and CDR 0 groups, zRTs across feature overlap levels zero, one, and two were best fit with a quadratic term (young adults: F (1, 29) = 19.12, p < .001; CDR 0: F (1, 29) = 20.28, p < .001). This was due to a larger difference in zRT between feature overlap one and two compared to zero and one (Table 4; Figure 4). The CDR 0.5 participants produced a linear increase in zRT across the three levels of feature overlap, F (1,29) = 111.72, p < .001, this increase was approximately equal between feature overlap level zero and one, and one and two, as indicated by the non-significant test for the quadratic term, F (1,29) = .03, p = .86 (Figure 4). An ANCOVA on the data from the older two groups with age as a covariate confirmed this interaction was not due to the difference in age between the two older groups, F (2,114) = 13.48, p < .001.
Table 4.
zRT for correct rejections by feature overlap and trial stage M (SD)
| Feature Overlap | |||
|---|---|---|---|
| Zero | One | Two | |
| Trial Stage |
|
||
| Young | |||
| Early | −.47 (.14) | −.20 (.12) | .23 (.17) |
| Middle | −.49 (.11) | −.25 (.12) | .21 (.19) |
| Late | −.47 (.14) | −.25 (.16) | .06 (.19) |
| CDR 0 | |||
| Early | −.42 (.14) | −.21 (.16) | .27 (.19) |
| Middle | −.45 (.11) | −.19 (.14) | .18 (.24) |
| Late | −.40 (.16) | −.18 (.19) | .11 (.26) |
| CDR 0.5 | |||
| Early | −.32 (.13) | −.06 (.18) | .17 (.21) |
| Middle | −.31 (.15) | −.15 (.15) | .00 (.18) |
| Late | −.32 (.17) | −.16 (.16) | −.02 (.20) |
Early refers to positions 2-4, Middle 5-7, Late 8-10. CDR = Clinical Dementia Rating CDR 0 = no dementia; CDR 0.5 = very mild Alzheimer’s disease
Figure 4).
Standardized response time (zRT) data as a function of feature overlap with the target and trial stage for young, CDR 0 and CDR 0.5 participants. Error bars represent ± one s.e.m.
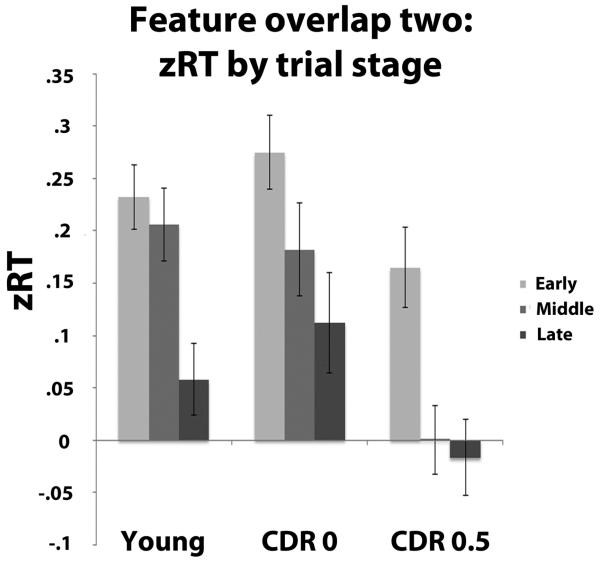
The initial three-way ANOVA also indicated a main effect of trial stage, F (2, 174) = 7.44, p = .001 and a feature overlap × trial stage interaction, F (4, 348) = 9.08, p < .001. zRTs became shorter for all groups as trial stage increased, but this was not true for all levels of feature overlap. The interaction was due to zRTs decreasing for feature overlap level two from the early to middle stage of the trial, t (89) = 3.0, p = .004 and from the middle to late stage of the trial, t (89) = 2.79, p = .006. While the three-way interaction was non-significant, F (8, 348) = 1.53, p = .14, group status did appear to influence the relationship between feature overlap level two and trial stage. Given that feature overlap two represented the most difficult condition, we investigated possible group differences at this level, and conducted a 3 (group) × 3 (feature overlap) ANOVA on the zRT data for feature overlap two. This analysis indicated a marginally significant group × trial stage interaction when testing for a quadratic effect for trial stage, F (2, 87) = 2.66, p = .08. As shown in Figure 5 this pattern is quite systematic, in that for the healthy younger adults there is a reliable difference between both the early and middle positions and the late position, t (29) = 3.3, p = .003, and t (29) = 2.79, p = .01 respectively, with no difference between the early and middle positions, t (29) = .61, p = .55. Turning to the older adults, response times seemed to linearly decrease across the trial positions. While there is no reliable difference between early and middle positions, t (29) = 1.41, p = .17, and middle and late positions, t (29) = 1.4, p = .17, the difference between early and late positions was significant, t (29) = 2.27, p = .03. For the CDR 0.5 individuals there is a reliable decrease between the early positions and both the middle, t (29) = 3.19, p = .003, and the late t (29) = 3.25, p = .003 positions. Thus, the increase in response latency for the high two-feature overlap items systematically changed across positions across groups, with a relatively late effect for the young adults, more linear increase for the CDR 0 individuals, and a relatively early effect for the CDR 0.5 individuals (see Figure 5).
