Abstract
Objectives
To determine time to menarche in girls and testicular volume increase in boys after removal of a histrelin implant, which causes profound hypothalamic-pituitary-gonadal axis suppression.
Study design
Medical records of patients treated with a histrelin implant were reviewed. Seventy-one patients (56 girls) treated with the histrelin implant were identified, of these patients, 37 explanted girls (68% naïve) and 6 explanted boys (83% naïve) were included in the analysis. Time to menarche after explantation in girls and time to testicular volume increase after explantation in boys were determined. Additional variables investigated included indication for and duration of treatment, history of menarche (girls), previous therapy, and age at beginning and end of histrelin treatment.
Results
Of the girls, 30 were treated for central precocious puberty (CPP), 26 had menarche at an average of 12.75 months after explantation. Of the 30, 7 were treated for other indications, of whom 6 had reached menarche. In girls with CPP, older age at explantation correlated with sooner menarche (P = .04). All boys achieved spontaneous testicular enlargement within 1 year of explantation.
Conclusions
This study documented resumption of puberty after histrelin explantation in treatment naïve and non-naïve boys and girls with and without CPP. Menarche in girls with CPP occurs within a similar timeframe to that observed after other treatment approaches.
Central precocious puberty (CPP) is defined as activation of the hypothalamic-pituitary-gonadal (HPG) axis before age 8 in girls or age 9 in boys, resulting in early secondary sexual development.1–3 Gonadotropin-releasing hormone agonists (GnRHas) are the standard of care for treating CPP.1 Although several formulations and routes of administration exist, the most widely used GnRHa in the US has been intramuscular depot leuprolide injections. Since 2007, a subcutaneous implant containing the potent GnRHa histrelin has been a popular alternative for the treatment of CPP.4 Made of a microporous hydrogel, the implant is surgically inserted just under the skin in the upper arm and continuously releases histrelin for at least 1 year.5 Studies in children with CPP have demonstrated complete HPG axis and sex steroid suppression as well as improvements in predicted adult height.4,6 In fact, stimulated luteinizing hormone (LH) levels are even lower in patients treated with the histrelin implant compared with those treated with depot leuprolide injections.4,7 Whether this will result in a delay in resumption of puberty after removal of the implant is unknown. Thus far, only limited information regarding HPG axis activation after treatment with the histrelin implant in girls with CPP is available. Thus, the primary aim of this study was to investigate resumption of puberty as manifested by menarche in girls and testicular volume increase in boys after histrelin explantation in a diverse group of patients with and without CPP. In addition, we sought to evaluate whether individual patient characteristics correlated with this timing.
Methods
After institutional review board approval, medical records of children followed in the pediatric endocrine clinic at Riley Hospital for Children who were treated with a histrelin implant were identified. Eligible patients were those in whom histrelin explantation and discontinuation of GnRHa therapy had occurred at least 1 month before the start of data collection. Primary outcome variables extracted from the medical records included the interval between explantation and menarche in girls and the interval between histrelin explantation and documentation of an increase in testicular volume in boys. A nurse placed phone calls to parents of girls who were postexplantation but premenarchal at the time of their last clinic visit, querying whether menarche had been achieved and, if so, in what month and year. Additional variables extracted from the medical records consisted of the indication for GnRHa treatment, the age at start of therapy, the total duration of GnRHa therapy, the number of implants used, history of GnRHa therapy before histrelin implant, history of menarche before therapy (girls), and age at final explantation. Criteria for GnRHa therapy in patients with CPP were based on a pubertal peak stimulated or random ultrasensitive LH level along with an advanced bone age and breast Tanner stage ≥II before age 8 in girls or testicular volume ≥4 cm2 before age 9 in boys.
Statistical Analyses
To determine the average time to menarche, the mean and 95% CIs were calculated for both the premenarchal and menarchal groups. The effects of continuous patient variables on the time to menarche were analyzed using linear regression analysis, and goodness of fit was reported as r2 with a corresponding P value. The effects of categorical patient variables on the time to menarche were analyzed using unpaired 2-tailed Student t-tests, and P values were reported along with group mean and SE values. A P value of < .05 was considered significant.
