INTRODUCTION
Repertoires of naturally occurring self-reactive antibodies (Ab) of different isotypes have been intensively studied during the last four decades (1–10). These autoantibodies have been termed “naturally occurring” as they are produced at birth in the absence of exposure to foreign antigens. The full repertoire of these autoantibodies develops by early childhood. Data in mice show that these naturally occurring autoantibodies have low binding affinity and are produced by a subset of B cells termed B1 cells as these B cells are the first to develop during ontogeny. These B1 cells produce predominantly IgM autoantibodies, independently of T cell help, and exhibit an enhanced response to innate immune signals such as TLR agonist. Hence B1 cells differ from B2 cells in that the response of B1 cells in-vivo can be driven by TLR agonists independently of their BCR specificity (11–14). Additionally, there is data to indicate that autoantibody producing B1 cells, unlike self-reactive T cells, are positively selected for their self-reactivity thus implying that natural autoantibodies are conserved by design (15). Further support for their importance comes from studies in mice demonstrating that B1 cells can contribute up to 80% of circulating IgM (16). However in both rodents and humans, these IgM autoantibodies decline with age, especially after the fifth decade in humans (17, 18).
Naturally occurring IgM autoantibodies are encoded by minimally or non-mutated germ line genes and are characteristically polyreactive with low binding affinity and therefore differ from disease-producing autoantibodies in that the latter are predominantly of the IgG isotype and bind with high affinity and specificity to the auto-antigen. IgM natural autoantibodies have been shown to be polyclonal with clones having specificity for different self-antigens, some of which have been identified e.g. clones producing IgM with specificity for leucocyte receptors (IgM-ALA), IgG (rheumatoid factor), complement components and neo-antigens that are exposed when lipids are oxidised or cells undergo apoptosis (7–10, 19, 20, 21). These naturally occurring antibodies, by virtue of being polyreactive, also cross-react with pathogen expressed molecules, including phosphorylcholine on streptococcus pneumoniae and other antigens expressed by various viruses and parasites (20,21). Hence, it has been suggested that these natural IgM antibodies are protective, serving as a first line of defense against infections and in addition, protecting the host from neo-antigen induced inflammatory responses. For example, natural IgM autoantibodies, specific for exposed neo-determinants such as phosphorylcholine (PC), present on apoptotic cells and oxidized lipids, have been shown to have anti-inflammatory properties in mouse models of arthritis and atherosclerosis (21). Potential mechanisms for inhibiting inflammation include masking of neo-antigens by natural IgM and rendering DC ineffective through DC phagocytosis of IgM coated apoptotic cells. Similarly, mice with B cells having a specific defect in IgM secretion, have an increased mortality when infected with either influenza virus or Streptococcus pneumoniae bacteria, even though their B cells and other immunoglobulin levels are normal (20). Such mice, unlike their wild-type counterpart, lack the protective natural IgM antibodies, which in their wild-type counterpart, increase rapidly after such infections.
In this review, we will present our observations on naturally occurring IgM anti-leucocyte autoantibodies (IgM-ALA) which were initially discovered because of their binding reactivity to lymphocytes (reviewed in 19). B1 lymphocytes producing IgM-ALA can be found in the umbilical cord blood in humans and mice and there is evidence to indicate that IgM-ALA secreting B1 cells are positively selected for their self-reactivity as gene-targeted mice, lacking the Thy-1 antigen (CD90), fail to develop B1 cells secreting IgM-ALA with specificity for the Thy-1 antigen on thymocytes (15,22–24). These IgM-ALA are present at low levels in normal individuals but increase during inflammatory disorders (e.g. sarcoidosis and end stage renal disease) and after various infections e.g. HIV and malaria (reviewed in Ref 19). Prior studies have demonstrated that IgM-ALA comprise a heterogeneous group of several antibodies each with specificity for a different leucocyte receptor, many of which are undefined (19). Some of these receptors contain phospholipids and glycolipids. IgM-ALA are not cytolytic at 37° even though these antibodies fix complement and are cytolytic at colder temperatures i.e. 18–20° (19). Both, the lack of cytolytic activity at body temperature and the observed increase in IgM-ALA with different inflammatory and infected states, prompted us to hypothesize that IgM-ALA are designed as such to regulate leucocyte function. Such a hypothesis, we argued, would favor the need for low affinity binding and for positively selecting for B1 cells despite their self-reactivity. Support for such a hypothesis also came from observations we and others made in renal and cardiac transplant recipients where the subset of patients with high levels of IgM-ALA, at time of transplant, had significantly less allograft rejections and better graft survival (19, 25). We investigated our hypothesis by initially doing in-vitro studies with polyclonal human IgM purified from human serum and monoclonal human IgM isolated from human umbilical cord B cell clones. Secondly, we studied the role of polyclonal murine IgM (purified from serum) in attenuating inflammation in murine models of renal ischemia reperfusion injury (IRI), cardiac allograft rejection and autoimmune mediated insulitis. Details of the studies briefly described in the next few pages can be obtained from our previously published manuscripts (19, 25–27).
