Abstract
Using a case of hyperglycemic hypertonic nonketosis we examine the changing composition of body fluid spaces to explore the distinction between dehydration with hypertonicity and volume depletion. These terms have specific meaning and their proper use guides therapy when pathophysiology disturbs the composition of various body fluid compartments.
Case Report
A 35 year-old African-American male with a history of obesity, hypertension, and recently diagnosed diabetes mellitus stopped metformin following an upper respiratory infection. Polyuria and polydipsia followed with the patient drinking large amounts of Gatorade until the onset of nausea, vomiting, and confusion. On presentation he was afebrile, unsteady, and lethargic weighing 155 kg at 6 feet of height. A supine blood pressure of 132/88 with a pulse of 90 beats/min changed on standing to a blood pressure of 121/90 with a pulse of 122 beats/min. Remainder of the physical examination was remarkable for posterior oropharyngeal erythema and dry mucous membranes. Initial laboratory studies are listed in Table 1.
Table 1. Initial Laboratory Studies.
| Parameter | Value |
|---|---|
| Blood Chemistries | |
| Sodium (mEq/L) | 139 |
| Potassium (mEq/L) | 4.3 |
| Chloride (mEq/L) | 103 |
| Bicarbonate (mEq/L) | 26 |
| BUN (mg/dL) | 25 |
| Creatinine (mg/dL) | 1.47 (1 month prior 0.9) |
| Glucose (mg/dL) | 1213 |
| Calcium (mg/dL) | 10.5 |
| Serum Osmolality (mOsm/kg) | 356 |
| Anion Gap (mEq/L) | 10 |
| Complete Blood Count | |
| Hemoglobin (g/dL) | 17.7 (1 month prior 16.1) |
| Hematocrit (%) | 57 (1 month prior 48) |
| WBC Count (× 103/μL) | 10.2 |
| Platelets (× 103/μL) | 202 |
| Urine Dipstick | |
| pH | 5.5 |
| Specific Gravity | 1.035 |
| Glucose | 4+ |
| Ketones | Negative |
| Urine Chemistries | |
| Sodium (mEq/L) | 54 |
| Potassium (mEq/L) | 33 |
| Other | |
| Rapid Strep Test | + |
Note: Conversion factors for units: urea nitrogen in mg/dL to mmol/L, × 0.357; serum creatinine in mg/dL to μmol/L, × 88.4; glucose in mg/dL to mM, × 0.05551; calcium in mg/dL to mmol/L, × 0.2495; hemoglobin g/dL to g/L × 10. No conversion is necessary for sodium, potassium, chloride, bicarbonate, and anion gap in mEq/L and mmol/L.
Introduction
Dehydration refers to a loss of total body water producing hypertonicity. Unfortunately, the word dehydration is often used interchangeably with volume depletion, which refers to something different, a deficit in extracellular fluid volume. The distinction between these two conditions is important as the type of fluids used for therapy and their rate of administration differs for each. Hypertonicity is the primary pathophysiologic feature of water deficiency and is preferred terminology over the now careless use of dehydration. Here we examine a patient with hyperglycemic hypertonic nonketosis (HHNK) to illustrate the concepts of volume depletion and hypertonicity and their role in designing rational fluid therapy.
Pathophysiology
Body Fluid Spaces
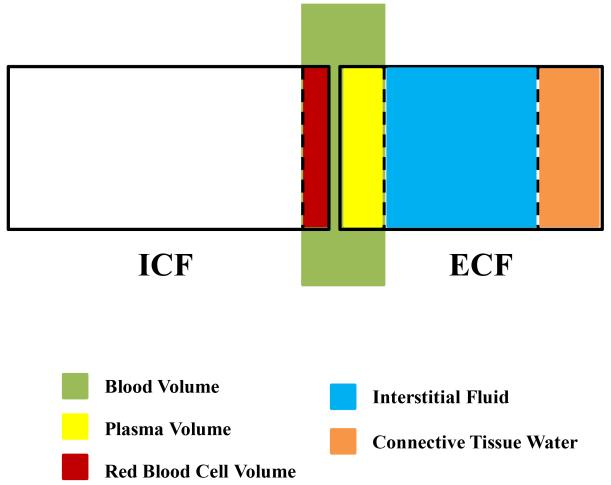
Total body water (TBH2O) represents about 45-60% of body weight depending on age, gender, and race (1, 2). TBH2O is further divided into an intracellular fluid compartment (ICF; about 55% of total body water) and an extracellular fluid compartment (ECF; about 45% of total body water) (3), which are proportional to the ratio of osmotically-active intracellular K+ to extracellular Na+ (4). The clinical term volume is bedside shorthand for ECF volume (ECFV). ECF can be subdivided into plasma volume representing 17% of the ECF, interstitial volume encompassing 50-60% of the ECF, and the remainder consisting of bone and connective tissue water (3). Blood volume is the sum of the extracellular plasma volume and the red blood cell volume (Figure 1).
Figure 1. Schematic Representation of Body Water Compartments.