Figure 5).
Differential decrease in standardized RTs for groups across trial stage at level two of feature overlap. Error bars represent ± one s.e.m.
3.4. Logistic regression analysis & ROC analysis
Logistic regression analyses revealed that a metric derived from the current task provided excellent discrimination between healthy older adults and those in the earliest stages of AD, surpassing discrimination performance using available standard neuropsychological measures. On the delayed recall measure, the CDR 0 (M = 13.46, SD = 4.77) and CDR 0.5 (M = 6.52, SD = 6.48) groups were significantly different t (49) = 4.37, p < .001 (see Table 5 for all neuropsychological task results). Also the two groups reliably differed on the episodic memory composite, t (49) = 4.84, p < .001, and the global cognition composite, t (49) = 4.46, p < .001, with the CDR 0 group having higher scores on both measures. In the first analysis, age and delayed recall were entered in step one, resulting in a significant amount of explained variability in CDR status, 2 (2, N = 51) = 17.7, p < .001. In the second step we entered d’ 10 , which explained a large amount of residual variance, 2 (1, N = 51) = 12.4, p < .001. Similar patterns were evident in the models incorporating the composite scores. When age and the episodic memory composite score were entered in step one, the result was significant, 2 (2, N = 51) = 19.6, p < .001. In step two of this model, the inclusion of d’10 explained a significant amount of the residual variance 2 (1, N = 51) = 9.4, p = .002. Including the global cognition composite and age also explained a large proportion of variability in CDR status 2 (2, N = 51) = 18.1, p < .001. The inclusion of d’10 again explained a significant amount of residual variance 2 (1, N = 51) = 10.8, p = .001. When entering age and d’10 in step one however, the additional explanatory power of either delayed recall, the episodic memory composite, or the global cognition composite was attenuated. The initial model with age and d’10 was significant, 2 (2, N = 51) = 25.8, p < .001, and the inclusion of the delayed recall measure did result in a significant increase in CDR discrimination 2 (1, N = 51) = 4.2, p = .04. When entering the episodic memory composite score in step two of a separate model, only a marginally significant increase in the amount of variance explained was observed, 2 (1, N = 51) = 3.2, p = .08; the inclusion of the global cognition composite, in a separate model, yielded a similar result, 2 (1, N = 51) = 3.08, p = .08. Thus, the discrimination performance in the most difficult condition on this task appears to be particularly sensitive to the transition between healthy aging and the earliest stages of AD. Table 6 depicts the classification rates and effect sizes for the models.
Table 5.