Human umbilical cord B cell clones produce IgM-ALA that exhibit leucocyte receptor specificity
In human studies, we initially wanted to determine if IgM-ALA exhibited receptor specificity. It was important to examine this question as natural IgM is polyreactive and could nonspecifically bind to carbohydrate moieties on leucocyte receptors. We approached this question by evaluating monoclonal IgM secreted by B cell clones generated from human umbilical cord blood. Initially, we observed that more than 90% of human B cell clones were IgM secreting and with about 10% of these IgM clones having IgM-ALA activity. This initial finding demonstrating that the vast majority of IgM monoclonal antibodies failed to bind to leucocytes, argued against the possibility that human IgM bound to leucocytes via Fcμ receptors. Secondly, these findings prompted us to identify if IgM-ALA monoclonal antibodies had leucocyte receptor specificity. We approached this question by interacting human IgM-ALA monoclonal antibodies with different human cell lines and observed that some of the IgM-ALA monoclonal antibodies only bound to receptors on T cells while other monoclonals bound to receptors expressed by all leucocytes or only to NK cells or B cells (19,25,26). These data clearly indicated that IgM-ALA had leucocyte receptor specificity and that binding of IgM-ALA to human leucocytes was not mediated via Fcμ receptors, which in humans is expressed by both B and T cells. We went on to show that a subset of human monoclonal IgM, having T cell reactivity, immune-precipitated CD4 from cell lysates.
Polyclonal IgM from different human sera differ in their repertoire for receptor binding and can regulate lymphocyte function in -vitro
Polyclonal IgM, purified from serum by size exclusion chromatography, and obtained from sera of normal individuals, HIV infected patients and end stage renal dialysis (ESRD) patients immune-precipitated CD3, CD4, CCR5 and CXCR4 from cell lysates of leucocyte cell lines (25,26). Interestingly, the repertoire of IgM-ALA varied among individuals especially patients. For example, certain HIV infected patients had high levels of IgM anti-CXCR4 and minimal anti-CCR5 while other patients had high anti-CCR5 reactivity and minimal anti-CXCR4 reactivity.
These IgM-ALA had functional activity, as in in-vitro studies, addition of polyclonal IgM, but not IgG or Waldenstrom IgM lacking IgM-ALA, inhibited several lymphocyte functions (19,25,26). IgM downregulated expression of CD4, CD2 and CD86 but not CD8 and CD28 on human peripheral blood lymphocytes (PBL) activated with alloantigens (MLR). Additionally, purified polyclonal IgM from normal, ESRD and HIV patients inhibited the increased production of TNF-α, IL-13 and IL-2 but not IFN-γ, IL-6, IL-8, MCP-1 and MIG when PBL were activated in an MLR. Both T cell proliferation, induced by anti-CD3 or by alloantigens, and Zap-70 phosphorylation induced by anti-CD3 were similarly inhibited by polyclonal IgM from normals and patients although more inhibitory activity was observed with patient IgM. Finally, polyclonal IgM inhibited binding of biotin labelled CXCL12 and CCL3 to their respective chemokine receptors and in addition inhibited chemotaxis induced by these chemokines in a dose dependent manner. Similar data as with pentameric IgM were also obtained using polyclonal monomeric IgM thus questioning nature’s purpose for designing the pentameric molecule.
In summary, the in-vitro data with human IgM would indicate that IgM-ALA regulates leucocyte function by downmodulating and blocking certain leucocyte receptors. IgM does not broadly inhibit production of all pro-inflammatory cytokines but regulates leucocyte activation, proliferation and chemotaxis to avert excess immunosuppression. The marked individual variation in the repertoire of IgM, with specificity to the different leucocyte receptors, observed in both normal and disease states, could potentially explain the differences in the vigor and character of inflammatory responses in different individuals exposed to the same inciting agent.