Results
Seventy-one patients (56 girls, 15 boys) treated with a histrelin implant were identified. Of these, 46 (40 girls, 6 boys) had undergone explantation. Of the girls, 2 were lost to follow-up and 1 resumed leuprolide injections, and thus 37 were included in the analysis. Of these, 30 (81%) were treated for CPP, 26 (87%) of whom had reached menarche, whereas 7 (19%) were treated for other indications, 6 (86%) of whom had achieved this milestone. Of the 6 boys, 1 was lost to follow-up, and of the 5 included in the final analysis, 3 (60%) were treated for CPP and 2 (40%) for other indications (Figure 1).
Figure 1.
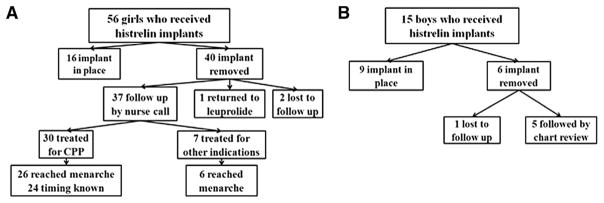
Flowchart of A, girls and B, boys treated with histrelin implant.
Time to Menarche in Girls with and without CPP
Of the 30 girls treated for CPP, 18 (60%) were naïve to GnRHa treatment at the time of initial histrelin implantation. Of the 26 who have reached menarche, the month and year were known for 24. These girls reached menarche on average 12.75 (95% CI 9.6–15.9) months after histrelin explantation (range 2–36 months). Menarche occurred at an average age of 12.94 (95% CI 10.5–15.3) years, which is nearly identical to that seen in the general population. In the 4 girls treated for CPP who have yet to achieve menarche, the average interval since explantation is 24.75 (95% CI 8.0–41.5) months, and these patients have an average age of 12.36 (95% CI 11.8–12.9) years. The clinical characteristics of girls treated for CPP are summarized in the Table (available at www.jpeds.com), and menarchal status is shown in Figure 2 (available at www.jpeds.com).
Table.
Clinical characteristics of girls with CPP (N = 30)
| Characteristics | Mean ± SD |
|---|---|
| Age at histrelin implant (yr) | 8.74 ± 1.37 |
| Age at treatment start (yr) | 8.1 ± 1.06 |
| Prior GnRHa use, n (%) | 12 (40%) |
| Duration of histrelin therapy (yr) | 2.41 ± 1.07 |
| Duration of GnRHa therapy (yr) | 2.89 ± 1.29 |
| Age at histrelin explantation (yr) | 11.15 ± 0.97 |
| History of menarche, n (%) | 7 (23%) |
| No. of girls treated with 1, 2, 3, and 5 implants for total treatment duration, n | 19, 5, 5, 1 |
| No. who have reached menarche, n (%) | 26 (87%) |
Figure 2.
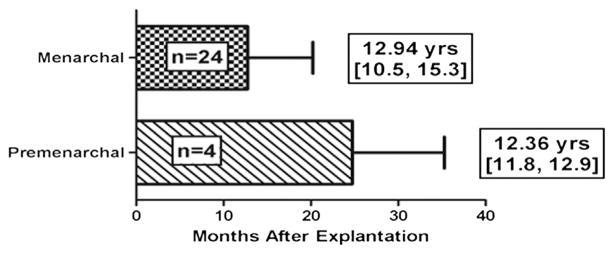
Time from histrelin explantation to menarche is indicated in the top bar and time since explantation for pre-menarchal girls in the bottom bar. Mean and 95% CIs are shown for age in years for the 24 girls who have reached menarche and in the 4 who are still premenarchal.
Indications for GnRHa treatment in the 7 girls without CPP included a poor prognosis for adult height in patients with normally timed puberty and idiopathic short stature (n = 2), growth hormone deficiency (n = 4), and profound hypothyroidism (n = 1). All of these girls were naïve to GnRHa treatment and 6 had achieved menarche on average 23.83 (95% CI 15.4–32.3) months since explantation at an average age of 16.71 (95% CI 15.2–18.2) years.