Tonicity
Why do we ever measure a serum Na+ clinically? Its usefulness lies only as a surrogate marker of tonicity. Tonicity is a descriptive physiologic term that refers to the volume behavior of cells in a solution; cell volume tends to expand as body fluids become hypotonic or shrink as surrounding fluids become hypertonic. Tonicity is different than serum osmolality because measurement of the latter reflects the totality of effective and ineffective osmoles in a liter of body fluid. Only effective osmoles trapped on either side of the cell membrane change cell volume; they obligate the hydration of their respective body space through transmembrane water flow until effective osmolality equalizes across all fluid compartments to establish tonicity. Ineffective osmoles, such as urea and alcohol, cross cell membranes and do not influence transmembrane water flow or change cell volume (5, 6). If the cell volume effects of tonicity cannot be quantitated directly and serum osmolality is an unreliable indicator (7), then serum Na+ becomes a useful surrogate marker of tonicity, and we can construct a thought equation to understand this surrogacy: Serum Na+ = Tonicity = Effective Osmols ÷ TBH2O = (TBNa+ + TBGlucose + TBK+) ÷ TBH2O.
In this thought equation, osmotically-active TBNa+ and its anions (not shown) plus glucose bathe the outside of cells and osmotically-active TBK+ and its anions are inside cells. These bulk solutes obligate water to hydrate one compartment or the other in proportion to available effective osmoles and, at equilibrium, serum Na+ roughly reflects net tonicity imposed by effective osmoles across all compartments. Lest we forget, intracellular K+ is an important determinant of steady state serum Na+ (6, 7), as osmotically active TBK+ is 20% more abundant than TBNa+, which explains why the ICF is slightly larger than the ECF (4). TBNa+, TBK+, and TBH2O are governed by diet and renal excretion, and to a lesser extent by losses from the gut, lungs, and skin. When the content of body solute, levels of anti-diuretic hormone, and extracellular volume are steady-state normal, urine Na+ and K+ excretion and the electrolyte-free water clearance primarily reflect dietary intake (6-8).
Serum Na+ and Hyperglycemia
In the setting of hyperglycemia, the relationship between TBGlucose and serum glucose can be mathematically expressed using a framework in which glucose is added to plasma and allowed to diffuse into a volume of distribution (VD) expressed as a fraction of total body water: Serum glucose = TBGlucose ÷ (VD × TBH2O). Based on this framework, a modified relationship for serum Na+ correcting for hyperglycemia may be derived as follows: Serum Na+G = (TBNa+ + TBK+ ÷ TBH2O) − Glucose × (1 - VD) ÷ 2. As glucose accumulates in the extracellular space, effective osmolality rises leading to a shift of body water from ICF to ECF to re-establish equilibrium at a new level of tonicity. A dilutional fall in serum Na+ ensues proportional to the change in glucose concentration and VD: ΔSerum Na+G = ΔGlucose × (1-VD) ÷ 2. VD is a complex function of insulin activity, glucose distribution time, ECFV, and glucose concentration itself, but under normal steady-state conditions encompasses the mannitol-penetrable ECF plus about 10% of ICF (VD ≅ 0.4) (3, 9, 10). The clinically relevant range of VD (0.3-0.5) translates into a 1.5-1.9 mEq/L (mean 1.7 mEq/L) fall in serum Na+ for every 100 mg/dL rise in serum glucose (11-14). At the bedside, the measured serum Na+ is corrected for its decline related to hyperglycemia so that it largely reflects TBNa+ and TBK+ relative to TBH2O: Serum Na+G = Measured serum Na+ + (1.7 × ΔGlucose/100 in mg/dL).
Blood Volume
Neurohormonal homeostatic mechanisms sense and defend effective circulating blood volume (ECBV), a poorly measurable quality of arterial filling determined primarily by blood volume, cardiac output, and vascular tone (15). Plasma volume, as a component of blood volume, represents the common link between ECFV and ECBV. Thus, ECFV and ECBV parallel one another normally, but diverge in many pathologic states; for example, edematous states such as congestive heart failure or cirrhosis, often exhibit diminished ECBV with expanded ECFV (15).
The defense of ECBV classically involves vasoconstriction, tachycardia, and improved myocardial contractility to maintain circulatory pressure and flow to vital organs. A less commonly appreciated response is transcapillary refill, which involves movement of interstitial fluid into the vascular space to replenish lost intravascular volume (16, 17). Transcapillary refill is observed routinely during dialytic ultrafiltration particularly using hemoconcentration-based blood volume monitoring (18). Kinetic studies after phlebotomy or ultrafiltration (10-20% blood volume loss) suggest the vascular refill rate is maximal immediately after a volume loss, recouping about 50% of lost fluid within 2 hours with an eventual plateau at 24 hours after about 75-80% of lost vascular volume is recovered (19-22). Rapid losses of blood volume draw primarily from blood volume alone, while slower losses recruit from about 75% of the ECF (plasma volume plus interstitial fluid volume) requiring 3 to 4-fold greater deficits to produce equivalent hemodynamic compromise.
Non-hemorrhagic fluid losses such as gastrointestinal, renal, or third spacing initially derive from the plasma volume but are usually slow enough to distribute across much of the ECF compartment, although there are exceptions (20, 23). Unlike hemorrhage, the ensuing hemoconcentration augments transcapillary refill and systemic vascular resistance (24, 25) and the extent of hemoconcentration quantifies volume deficits in the absence of blood loss (26).