Murine polyclonal IgM regulate murine NK, NKT and T cells in-vitro
Like human IgM, serum purified polyclonal murine IgM immunoprecipitates several different membrane receptors present in cell lysates from leucocyte cell lines. Additionally, with flow cytometry, we could demonstrate higher binding of IgM to membrane receptors expressed by unactivated DC, neutrophils and B cells and low binding to unactivated T cells. However, IgM binding to all leucocytes, including T cells, was increased to high levels after cells were activated.
We next determined if polyclonal murine IgM, purified from serum by size exclusion chromatography, inhibited lymphocyte function in-vitro (26). In these studies, polyclonal murine IgM markedly decreased (>80%) the production of IFN- by NK and NKT cells when activated with α-galalactosyl-ceramide. Similarly, IgM was tested on T cells activated with either LPS/anti-CD3 or with alloantigens (MLR). In the in-vitro T cell activation, different cytokines were added to the cultures to optimize conditions for T cell differentiation into TH-1, TH-2, TH-17 and Treg cells. In these in-vitro studies, we showed that IgM inhibited T cell proliferation and differentiation into TH-1 and TH-17 cells even when IgM was added 2 days after activation. Importantly, polyclonal IgM, when pre-adsorbed with leucocytes, failed to inhibit T cell proliferation and differentiation indicating that the inhibitory effect of polyclonal IgM is mediated by the IgM-ALA subset. Additionally, IgM induced differentiation of FOXp3+ Tregs from CD4+CD25− FOXp3- T cells and prevented flow sorted FOXp3+ Tregs from differentiating into TH-17 cells under TH-17 cytokine conditions. Murine polyclonal IgG, isolated from the same sera, had no inhibitory effect in these in-vitro studies.
In summary, these in-vitro studies indicated that IgM-ALA by binding to leucocyte receptors, inhibit the pro-inflammatory function of NK, NKT and T cells and promotes Treg differentiation to suppress inflammation. It is possible that some of the IgM-ALA mediated inhibitory effects on murine leucocytes could involve Fcα/μ receptors present on splenic DC (28). However the lack of IgM mediated TH-1 and TH-17 inhibition, using IgM depleted of IgM-ALA, would argue against this mechanism. Similarly, the participation of Fcμ receptors, mediating these inhibitory effects on DC and T cells, is highly unlikely, as in the mouse, these receptors are not expressed by DC and T cells but are highly expressed on B cells(29).
Both, the strong association between high IgM-ALA levels and reduced rejection after human allograft transplantation and the in-vitro studies demonstrating that human and murine IgM regulate lymphocyte function via specific binding of IgM-ALA to leucocyte receptors, prompted us to use murine models to determine if IgM-ALA attenuates inflammation in-vivo. In these murine models, we tested the regulatory role of IgM on inflammation mediated by innate immune mechanisms i.e. renal ischemic reperfusion injury (IRI), by adaptive immune mechanisms i.e. cardiac allograft transplantation and by autoimmune mediated mechanisms i.e. insulitis in NOD mice (26,27).
