Abstract
Although highly active antiretroviral therapy has improved survivorship dramatically and decreased the incidence of cytomegalovirus retinitis among patients with AIDS, other ophthalmic complications continue to occur. One complication observed in ~12% of HIV-infected patients is a presumed neuroretinal disorder (NRD), manifested as decreased contrast sensitivity and associated with vague subjective complaints of hazy vision. Pathologically, patients with AIDS even without ocular opportunistic infections have loss of optic nerve axons, suggestive of mitochondrial dysfunction. We explored whether variation in mitochondrial DNA was associated with time to NRD in HIV-infected patients in the Longitudinal Study of Ocular Complications of AIDS cohort. Within the Western European, or “N”, mitochondrial DNA macrohaplogroup, haplogroup J, was associated with 80% decrease in the risk of progression to NRD during the study (hazard ratio = 0.20, P = 0.039) and suggested an independent association with protection against NRD in a cross-section of all patients taken at enrollment (1.5% vs. 8.9% in patients with vs. without haplogroup J, respectively, P = 0.05). These data suggest that mitochondrial genotype may influence propensity to develop HIV-associated NRD in patients with AIDS.
Keywords: AIDS, mitochondrial DNA, neuroretinal disorder
INTRODUCTION
Ocular complications are common among patients with AIDS.1–3 Historically, the primary cause of visual loss in patients with AIDS is an ocular opportunistic infection, particularly cytomegalovirus (CMV) retinitis.1–5 However, the most frequently encountered ocular finding has been microangiopathy consisting of cotton wool spots with or without intraretinal hemorrhages.1 This microangiopathy typically does not cause visual loss in and of itself unless the pathology encroaches on the central fovea.1 A subtle cause of visual dysfunction is a presumed neuroretinal disorder (NRD), manifested by abnormal contrast sensitivity, color vision, and visual fields.6–9 Histopathologic studies from the era before the advent of highly active antiretroviral therapy (HAART) demonstrated substantial loss of optic nerve axons, consistent with an HIV-associated NRD.10 Approximately 12% of eyes in patients with AIDS will have low contrast sensitivity and a higher proportion will have abnormalities of visual fields despite HAART therapy.9 The prevalence of abnormal contrast sensitivity is increased among patients with low CD4+ T-cell counts, particularly those with counts less than 100 cells per microliter.9 This abnormality of contrast sensitivity is evident even among patients with normal visual acuity (20 of 20 or better), and often is substantial enough to impair reading speed.11 The pathogenesis of the HIV-associated NRD is unknown. Proposed mechanisms include (1) direct HIV infection of neural tissue; (2) bystander damage as a consequence of the immunological response to HIV infection; and (3) cumulative damage to the retina and optic nerve from the microangiopathy.
The relationship between axonal health and mitochondrial function has been observed in various genetic and acquired eye diseases including Leber Hereditary Optic Neuropathy (LHON).12 LHON has been associated with several mitochondrial DNA (mtDNA) mutations,13–16 for which pathology can be exacerbated by environmental factors or background mtDNA haplogroup,17,18 Further, among patients infected with HIV, abnormalities in mitochondrial function have been associated with mtDNA depletion,19 disruption of energy production, antioxidant enzyme de-ficiency,20 and increased oxidative damage were observed pretherapy.21 These factors suggest mitochondrial genotypes may influence NRD susceptibility in HIV-1–positive patients; hence, we evaluated whether mtDNA haplogroup was associated with occurrence of HIV-related NRD in patients in the Longitudinal Study of the Ocular Complications (LSOCA) cohort.
METHODS
Cohort
The LSOCA is a multicenter prospective cohort study designed to collect data on the incidence, prevalence, and complications resulting from AIDS-related ocular morbidities during the era of HAART. All patients in the study have been diagnosed with AIDS.2 This analysis focuses on European American patients who did not have CMV at study entry and did not develop CMV retinitis during follow-up. Enrollment in LSOCA began in September 1998. Eighty-four percent of the participants in this analysis were receiving HAART at entry. Details of the design and implementation of this prospective study have been described previously.2
Genotyping
DNA samples were extracted from white blood cells for each subject. We used 6 haplotype-tagging single nucleotide polymorphisms (SNPs) initially to classify individuals as mitochondrial macrohaplogroups N, M, and L. Individuals who had either L or M macrohaplogroups (found in persons with ancestry from Africa and East Asia) were excluded from the study. Haplogroups within the Western European (N) subset were further parsed with SNPs in the Mitochondrial Haplogrouping by the Candidate Functional Variants approach described by Hendrickson et al.22 Genotyping was performed by TaqMan Assays-by-Design (Applied Biosystems Inc, Foster City, CA). Thermocycling conditions were an initial 95°C hold for 3 minutes, followed by 30 cycles of 92°C for 15 seconds, and 56°–62°C annealing for 1 minute, depending on primer specificity.