When net fluid loss is isotonic, it draws completely from the ECF and thus the volume of fluid loss exactly equals the volume deficit. Conversely, when there is pure water loss, ECF tonicity rises causing rapid translocation of water from the larger intracellular compartment to establish a new elevated level of body tonicity. Thus, pure water loss leads to hypertonicity and contraction of all body water compartments proportional to their share of total body water (27). Theoretically, the concept of isotonic or pure water loss is attractive, but such losses rarely occur in isolation. Most non-hemorrhagic fluid losses are hypotonic, but can be partitioned into isotonic and pure water components to apply the theoretical framework. Moreover, considering hypotonic losses as part isotonic and part pure water distinguishes volume depletion from hypertonicity, helps recognize a predominant abnormality, and allows for an appropriate intervention combining isotonic saline and free water repletion at safe rates for therapy. To crystallize these concepts, we contrast 1L fluid losses of varying composition and their effects on body fluid compartments in Table 2.
Table 2.
Body Fluid Compartments and Serum Na+ with Hypothetical 1 L Fluid Losses.
| Body Fluid Compartment |
Decrease in Volume of Respective Compartment (mL) | ||||
|---|---|---|---|---|---|
|
| |||||
| Acute Blood Loss |
Slow Blood Loss |
Isotonic Fluid |
Pure Water | Half-Isotonic Fluid |
|
|
| |||||
| Intracellular | 400 | 400 | 0 | 550 | 275 |
|
| |||||
| Extracellular | 600 | 600 | 1000 | 450 | 725 |
| Interstitial | 0 | 1250 | 750 | 375 | 562.5 |
| Plasma | 600 | −650* | 250 | 75 | 162.5 |
|
| |||||
| Blood | 1000 | 250 | 250 | 125 | 187.5 |
|
| |||||
| Hct (%) | 40 | 33.7 | 42.1 | 40 | 41 |
|
| |||||
| Δ Serum Na+ | 0 | 0 | 0 | ⇑ 3.6 mEq/L | ⇑ 1.8 mEq/L |
Assuming TBH2O = 40L, blood volume = 5L, baseline % Hct = 40 & serum Na+ = 140 mEq/L
Transcapillary refill expands plasma volume at the expense of interstitial fluid in the setting of slow blood loss.
Clinical Features
Volume Depletion
Volume depletion is diagnosed at the bedside with corroboration from laboratory studies. A common approach is to interrogate the status of ECBV defense mechanisms using postural or baseline changes in heart rate and blood pressure or accompanying symptoms such as orthostatic presyncope. Orthostatic changes in heart rate or blood pressure do not become evident in normal subjects until 15-20% of blood volume is removed acutely (28, 29). Assuming a 15% fall in blood volume as a minimal threshold for clinically detectable volume depletion, a non-hemorrhagic, isotonic loss of about 15% of ECF amounting to 7% of TBH2O is required. In contrast, a pure water deficit equivalent to 15% of TBH2O is needed to reach the same hemodynamic threshold. Consequently, isotonic losses are about 2-fold more potent than pure water losses at depleting blood volume. Indeed, isotonic losses alter systemic hemodynamics, reduce blood volume and GFR, and leave body tonicity unchanged. Conversely, an equivalent pure water deficit does not measurably alter blood volume or GFR, while hypernatremia and hypertonicity are prominent (30-34).
Clinicians often use the renal response to hypovolemia to adjudicate a clinical impression of volume depletion. As blood volume and ECBV fall, initial intrarenal events maintain renal blood flow (RBF) and GFR primarily through prostaglandin effects on afferent arteriolar tone despite systemic vasoconstriction. As ECBV declines further, angiotensin II-mediated efferent arteriolar vasoconstriction reduces renal blood flow, but preserves GFR leading to a rise in filtration fraction, which contributes to enhanced proximal tubular sodium and urea reabsorption. Eventually the mechanisms combating afferent arteriolar vasoconstriction fail leading to a precipitous fall in RBF and GFR (35).
In humans, RBF begins to fall at around 10% blood loss and GFR falls at about 20% blood loss (36-39). Thus, a rise in serum creatinine or oliguria related solely to non-hemorrhagic hypovolemia anticipates a 15-20% deficit in ECF. Vascular disease from hypertension or diabetes, cardiac dysfunction, chronic kidney disease, or medications interfering with compensatory angiotensin or prostaglandin systems, will exhibit GFR declines at lower levels of volume depletion (35).
Hypertonicity
Hypertonicity usually results from a disproportionate fall in TBH2O relative to TBNa+ and TBK+ producing hypernatremia. With pure water loss, extracellular tonicity rises and draws fluid from the intracellular compartment which, given its larger size, bears more of this loss. Thus, hypertonicity in many respects requires intracellular volume contraction, while volume depletion is a disorder of blood volume contraction. Brain cell function is particularly sensitive to crenation and neurologic symptoms predominate following hypertonicity. Only extraordinary pure water losses producing serum Na+ concentrations >170 mEq/L risk hemodynamic alterations (27), and neurologic symptoms are often apparent before hypertonicity progresses to this point.