Time to Testicular Volume Increase in Boys
Of the 5 boys, 4 (80%) were naïve to GnRHa therapy. All demonstrated a spontaneous increase in testicular volume within 1 year after histrelin explantation. The 3 boys treated for idiopathic CPP showed the most significant increases in testicular volume, reaching nearly adult size within 1 year after removal of the histrelin implant. Of the boys treated for other indications, 1 had growth hormone deficiency and the other had familial male precocious puberty with secondary CPP. All boys who were GnRHa naïve experienced a decrease in testicular volume during treatment with the histrelin implant (Figure 3).
Figure 3.
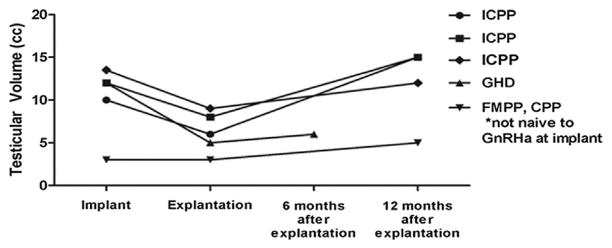
Testicular volumes and indications for treatment are illustrated for each of the boys in the study. GHD, growth hormone deficiency; ICPP, idiopathic CPP.
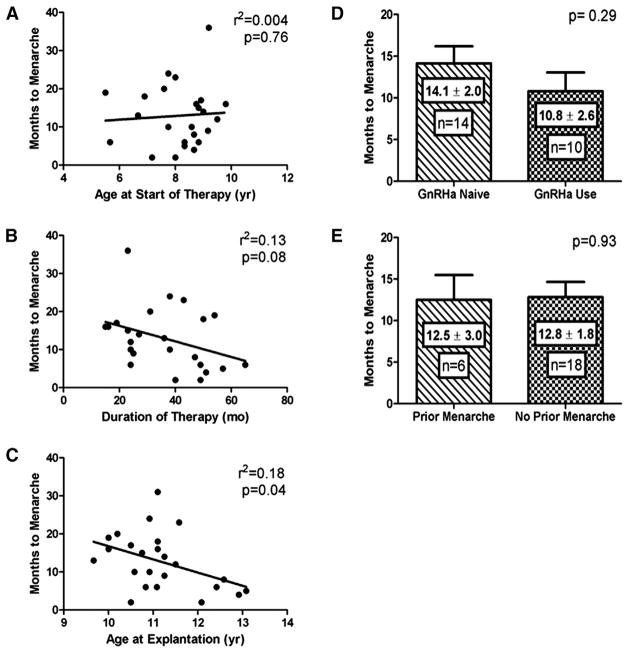
Influence of Patient Characteristics on Timing of Menarche in Girls with CPP
There was no significant correlation between age at initiation of GnRHa therapy (depot leuprolide or histrelin) and time to menarche after histrelin explantation (P = .76; Figure 4, A). However, a negative trend was noted between total duration of GnRHa therapy and time to menarche (P = .08; Figure 4, B). Age at histrelin explantation was inversely correlated with time to menarche (P = .04; Figure 4, C). Additional analyses revealed no effect of the number of histrelin implants used or if implants were left in place for 1 vs 2 years (data not shown). There was no correlation between prior treatment status (naïve vs non-naïve) or menarchal history and time to menarche (Figure 4, D and E).
Figure 4.
The effect of individual variables on the timing to menarche in girls with CPP is illustrated as follows: A, age at GnRHa start, B, duration of GnRHa therapy, C, age at histrelin explantation, D, history of prior menarche, and E, depot GnRHa use before histrelin implant.
Discussion
The histrelin implant for treatment of CPP has been steadily increasing in popularity. Because it obviates monthly intramuscular injections, it is preferred by children and their parents.4,6 However, important questions remain regarding its use, not the least of which pertains to reproductive function after discontinuation. Until now, minimal information on this issue has been available.