IgM-ALA inhibits in-vivo inflammation mediated by innate immune mechanisms in renal IRI
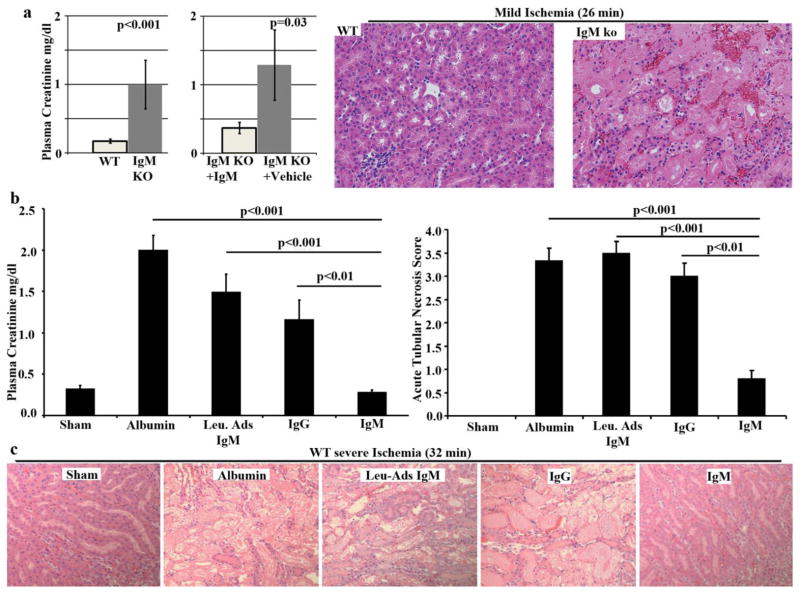
Because polyclonal murine IgM inhibited the in-vitro activation of NK and NKT cells by α-galalactosyl-ceramide, we proceeded to determine if polyclonal IgM inhibited the in-vivo inflammatory response that occurs after kidney ischemia reperfusion injury (IRI) and where the inflammation is initiated by DC activation of NK and NKT cells. We used two approaches to test the protective role of IgM in the suppression of this innate inflammatory response. First, we performed renal IRI in B6/S4-IgMko mice that lack circulating IgM but have normal levels of other immunoglobulins. These mice have normal or increased levels of Tregs, B regs and IL-10 and their normal functioning B cells express membrane IgM but are unable to secrete the IgM. Unlike their WT counterpart, we demonstrated that these mice are very sensitive to renal ischemia, developing acute kidney injury with ischemia times that are insufficient to cause kidney injury in their WT counterpart (Fig 1). Replenishing IgM in the IgMko mice, to achieve serum levels similar to that in their WT counterparts, protected these IgMko mice from developing renal IRI with mild ischemia, thus indicating that sensitivity to ischemia in the IgMko mice resulted from a lack of circulating IgM (Fig 1).
Figure 1.
Fig. 1a – B6/S4-IgMko mice are more sensitive to renal IRI when compared to their WT counterparts. In Fig. 1a, kidneys from B6/S4-IgMko mice and their WT counterparts were subjected to mild ischemia (26 min) and then reperfused. Data depict 24 hr plasma creatinine comparing WT mice with B6/S4-IgMko mice, and B6/S4-IgMko pretreated with 240μg IgM, 24 hrs before ischemic injury. Representative data from one of four experiments is also presented comparing H&E staining of renal outer medulla between B6/S4-IgMko and WT after 24 hours of reperfusion. Fig. 1b and 1c - Polyclonal IgM but not leucocyte adsorbed IgM (Leu-Ads IgM) protects against renal IRI in WT-B6 mice. In these studies WT-B6 mice were pretreated with equal quantities (150μg in 0.75ml) of IgM or Leu-Ads IgM or IgG, 24 hrs before subjecting the kidneys to severe ischemia (32 mins). Kidneys were reperfused for 24 hrs prior to determining plasma creatinine and obtaining kidneys for histology. Control mice were pretreated with 0.75 ml RPMI containing 150μg bovine albumin to exclude variables such as volume/colloid that can protect against ischemic injury. 5 to 7 mice were used for each group in Fig. 1. A student’s t- test was used to calculate p values in Fig. 1a while in Fig. 1b, a two way ANOVA test was used. Values are mean ± SEM.
In the second approach, normal purified polyclonal IgM was administered intravenously to wild type C57BL6 (WT-B6) mice to increase circulating IgM by about 30 to 50% and to determine if increasing IgM would protect these WT-B6 from severe renal ischemia. These studies clearly indicated that increasing circulating IgM levels protected mice from severe renal IRI (Fig 1). We next determined that this protection was mediated by IgM-ALA as administering similar quantity of polyclonal IgM pre-adsorbed with splenic leucocytes, failed to protect these WT-B6 mice from severe renal IRI (Fig 1).
Further studies were performed to determine the mechanism by which polyclonal IgM protects against renal IRI. On histology, protected kidneys subjected to ischemia, exhibited minimal inflammatory response after severe ischemic injury and the cortico-medullary tubules were well preserved (26). We wanted to determine how IgM interrupts the ischemia induced inflammatory process.
