Abstract
This study assessed long-lasting consequences of repeated ethanol exposure during two different periods of adolescence on 1) baseline levels of social investigation, play fighting, and social preference and 2) sensitivity to the social consequences of acute ethanol challenge. Adult male and female Sprague-Dawley rats were tested 25 days after repeated exposure to ethanol (3.5 g/kg intragastrically [i.g.], every other day for a total of 11 exposures) in a modified social interaction test. Early-mid adolescent intermittent exposure (e-AIE) occurred between postnatal days (P) 25–45, whereas late adolescent intermittent exposure (l-AIE) was conducted between P45–65. Significant decreases in social investigation and social preference were evident in adult male rats, but not their female counterparts following e-AIE, whereas neither males nor females demonstrated these alterations following l-AIE. In contrast, both e-AIE and l-AIE produced alterations in sensitivity to acute ethanol challenge in males tested 25 days after adolescent exposure. Ethanol-induced facilitation of social investigation and play fighting, reminiscent of that normally seen during adolescence, was evident in adult males after e-AIE, whereas control males showed an age-typical inhibition of social behavior. Males after l-AIE were found to be insensitive to the socially suppressing effects of acute ethanol challenge, suggesting the development of chronic tolerance in these animals. In contrast, females showed little evidence for alterations in sensitivity to acute ethanol challenge following either early or late AIE. The results of the present study demonstrate a particular vulnerability of young adolescent males to long-lasting detrimental effects of repeated ethanol. Retention of adolescent-typical sensitivity to the socially facilitating effects of ethanol could potentially make ethanol especially appealing to these males, therefore promoting relatively high levels of ethanol intake later in life.
Keywords: adolescence, ethanol exposure, social behavior, anxiety, social facilitation, sex differences
Introduction
In humans, initiation of alcohol use occurs predominantly during early adolescence (Faden, 2006), with high drinking levels seen in some individuals. For instance, approximately 5.1% of 8th graders, 15.6% of 10th graders, and 23.7% of high school seniors in the United States reported a binge pattern of drinking (5+ drinks in a row) in the previous 2 weeks (Johnston, O’Malley, Bachman, & Schulenberg, 2013). Adolescents who engage in even episodic heavy drinking at an early age are more likely to develop alcohol use disorders later in life (Bonomo, Bowes, Coffey, Carlin, & Patton, 2004; Grant, Stinson, & Harford, 2001; Hingson, Heeren, & Winter, 2006) and to experience a number of long-lasting adverse psychosocial consequences (Wells, Horwood, & Fergusson, 2004). Although causal relationships remain to be firmly established, binge patterns of alcohol consumption that result in blood alcohol levels of 80 mg/dL and higher are thought to be harmful to the developing adolescent brain (Bava & Tapert, 2010; Silveri, 2012). According to general principles of neurobehavioral toxicology, the brain is often especially vulnerable to long-lasting effects of drugs during its development, with the periods of pronounced developmental changes in brain regions sensitive to a certain drug defining the critical periods for induction of lasting consequences of that drug (Adams et al., 2000). During adolescence, marked changes occur in brain circuits implicated in responsiveness to social and emotional stimuli (Blakemore, 2012), with these brain circuits being sensitive to alcohol as well (Vilpoux, Warnault, Pierrefiche, Daoust, & Naassila, 2009).
It is not surprising, therefore, that alcohol-dependent adolescents have been found to exhibit enhanced negative emotionality, characterized by depression, anxiety, and enhanced stress reactivity (Martin, Lynch, Pollock, & Clark, 2000). Alcohol-related problems are particularly common in adolescents with social anxiety (Buckner, Eggleston, & Schmidt, 2006; Gilles, Turk, & Fresco, 2006). Although social anxiety often precedes alcohol use and abuse (Buckner et al., 2008), causal relationships are still not well understood (e.g., Morris, Stewart, & Ham, 2005). Indeed, it is not clear whether chronic alcohol use during adolescence can enhance or even elicit social anxiety or, in contrast, whether alcohol is more appealing for socially anxious adolescents due to its anxiolytic effects (Carrigan & Randall, 2003). Human studies of underage drinking, however, do not permit systematic manipulation of critical variables, due to ethical considerations that preclude administration of alcohol to underage adolescents. Similarities found between human adolescents and adolescents of various mammalian species in terms of developmental history and behavioral changes, as well as neural and hormonal alterations (Spear, 2000, 2011; Spear & Varlinskaya, 2010), provide reasonable justification for the use of animal models for the assessments of consequences of repeated ethanol exposure during adolescence on anxiety-related behavior under social circumstances, as well as sensitivity to the socially anxiolytic effects of ethanol.