Many studies have investigated the follow-up of girls with CPP treated with injectable depot GnRHa preparations. Depot leuprolide has been the most commonly used GnRHa in the US, and one study found the time to menarche to be 18 ± 6 months after ending therapy at an average age of 12.9 ± 0.9 years of age.8 Studies evaluating other depot GnRHa preparations have found remarkably consistent results.1,9,10 The average time to menarche after the histrelin implant in the girls in our study is similar to what has been observed after depot GnRHa formulations, although perhaps with greater individual variability. As 4 of our girls have not yet reached this milestone, the average interval since explantation will only increase. Although much less data regarding HPG-axis activation in boys with CPP after receiving depot GnRHa are available, pubertal LH and testosterone concentrations have been documented at <1 year after discontinuation of therapy.11,12 Thus, the finding of testicular enlargement at the first follow-up visit in all of the boys in our study is consistent with these results. Only 1 other study evaluating time to menarche after histrelin explantation has been reported.13 In 11 girls with CPP, all except 2 of whom had been previously treated with depot injections, menarche occurred at an average of 9.3 months after histrelin explantation. Although our study involved more patients, the majority of whom were naïve to previous GnRHa treatment, our results are likely more generalizable to the typical clinical setting. Novel aspects of our study include information about pubertal resumption after use of the histrelin implant in boys as well as in children treated for indications other than CPP, neither of which have previously been reported. Although efficacy remains unproven, GnRHa have been prescribed with the hope of increasing ultimate stature in children without CPP and a variety of conditions in which a poor prognosis for adult height is present.14–16
In evaluating the effects of individual patient characteristics on the time to menarche in girls with CPP, only age at explantation was found to be significantly correlated. We found an inverse relationship between age and time to menarche after explantation. This makes sense intuitively, as girls who are older at the time of explantation are closer to the natural age of menarche and therefore more likely to achieve this sooner. None of the other analyzed variables were correlated. These findings are in contrast to several previous studies in which girls who had achieved menarche before GnRHa treatment and those who had been treated for a longer duration reached menarche sooner than other girls after discontinuing GnRHa therapy.11,17,18 This may be due to differences between the histrelin implant and depot GnRHa preparations or to individual patient variables. Alternatively, the lack of effect of prior menarche may be due to the small number of postmenarchal girls in our sample. The finding that prior GnRHa use had no influence on time to menarche after explantation may arise from the fact that the total length of treatment was not standardized in this analysis.
Limitations of our study include that it is primarily retrospective and involved a heterogeneous group of patients in whom criteria for treatment varied. Additionally, time to menarche in girls was based on parent report. However, as menarche is a highly anticipated and memorable milestone in girls treated with GnRHa, this information is likely reliable. Because boys were only examined at 6–12 months after explantation, the precise time at which pubertal progression ensued is unknown. Regardless, the fact that several already demonstrated adult testicular volumes at the first follow-up visit implies that it was likely quite soon after the histrelin implant was removed. Last, the inclusion of the patient with familial male precocious puberty is problematic. Nonetheless, given the dearth of information about boys, this child’s clinical trajectory after treatment with the histrelin implant is still valuable. Our findings should be considered preliminary pending further investigation, particularly in boys.
Acknowledgments
E.E. participated in a sponsored clinical trial (NCT00779103) involving the histrelin implant.
Glossary
- CPP
Central precocious puberty
- GnRHa
Gonadotropin-releasing hormone agonist
- HPG
Hypothalamic-pituitary-gonadal
- LH
Luteinizing hormone
Footnotes
The other authors declare no conflicts of interest.
Portions of this study were presented as a platform presentation at the annual meeting of the Pediatric Endocrine Society, May 6, 2013, Washington, DC.