Damage-associated molecular patterns (DAMPS) and glycolipids released from ischemic tubules and endothelial cells are taken up by resident DC which get activated and in turn activate NK and NKT cells either locally or in the spleen. Activated NK and NKT cells then activate the inflammatory effector cells, predominantly granulocytes and macrophages, which infiltrate the ischemic kidney and mediate the tubular damage. Inflammatory effector cells are attracted to ischemic tubular cells as a result of increased tubular and endothelial cell chemokine production. Both ischemia and the release of DAMPS, leads to TLR up-regulation and activation on tubular and endothelial cells with resulting NF-kβ mediated chemokine production.
Based on the in-vitro studies, polyclonal IgM could possibly inhibit NK and NKT cell mediated activation of inflammatory effectors. Secondly, we wanted to determine if IgM inhibited TLR up-regulation and chemokine production from ischemic tubules and endothelial cells. To examine this question, we evaluated kidneys at about 3 hours post ischemia i.e. before infiltration of inflammatory effectors. Immuno-histochemical staining of tissue sections revealed that IgM pretreated mice had significantly less TLR up-regulation on both tubular and endothelial cells at 3 hours post-ischemia and in addition, these cells produced less chemokines. Both these histological observations were confirmed by mRNA levels (26). These studies would therefore indicate that IgM in-vivo protects against renal IRI by inhibiting TLR upregulation of ischemic tubules and endothelial cells. It is also possible that IgM also inhibits NK and NKT cell activation in-vivo.
Polyclonal IgM inhibits in-vivo inflammation mediated by adaptive immune mechanisms in allograft transplantation
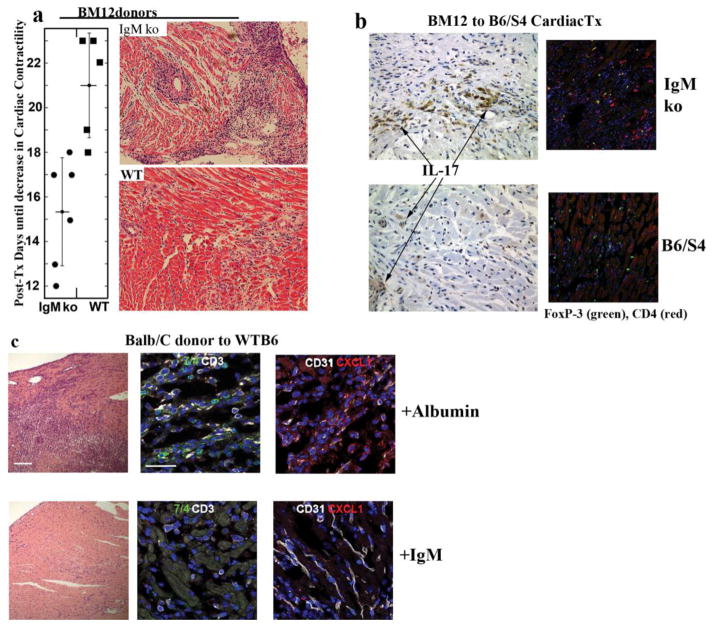
Because the in vitro studies demonstrated that IgM inhibited alloantigen-activated T cell proliferation and differentiation into Th-1 and Th-17, we performed studies aimed at determining whether IgM could also inhibit allograft rejection, which is an in vivo model of inflammation mediated by alloantigen-activated T cells (26). Two approaches were used. First, cardiac transplants were performed in B6/S4-IgMko mice using B6-bm12 donor hearts, which are mostly incompatible at the MHC class II locus (Ia). In this transplant model, there is a chronic from of cellular rejection and a vasculopathy that is initiated by a T cell-mediated inflammatory process not involving anti-MHC Abs. As a result, a rejection-induced decrease in cardiac function is detected between days 17 and 28, when, with finger palpation, one can clearly detect a diminution in cardiac contractility. However, cardiac allograft ceases having a heart beat at 2–3 mo in this model. In Fig 2, one can observe that rejection (defined by a decrease in cardiac contractility) occurs significantly earlier (i.e., between days 10 and 18), when B6-bm12 donor hearts are transplanted into B6/S4-IgMko recipients. Furthermore, Fig 2, clearly shows that, by day 10, the rejection-induced inflammatory process in cardiac allografts is severe when transplanted into B6/S4-IgMko recipients but is minimal in WT-B6/S4 recipients. Additionally, cardiac allografts in B6/S4-IgMko recipients cease having a heart beat in 2–3 weeks, which is significantly earlier compared with their WT-B6/S4 counterparts, in which cessation of heart beat occurs after >2 mo.