Psychometric data as a function of the two older adult groups M (SD)
| Task | CDR 0 | CDR 0.5 | p-value |
|---|---|---|---|
| Logical Memory Story A-Immediatea | 13.92 (4.77)_ | 8.44 (5.58) | < .001 |
| Logical Memory Story A-Delayed | 13.46 (4.59) | 6.52 (6.47) | < .001 |
| Selective Reminding Taska | 34.07 (6.67) | 21.46 (10.71) | < .001 |
| Paired Associate Recalla | 14.81 (4.57) | 10.72 (4.74) | .004 |
| Informationb | 20.46 (5.37) | 17.63 (5.64) | .07 |
| Boston Naming Testb | 54.75 (5.68) | 48.96 (10.04) | .02 |
| Animal Fluencyb | 22.37 (6.49) | 15.74 (5.70) | < .001 |
| Mental Controlc | 6.92 (2.13) | 6.81 (2.02) | .86 |
| Digit Forwardc | 6.50 (1.14) | 6.52 (0.89) | .95 |
| Digit Backwardc | 4.83 (1.13) | 4.19 (0.92) | .03 |
| Letter Fluencyc | 33.58 (9.84) | 27.85 (9.95) | .04 |
| Block Designd | 32.91 (8.85) | 23.44 (5.12) | < .001 |
| Digit Symbol Codingd | 51.42 (10.53) | 38.54 (10.90) | < .001 |
| Trail Making A*d | 32.30 (11.42) | 46.70 (21.79) | .002 |
| Trail Making B*d | 86.92 (35.08) | 117.00 (48.97) | .02 |
Higher scores indicate worse performance;
tasks in episodic memory composite,
tasks in semantic memory composite,
tasks in working memory composite,
tasks in visual spatial composite
Table 6.
Logistic regression analyses predicting healthy aging vs. very mild Alzheimer’s disease
| 1st step variables_____ | CCR | R 2 | 2nd step variable | CCR | R2______ |
|---|---|---|---|---|---|
| Age & delayed recall | 74.5 | .39 | d’10 | 82.4 | .59 |
| Age & episodic memory | 70.6 | .43 | d’10 | 78.4 | .58 |
| Age & global cognition | 74.5 | .40 | d’10 | 80.4 | .58 |
| Age & d’10 | 78.4 | .53 | delayed recall | 82.4 | .59 |
| Age & d’10 | 78.4 | .53 | episodic memory | 78.4 | .58 |
| Age & d’10 | 78.4 | .53 | global cognition | 80.4 | .58 |
Episodic memory and global cognition refer to composite scores calculated from neuropsychological tests. CCR = correct classification rate; R2 refers to Nagelkerke’s R2
Finally, we conducted an ROC analysis to determine the optimal cutoff score at d’10 which separates the two older adult groups. The analysis indicated the best cutoff score at position ten for classifying the two groups was a d’ of 2.74, which had a median sensitivity of 1.0 and a median specificity of 0.63.
4. Discussion
In this study we employed a task that has been shown to be sensitive to hippocampal function to assess changes in related cognitive abilities across the lifespan, and evaluated whether performance could distinguish healthy aging from very mild AD. Healthy aging was associated with reduced accuracy on this task. Subjects with very mild AD showed more marked impairment, both quantitatively and qualitatively, than the cognitively intact older adults. The AD-related impairment was coupled with an additional decrease, not seen in other older adults, in signal detection as a function of interference, specifically the number of intervening stimuli since the sample stimulus was presented. Classification (using logistic regression models) of CDR 0 vs. CDR 0.5 participants based on performance measures was highly accurate. Finally, while FA rates increased as a function of feature overlap with the target for all participants, FA rates increased more with feature overlap for healthy older adults than young adults, and for AD patients relative to healthy older adults. Together, these findings support the hypothesis that tasks assessing relational processing, even at short delays, are sensitive to the earliest stages of AD.
The results from the signal detection analyses are informative as to whether AD is an extreme along the trajectory of healthy aging or, as suggested by multifactorial models of brain aging (Buckner, 2004), a qualitatively different state, In addition to being less accurate overall, the d’ data from the CDR 0.5 participants displayed a unique pattern characterized by decreasing accuracy as the number of interfering stimuli increased. This pattern of behavioral results indicates that AD carries with it an additional memory impairment that may be centered on sensitivity to interference. Therefore, these data suggest the cognitive manifestations of AD seem to be due to aging together with an additional factor due to a disease state.
Considering the neural substrates underlying these differences in task performance due to AD, it is likely that the hippocampus and possibly the MTL cortex are involved. In an eye-tracking version of an experiment using these stimuli, impairments in MTL amnesic patients were only observed after a sufficient number of objects (greater than six) had been fixated, potentially indicating a degrading representation due to increased interference (Warren et al., 2011). Structural MRI studies of healthy aging and mild AD report that hippocampal volume and MTL cortical thickness correctly categorize individuals with a high level of accuracy, indicating substantial tissue loss in these regions occurs in the earliest stages of AD (Desikan et al., 2009; Dickerson et al., 2009; Head, Snyder, Girton, Morris, & Buckner, 2005). Given these anatomical findings and the observed decline in d’ at longer latencies (similar to that observed in amnesic patients), the effect here may be related to hippocampal atrophy. This interpretation would be consistent with animal models that suggest hippocampal lesions result in a disproportionate deficit in the hit rate (which was observed here in the CDR 0.5 group) as opposed to the false alarm rate (Fortin et al., 2004).