The social interaction test has been used extensively for the assessment of anxiety-like behavior in laboratory rodents (File, 1980; File & Hyde, 1978; File & Seth, 2003). In the conventional social interaction test, a pair of rats is placed into a testing chamber, and overall time spent in social interactions is used as a dependent variable (File, 1980). Yet, the discrete behavioral acts summed together for these assessments (e.g., social investigation and play fighting) reflect behaviorally distinctive and differentially regulated forms of interactive social behaviors with separable ontogenetic patterns (Vanderschuren, Niesink, & Van Ree, 1997; Varlinskaya & Spear, 2008; Varlinskaya, Spear, & Spear, 1999) and differential responsiveness to seemingly anxiogenic manipulations (Doremus-Fitzwater, Varlinskaya, & Spear, 2009). For instance, play fighting exhibits an inverted U-shaped ontogenetic pattern that peaks around P30–35, whereas social investigation increases ontogenetically and represents a more adult-typical form of social interactions (Panksepp, 1981; Vanderschuren et al., 1997; Varlinskaya et al., 1999). Play fighting, but not social investigation, is drastically increased by deprivation from social contact via isolate housing throughout the entire adolescent period (Vanderschuren et al., 1997; Varlinskaya & Spear, 2008), whereas social investigation is exclusively decreased by prior history of exposure to non-social stressors (Doremus-Fitzwater et al., 2009; Varlinskaya, Doremus-Fitzwater, & Spear, 2010). Taken together, these findings suggest that play fighting and social investigation may be mediated via different neural systems, and hence may be differentially vulnerable to alterations induced by intermittent ethanol exposure during adolescence. Modification of the social interaction test, allowing an experimental animal to freely move toward or away from a non-manipulated social partner in a 2-compartment testing apparatus, permits assessment of social motivation via a preference/avoidance coefficient in addition to measuring the frequency of play fighting and social investigation (Varlinskaya et al., 1999). Using this modified social interaction test, we have found decreases in social preference and/or social investigation to reflect anxiety-like alterations in social interactions (Doremus-Fitzwater et al., 2009; Morales, Varlinskaya, & Spear, 2013a; Varlinskaya et al., 2010; Varlinskaya & Spear, 2012).
Using a rat model of adolescence and the modified social interaction test, we have shown that repeated ethanol exposure (1.0 g/kg intraperitoneally [i.p.] for 7 days) increases anxiety-like behavior under social circumstances in adolescent but not adult animals, as indexed by decreases in social preference (Varlinskaya & Spear, 2007). Furthermore, these adolescent animals became unusually sensitive to the anxiolytic effects of ethanol, with the decreases in social preference effectively reversed by acute ethanol challenge. Taken together, these findings suggest that increases in anxiety-like behavior and sensitivity to the socially anxiolytic effects of ethanol associated with repeated ethanol exposure are typical for the adolescent developmental period, with no such alterations evident following adult ethanol exposure. In that study, however, animals were tested 48 h following the last ethanol exposure, and hence it is not clear whether the social anxiety-like behavior and changes in ethanol sensitivity induced by repeated ethanol in adolescents are short-lasting and associated merely with ethanol withdrawal or whether these effects can persist for a long time and be detected later in life. In order to assess whether social consequences of adolescent intermittent exposure to ethanol (AIE) are persistent and continue into adulthood (i.e., 25 days after repeated exposure to ethanol), the present study investigated consequences of AIE on 1) baseline levels of play fighting, social investigation, and social preference and 2) sensitivity to the social consequences of acute ethanol challenge in adult male and female rats. Plasma corticosterone (CORT) levels were measured in order to assess possible relationships between anxiety-like behavioral alterations, sensitivity to acute ethanol challenge, and responsiveness of the hypothalamus-pituitary-adrenal (HPA) axis following AIE.
Animals were exposed to ethanol either during early-mid adolescence [postnatal days (P) 25–45; Experiment 1] or late adolescence (P45–65; Experiment 2) and tested in adulthood, 25 days following exposure, in order to assess whether exposure relatively early in adolescence has more detrimental effects than exposure later in adolescence. Rats were exposed to 3.5 g/kg ethanol i.g. every 48 h for a total of 11 exposures, with control animals either receiving water i.g. or left non-exposed. Both of these control groups were included, given that the repeated gavage process may be relatively stressful and might influence the target measures. Indeed, in prior work we have found that repeated stress alters both social behavior and responsiveness to acute ethanol challenge in adolescents and adults (Varlinskaya et al., 2010), with stressed animals demonstrating anxiety-like behavioral alterations, indexed via significant decreases in social investigation and/or social preference, and enhanced sensitivity to socially anxiolytic effects of ethanol.
Materials and Methods
Subjects
Male and female Sprague-Dawley rats bred and reared in our colony at Binghamton University were used as experimental subjects (n = 479) and social partners (n = 479). All animals were housed in a temperature-controlled (22 °C) vivarium maintained on a 14-h/10-h light/dark cycle (lights on at 7:00 AM) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Litters were culled to 10 (5 male and 5 female) pups on postnatal day (P) 1 and housed with their mothers in standard maternity cages with pine shavings as bedding material. Pups were weaned on P21 and placed into standard plastic cages together with their same-sex littermates. In all respects, maintenance and treatment of the animals were in accord with guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Experimental Design
The design for both experiments was a 3 (exposure: no exposure, water, ethanol) × 5 (test condition: no injection, saline, 0.5, 0.75, 1.0 g/kg ethanol injection) × 2 (sex) factorial. Possible effects of adolescent exposure on baseline levels of social behavior were assessed in animals that were not injected prior to testing, whereas AIE-associated changes in sensitivity to the social consequences of acute ethanol were assessed in animals acutely challenged with one of the four doses of ethanol. Same-sex littermates housed together were assigned semi-randomly to different acute test conditions to avoid the possible confounding of litter with social testing effects (Holson & Pearce, 1992; Zorrilla, 1997).
Intermittent Ethanol Exposure
Animals were exposed to ethanol i.g. (3.5 g/kg, 25% solution in tap water) every other day (11 exposures) either during early-mid adolescence (P25–P45, Experiment 1) or during late adolescence-young adulthood (P45–P65, Experiment 2). Controls were given an isovolumetric amount of tap water by gavage on these exposure days. An additional control group of non-exposed animals was included in each experiment as well.