Figure 2.
Fig. 2a – Allograft rejection is more rapid and severe in B6/S4-IgMko. In Fig. 2a, B6/S4-IgMko mice and their WT counterparts received cardiac allografts from B6-bm-12 donors that are only incompatible at the MHC-Ia locus. Fig. 2a depicts the post transplant day when cardiac contractility was found to be decreased by finger palpation. Fig. 2a also presents data on B6-bm12 cardiac allograft histology on Day 10 post transplant. This is a representative example from 3 mice in each group that were sacrificed on day 10 to 12 post transplant. Fig 2b – More severe rejection in B6/S4-IgMko is not due to deficient Treg function. Severe cardiac allograft rejection in B6/S4-IgMko is associated with abundant TH-17 cells as well as with abundant CD4+Foxp3+ T cells. B6-bm12 cardiac allografts from mice in Fig. 2b were examined by immunofluorescence staining for CD4+Foxp3+ cells and by immunohistochemistry for TH-17 cells (stained brown). With immunofluoresence microscopy 12 fields (x400 magnification) were evaluated on each slide to quantitate Foxp3+ cells and IL-17+ cells. The average number of Foxp3+ cells in each field for WT-B6 and IgMko was 17.5 and 18.6 respectively. Representative examples from one of the 3 mice in each group are depicted. Fig. 2c Polyclonal IgM protects WT-B6 mice from allograft rejection. In these studies WT-B6 mice received cardiac allografts from Balb/c donors that are fully MHC incompatible. One group of mice received 175μg IgM on Day 1, 3 and 5 after the cardiac transplant. The control group received 3 doses of RPMI with 175μg bovine albumin. Mice were sacrificed on Day 6. H&E and immunofluorescence staining were performed on cardiac allografts. In middle panel, sections were stained for neutrophils (7/4, green) and CD3+ T cells (white). In panel to the right, capillary endothelial cells were labeled with CD31 (anti-PCAM, white) and CXCL-1 (red). Representative examples from one of the three mice in each group are depicted. Note that the CD31 staining capillaries were well preserved in mice receiving polyclonal IgM.
In the second approach, we wanted to determine whether IgM, when administered to WT-B6 mice, inhibited the severe and rapid rejection that occurs in the setting of fully MHC-incompatible donor hearts (i.e., from BALB/c donors). In this model, rejection in WT-B6 recipients is detectable by day 5 with finger palpation, and the heart ceases having a heart beat by days 7–9 (26). In these studies, 175 μg IgM was administered 24 h after ascertaining that cardiac surgery was successful, and the dose of IgM was repeated on days 3 and 5. Mice were euthanized on day 6. Fig 2 clearly shows that IgM inhibited the severe inflammation in the cardiac allograft induced by rejection on day 6, as detected by H&E staining and immunofluorescence staining for neutrophils (7/4) and T cells (CD3). Importantly, with immunohistochemistry, this lack of leukocyte infiltration in the cardiac parenchyma of IgM-treated recipients was also associated with decreased CXCL1 production and with no or minimal fragmentation of capillaries, as identified by the endothelial cell marker CD31 (Fig 2).
Polyclonal IgM inhibits in-vivo inflammation mediated by auto-immune mechanisms
Because the in-vitro studies demonstrated that polyclonal IgM inhibited anti-CD3 activated T cell proliferation and differentiation into Th-1 and Th-17cells, we performed studies aimed at determining whether IgM could also inhibit T cell mediated auto-immune insulitis that results in islet cell destruction and diabetes mellitus (DM) in NOD mice (27). In these mice, the autoimmune inflammatory process begins spontaneously around 4 to 5 weeks after birth and the initial phase is characterized by a silent and non-destructive infiltration of the perivascular and periductal regions in the pancreas as well as the peripheral islet regions by a heterogeneous mixture of CD4 and CD8 T cells, B cells, macrophages and DC (peri-insulitis). In the invasive phase that begins at 8–12 weeks of age, the immune infiltrate enters the islet inducing beta cell destruction (insulitis). Significant destruction first becomes evident around 12 to 13 weeks of age with mice exhibiting overt diabetes (DM).