Clinical Assessment of NRD
Among patients without NRD at enrollment, NRD date was defined as the first date when a patient had log unit contrast sensitivity less than −1.5, in either eye. Clinical methods for assessing NRD are detailed by Freeman et al.9
Statistical Analysis
Association between mitochondrial haplogroup and time to NRD was analyzed with the Cox Proportional Hazards model. Time to event was calculated with staggered entry, so that we could estimate time to NRD from AIDS diagnosis, even though approximately 80% of patients were diagnosed with AIDS before study entry.23 Only patients with a Snellen equivalent visual acuity of 20 of 20 or better were included in survivorship analyses to avoid cases of decreased contrast sensitivity attributable to other major ocular complications, such as cataracts or glaucoma. Models were adjusted for square root of nadir CD4+ T-cell count, highest log10 HIV-1 load, age, and gender. Haplogroups were tested against all other possible genotypes. Rare, loosely associated haplogroups R*, HV*, and JT* were excluded from individual analyses but included in controls. Significance was based on the log-likelihood χ2 test (P ≤ 0.05) and was not adjusted for multiple tests. Patients with NRD at enrollment were excluded from survivorship analysis; therefore, significant results were retested by a cross-sectional analysis of patients with and without NRD at time of enrollment by logistic regression. For cross-sectional analyses, the criterion for visual acuity was relaxed to a corrected 20 of 25 Snellen equivalent or better, as only 19 patients had NRD at enrollment with the more stringent cut-off of 20 of 20 or better. All analyses were performed with SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
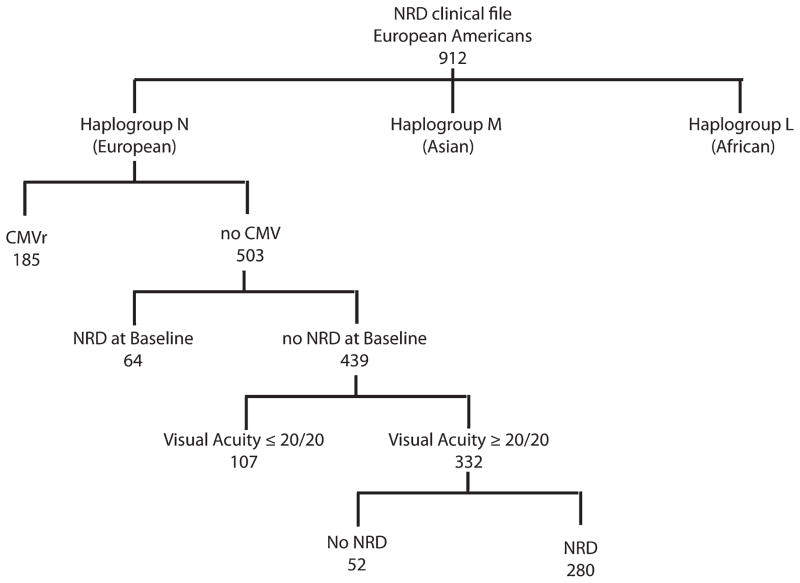
In the LSOCA cohort, 688 patients who self-identified as “white” had a Western European, or “N”, mitochondrial macrohaplogroup (96%). Of these, 503 patients did not have infectious retinitis before or during the study. Clinical characteristics of these patients are shown in Table 1. Four hundred thirty-nine participants had neither CMV retinitis nor NRD at enrollment, 19.6% of whom subsequently developed NRD as shown in Figure 1.
TABLE 1.
Clinical Characteristics of LSOCA Participants in This Analysis (n = 503)
| Demographics | |
| European American race (%) | 100 |
| Male gender (%) | 91 |
| Age (yrs), mean ± SD | 44 ± 8 |
| Immunology and virology [median (25th percentile, 75th percentile)] | |
| Nadir CD4+ T-cell count (copies/mL) | 54 (19, 121) |
| CD4+ T-cell count (cells/mL) | 210 (82, 363) |
| Peak HIV viral load (log10 cells/mL) | 5.3 (4.7, 5.7) |
| HIV viral load (log10 counts/mL) | 2.9 (2.3, 4.6) |
| AIDS history | |
| Time since AIDS diagnosis (yrs) | |
| Mean ± SD | 5.1 ± 3.7 |
| Median (25th percentile, 75th percentile) | 4.7 (2.1, 7.2) |
| HAART use (%) | 84 |
FIGURE 1.