Procedure
Social testing occurred during adulthood 25 days after the last adolescent exposure (i.e., on P70 in Experiment 1, P90 in Experiment 2). On test day, animals were taken from their home cage, injected i.p. with saline or ethanol or left non-injected, and placed individually in the testing apparatus for 30 min. The testing apparatus (45 × 30 × 30 cm) was composed of Plexiglas® (Binghamton Plate Glass, Binghamton, NY) and was divided into two equally sized compartments by a clear Plexiglas® partition with an aperture (9 × 7 cm) to allow movements of the animals between compartments. A social partner of the same age and sex was then introduced for a 10-min test period. Partners were always unfamiliar with both the test apparatus and the experimental animal, were not socially deprived prior to the test (Varlinskaya & Spear, 2002, 2006, 2008), and were experimentally and drug-naive. Weight differences between test subjects and their partners were minimized as much as possible, with this weight difference not exceeding 20 g at P70 or 30 g at P90, and test subjects always being heavier than their partners. In order to differentiate experimental animals from their social partners during the test, each experimental animal was marked with a vertical black line across the back.
During the 10-min test session, the behavior of the animals was recorded by a video camera (Panasonic model AF-X8, Secaucus, NJ), with real time being directly recorded onto the videotape for later scoring (Easy Reader II Recorder; Telcom Research TCG 550, Burlington, Ontario). All testing procedures were conducted between 9:00 AM and 1:00 PM under dim light (15–20 lux). Trunk blood samples were collected immediately after the test for determination of corticosterone levels.
Behavioral Measures
The frequencies of social investigation and play fighting were analyzed from video recordings (Vanderschuren et al., 1997; Varlinskaya & Spear, 2002, 2006, 2008) by a trained experimenter without knowledge of the experimental condition of any given animal. Social investigation was defined as the sniffing of any part of the body of the partner. Play fighting was scored as the sum of the frequencies of the following behaviors: pouncing or playful nape attack (experimental subject lunges at the partner with its forepaws extended outward); following and chasing (experimental animal rapidly pursues the partner); and pinning (the experimental subject stands over the exposed ventral area of the partner, pressing it against the floor). Play fighting can be distinguished from serious fighting in the laboratory rat by the target of the attack — during play fighting, snout or oral contact is directed towards the partner’s nape, whereas during serious fighting the partner’s rump is the object of the attack (Pellis & Pellis, 1987). Aggressive behavior (serious fighting) was not analyzed in these experiments, since subjects did not exhibit serious attacks or threats.
Social preference/avoidance was analyzed by separately measuring the number of crossovers demonstrated by the experimental subject toward, as well as away from, the social partner. Social motivation was assessed by means of a coefficient of preference/avoidance [coefficient (%) = (crossovers to the partner - crossovers away from the partner)/(total number of crosses both to and away from the partner) × 100]. Social preference was defined as positive values of the coefficient, while social avoidance was associated with negative values (Varlinskaya et al., 1999).
Acute Ethanol Challenge
Ethanol was injected i.p. as a 12.6% (v/v) solution in saline (0.9%, w/v) at doses of 0, 0.5, 0.75, and 1.0 g/kg. Ethanol challenge dose was varied by altering the volume of the 12.6% ethanol solution to avoid concentration-induced differences in ethanol absorption rate (see Linakis & Cunningham, 1979). Control animals were injected with isotonic saline at a volume equal to that of the highest dose of ethanol administered (i.e., 1% of animal’s body weight). All solutions were injected at room temperature. Similar to our previous work (Varlinskaya et al., 2010; Varlinskaya & Spear, 2002, 2006, 2012), the i.p. route of ethanol administration was employed in this study, given that it produces little variability in blood ethanol levels and has been the most commonly used route of administration in neuropharmacological studies of acute ethanol effects.
Blood Ethanol Determination
For analysis of blood ethanol content, trunk blood samples were collected immediately after behavioral testing using heparinized tubes. Blood samples were then rapidly frozen and maintained at −80 °C. Samples were assessed for BECs via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μL aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 min and then extracted and injected a 1.0 mL sample of the gas headspace into the chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software, which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
Corticosterone Determination
Trunk blood samples were collected using heparinized tubes and were then centrifuged at 2 °C for 20 min at 3000 rpm. Plasma samples were frozen and kept in a −80 °C freezer until the time of assay. Plasma corticosterone (CORT) levels were analyzed by radioimmunoassay (RIA) using RIA kits obtained from INC Biomedicals, Inc. (Orangeburg, NY).
Data Analyses
Baseline levels of social investigation, social preference, and play fighting along with CORT levels and body weights were examined in non-injected animals using separate 3 (adolescent exposure: no exposure, water, ethanol) × 2 (sex) analyses of variance (ANOVAs). Where significant main effects of sex were evident, planned ANOVAs within each sex were conducted to explore consequences of adolescent exposure, with main effects of adolescent exposure further examined by Fisher planned comparisons. The effects of acute ethanol challenge on social investigation, social preference, play fighting, and CORT levels were analyzed separately using 3 (adolescent exposure) × 4 (ethanol challenge dose: 0, 0.5, 0.75, 1.0 g/kg) × 2 (sex) ANOVAs. Where significant main effects and interactions involving sex were evident, data were analyzed separately for males and females to further explore consequences of adolescent exposure on responsiveness to acute ethanol challenge. These analyses were followed by post hoc tests in order to determine the locus of significant main effects and interactions within each sex using Fisher planned LSD tests to avoid inflating the possibilities of Type II errors (see Carmer & Swanson, 1973). These planned tests included comparisons between animals challenged with the various doses of ethanol and saline-challenged animals, as well as among non-exposed, water- and ethanol-exposed animals at each challenge dose for each of the social behavioral measures. Significance was set at p < .05, and all data are expressed as mean ± standard error (M ± SEM).