Most nucleated cells acclimate to hypertonicity by accumulating electrolyte osmoles initially followed by organic osmoles chronically. These osmoles pull water back into the intracellular compartment partially restoring cell volume (40). If the progression of hypertonicity eclipses intracellular osmolyte accumulation, severe neurologic symptoms ensue with seizures, coma, and central pontine myelinosis as the most dreaded complications. If hypertonicity develops slowly, neurons acclimate, maintain cell volume, and patients exhibit only mild neurologic symptoms or may even present asymptomatically. However, rapid correction of chronic, compensated hypertonicity may precipitate cerebral edema when osmotic entry of water into brain cells outstrips their short-term ability to shed accumulated organic osmoles (41).
Hyperglycemic hypertonicity behaves differently from hypernatremic hypertonicity. Organic osmolytes in animal studies accumulate and dissipate quickly in hyperglycemia alone compared to hypernatremia, which may explain the relative rarity of treatment-related cerebral edema in HHNK (42-45). High dose insulin strongly promotes entry of osmoles into cells, such as glucose and K+, and may predispose to cerebral edema (44). The greater prevalence of cerebral edema in diabetic ketoacidosis compared to HHNK suggests its pathogenesis relates more to metabolic derangements from acidosis rather than dysregulation of cell volume (44-46).
The homeostatic response to hypertonicity is ADH-mediated urinary water conservation and stimulation of thirst seeking water. ADH release and thirst are much more sensitive to hypertonicity compared to hypovolemia (7). ADH also increases distal nephron reabsorption of urea and recycling to improve the efficiency of water reabsorption leading to mild azotemia (47). Conversely, since vascular volume is typically maintained, GFR and serum creatinine are unchanged initially. Oliguria is evident early in hypertonicity (<5% increase over set point), while appearing late in hypovolemia as deficits of at least 10% ECBV are required to stimulate ADH release and raise urine osmolality (33, 34, 48). We summarize the contrasting clinical features of volume depletion and hypertonicity in Table 3.
Table 3. Clinical Features of Volume Depletion and Hypertonicity.
| Volume Depletion | Hypertonicity | |
|---|---|---|
|
| ||
| History (34, 48) | ||
| Altered Mentation | + | +++ |
| Orthostasis | ++ | 0 |
| Thirst | + | +++ |
|
| ||
| Physical Examination (48, 57-61) | ||
| Orthostatic/Supine Tachycardia | ++ | 0 |
| Diminished skin turgor | ++ | + |
| Dry mucous membranes or axillae | + | +++ |
| Longitudinal tongue furrows | + | +++ |
| Oliguria | ++ | +++ |
|
| ||
| Laboratory Studies (6, 48, 62) | ||
| Hypernatremia & Plasma Hypertonicity | 0 | +++ |
| Elevated BUN | +++ | + |
| Elevated serum creatinine | ++ | 0 |
| Elevated urine osmolality | ++ | +++ |
| Diminished urine Na+ | +++ | 0 |
| Hemoconcentration | + | 0 |
|
| ||
| Treatment (6, 48) | ||
| Fluid Type | Isotonic Saline | Free Water |
| Rate of Administration | Fast | Slow |
Rational Approach to Fluid Therapy
A rationale approach to therapy begins with estimation of the volume and water deficits, as these deficits are replenished with rapid isotonic saline administration and slow free water repletion, respectively. With hyperglycemia, glucose overwhelms renal reabsorption and an osmotic diuresis ensues producing a slightly hyperosmolar urine (~400-500 mOsm/kg). Glucose accounts for about 50-60% of this urine osmolality and combined urinary Na+ + K+ concentration is in the range of 50-100 mEq/L (49-52). Only the use of electrolyte-free water clearance rather than osmolar free water clearance correctly identifies significant urinary free water losses (6, 53).
The eventual net loss of isotonic fluid and pure water will depend on the patient’s oral intake. Polydipsia may drive enough water intake to nullify the free water deficit and even on rare occasions produce hypotonicity (43). The only way to clearly recognize this situation is to calculate the hyperglycemia corrected serum Na+G, since a value near or less than 140 mEq/L suggests normal water balance or water excess, respectively. Functionally anephric dialysis patients often present in this manner, as urinary water losses are negligible, while water intake continues unabated. The therapy in this situation is primarily judicious insulin therapy (54). Conversely, previous food and salt intake may minimize the isotonic loss and produce a pure water deficit. In most patients with HHNK, both isotonic and free water losses are present with a clinical constellation of hemodynamic compromise and neurologic symptoms (43). The volume deficit can be adjudicated clinically as our patient exhibited orthostasis and reduced GFR consistent with about a 20% fall in blood volume and ECFV. Given our patient’s morbid obesity, TBH2O is estimated using anthropomorphic equations (2) rather than weight based rules of thumb: total body water (L) = 2.447–0.09516 age (years) + 0.1074 height (cm) + 0.3362 weight (kg) = 70L. Since ECF is 45% of TBH2O, the patient’s volume deficit is about 6-6.5L (0.45 × 70L × 0.2). His pure water deficit is calculated using hyperglycemia corrected serum Na+G in the oft cited formula: Water Deficit = TBH2O × [(serum Na+G ÷ 140) − 1] (6) where the corrected serum Na+G is 158 mEq/L (139 mEq/L + 1.7 × 11.1) with a water deficit of 9L.