In these studies, we wanted to determine the effect of increasing IgM levels on development of DM. NOD mice were administered bi-weekly intra-peritoneal polyclonal IgM (50μg/dose) beginning either at 5 or 11 weeks of age and ending when mice were 18weeks old. At 25 weeks of age, 80% of control mice(n=30) became diabetic, while 0% of mice (n=30) treated with IgM beginning at 5 weeks developed DM. Importantly, only 20% of pre-diabetic mice (n=20) treated with IgM beginning at 11 weeks of age developed DM at 25 weeks of age. This latter observation is particularly notable as prior studies using co-stimulatory blockade failed to prevent DM in this murine model. Histological examination of pancreas at 18 to 25 weeks of age, revealed no or minimal insulitis in NOD mice treated with IgM beginning at 5 weeks of age.
Bone marrow DC (BMDC) pretreated with IgM in-vitro protect against renal IRI
Prior observations demonstrating high IgM binding to DC prompted us to evaluate whether the in-vivo anti-inflammatory effects of higher IgM levels were mediated via IgM regulation of DC. To examine this question, we cultured BMDC with IgM in the presence of LPS for 48 hours and after washing, administered 0.5×106 BMDC intravenously into mice. 24 hours later, mice were subjected to severe IRI. Preliminary data indicates that mice infused with IgM/LPS pre-treated BMDC were protected to a similar extent as with intravenous IgM, while mice infused with LPS or IgG/LPS pretreated BMDC were not protected. Similar data were obtained when murine BMDC were pre-treated with human IgM. Hence, one mechanism for IgM mediated protection could involve regulation of DC and these observations raise the possibility of cell based therapy requiring the use of less IgM and obviating the need for intravenous IgM therapy.
Summary
Several investigators have demonstrated that natural IgM has anti-inflammatory properties and have proposed different mechanisms depending upon the specificity of these natural IgM autoantibodies. A monoclonal murine IgM autoantibody with specificity for phosphorylcholine (PC) present on oxidized lipids and apoptotic cells prevents atherosclerotic disease in mice and also inhibits inflammation in a murine model of arthritis (reviewed in 21). Similarly, natural IgM autoantibodies have been shown to protect mice from influenza virus infection and strept pneumococcal infection (reviewed in ref 20). In our studies we show that polyclonal natural IgM protects mice from inflammation induced injury that occurs after renal ischemia reperfusion injury (IRI), autoimmune diabetes in NOD mice and cardiac allograft rejection. In our in-vivo model of renal IRI, we show that protection is mediated by IgM-ALA autoantibodies. It is important to stress that in all these in-vivo inflammatory models, prevention or control of inflammation required natural IgM, even though mice had the normal repertoire of other suppressive mechanisms including Tregs, B regs and IL-10. Particularly interesting is the control of renal IRI induced inflammation with DC pretreated in-vitro with IgM-ALA. The latter may have relevance for cell based therapy.
References
- 1.Steele EJ, Cunningham AJ. High proportion of Ig producing cells making autoantibody in normal mice. Nature. 1978;274:483–486. doi: 10.1038/274483a0. [DOI] [PubMed] [Google Scholar]
- 2.Dighiero G, Guilbert B, Fermand JP, Lymberi P, Danon F, Avrameas S. Thirty-six human monoclonal immunoglobulins with antibody activity against cytoskeleton proteins, thyroglobulin and native DNA: immunologic studies and clinical correlations. Blood. 1983;62:264–270. [PubMed] [Google Scholar]
- 3.Hardy RR, Hayakawa K. Development and physiology of Ly-1 B and its human homolog Leu-1 B. Immunol Rev. 1986;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Burastero SE, Ueki Y, Larrick JW, Notkins AL, Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus: frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto’s disease and systemic lupus erythematosus. J Immunol. 1988;141:4165–4172. [PubMed] [Google Scholar]
- 5.Mouthon L, Lacroix-Desmazes S, Nobrega A, Barreau C, Coutinho A, Kazatchkine MD. The self reactive antibody repertoire of normal human serum IgM is acquired in early childhood and remains conserved throughout life. Scand J Immunol. 1996;44:243–251. doi: 10.1046/j.1365-3083.1996.d01-306.x. [DOI] [PubMed] [Google Scholar]