Results
Experiment 1. Social consequences of early adolescent intermittent ethanol exposure (e-AIE)
In Experiment 1, animals were exposed to ethanol during early-mid adolescence (P25–P45) and tested as young adults on P70. Two hundred thirty-nine animals served as experimental subjects, with 8 animals placed in each of the 30 experimental groups, except for the water-exposed, non-injected female group, where 7 animals were included.
Baseline Social Behavior and Corticosterone Levels
There was a significant main effect of sex for social investigation, F(1,41) = 10.96, p < .01; social preference, F(1,41) = 4.32, p < .05; play fighting, F(1,41) = 4.83, p < .05; CORT levels, F(1,41) = 18.08, p < .0001; and body weights, F(1,41) = 309.28, p < .0001. In general, females showed lower levels of social investigation, social preference, and play fighting than males, whereas CORT levels were significantly higher in females relative to their male counterparts. Body weights of females were also significantly lower than those of males. Given the observed sex differences that coincided with sex-related differences in social interactions and CORT levels reported previously (Handa, Burgess, Kerr, & O’Keefe, 1994; Johnston & File, 1991; Stack et al., 2010), data were analyzed separately for males and females.
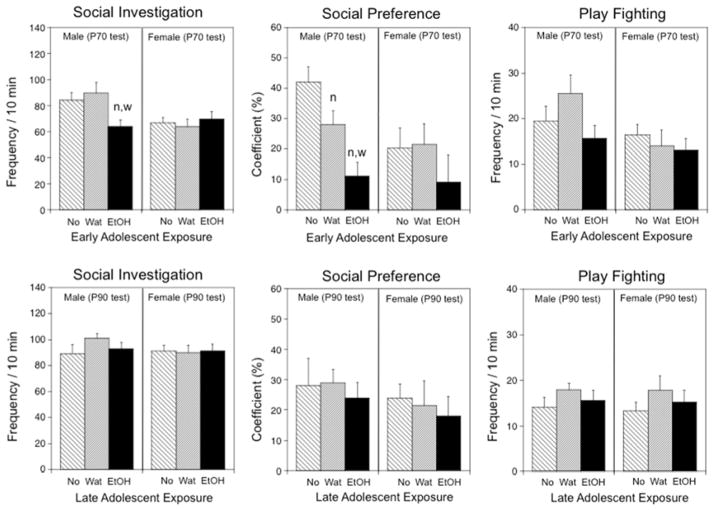
In males tested at P70, social investigation and social preference were affected by prior early adolescent exposure, F(2,21) = 4.25, p < .05 and F(2,21) = 10.41, p < .001, respectively (see Fig. 1, top panels). Both measures were decreased by e-AIE. Levels of social investigation were decreased by about 25% in ethanol-exposed males when compared to both control groups, whereas they demonstrated a 3–4-fold decrease in social preference relative to these controls. Social preference was also significantly lower in water-exposed males than in their non-exposed counterparts. Play fighting demonstrated by males, however, was not affected by e-AIE. In females, early adolescent exposure did not alter social behavior at the P70 test. CORT levels did not differ as a function of adolescent exposure in either males (overall mean = 236.3 ± 20.9 ng/mL) or females (overall mean = 461.2 ± 47.5 ng/mL). Early adolescent exposure also had no effect on body weights of males (overall mean = 405.8 ± 7.5 g) or females (overall mean = 265.6 ± 5.4 g) when assessed at testing on P70.
Figure 1.
The impact of early (top panels) and late (bottom panels) adolescent intermittent ethanol exposure on social investigation, social preference, and play fighting during a 10-min social interaction test in male and female rats when tested 25 days following e-AIE in Experiment 1 (on P70) or l-AIE in Experiment 2 (on P90). Significant differences relative to the non-exposed control group are indicated with “n” (p < .05), whereas significant differences (p < .05) relative to the water-exposed group are indicated with “w”.
Acute Ethanol Challenge
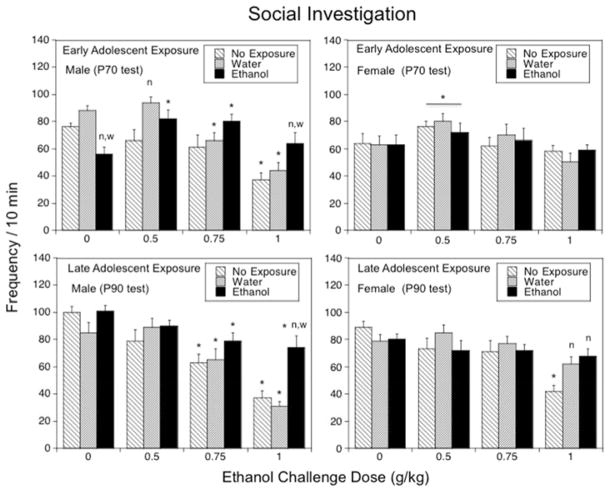
The overall ANOVA of social investigation revealed a significant sex × early adolescent exposure × ethanol challenge dose interaction, F(6,168) = 2.196, p < .05, suggesting sex-related differences in the consequences of early adolescent exposure on sensitivity to acute ethanol challenge. Indeed, in males, the analysis of social investigation revealed a significant interaction of early adolescent exposure and ethanol challenge dose, F(6,84) = 5.00, p < .001 (see Fig. 2, top left panel). When challenged with saline, e-AIE males demonstrated significantly less social investigation than non-exposed and water-exposed controls. The e-AIE-associated social deficit seen in saline-challenged animals was reversed by ethanol, with doses of 0.5 and 0.75 g/kg significantly facilitating social investigation (~47% and 42% increases, respectively) in ethanol-exposed males relative to their saline-challenged counterparts and restoring social investigation to levels comparable to those of non-exposed and water-exposed controls challenged with saline. These ethanol-induced increases in social investigation at P70 were seen exclusively in e-AIE males, whereas non-exposed and water-exposed controls showed an age-typical inhibition of social investigation following acute ethanol challenge. Social investigation was significantly decreased in water-exposed males following 0.75 and 1.0 g/kg ethanol, whereas non-exposed males demonstrated a significant decrease of social investigation only following the highest dose. At the highest ethanol challenge dose tested, ethanol-exposed males showed significantly more social investigation than the non-exposed and water-exposed control groups.