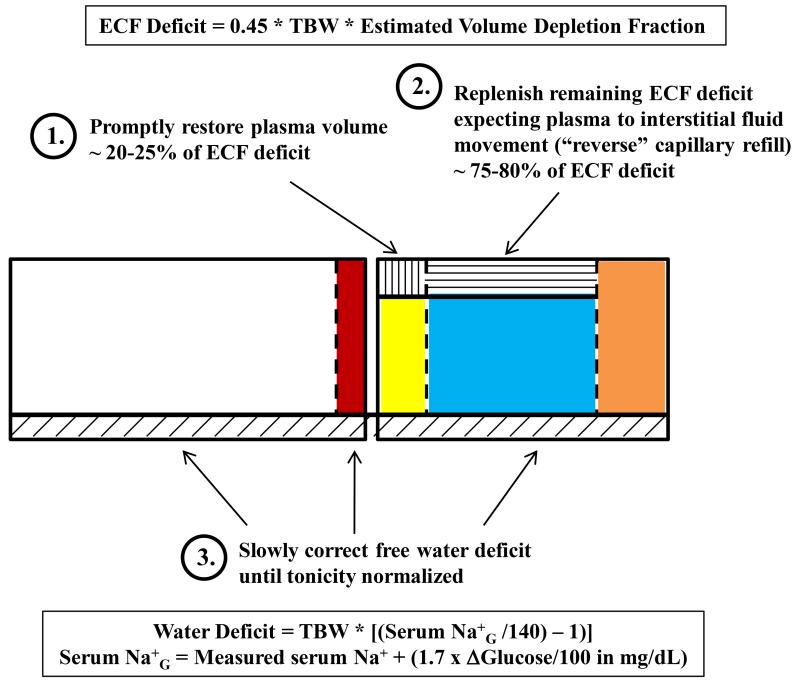
Management of HHNK typically occurs in three overlapping phases: ECBV restoration, repletion of ECF deficits and correction of hyperglycemia, and correction of free water deficits (Figure 2). Other electrolyte abnormalities particularly TBK+ depletion should also be addressed concurrently and partially before the administration of insulin with frank hypokalemia (46). To avoid end-organ ischemic injury, remedy of ECBV depletion takes precedence over correction of hypertonicity. The rate of correction is always a point of contention, but a rational approach is to replenish plasma volume quickly and then slowly replace the interstitial fluid as transcapillary refill reverses direction over the next 24 hours. Since transcapillary refill replenishes 75-80% of lost vascular volume, the plasma volume deficit is about 20-25% (100% minus 75-80%) of the total ECF deficit or around 1-1.5L. Administration of this volume as a bolus of normal saline in the first 1-2 hours is reasonable. Relatively robust isotonic saline administration may be continued for ECFV repletion (~5L over 24 hours = ~200 mL/hr) and compensation for isotonic urinary losses (half of urine volume assuming a urine Na+ + K+ about half of the serum Na+).
Figure 2. Body Fluid Derangements and Fluid Therapy Strategy in HHNK with Volume Depletion & Hypertonicity.
Circulating volume depletion is the overriding initial therapeutic goal followed by interstitial fluid depletion which account for 1/4th and 3/4th of the ECFV deficit respectively. Correction of tonicity with free water or hypotonic solutions is conducted slowly ensuring serum Na+G falls at a rate < 10 mEq/day.
Low dose insulin therapy (0.1 units/kg/hour) should only be started after hemodynamic stabilization as the resulting intracellular glucose shift will also drive extracellular fluid back into cells further compromising ECFV (43, 46). Insulin therapy should continue until the serum glucose is around 300 mg/dL, a slightly hyperglycemic target designed to maintain mild hypertonicity to mitigate risk of cerebral edema (46). At this point, the insulin rate is lowered and solutions containing dextrose are used to avoid hypoglycemia. The rate of dextrose infusion to keep serum glucose constant can be calculated to match the Glucose Burn Rate: 5% dextrose infusion rate (mL/hour) ≅ average hourly change in serum glucose (mg/dL/hour) × 0.08 × TBH2O. The required 5% dextrose infusion rate is often surprising. A relatively low fall in serum glucose of 50 mg/dL/hour (55) in our patient requires a 5% dextrose infusion rate of 250-300 mL/hour to simply maintain serum glucose constant. Infusions of 10% or 50% dextrose may be used if high infusion rates are not desired.
As volume resuscitation proceeds, GFR will often recover before hyperglycemia, leading to recrudescent polyuria. A common misconception is that isotonic saline will correct hypertonicity since infusate tonicity is less than body tonicity. While a minor salutary effect is possible in the short run, significant osmotic diuresis with hypotonic urine will lead to salination and worsening hypertonicity if unattended. For example, 1L of isotonic saline may be excreted as 2L of hypotonic urine producing a net free water loss of 1L. Thus, a transition to hypotonic fluids is eventually necessary.
A significant rise in urine volume typically indicates improving GFR and the excretion of hypotonic urine. 0.45% saline is useful initially to match hypotonic urine losses, continue volume repletion, and begin free water correction. A relatively slow correction of the free water deficit to reduce serum Na+G by <10 mEq/day is often suggested to minimize risk of treatment induced cerebral edema (56). Since serum Na+G is 158 mEq/L in our patient, the increment of 18 mEq/L should be normalized over 48 hours or more. Free water administration of about 150-200 mL/hour in excess of on-going urinary (about ½ urinary volume) and insensible losses (30-50 mL/hour) will be required. About 400-500 mL/hour of half-isotonic saline will provide the equivalent of 200-250 mL/hour of free water and isotonic saline. As euvolemia is achieved, a switch should be made to D5W at about 150 mL/hour in excess of on-going free water losses to correct residual hypernatremia (serum Na+G >140 mEq/L).