The group differences in the FA rate data across the duration of a trial, where increasing feature overlap caused disproportionate increases in FA rates for the two older adult groups, could be attributable to several candidate mechanisms. For instance, atrophy in brain regions implicated in source monitoring and inhibition, such as the prefrontal cortex (PFC), may play a role in the FA effects since PFC volume decreases in healthy aging and this area is also damaged in AD (Dickerson et al., 2009; Mesulam, 2000; Raz et al., 2005). Additionally, FA rates on this task in both older adult groups may also partially be explained from a pattern separation framework centered on MTL integrity. Healthy older adults (relative to young adults) and amnestic mild cognitive impairment patients (relative to healthy older adults) are more likely to endorse highly similar lures as “old,” demonstrating a shift towards pattern completion. Specifically, older adults and amnestic mild cognitive impairment patients need objects to be more perceptually distinct from a previously seen item held in memory in order for them to distinguish the current item from the internal representation. When the current object is highly similar to the remembered object, older adults tend to treat these two stimuli as the same, and therefore erroneously endorse the current item as “old.” In the current study, parametric increases in perceptual similarity of these novel stimuli caused all participants to endorse more similar items as “old” more frequently, but the slope of the lines across the levels of feature overlap was steeper for CDR 0 participants than young adults, with the CDR 0.5 participants showing even steeper increases in false alarms. Thus, the false-alarm rate data here are broadly consistent with previous work on the topic, and may be reflective of a weakness in pattern separation with aging, causing older adults to less reliably distinguish perceptually similar items and therefore move toward pattern completion (Stark, Yassa, Lacy, & Stark, 2013; Yassa et al., 2010). From this perspective, the integrity of the entorhinal cortex and dentate gyrus may be important, as performance on pattern separation tasks is linked to these areas, and they are impacted by Alzheimer’s disease and normal aging, respectively (Small, Schobel, Buxton, Witter, & Barnes, 2011; Yassa, Mattfeld, Stark, & Stark, 2011).
One unexpected result with the FA rate data was the main effect of trial position, driven largely by increases in FAs in the middle portion of a trial relative to early, in the two older adult groups. One speculative possibility accounting for this is that participants realized the probability of target appearance by trial position, and became more likely to indicate a match in the middle portion of a trial in anticipation of the potential target. This effect may have been absent in the young adult group due to the overall low levels of FAs, and it may have been noisier in the CDR 0.5 group due to the requirement to extract the probability of when the target appears, a task that some may have been too impaired to achieve.
The zRT data for the young and CDR 0 groups are intriguing because both of these groups remained as accurate in identifying the matching stimulus at position six as they were at position ten, but like the CDR 0.5 participants, both groups showed reduced zRTs to stimuli sharing two features with the target as the trial continued. This modulation of zRT by trial length was different for each of the three groups, with the CDR 0.5 group showing an early decrease in zRT, the young adults a late decrease, and the CDR 0 participants a gradual reduction in zRT (Figure 5). Since zRTs didn’t decrease for all lure types, this effect is likely linked to the underlying mnemonic representation and decision making process, rather than generically faster responding at the end of a trial. Previous work using these stimuli while eye-movement data were recorded suggest that reduced viewing time to highly similar lures is indicative of reductions in the internal representation of the target, as the viewed stimulus provides less of a match with the individual’s target representation and is thus easier to reject (Warren et al., 2011). Therefore, it is possible that all groups experienced some degradation of the internal representation of the target stimulus as more interference occurred, but the amount of degradation that occurred in the young and CDR 0 groups was not enough to affect accuracy. It may also be that the zRT and d’ metrics represent two different measures of the mnemonic representation of the target stimulus, as the zRT metric is composed solely of correct rejections, whereas the d’ measure takes into account all responses.