Figure 2.
The impact of early (Experiment 1, top panels) and late (Experiment 2, bottom panels) adolescent intermittent ethanol exposure on acute ethanol-induced alterations in social investigation demonstrated by adult male and female rats during a 10-min social interaction test conducted 25 days following the exposure period. Asterisks (*) indicate significant ethanol dose differences when compared with the corresponding saline control (p < .05); significant differences relative to the non-exposed control group are indicated with “n” (p < .05), whereas significant differences relative to the water-exposed control group are indicated with “w” (p < .05).
In females, social investigation differed only as a function of ethanol challenge dose, F(3,84) = 5.31, p < .01 (see Fig. 2, top right panel): the dose of 0.5 g/kg ethanol induced significant, although not pronounced (~21%) increases in social investigation, when collapsed across early adolescent exposure condition. No inhibitory effects of acute ethanol were evident across the tested dose range in female subjects.
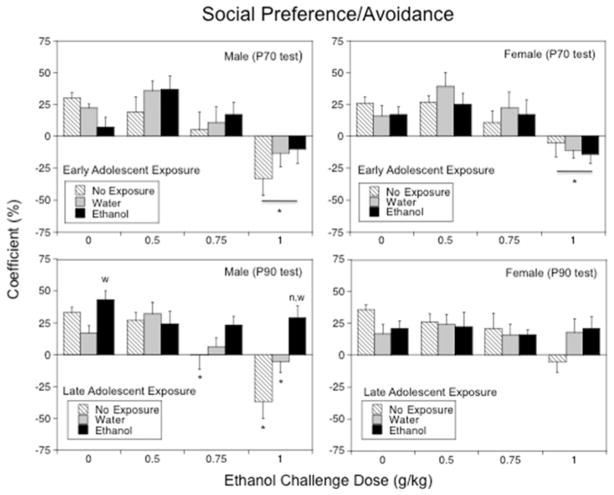
The coefficient of social preference/avoidance differed as a function of ethanol challenge dose only, F(1,112) = 17.25, p < .0001, with no significant main effects and interactions involving sex or adolescent exposure evident for this behavioral measure. The 1.0 g/kg challenge dose transformed social preference into social avoidance (Fig. 3, top panels) in experimental animals regardless of sex or adolescent exposure.
Figure 3.
The impact of early (Experiment 1, top panels) and late (Experiment 2, bottom panels) adolescent intermittent ethanol exposure on acute ethanol-induced alterations in social preference demonstrated by adult male and female rats during a 10-min social interaction test conducted 25 days following the exposure period. Asterisks (*) indicate significant ethanol dose differences when compared with the corresponding saline control (p < .05); significant differences relative to the non-exposed control group are indicated with “n” (p < .05), whereas significant differences relative to the water-exposed control group are indicated with “w” (p < .05).
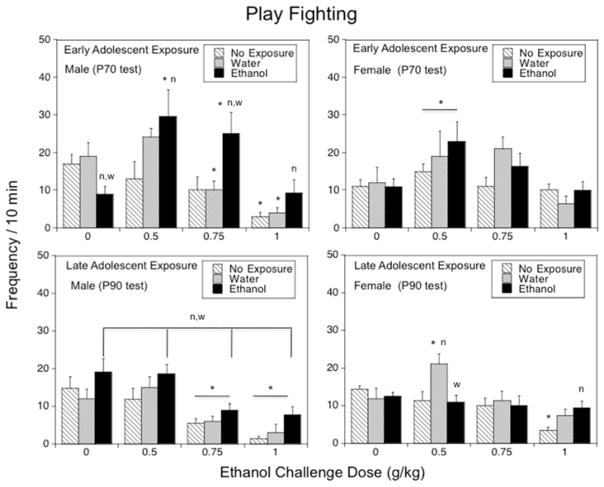
The overall ANOVA of play fighting revealed a significant 3-way interaction, F(6,168) = 2.17, p < .05, suggesting sex-related differences in the responsiveness to adolescent exposure. In males, changes in play fighting induced by acute ethanol challenge differed as a function of early adolescent exposure [early adolescent exposure × ethanol challenge dose, F(6,84) = 3.26, p < .01]. When challenged with saline, males exposed to ethanol during early adolescence showed significant decreases in play fighting relative to non-exposed and water-exposed males. Acute ethanol (0.5 and 0.75 g/kg) significantly enhanced play fighting in the ethanol-exposed group (see Fig. 4, top left panel), with ~200–250% increases in play fighting over saline-challenged animals after doses of 0.5 g/kg and 0.75 g/kg ethanol. In contrast, non-exposed and water-exposed controls showed a significant ethanol-induced inhibition of this adolescent-characteristic form of social interactions at doses of 0.75 g/kg (water-exposed males) and 1.0 g/kg (both control groups). Ethanol-exposed males demonstrated significantly more play fighting than non-exposed males at doses of 0.5 and 1.0 g/kg, with these differences between ethanol- and water-exposed males evident at 0.75 g/kg ethanol only. In females, the dose of 0.5 g/kg ethanol induced a 66% increase in play fighting, when collapsed across adolescent exposure condition [main effect of ethanol challenge dose, F(3,84) = 5.82, p < .01].