Table 4. Definition of Key Terms
- ADH (Anti-Diuretic hormone)
Also known as vasopression. Hormone released from posterior pituitary primarily in response to hypertonicity and hypovolemia.
- Effective Osmoles
Molecules in a solution which lead to osmotic water movement across a semi-permeable (cell) membrane. Examples include Na+, K+, glucose, and mannitol.
- Ineffective Osmoles
Molecules in a solution which do not produce water movement since they effectively cross a semi-permeable membrane. Examples include urea and ethanol.
- Hyperosmolality
Refers to lab measurement of dissolved molecules in a liter of body fluid including both effective and ineffective osmoles. Operationally defined as > 290 mOsm/kg in patients.
- Hypertonicity
A measure of effective osmoles in a fluid compartment which produces water movement into the given compartment.
- HHNK (Hyperglycemic Hypertonic Non-Ketosis)
Diabetic complication typically observed in type II diabetes mellitus characterized by severe hyperglycemia, neurologic manifestations related to hypertonicity, and volume depletion related to urinary sodium losses. Ketoacidosis is minimal. Historically, HHNK was denoted as hyperglycemic hyperosmolar non-ketosis, but hypertonicity, not hyperosmolarity is the critical pathophysiologic feature.
- ICF (Intracellular Fluid)
Consists of all fluid volume within cells including red blood cells.
- ECF (Extracellular Fluid)
Body water residing outside cells including plasma, secretory fluids (intestinal, pleural, peritoneal, CSF, etc), interstitial fluid, and connective tissue water.
- TBH2O (Total Body Water)
Sum of ICF and ECF and accounts for all body water.
- TBNa (Osmotically Active Total Body Sodium)
Refers to body sodium which contributes to body tonicity. Technically different from total body sodium which includes an osmotically inactive pool of sodium residing primarily in bone and possibly skin.
- TBK (Osmotically Active Total Body Potassium)
Refers essentially to total body content of potassium. Very little potassium is osmotically inactive.
- ECFV (Extracellular Fluid Volume)
The absolute amount of fluid in the extracellular fluid compartment.
- ECBV (Effective Circulating Blood Volume)
Term to denote an ill-defined quality of arterial filling which often but not always parallels ECFV.
- Oliguria
Reduced urine output insufficient to eliminate metabolic by-products. Often arbitrarily defined as < 400 mL/day.
References
- 1.Chumlea WC, Guo SS, Zeller CM, et al. Total body water reference values and prediction equations for adults. Kidney Int. 2001;59:2250–2258. doi: 10.1046/j.1523-1755.2001.00741.x. [DOI] [PubMed] [Google Scholar]
- 2.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 3.Edelman IS, Leibman J. Anatomy of body water and electrolytes. Am J Med. 1959;27:256–277. doi: 10.1016/0002-9343(59)90346-8. [DOI] [PubMed] [Google Scholar]
- 4.Edelman IS, Leibman J, O’Meara MP, Birkenfeld LW. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37:1236–1256. doi: 10.1172/JCI103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gennari FJ. Current concepts. Serum osmolality. Uses and limitations. N Engl J Med. 1984;310:102–105. doi: 10.1056/NEJM198401123100207. [DOI] [PubMed] [Google Scholar]
- 6.Mange K, Matsuura D, Cizman B, et al. Language guiding therapy: the case of dehydration versus volume depletion. Ann Intern Med. 1997;127:848–853. doi: 10.7326/0003-4819-127-9-199711010-00020. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg M. Hyponatremia. Med Clin North Am. 1981;65:251–269. doi: 10.1016/s0025-7125(16)31523-1. [DOI] [PubMed] [Google Scholar]
- 8.Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19:1076–1078. doi: 10.1681/ASN.2007091042. [DOI] [PubMed] [Google Scholar]
- 9.Ferrannini E, Smith JD, Cobelli C, Toffolo G, Pilo A, DeFronzo RA. Effect of insulin on the distribution and disposition of glucose in man. J Clin Invest. 1985;76:357–364. doi: 10.1172/JCI111969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquez JA. Theory of production rate calculations in steady and non-steady states and its application to glucose metabolism. Am J Physiol. 1992;262:E779–790. doi: 10.1152/ajpendo.1992.262.6.E779. [DOI] [PubMed] [Google Scholar]
- 11.Katz MA. Hyperglycemia-induced hyponatremia--calculation of expected serum sodium depression. N Engl J Med. 1973;289:843–844. doi: 10.1056/NEJM197310182891607. [DOI] [PubMed] [Google Scholar]
- 12.Oh MS. Pathogenesis and diagnosis of hyponatremia. Nephron. 2002;92(Suppl 1):2–8. doi: 10.1159/000065370. [DOI] [PubMed] [Google Scholar]