- 6.Clarke SH, Arnold LW. B-1 cell development: evidence for an uncommitted immunoglobulin (Ig)M+ B cell precursor in B-1 cell differentiation. J Exp Med. 1998;187:1325–1334. doi: 10.1084/jem.187.8.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236:77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 8.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236:81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 9.Kasaian MT, Casali P. Autoimmunity-prone B-1 (CD5 B) cells, natural antibodies and self recognition. Autoimmunity. 1993;15:315–329. doi: 10.3109/08916939309115755. [DOI] [PubMed] [Google Scholar]
- 10.Rieben R, Roos A, Muizert Y, Tinguely C, Gerritsen AF, Daha MR. Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in-vitro and in-vivo in a rat model of acute inflammation. Blood. 1999;93:942–48. [PubMed] [Google Scholar]
- 11.Murakami M, Tsubata T, Shinkura R, Nisitani S, Okamoto M, Yoshioka H, Usui T, et al. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J Exp Med. 1994;180:111–121. doi: 10.1084/jem.180.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nisitani S, Tsubata T, Murakami M, Honjo T. Administration of interleukin-5 or -10 activates peritoneal B-1 cells and induces autoimmune hemolytic anemia in anti-erythrocyte autoantibody-transgenic mice. Eur J Immunol. 1995;25:3047–3052. doi: 10.1002/eji.1830251110. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Tung JW, Ghosn EE, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci USA. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayakawa K, Asano M, Shuiton SA, Gui M, Allman D, Stewart CL, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 16.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love SD, Lee W, Nakamura YC, Platt JL, Bollinger RR, Parker W. Natural anti-carbohydrate IgM in mice: dependence on age and strain. J Immunol Methods. 2000;246:61–68. doi: 10.1016/s0022-1759(00)00296-9. [DOI] [PubMed] [Google Scholar]
- 18.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70−. J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobo PI, Schlegel KH, Spencer CE, Okusa MD, Chisholm C, McHedlishvili N, et al. Naturally occurring IgM anti-leukocyte autoantibodies (IgM-ALA) inhibit T cell activation and chemotaxis. J Immunol. 2008;180:1780–1791. doi: 10.4049/jimmunol.180.3.1780. [DOI] [PubMed] [Google Scholar]
- 20.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 21.Grönwall C, Vas J, Silverman GJ. Protective roles of natural IgM antibodies. Front Immun. 3:66. doi: 10.3389/fimmu.2012.00066. 20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ailus K, Palosuo T. IgM class autoantibodies in human cord blood. J Reprod Immunol. 1995;29:61–67. doi: 10.1016/0165-0378(95)00933-c. [DOI] [PubMed] [Google Scholar]
- 23.Barbouche R, Forveille M, Fischer A, Amraveas S, Durandy A. Spontaneous IgM autoantibody production In-vitro by B lymphocytes of normal human neonates. Scand J Immunol. 1992;35:659–667. doi: 10.1111/j.1365-3083.1992.tb02972.x. [DOI] [PubMed] [Google Scholar]
- 24.Cervenak J, Kiss K, Uher F. Partial characterization of two lymphocyte-specific natural autoantibodies isolated from newborn mice. Acta Microbiol Immunol. 1999;46:53–62. [PubMed] [Google Scholar]
- 25.Lobo PI, Schlegel KH, Vengal J, Okusa MD, Pei H. Naturally occurring IgM anti-leukocyte autoantibodies inhibit T-cell activation and chemotaxis. J Clin Immunol. 2010;30(suppl 1):S31–S36. doi: 10.1007/s10875-010-9412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobo PI, Bajwa A, Schlegel KH, Vengal J, Lee SJ. Natural IgM anti-leukocyte autoantibodies attenuate excess inflammation mediated by innate and adaptive immune mechanisms involving Th-17. J Immunol. 2012;188:1675–1785. doi: 10.4049/jimmunol.1101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chhabra P, Schlegel K, Okusa MD, Lobo PI, Brayman KL. Naturally occurring immunoglobulin M, (nIgM) autoantibodies prevent autoimmune diabetes and mitigate inflammation after transplantation. Annals of Surgery. 2012;256(4):634–641. doi: 10.1097/SLA.0b013e31826b4ba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuno K, Kang DW, Tahara K, Torii I, Kubagawa HM, Ho KJ, et al. Unusual biochemical features and follicular dendritic cell expression of human Fcα/μ receptor. Eur J Immunol. 2007;37:3540–3550. doi: 10.1002/eji.200737655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. International Immunology. 2009;22(3):149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]