Figure 4.
The impact of early (Experiment 1, top panels) and late (Experiment 2, bottom panels) adolescent intermittent ethanol exposure on acute ethanol-induced alterations in play fighting demonstrated by adult male and female rats during a 10-min social interaction test conducted 25 days following the exposure period. Asterisks (*) indicate significant ethanol dose differences when compared with the corresponding saline control (p < .05); significant differences relative to the non-exposed control group are indicated with “n” (p < .05), whereas significant differences relative to the water-exposed control group are indicated with “w” (p < .05).
CORT levels differed as a function of sex, F(1,168) = 92.17, p < 0.0001, with females demonstrating substantially higher levels than males (see Table 1). Further analysis revealed that in males, CORT levels differed as a function of early adolescent exposure, F(2,84) = 4.25, p < .05, with the e-AIE males generally demonstrating significantly blunted CORT response (215.44 ± 22.10 ng/mL) relative to their non-exposed counterparts (293.03 ± 20.83 ng/mL). The ANOVA also revealed a significant main effect of ethanol challenge dose, F(3,84) = 12.41, p < .0001, with significant increases in CORT levels evident in males following challenge with the dose of 1.0 g/kg relative to saline-challenged animals (354.79 ± 14.53 ng/mL versus 214.92 ± 20.70 ng/mL). In females, when collapsed across ethanol challenge dose, CORT responses differed as a function of early adolescent exposure as well, F(2,84) = 4.81, p < .05. However, significant differences were evident between non-exposed and water-exposed controls, with non-exposed females demonstrating significantly higher CORT levels relative to water-exposed females (613.53 ± 52.30 ng/mL versus 426.91 ± 43.31 ng/mL). Ethanol induced significant increases in CORT levels [main effect of ethanol challenge dose, F(3,84) = 6.63, p < .001] relative to saline-challenged animals (396.71 ± 48.69 ng/mL) in P70 females at doses of 0.75 (599.46 ± 50.66 ng/mL) and 1.0 g/kg (645.83 ± 44.62 ng/mL), when data were collapsed across early adolescent exposure condition.
Table 1.
Corticosterone levels (ng/mL) following acute ethanol challenge in Experiment 1.
| Sex | Ethanol Dose (g/kg) | No Exposure | Water Exposure | Ethanol Exposure |
|---|---|---|---|---|
| Male | 0 | 281.9 ± 23.0 | 179.3 ± 36.7 | 181.8 ± 37.1 n |
| 0.5 | 242.8 ± 64.0 | 151.0 ± 39.4 | 142.9 ± 36.0 n | |
| 0.75 | 280.0 ± 32.5 | 326.5 ± 34.5 | 200.3 ± 50.4 n | |
| 1.0 | 376.9 ± 28.0 * | 359.6 ± 29.1 * | 336.9 ± 19.8 * n | |
| Female | 0 | 418.9 ± 101.5 | 349.5 ± 60.1 n | 421.8 ± 94.7 |
| 0.5 | 573.8 ± 104.2 | 321.8 ± 108.1 n | 342.4 ± 69.7 | |
| 0.75 | 720.6 ± 91.8 * | 461.4 ± 87.4 * n | 616.4 ± 66.1 * | |
| 1.0 | 740.9 ± 96.6 * | 575.0 ± 67.4 * n | 621.6 ± 59.7 * |
Asterisks (*) indicate significant ethanol dose differences relative to the corresponding saline control (p < .05), with data collapsed across adolescent exposure condition. Significant differences relative to the non-exposed control group are indicated by “n” (p < .05), with data collapsed across ethanol dose.
BECs differed as a function of sex, F(1,126) = 7.59, p < .01, with females demonstrating slightly but significantly lower BECs than males (see Table 2). BECs increased in a dose-dependent fashion in males and females [main effects of ethanol dose, F(2,63) = 167.68, p < 0.0001 and F(2,63) = 139.92, p < 0.0001, respectively], but did not differ as a function of early adolescent exposure.
Table 2.
Blood ethanol concentrations (mg/dL) following acute ethanol challenge in Experiment 1.
| Sex | Ethanol Dose (g/kg) | No Exposure | Water Exposure | Ethanol Exposure |
|---|---|---|---|---|
| Male | 0.5 | 20.9 ± 3.1 | 16.9 ± 4.8 | 19.1 ± 3.3 |
| 0.75 | 52.3 ± 3.2 | 53.9 ± 3.1 | 50.7 ± 6.9 | |
| 1.0 | 79.4 ± 6.3 | 86.0 ± 2.9 | 90.7 ± 3.7 | |
| Female | 0.5 | 17.0 ± 3.4 | 14.0 ± 2.7 | 13.8 ± 3.4 |
| 0.75 | 43.4 ± 5.4 | 41.6 ± 5.0 | 47.5 ± 8.5 | |
| 1.0 | 70.8 ± 5.4 | 84.9 ± 1.8 | 81.4 ± 2.9 |
Experiment 2. Social consequences of late adolescent intermittent ethanol exposure (l-AIE)
In Experiment 2, animals were exposed to ethanol during late adolescence/early adulthood (P45–P65) and tested as adults on P90, with 240 experimental subjects tested (n = 8 per group).