- 13.Moran SM, Jamison RL. The variable hyponatremic response to hyperglycemia. West J Med. 1985;142:49–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Tzamaloukas AH, Ing TS, Siamopoulos KC, et al. Body fluid abnormalities in severe hyperglycemia in patients on chronic dialysis: review of published reports. J Diabetes Complications. 2008;22:29–37. doi: 10.1016/j.jdiacomp.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Schrier RW. Decreased effective blood volume in edematous disorders: what does this mean? J Am Soc Nephrol. 2007;18:2028–2031. doi: 10.1681/ASN.2006111302. [DOI] [PubMed] [Google Scholar]
- 16.Gauer OH, Henry JP, Behn C. The regulation of extracellular fluid volume. Annu Rev Physiol. 1970;32:547–595. doi: 10.1146/annurev.ph.32.030170.002555. [DOI] [PubMed] [Google Scholar]
- 17.Drucker WR, Chadwick CD, Gann DS. Transcapillary refill in hemorrhage and shock. Arch Surg. 1981;116:1344–1353. doi: 10.1001/archsurg.1981.01380220088014. [DOI] [PubMed] [Google Scholar]
- 18.Ishibe S, Peixoto AJ. Methods of assessment of volume status and intercompartmental fluid shifts in hemodialysis patients: implications in clinical practice. Semin Dial. 2004;17:37–43. doi: 10.1111/j.1525-139x.2004.17112.x. [DOI] [PubMed] [Google Scholar]
- 19.Ebert RV, Stead EA, Gibson JG. Response of Normal Man to Acute Blood Loss. Arch Intern Med. 1941;68:578–590. [Google Scholar]
- 20.Koomans HA, Geers AB, Mees EJ. Plasma volume recovery after ultrafiltration in patients with chronic renal failure. Kidney Int. 1984;26:848–854. doi: 10.1038/ki.1984.227. [DOI] [PubMed] [Google Scholar]
- 21.Moore FD. The Effects of Hemorrhage on Body Composition. N Engl J Med. 1965;273:567–577. doi: 10.1056/NEJM196509092731101. [DOI] [PubMed] [Google Scholar]
- 22.Riddez L, Hahn RG, Brismar B, Strandberg A, Svensen C, Hedenstierna G. Central and regional hemodynamics during acute hypovolemia and volume substitution in volunteers. Crit Care Med. 1997;25:635–640. doi: 10.1097/00003246-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Kavic SM, Frehm EJ, Segal AS. Case studies in cholera: lessons in medical history and science. Yale J Biol Med. 1999;72:393–408. [PMC free article] [PubMed] [Google Scholar]
- 24.Grayson TL, White JE, Moyer CA. Oxygen Consumptions; Concentrations of Inorganic Ions in Urine, Serum and Duodenal Fluid, Hematocrits, Urinary Excretions; Pulse Rates and Blood Pressure during Duodenal Depletions of Sodium Salts in Normal and Alcoholic Man. Ann Surg. 1963;158:840–858. doi: 10.1097/00000658-196311000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reubi FC. Hemodynamic changes in isotonic dehydration. Contrib Nephrol. 1980;21:55–61. doi: 10.1159/000385247. [DOI] [PubMed] [Google Scholar]
- 26.Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev. 1985;65:149–209. doi: 10.1152/physrev.1985.65.1.149. [DOI] [PubMed] [Google Scholar]
- 27.Feig PU, McCurdy DK. The hypertonic state. N Engl J Med. 1977;297:1444–1454. doi: 10.1056/NEJM197712292972608. [DOI] [PubMed] [Google Scholar]
- 28.Knopp R, Claypool R, Leonardi D. Use of the tilt test in measuring acute blood loss. Ann Emerg Med. 1980;9:72–75. doi: 10.1016/s0196-0644(80)80333-7. [DOI] [PubMed] [Google Scholar]
- 29.McGee S, Abernethy WB, 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022–1029. doi: 10.1001/jama.281.11.1022. [DOI] [PubMed] [Google Scholar]
- 30.McCance RA. Medical Problems in Mineral Metabolism. III. Experimental Human Salt Deficiency. Lancet. 1936;227:823–830. [Google Scholar]
- 31.McCance RA. The effect of salt deficiency in man on the volume of the extracellular fluids, and on the composition of sweat, saliva, gastric juice and cerebrospinal fluid. J Physiol. 1938;92:208–218. doi: 10.1113/jphysiol.1938.sp003595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCance RA, Widdowson EM. The secretion of urine in man during experimental salt deficiency. J Physiol. 1937;91:222–231. doi: 10.1113/jphysiol.1937.sp003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCance RA, Young WF, Black DA. The secretion of urine during dehydration and rehydration. J Physiol. 1944;102:415–428. doi: 10.1113/jphysiol.1944.sp004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadal JW, Pedersen S, Maddock WG. A Comparison between Dehydration from Salt Loss and from Water Deprivation. J Clin Invest. 1941;20:691–703. doi: 10.1172/JCI101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badr KF, Ichikawa I. Prerenal failure: a deleterious shift from renal compensation to decompensation. N Engl J Med. 1988;319:623–629. doi: 10.1056/NEJM198809083191007. [DOI] [PubMed] [Google Scholar]
- 36.Lauson HD, Bradley SE, Cournand A, Andrews VV. The Renal Circulation in Shock. J Clin Invest. 1944;23:381–402. doi: 10.1172/JCI101506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lombardo TA, Eisenberg S, Oliver BB, Viar WN, Eddleman EE, Jr., Harrison TR. Effects of bleeding on electrolyte excretion and on glomerular filtration. Circulation. 1951;3:260–270. doi: 10.1161/01.cir.3.2.260. [DOI] [PubMed] [Google Scholar]