A breakdown of the phenotypes, mitochondrial genotypes, and sample numbers of the LSOCA patients in the study.
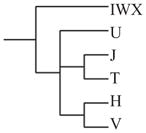
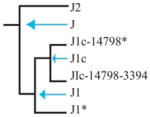
Individuals within macrohaplogroup N were analyzed as major European haplogroups IWX, U, J, T, H, and V. Haplogroup J was associated with delayed progression to NRD (hazard ratio = 0.20; 95% confidence interval = 0.03 to 1.48; P = 0.039) as shown in Table 2. Of the 29 patients with haplogroup J, only 1 developed NRD during the study. Based on cross-sectional analysis of NRD, 1.5% of the 31 patients with NRD at enrollment were in haplogroup J, versus 8.9% of controls (odds ratio = 0.39; 95% confidence interval = 0.15 to 1.00; P = 0.05). To identify the potential source of this association, we parsed the haplogroups within J further into subhaplogroups (Table 3). Within J, all subhaplogroups were consistently protective; however, only J1 reached borderline significance (P = 0.059). This result likely is due to the loss of power resulting from subdividing haplogroup J into much smaller subgroups.
TABLE 2.
Association Between NRD and the Major European Haplogroups
| f (%) | n (Affected) | HR | 95% CI | P | |
|---|---|---|---|---|---|
|
8.1 | 25 (2) | 0.52 | (0.12 to 2.19) | 0.328 |
| 22.9 | 71 (11) | 1.04 | (0.53 to 2.06) | 0.903 | |
| 9.7 | 29 (1) | 0.20 | (0.03 to 1.48) | 0.039 | |
| 11.7 | 35 (7) | 1.46 | (0.65 to 3.28) | 0.382 | |
| 41.8 | 130 (21) | 1.12 | (0.62 to 2.02) | 0.704 | |
| 2.6 | 8 (2) | 1.63 | (0.39 to 6.78) | 0.530 |
Bold values are significant at α = 0.05.
Basic phylogenetic relationships between the haplogroups are shown on the left side of the table. N (affected) represents the number of individuals with each haplogroup, whereas the number with that particular haplogroup that have NRD.
TABLE 3.
Association Between NRD and Haplogroups Within J
| f (%) | n (Affected) | HR | 95% CI | P | ||
|---|---|---|---|---|---|---|
|
J2 | 1.0 | 3 (0) | Undefined | 0.365 | |
| J | 9.7 | 29 (1) | 0.20 | (0.03 to 1.48) | 0.039 | |
| J1c-14798* | 5.1 | 15 (1) | 0.35 | (0.05 to 2.56) | 0.218 | |
| J1c | 7.0 | 21 (1) | 0.28 | (0.04 to 2.07) | 0.124 | |
| JIc-14798-3394 | 2.1 | 6 (0) | Undefined | — | 0.243 | |
| J1 | 8.7 | 26 (1) | 0.22 | (0.03 to 1.64) | 0.059 | |
| J1* | 1.7 | 5 (0) | Undefined | — | 0.216 |
Bold values are significant at α = 0.05.
Nodes are indicated in italics. Data shown for the J haplogroup are as in Table 2. Significant (P ≤ 0.05) associations are shown in boxes.
One should note that P values are based on log-likelihood χ2 tests, whereas the confidence intervals shown are computed with the Wald statistic. The log-likelihood statistic is more accurate in extreme cases such as here. The frequency shown is the frequency of the haplogroup in the analysis shown. Rare, loosely associated haplogroups that are incorporated in R*, HV* and JT* were excluded from individual analyses but included in controls; therefore, the frequencies do not add up to 100%.
We also examined mitochondrial haplogroups reported previously to be associated with AIDS progression in untreated patients22 and with lipoatrophy24 and peripheral neuropathy25 in patients on HAART. Within these haplogroups, 2 additional significant associations between mitochondrial haplogroups and progression to NRD are reported in Table 4. No individuals in haplogroup U5a1-15218, previously shown to be associated with accelerated AIDS progression,22 or haplogroup H3, associated with delayed AIDS progression,22 developed NRD in our study (P = 0.046 and 0.028, respectively). However, neither of these haplogroups had a significant effect on the prevalence of NRD at enrollment. No other significant NRD associations were observed.