Baseline Social Behavior and Corticosterone Levels
No effects of sex or l-AIE on social investigation, social preference, or play fighting were seen in animals tested at P90 (see Fig. 1, bottom panels). CORT levels differed as a function of sex, F(1,42) = 102.9, p < .0001, with females demonstrating CORT levels at least 2 times higher than their male counterparts. CORT responses did not differ as a function of late adolescent exposure in either males (overall mean = 329.2 ± 18.4 ng/mL) or females (overall mean = 762.0 ± 40.4 ng/mL). Body weights also differed as a function of sex, F(1,42) = 526.29, p < .0001, although there was no effect of late adolescent exposure on body weights of males (overall mean = 493.9 ± 8.4 g) or females (overall mean = 300.5 ± 4.8 g) at testing on P90.
Acute Ethanol Challenge
The overall ANOVA of social investigation revealed significant sex × late adolescent exposure [F(2,167) = 3.19, p < .05] and sex × acute ethanol challenge dose [F(3,167) = 4.23, p < .01] interactions, suggesting sex differences in the effects of late adolescent exposure. Further analyses performed separately in males and females demonstrated that the effects of acute ethanol challenge on social investigation differed among males with different histories of late adolescent exposure [late adolescent exposure × ethanol challenge dose interaction, F(6,84) = 2.73, p < .005]. Whereas males in all exposure conditions demonstrated significant decreases in social investigation at 0.75 and 1.0 g/kg ethanol relative to their saline-challenged counterparts, the inhibitory effect of the highest dose was significantly less evident in males exposed to ethanol during late adolescence relative to non-exposed and water-exposed control conditions (Fig. 2, bottom left panel). In females, late adolescent exposure interacted with ethanol challenge dose as well, F(6,83) = 2.49, p < .05, with water- and ethanol-exposed females being insensitive to the socially suppressing effects of ethanol evident in non-exposed females following the dose of 1.0 g/kg (Fig. 2, bottom right panel). Social investigation of non-exposed females at 1.0 g/kg ethanol was also significantly lower than in their water-exposed and ethanol-exposed counterparts.
The overall ANOVA of the preference/avoidance coefficient revealed significant interactions of sex × late adolescent exposure [F(2,167) = 3.97, p < .05] and sex × acute ethanol challenge dose [F(3,167) = 2.71, p < .05]. Given these sex differences, data were analyzed separately for males and females. In males, the ANOVA of the preference/avoidance coefficient revealed a significant late adolescent exposure × ethanol dose interaction, F(3,84) = 3.76, p < .001 (Fig. 3, bottom left panel). When challenged with saline, l-AIE males demonstrated almost 2.5 times greater social preference than water-exposed controls. Significant ethanol-induced decreases in social preference and transformation into social avoidance were evident in non-exposed males at the doses of 0.75 and 1.0 g/kg and in water-exposed males following the highest ethanol dose. Ethanol-exposed males, however, were not affected by acute ethanol challenge and demonstrated significantly higher values of the coefficient than non-exposed and water-exposed control groups following the dose of 1.0 g/kg. In females, the coefficient was not significantly affected either by late adolescent exposure or ethanol challenge dose (Fig. 3, bottom right panel).
Similarly, significant sex × late adolescent exposure [F(2,167) = 3.76, p < .05] and sex × acute ethanol challenge dose [F(3,167) = 2.76, p < .05] interactions were evident for play fighting, with further analyses performed separately for each sex. In males, play fighting differed as a function of late adolescent exposure [F(2,84) = 6.71, p < .01] and ethanol challenge dose [F(3,84) = 16.11, p < .0001]. Play fighting was significantly suppressed by the doses of 0.75 and 1.0 g/kg relative to saline-challenged males regardless of adolescent exposure condition (see Fig. 4, bottom left panel), whereas, when collapsed across challenge dose, l-AIE males showed significantly more play fighting than their non-exposed and water-exposed counterparts (play fighting frequency: 13.87 ± 1.55, 8.38 ± 1.41, and 8.59 ± 1.38, respectively). In females, the ANOVA of play fighting revealed a significant late adolescent exposure × ethanol challenge dose interaction, F(6,83) = 2.78, p < .05 (Fig. 4, bottom right panel). Water-exposed females exhibited an ~80% increase in play fighting at 0.5 g/kg ethanol relative to their saline-challenged counterparts, differing significantly from non-exposed and ethanol-exposed females challenged with the same ethanol dose. Non-exposed females demonstrated significant social inhibition at the highest dose relative to their saline-challenged counterparts and ethanol-exposed females challenged with 1 g/kg ethanol.
The overall ANOVA of CORT levels revealed significant main effects of sex [F(1,167) = 492.04, p < .0001] and acute challenge dose [F(3,167) = 4.71, p < .01], along with a sex × late adolescent exposure interaction, F(2,167) = 4.38, p < .05. There was no effect of late adolescent exposure on CORT levels in males (Table 3), although there was an effect of ethanol challenge dose, F(3,84) = 7.26, p < .0001, with CORT levels significantly elevated following the dose of 1.0 g/kg relative to saline (412.29 ± 17.79 ng/mL and 353.38 ± 18.12 ng/mL, respectively). In contrast, the CORT response differed as a function of late adolescent exposure in females, F(2,83) = 5.63, p < .01, with no effects of ethanol challenge dose seen for this measure. CORT levels were significantly blunted in water-exposed and ethanol-exposed females relative to their non-exposed counterparts (723.58 ± 25.96 ng/mL, 756.19 ± 30.97 ng/mL, and 855.09 ± 28.89 ng/mL, respectively, with data collapsed across ethanol challenge dose).