- 38.Stone AM, Stahl WM. Renal effects of hemorrhage in normal man. Ann Surg. 1970;172:825–836. doi: 10.1097/00000658-197011000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiggins WS, Manry CH, Lyons RH, Pitts RF. The effect of salt loading and salt depletion on renal function and electrolyte excretion in man. Circulation. 1951;3:275–281. doi: 10.1161/01.cir.3.2.275. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 41.McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–1266. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- 42.Matz R. Management of the hyperosmolar hyperglycemic syndrome. Am Fam Physician. 1999;60:1468–1476. [PubMed] [Google Scholar]
- 43.McCurdy DK. Hyperosmolar hyperglycemic nonketotic diabetic coma. Med Clin North Am. 1970;54:683–699. [PubMed] [Google Scholar]
- 44.Pollock AS, Arieff AI. Abnormalities of cell volume regulation and their functional consequences. Am J Physiol. 1980;239:F195–205. doi: 10.1152/ajprenal.1980.239.3.F195. [DOI] [PubMed] [Google Scholar]
- 45.Zeitler P, Haqq A, Rosenbloom A, Glaser N. Hyperglycemic Hyperosmolar Syndrome in Children: Pathophysiological Considerations and Suggested Guidelines for Treatment. J Pediatr. 2010 doi: 10.1016/j.jpeds.2010.09.048. [DOI] [PubMed] [Google Scholar]
- 46.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bankir L, Bouby N, Trinh-Trang-Tan MM, Ahloulay M, Promeneur D. Direct and indirect cost of urea excretion. Kidney Int. 1996;49:1598–1607. doi: 10.1038/ki.1996.232. [DOI] [PubMed] [Google Scholar]
- 48.Leaf A. Dehydration in elderly. N Engl J Med. 1984;311:791–792. doi: 10.1056/NEJM198409203111209. [DOI] [PubMed] [Google Scholar]
- 49.Atchley DW, Loeb RF, Richards DW, Benedict EM, Driscoll ME. ON DIABETIC ACIDOSIS: A Detailed Study of Electrolyte Balances Following the Withdrawal and Reestablishment of Insulin Therapy. J Clin Invest. 1933;12:297–326. doi: 10.1172/JCI100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brodsky WA, Rapoport S, West CD. The mechanism of glycosuric diuresis in diabetic man. J Clin Invest. 1950;29:1021–1032. doi: 10.1172/JCI102333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seldin DW, Tarail R. The metabolism of glucose and electrolytes in diabetic acidosis. J Clin Invest. 1950;29:552–565. doi: 10.1172/JCI102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gennari FJ, Kassirer JP. Osmotic diuresis. N Engl J Med. 1974;291:714–720. doi: 10.1056/NEJM197410032911408. [DOI] [PubMed] [Google Scholar]
- 53.Rose BD. New approach to disturbances in the plasma sodium concentration. Am J Med. 1986;81:1033–1040. doi: 10.1016/0002-9343(86)90401-8. [DOI] [PubMed] [Google Scholar]
- 54.Tzamaloukas AH, Ing TS, Siamopoulos KC, et al. Pathophysiology and management of fluid and electrolyte disturbances in patients on chronic dialysis with severe hyperglycemia. Semin Dial. 2008;21:431–439. doi: 10.1111/j.1525-139X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 55.Rosenthal NR, Barrett EJ. An assessment of insulin action in hyperosmolar hyperglycemic nonketotic diabetic patients. J Clin Endocrinol Metab. 1985;60:607–610. doi: 10.1210/jcem-60-3-607. [DOI] [PubMed] [Google Scholar]
- 56.Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493–1499. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter CC. Skin turgor. Lancet. 1981;1:675. doi: 10.1016/s0140-6736(81)91604-4. [DOI] [PubMed] [Google Scholar]
- 58.Dorrington KL. Skin turgor: do we understand the clinical sign? Lancet. 1981;1:264–266. doi: 10.1016/s0140-6736(81)92097-3. [DOI] [PubMed] [Google Scholar]
- 59.Hew-Butler T, Noakes TD, Soldin SJ, Verbalis JG. Acute changes in arginine vasopressin, sweat, urine and serum sodium concentrations in exercising humans: does a coordinated homeostatic relationship exist? Br J Sports Med. 2010;44:710–715. doi: 10.1136/bjsm.2008.051771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh NP, Montague JC, Callow N, Rowlands AV. Saliva flow rate, total protein concentration and osmolality as potential markers of whole body hydration status during progressive acute dehydration in humans. Arch Oral Biol. 2004;49:149–154. doi: 10.1016/j.archoralbio.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Lapides J, Bourne RB, Maclean LR. Clinical Signs of Dehydration and Extracellular Fluid Loss. JAMA. 1965;191:413–415. doi: 10.1001/jama.1965.03080050059022. [DOI] [PubMed] [Google Scholar]
- 62.Miller TR, Anderson RJ, Linas SL, et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89:47–50. doi: 10.7326/0003-4819-89-1-47. [DOI] [PubMed] [Google Scholar]