The finding that the d’10 measure was able to further differentiate very mild AD from aging after accounting for delayed memory, episodic memory, or global cognition is informative in defining the cognitive deficits of very early AD. Rather than impairment in delayed episodic memory being the quintessential symptom of very mild AD, the results here indicate an impairment in relational processing to be at least as sensitive to very mild AD. This result is consistent with early pathology occurring in the hippocampus, and that structure’s role in relational processing irrespective of delay (Voss et al., 2011; Warren et al., 2012). It is conceivable that the memory demands in this task, particularly accuracy late in a trial, are more related to the cognitive construct of “episodic memory,” given recent results indicating hippocampal amnesic patients are impaired on not-easily-rehearsed items only when working memory capacity is exceeded (Jeneson & Squire 2011; Jeneson, Wixted, Hopkins, & Squire, 2012). However, the analysis of the hit rate on catch trials hints at this not being the case, as the CDR 0.5 group was already showing an impairment that became exacerbated with the presentation of more lure stimuli. To be sure, our study was not adequately powered to assess accuracy early in the trial, nor do hippocampal changes occur in isolation with AD; thus, future studies incorporating more targets before working-memory capacity would be exceeded coupled with hippocampal volumes may inform this debate. Nonetheless, we interpret our results as evidence of a relational processing impairment not limited to long temporal delays in very mild AD, and this information may have practical value for the neuropsychological assessment of suspected AD.
Considering the additional early manifestations of AD in the cortex, a non-mutually exclusive possibility explaining the improved classification rate for the two older adults groups involves the earliest stages of AD presenting with broader cognitive impairments, which this task may also be able to assess. For instance, a large body of evidence suggests that attentional processes decline in very early AD, including failures of inhibition and cognitive control (e.g., Balota et al., 2009; Budson, Daffner, Desikan, & Schacter, 2000; Monti, Weintraub, & Egner, 2010; Hutchison, Balota & Duchek, 2010; Spieler, Balota, & Faust, 1996, Tse, Balota, Duchek, Yap, & McCabe et al., 2011; Twamley, Ropacki, & Bondi, 2006). Thus it is possible that the attentional demands in this paradigm, including sustained attention across the ten stimulus presentations on each trial, and inhibiting a “yes” response to stimuli that shared a majority of perceptual features with the target, caused the CDR 0.5 group to perform worse. This in turn may have carried unique variance between the two older adult groups that improved classification beyond what was provided by the delayed recall, episodic memory, or global cognition factor scores. The notion of cognitive manifestations of very mild AD extending beyond the domain of episodic memory meshes well with the full neuropathological picture of very mild AD, given that the earliest and greatest areas of amyloid deposition occur in neocortical sites, namely the default mode network (Buckner, 2004; Duchek et al., 2013). Moreover, metabolism dysfunction observed via [18F] fluorodeoxyglucose positron emission tomography in those with very early Alzheimer’s disease is strongest in the posterior cingulate cortex, precuneus, and temporoparietal regions (Ewers, Sperling, Klunk, Weiner, & Hampel, 2011). Thus, given the multiple types and locations of pathology in very early AD, tasks that tax both the MTL system and its interaction with broader cortical networks may be more sensitive to the earliest stages of AD than domain-specific neuropsychological tasks. Future investigations could be designed to address this hypothesis by evaluating differences in the neuropsychological profiles of amnesic patients with focal hippocampal damage and patients with very mild AD.
In summary, we report results using a novel task for the discrimination of healthy aging from very mild AD. Healthy older adults were less accurate on this task than young adults, and those with very mild AD showed further reduced accuracy, and additionally became less accurate as a function of interference. This effect was not observed in the young and healthy older adult groups, suggesting it may be an exclusive part of the cognitive phenotype in very mild AD, and indicates that this phenotype may be the product of both aging and an additional disease state. Furthermore, a metric derived from task performance explained additional variance in logistic regression models predicting CDR status after accounting for episodic memory or global cognition, suggesting this task may be uniquely sensitive to a portion of variance in the cognitive manifestation of very mild AD that is not captured by standard neuropsychological tasks.
Highlights.