TABLE 4.
Association Between NRD and Previously Reported Associations MtDNA Haplogroup and AIDS Progression in Untreated Patients22 and With Lipoatrophy24 and Peripheral Neuropathy25 in Patients on HAART
| f (%) | n (affected) | HR | 95% CI | P | |
|---|---|---|---|---|---|
| IWX | 8.1 | 25 (2) | 0.52 | (0.12 to 2.19) | 0.328 |
| W | 2.9 | 9 (0) | Undefined | — | 0.124 |
| U5a1-15218 | 3.6 | 11 (0) | Undefined | — | 0.046 |
| Uk | 10.5 | 32 (6) | 1.27 | (0.53 to 3.01) | 0.604 |
| J | 9.7 | 29 (1) | 0.20 | (0.03 to 1.48) | 0.039 |
| T | 11.7 | 35 (7) | 1.46 | (0.65 to 3.28) | 0.382 |
| H | 41.8 | 130 (21) | 1.12 | (0.62 to 2.02) | 0.704 |
| H1 | 12.6 | 39 (7) | 1.14 | (0.50 to 2.00) | 0.763 |
| H3 | 3.9 | 12 (0) | Undefined | — | 0.028 |
| H4 | 2.3 | 7 (2) | 1.56 | (0.37 to 6.56) | 0.567 |
| H5 | 2.3 | 7 (1) | 0.88 | (0.12 to 6.48) | 0.896 |
Bold values are significant at α = 0.05.
Only AIDS associations reported as “strong” by Hendrickson et al22 (ie, the haplogroups were consistently significant in greater than 2 tests) are shown.
DISCUSSION
We examined the genetic association of HIV-associated NRD with mtDNA haplogroups in European-American patients in the LSOCA cohort. The etiopathogenesis of HIV-related NRD is unknown. However, because both AIDS progression and HAART side effects are correlated with mitochondrial dysfunction, we also examined the effect of previously reported mtDNA haplogroups associated with AIDS progression22 and with mitochondrial toxicities in HAART patients24,26 on the incidence and prevalence of HIV-NRD.
Among Western European N macrohaplogroups, J was consistently protective in both a survivorship analysis of time-to-NRD in patients with no prior NRD diagnosis at enrollment and also in a cross-sectional analysis that compared patients who entered the study with NRD to those who did not. In previous studies, haplogroup J was associated with accelerated AIDS progression in untreated patients. However, in patients on HAART, J seemed to be protective against lipoatrophy, a drug side effect related to mitochondrial toxicity.22,25 In the case of NRD, the protective role of J seems consistent with post-therapy studies, which implies that extent of drug-related mitochondrial dysfunction is limited by the J haplogroup. Unfortunately, sufficient DNAs are not available from NRD patients from the pre-HAART era to determine whether the J haplogroup is also protective against NRD in untreated patients. Furthermore, the relationship between mitochondrial dysfunction and the etiopathogenesis of neither lipoatrophy nor NRD is well understood.
LHON is another ocular complication that has been observed in some patients with HIV. LHON is associated with several mtDNA mutations with incomplete penetrance13–16 that can be triggered by environmental factors, including antiretroviral therapy21,27–29 or background mtDNA haplogroup.17,18 In previous studies in uninfected patients, haplogroup J seemed to exacerbate mild LHON mutations.17 In our study, haplogroup J was protective against NRD, which suggests that there is no direct relationship between these 2 disorders.
Analysis of other previously reported mtDNA haplogroup associations with AIDS and HAART-related side effects uncovered 2 additional NRD associations. No patients in either haplogroup H3 or U5a1-15218 developed NRD during the study of 12 and 11 patients with those haplogroups, respectively. Among untreated patients infected with HIV, those with the H3 haplogroup progress to AIDS more slowly than other patients. H has also been suspected to increase the survival rate of individuals with sepsis.30 These data may suggest that haplogroup H3 provides overall protection from disease that in turn also prevents NRD. However, U5a1-15218, which has been associated with accelerated AIDS progression,22 was also protective against NRD in our study. Neither the H3 nor the U5a1-15218 association was replicated in the cross-sectional analysis of NRD at enrollment; therefore, both associations should be repeated in independent cohorts for validation.