References
- 1.Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, Antoniazzi F, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123:e752–62. doi: 10.1542/peds.2008-1783. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua JS. Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab. 2013;98:2198–207. doi: 10.1210/jc.2013-1024. [DOI] [PubMed] [Google Scholar]
- 3.Nebesio TD, Eugster EA. Current concepts in normal and abnormal puberty. Curr Probl Pediatr Adolesc Health Care. 2007;37:50–72. doi: 10.1016/j.cppeds.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Eugster EA, Clarke W, Kletter GB, Lee PA, Neely EK, Reiter EO, et al. Efficacy and safety of histrelin subdermal implant in children with central precocious puberty: a multicenter trial. J Clin Endocrinol Metab. 2007;92:1697–704. doi: 10.1210/jc.2006-2479. [DOI] [PubMed] [Google Scholar]
- 5.Lewis KA, Eugster EA. Experience with the once-yearly histrelin (GnRHa) subcutaneous implant in the treatment of central precocious puberty. Drug Design Dev Therapy. 2009;3:1–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch HJ, Gillis D, Strich D, Chertin B, Farkas A, Lindenberg T, et al. The histrelin implant: a novel treatment for central precocious puberty. Pediatrics. 2005;116:e798–802. doi: 10.1542/peds.2005-0538. [DOI] [PubMed] [Google Scholar]
- 7.Rahhal S, Clarke WL, Kletter GB, Lee PA, Neely EK, Reiter EO, et al. Results of a second year of therapy with the 12-month histrelin implant for the treatment of central precocious puberty. Int J Pediatr Endocrinol. 2009;2009:812517. doi: 10.1155/2009/812517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neely EK, Lee PA, Bloch CA, Larsen L, Yang D, Mattia-Goldberg C, et al. Leuprolide acetate 1-monthdepot for centralprecocious puberty:hormonal suppression and recovery. Int J Pediatr Endocrinol. 2010;2010:398639. doi: 10.1155/2010/398639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heger S, Partsch CJ, Sippell WG. Long-term outcome after depot gonadotropin-releasing hormone agonist treatment of central precocious puberty: final height, body proportions, body composition, bone mineral density, and reproductive function. J Clin Endocrinol Metab. 1999;84:4583–90. doi: 10.1210/jcem.84.12.6203. [DOI] [PubMed] [Google Scholar]
- 10.Cassio A, Bal MO, Orsini LF, Balsamo A, Sansavini S, Gennari M, et al. Reproductive outcome in patients treated and not treated for idiopathic early puberty: long-term results of a randomized trial in adults. J Pediatr. 2006;149:532–6. doi: 10.1016/j.jpeds.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka T, Niimi H, Matsuo N, Fujieda K, Tachibana K, Ohyama K, et al. Results of long-term follow-up after treatment of central precocious puberty with leuprorelin acetate: evaluation of effectiveness of treatment and recovery of gonadal function. The TAP-144-SR Japanese Study Group on Central Precocious Puberty. J Clin Endocrinol Metab. 2005;90:1371–6. doi: 10.1210/jc.2004-1863. [DOI] [PubMed] [Google Scholar]
- 12.Bertelloni S, Baroncelli GI, Ferdeghini M, Menchini-Fabris F, Saggese G. Final height, gonadal function and bone mineral density of adolescent males with central precocious puberty after therapy with gonadotropin-releasing hormone analogues. Eur J Pediatr. 2000;159:369–74. doi: 10.1007/s004310051289. [DOI] [PubMed] [Google Scholar]
- 13.Gillis D, Karavani G, Hirsch HJ, Strich D. Time to menarche and final height after histrelin implant treatment for central precocious puberty. J Pediatr. 2013;163:532–6. doi: 10.1016/j.jpeds.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Carel JC. Management of short stature with GnRH agonist and co-treatment with growth hormone: a controversial issue. Mol Cell Endocrinol. 2006;254–255:226–33. doi: 10.1016/j.mce.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Balducci R, Toscano V, Mangiantini A, Municchi G, Vaccaro F, Picone S, et al. Adult height in short normal adolescent girls treated with gonadotropin-releasing hormone analog and growth hormone. J Clin Endocrinol Metab. 1995;80:3596–600. doi: 10.1210/jcem.80.12.8530605. [DOI] [PubMed] [Google Scholar]
- 16.Pasquino AM, Pucarelli I, Roggini M, Segni M. Adult height in short normal girls treated with gonadotropin-releasing hormone analogs and growth hormone. J Clin Endocrinol Metab. 2000;85:619–22. doi: 10.1210/jcem.85.2.6387. [DOI] [PubMed] [Google Scholar]
- 17.Lee PA, Houk CP. Gonadotropin-releasing hormone analog therapy for central precocious puberty and other childhood disorders affecting growth and puberty. Treat Endocrinol. 2006;5:287–96. doi: 10.2165/00024677-200605050-00003. [DOI] [PubMed] [Google Scholar]
- 18.Arrigo T, De Luca F, Antoniazzi F, Galluzzi F, Iughetti L, Pasquino AM, et al. Menstrual cycle pattern during the first gynaecological years in girls with precocious puberty after gonadotropin-releasing hormone analogue treatment. Eur J Pediatr. 2007;166:73–4. doi: 10.1007/s00431-006-0207-z. [DOI] [PubMed] [Google Scholar]