We report a novel task involving short delay, relational memory processing
This task is sensitive to the earliest stages of clinical Alzheimer’s disease
Alzheimer’s disease has an additional memory impairment compared to aging
This additional memory impairment is seemingly due to interference
Measures from this task improve classification of patients from controls
Acknowledgments
This work was supported by NIA grants PO1 AGO3991, and NIA PO1 AGO26276 (D.A.B. Project Director, J.C. Morris P.I.) and NIMH grant R01-MH062500 awarded to N.J.C. Funding sources had no role in the development, execution, or reporting of this project. We thank John Morris and the clinicians at the Charles F. and Joanne Knight Alzheimer’s Disease Research Center (Knight ADRC) for their careful diagnostic evaluations of the older adults, as well as Devon McIntyre, Allison Midden, Ari Pence, and Inge Karosevica for their help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armitage SG. An analysis of certain psychological tests used in the evaluation of brain injury. Psych Mono. 1946;60:1–48. [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, McDermott KB, Yerys BE. Veridical and false memory in healthy older adults and in Dementia of the Alzheimer’s Type. Cognitive Neuropsychology. 1999;16:361–384. doi: 10.1080/026432999380834. [Google Scholar]
- Balota DA, Tse CS, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: The power of errors in Stroop color naming. Psychology and Aging. 2010;25:208–218. doi: 10.1037/a0017474. doi:10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45(13):2963–74. doi: 10.1016/j.neuropsychologia.2007.05.023. doi:10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Miller JP, Storandt M, Rubin EH, Morris JC, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of neurology. 1998;55(3):326–35. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. doi:10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relatioship between default activity, amyloid, and memory. Journal of neuroscience. 2005;25(24):7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: gist-based memory distortion in Alzheimer’s disease. Neuropsychology. 2000;14(2):277–87. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek J, Berg L. Reliability of the Washington University Clinical Dementia Rating. Archives of neurology. 1988;45(1):31–2. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210(4466):207–10. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. MIT Press; Cambridge: 1993. [Google Scholar]
- Corkin S. Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia. 1968;6(3):255–265. doi:10.1016/0028-3932(68)90024-9. [Google Scholar]
- Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, Fischl B. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer’s disease. Brain. 2009;132(Pt 8):2048–57. doi: 10.1093/brain/awp123. doi:10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. doi:10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Thomas JB, Snyder AZ, Rich P, Benzinger TL, Ances BM. Relationship between Stroop performance and resting state functional connectivity in cognitively normal older adults. Neuropsychology. 2013;27:516–528. doi: 10.1037/a0033402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Multiple memory systems in the brain. Oxford University Press; New York: 2001. [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends in neurosciences. 2011;34(8):430–42. doi: 10.1016/j.tins.2011.05.005. doi:10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychological bulletin. 1999;125(6):777–99. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;421:188–91. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2nd ed Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Graf P, Schacter DL. Implicit and explicit memory for new associations in normal and amnesic subjects. Journal of experimental psychology. Learning, memory, and cognition. 1985;11(3):501–18. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. Society. 1966;Vol. 1:521. doi:10.1901/jeab.1969.12-475. [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900–3. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. The Journal of neuroscience. 2008;28(1):116–24. doi: 10.1523/JNEUROSCI.3086-07.2008. doi:10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. The Journal of neuroscience. 2006;26(32):8352–9. doi: 10.1523/JNEUROSCI.5222-05.2006. doi:10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer’s disease. Cerebral cortex. 2005;15(6):732–9. doi: 10.1093/cercor/bhh174. doi:10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Hutchison KA, Balota DA, Duchek JM. The utility of Stroop task switching as a marker for early-stage Alzheimer’s disease. Psychology and Aging. 2010;25:545–559. doi: 10.1037/a0018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Langford ZD, Galvin JE. Cognitive profiles in dementia: Alzheimer disease vs healthy brain aging. Neurology. 2008;71(22):1783–9. doi: 10.1212/01.wnl.0000335972.35970.70. doi:10.1212/01.wnl.0000335972.35970.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of neurology. 2009;66(10):1254–9. doi: 10.1001/archneurol.2009.158. doi:10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Graham KS. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005;15(6):782–97. doi: 10.1002/hipo.20101. doi:10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, Graham KS. Perceptual deficits in amnesia: challenging the medial temporal lobe “mnemonic” view. Neuropsychologia. 2005;43(1):1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. doi:10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cerebral cortex. 2008;18(3):683–96. doi: 10.1093/cercor/bhm104. doi:10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Aging, Alzheimer’s disease, and dementia. In: Mesulam MM, editor. Principles of behavioral and cognitive neurology, second edition. Oxford University Press; New York, NY: 2000. pp. 439–507. [Google Scholar]