The P values as presented are moderate and conclusions must be tempered by the multiple comparisons problem, which occurs when testing multiple haplogroups for associations even when the tests are nonindependent. Further, censoring due to death may bias the results if patients who died did not have similar risks for NRD as patients who remained alive and under continuing surveillance. In a previous study of 5 US AIDS cohorts, we found that European haplogroups did not show significant population structure.22 In the case of LSOCA, although the multicenter data collection approach is similar to the previous cohorts we have tested, genome-wide nuclear SNP data is unavailable for a direct test. Therefore, we are unable to rule out population structure as a potential reason for the observed associations between mitochondrial haplogroups and NRD, and it is therefore important to replicate these results in other cohorts.
In conclusion, we found haplogroup J was protective against NRD in a cross-section of patients at study enrollment and in survivorship analyses of patients followed from baseline, suggesting that mtDNA haplogroup influences the propensity to NRD in patients with AIDS. Although more work is needed to validate the observations and to elucidate the mechanisms by which these associations occur, these data again suggest that mitochondrial genotype is an important factor in health and quality of life of persons infected with HIV.
Acknowledgments
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. Longitudinal Studies of the Ocular Complications of AIDS is supported by cooperative agreements from the National Eye Institute to The Mount Sinai School of Medicine (U10 EY 08052), The Johns Hopkins University Bloomberg School of Public Health (U10 EY 08057), and the University of Wisconsin, Madison School of Medicine (U10 EY 08067).
Mike Malasky and Mary McNally of the Laboratory of Genomic Diversity performed genotyping of patients. Bailey Kessing and Jennifer Troyer provided database support, and Efe Sezgin provided useful discussions.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health.
References
- 1.Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–683. [PMC free article] [PubMed] [Google Scholar]
- 2.Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114:780–786. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114:787–793. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 4.Thorne JE, Jabs DA, Kempen JH, et al. Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy 1. Ophthalmology. 2006;113:1432–1440. doi: 10.1016/j.ophtha.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Thorne JE, Holbrook JT, Jabs DA, et al. Effect of cytomegalovirus retinitis on the risk of visual acuity loss among patients with AIDS. Ophthalmology. 2007;114:591–598. doi: 10.1016/j.ophtha.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Mueller AJ, Plummer DJ, Dua R, et al. Analysis of visual dysfunctions in HIV-positive patients without retinitis. Am J Ophthalmol. 1997;124:158–167. doi: 10.1016/s0002-9394(14)70780-9. [DOI] [PubMed] [Google Scholar]
- 7.Shah KH, Holland GN, Yu F, et al. Contrast sensitivity and color vision in HIV-infected individuals without infectious retinopathy. Am J Ophthalmol. 2006;142:284–292. doi: 10.1016/j.ajo.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Plummer DJ, Marcotte TD, Sample PA, et al. Neuropsychological impairment-associated visual field deficits in HIV infection. HNRC Group. HIV Neurobehavioral Research Center. Invest Ophthalmol Vis Sci. 1999;40:435–442. [PubMed] [Google Scholar]
- 9.Freeman WR, Van Natta ML, Jabs D, et al. Vision function in HIV-infected individuals without retinitis: report of the studies of ocular complications of AIDS Research Group. Am J Ophthalmol. 2008;145:453–462. doi: 10.1016/j.ajo.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenhula WN, Xu S, Madigan MC, et al. Morphometric comparisons of optic nerve axon loss in acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:14–20. doi: 10.1016/s0002-9394(14)75746-0. [DOI] [PubMed] [Google Scholar]
- 11.West SK, Rubin GS, Broman AT, et al. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol. 2002;120:774–780. doi: 10.1001/archopht.120.6.774. [DOI] [PubMed] [Google Scholar]
- 12.Carelli V, Ross-Cisneros FN, Sadun AA. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochem Int. 2002;40:573–584. doi: 10.1016/s0197-0186(01)00129-2. [DOI] [PubMed] [Google Scholar]
- 13.Carelli V, Ghelli A, Bucchi L, et al. Biochemical features of mtDNA 14484 (ND6/M64V) point mutation associated with Leber’s hereditary optic neuropathy. Ann Neurol. 1999;45:320–328. [PubMed] [Google Scholar]
- 14.Fauser S, Luberichs J, Besch D, et al. Sequence analysis of the complete mitochondrial genome in patients with Leber’s hereditary optic neuropathy lacking the three most common pathogenic DNA mutations. Biochem Biophys Res Commun. 2002;295:342–347. doi: 10.1016/s0006-291x(02)00672-1. [DOI] [PubMed] [Google Scholar]
- 15.Valentino ML, Barboni P, Ghelli A, et al. The ND1 gene of complex I is a mutational hot spot for Leber’s hereditary optic neuropathy. Ann Neurol. 2004;56:631–641. doi: 10.1002/ana.20236. [DOI] [PubMed] [Google Scholar]
- 16.Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 17.Brown MD, Starikovskaya E, Derbeneva O, et al. The role of mtDNA background in disease expression: a new primary LHON mutation associated with Western Eurasian haplogroup J. Hum Genet. 2002;110:130–138. doi: 10.1007/s00439-001-0660-8. [DOI] [PubMed] [Google Scholar]
- 18.Torroni A, Petrozzi M, D’Urbano L, et al. Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet. 1997;60:1107–1121. [PMC free article] [PubMed] [Google Scholar]
- 19.Miura T, Goto M, Hosoya N, et al. Depletion of mitochondrial DNA in HIV-1-infected patients and its amelioration by antiretroviral therapy. J Med Virol. 2003;70:497–505. doi: 10.1002/jmv.10423. [DOI] [PubMed] [Google Scholar]
- 20.Jaruga P, Jaruga B, Gackowski D, et al. Supplementation with antioxidant vitamins prevents oxidative modification of DNA in lymphocytes of HIV-infected patients. Free Radic Biol Med. 2002;32:414–420. doi: 10.1016/s0891-5849(01)00821-8. [DOI] [PubMed] [Google Scholar]
- 21.Mackey DA, Fingert JH, Luzhansky JZ, et al. Leber’s hereditary optic neuropathy triggered by antiretroviral therapy for human immunodeficiency virus. Eye. 2003;17:312–317. doi: 10.1038/sj.eye.6700362. [DOI] [PubMed] [Google Scholar]
- 22.Hendrickson SL, Hutcheson HB, Ruiz-Pesini E, et al. Mitochondrial DNA haplogroups influence AIDS progression. AIDS. 2008;22:2429–2439. doi: 10.1097/QAD.0b013e32831940bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarwater PM, Mellors J, Gore ME, et al. Methods to assess population effectiveness of therapies in human immunodeficiency virus incident and prevalent cohorts. Am J Epidemiol. 2001;154:675–681. doi: 10.1093/aje/154.7.675. [DOI] [PubMed] [Google Scholar]
- 24.Hendrickson SL, Kingsley L, Ruiz-Pesini E, et al. Mitochondrial DNA haplogroups influence lipoatrophy after highly active anti-retroviral therapy. J Acquir Immune Defic Syndr. 2009;51:111–116. doi: 10.1097/QAI.0b013e3181a324d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulgan T, Haas DW, Haines JL, et al. Mitochondrial haplogroups and peripheral neuropathy during antiretroviral therapy: an adult AIDS clinical trials group study. AIDS. 2005;19:1341–1349. doi: 10.1097/01.aids.0000180786.02930.a1. [DOI] [PubMed] [Google Scholar]
- 26.Hulgan T, Tebas P, Canter JA, et al. Hemochromatosis gene polymorphisms, mitochondrial haplogroups, and peripheral lipoatrophy during antiretroviral therapy. J Infect Dis. 2008;197:858–866. doi: 10.1086/528697. [DOI] [PubMed] [Google Scholar]
- 27.Warner JE, Ries KM. Optic neuropathy in a patient with AIDS. J Neuroophthalmol. 2001;21:92–94. doi: 10.1097/00041327-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Shaikh S, Ta C, Basham AA, et al. Leber hereditary optic neuropathy associated with antiretroviral therapy for human immunodeficiency virus infection. Am J Ophthalmol. 2001;131:143–145. doi: 10.1016/s0002-9394(00)00716-9. [DOI] [PubMed] [Google Scholar]
- 29.Luke C, Cornely OA, Fricke J, et al. Late onset of Leber’s hereditary optic neuropathy in HIV infection. Br J Ophthalmol. 1999;83:1204–1205. doi: 10.1136/bjo.83.10.1194k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baudouin SV, Saunders D, Tiangyou W, et al. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet. 2005;366:2118–2121. doi: 10.1016/S0140-6736(05)67890-7. [DOI] [PubMed] [Google Scholar]