- Milner B, Corkin S, Teuber H-L. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H. M. Neuropsychologia. 1968;6(3):215–234. doi:10.1016/0028-3932(68)90021-3. [Google Scholar]
- Monti JM, Weintraub S, Egner T. Differential age-related decline in conflict-driven task-set shielding from emotional versus non-emotional distracters. Neuropsychologia. 2010;48(6):1697–706. doi: 10.1016/j.neuropsychologia.2010.02.017. doi:10.1016/j.neuropsychologia.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Annals of Neurology. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. The journal of neuoscience. 2006;26(17):4596–601. doi: 10.1523/JNEUROSCI.1923-05.2006. doi:10.1523/JNEUOSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekema C, Kessels RP, Mars RB, Petersson KM, Fernández G. The right hippocampus participates in short-term memory maintenance of object-location associations. Neuroimage. 2006;33(1):374–82. doi: 10.1016/j.neuroimage.2006.06.035. doi:10.1016/j.neuroimage.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31(5):865–73. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereberal cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. doi:10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Riggs L, Moses SN, Bardouille T, Herdman AT, Ross B, Ryan JD. A complementary analytic approach to examining medial temporal lobe sources using magnetoencephalography. NeuroImage. 2009;45(2):627–42. doi: 10.1016/j.neuroimage.2008.11.018. doi:10.1016/j.neuroimage.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Muller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP. Disorders of memory in Alzheimer’s disease. In: Cermak LS, editor. Handbook of neuropsychology. second edition Vol.2: Memory and its disorders. Elsevier; Amsterdam: 2000. pp. 155–195. [Google Scholar]
- Sidman M, Stoddard L, Mohr J. Some additional quantitative observations of immediate memory in a patient with bilateral hippocampal lesions. Neuropsychologia. 1968;6(3):245–54. doi: 10.1016/0028-3932(68)90023-7. [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature Reviews Neuroscience. 2011;12(10):585–601. doi: 10.1038/nrn3085. doi:10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia : the journal of the Alzheimer’s Association. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. doi:10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of experimental psychology. Human perception and performance. 1996;22(2):461–79. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;1–8 doi: 10.1016/j.neuropsychologia.2012.12.014. doi:10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–73. doi: 10.1212/01.wnl.0000228231.26111.6e. doi:10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone LG. Examiner Manual for the SRA Primary Mental Abilities Test. Science Research Associates; Chicago: 1949. [Google Scholar]
- Tse CS, Balota DA, Yap MJ, Duchek JM, McCabe DP. Effects of healthy aging and early stage dementia of the Alzheimer’s type on components of response time distributions. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SAL, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society. 2006;12:707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):E402–9. doi: 10.1073/pnas.1100225108. doi:10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Jensen U, Tranel D, Cohen NJ. Hiding in plain view: lesions of the medial temporal lobe impair online representation. Hippocampus. 2012;22(7):1577–88. doi: 10.1002/hipo.21000. doi:10.1002/hipo.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Observing degradation of visual representations over short intervals when medial temporal lobe is damaged. Journal of cognitive neuroscience. 2011;23(12):3862–73. doi: 10.1162/jocn_a_00089. doi:10.1162/jocn_a_00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JM, Balota DA, Sergent-Marshall SD. Semantic, phonological, and hybrid veridical and false memories in healthy older adults and in individuals with dementia of the Alzheimer type. Neuropsychology. 2001;15(2):254–268. doi:10.1037//0894-4105.15.2.254. [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, Cohen NJ. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus. 2013 doi: 10.1002/hipo.22115. doi:10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual: Wechsler Adult Intelligence Scale. Psychological Corporation; New York: 1955. [Google Scholar]
- Wechsler D, Stone CP. Manual: Wechsler Memory Scale. Psychological Corporation; New York: 1973. [Google Scholar]
- Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2012;2(4):a006171. doi: 10.1101/cshperspect.a006171. doi:10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren WA. Sparing of short-term memory in an amnesic patient: Implications for strength theory of memory. Neuropsychologia. 1968;6(3):235–244. doi:10.1016/0028-3932(68)90022-5. [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8873–8. doi: 10.1073/pnas.1101567108. doi:10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. NeuroImage. 2010;51(3):1242–52. doi: 10.1016/j.neuroimage.2010.03.040. doi:10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]