Table 3.
Corticosterone levels (ng/mL) following acute ethanol challenge in Experiment 2.
| Sex | Ethanol Dose (g/kg) | No Exposure | Water Exposure | Ethanol Exposure |
|---|---|---|---|---|
| Male | 0 | 362.0 ± 28.2 | 317.0 ± 29.2 | 388.1 ± 35.8 |
| 0.5 | 261.3 ± 25.4 | 381.6 ± 40.7 | 377.8 ± 32.8 | |
| 0.75 | 427.9 ± 32.5 | 385.8 ± 23.6 | 369.6 ± 27.7 | |
| 1.0 | 404.0 ± 27.2 * | 377.6 ± 25.8 * | 455.3 ± 21.5 * | |
| Female | 0 | 912.0 ± 48.9 | 755.0 ± 36.6 n | 694.4 ± 67.1 n |
| 0.5 | 769.3 ± 90.3 | 622.8 ± 43.1 n | 790.2 ± 57.9 n | |
| 0.75 | 849.0 ± 39.7 | 713.1 ± 59.6 n | 762.1 ± 84.9 n | |
| 1.0 | 890.1 ± 34.1 | 814.8 ± 48.9 n | 778.0 ± 33.9 n |
Asterisks (*) indicate significant ethanol dose differences relative to the corresponding saline control (p < .05), with data collapsed across adolescent exposure condition. Significant differences relative to the non-exposed control group are indicated by “n” (p < .05), with data collapsed across ethanol dose.
The overall ANOVA of BECs revealed a significant sex × ethanol challenge dose interaction, F(2,126) = 3.57, p < .05, with females demonstrating lower BECs than males at the dose of 0.75 g/kg. Further analyses revealed that BECs increased in a dose-dependent fashion in males and females [main effects of ethanol dose, F(2,63) = 204.9, p < 0.0001 and F(2,63) = 131.8, p < 0.0001, respectively], but did not differ as a function of late adolescent exposure (see Table 4).
Table 4.
Blood ethanol concentrations (mg/dL) following acute ethanol challenge in Experiment 2.
| Sex | Ethanol Dose (g/kg) | No Exposure | Water Exposure | Ethanol Exposure |
|---|---|---|---|---|
| Male | 0.5 | 27.9 ± 2.4 | 21.5 ± 1.9 | 22.8 ± 2.7 |
| 0.75 | 65.6 ± 3.0 | 62.5 ± 6.0 | 58.1 ± 2.8 | |
| 1.0 | 92.5 ± 2.5 | 82.5 ± 6.6 | 88.9 ± 3.8 | |
| Female | 0.5 | 25.5 ± 3.3 | 20.2 ± 2.3 | 20.6 ± 3.4 |
| 0.75 | 56.1 ± 3.6 | 50.1 ± 7.2 | 46.1 ± 3.4 | |
| 1.0 | 90.0 ± 3.8 | 94.5 ± 8.6 | 80.1 ± 6.4 |
Discussion
The social consequences of early AIE (Experiment 1) differed in males and females. Social anxiety-like behavioral alterations were evident in adult male rats following e-AIE, indexed via significant decreases in social investigation and social preference relative to their water-exposed and non-exposed counterparts. In contrast, females were insensitive to e-AIE and showed no significant changes in social behavior and social motivation. Males and females exposed to ethanol during late adolescence (l-AIE) and tested as adults under baseline, no injection condition showed no social anxiety-like behavioral alterations (Experiment 2), suggesting that early, but not late adolescence is the critical period for induction of long-lasting social consequences by repeated ethanol. In contrast, both e-AIE and l-AIE produced alterations in sensitivity to the social consequences of acute ethanol challenge in males tested 25 days after adolescent exposure. However, these alterations in sensitivity to acute ethanol differed as a function of AIE timing as well as the social measure under investigation. In contrast, AIE females showed very little changes, if any, in sensitivity to the social consequences of acute ethanol challenge.
Binge patterns of drinking are common for human adolescents (Windle et al., 2008), and this high-risk drinking is associated with alcohol-related and mental health problems later in life (Chou & Pickering, 1992; Courtney & Polich, 2009; Hill, White, Chung, Hawkins, & Catalano, 2000). Early onset of heavy binge drinking is viewed as a highly reliable predictor of lifetime prevalence of alcohol use disorders. Indeed, young individuals who begin drinking at 14 years of age and even earlier are 4 times more likely to become alcohol-dependent relative to those who started drinking at 20 years of age and later (Dawson, Goldstein, Chou, Ruan, & Grant, 2008; DeWit, Adlaf, Offord, & Ogborne, 2000; Ehlers, Slutske, Gilder, Lau, & Wilhelmsen, 2006). The causality between adolescent binge drinking and negative mental health outcomes, including anxiety disorders, is still not well understood. The results of the present study provide some information suggesting that in the rat model of adolescence, repeated ethanol exposure that mimics the binge pattern of adolescent drinking may have detrimental consequences in terms of the emergence of anxiety-like behavior evident later in life under social circumstances.
In Experiment 1, only male rats were affected by e-AIE, whereas ethanol-exposed females demonstrated no changes in social behavior and social preference when tested as adults. To a large extent, the studies that have assessed long-lasting anxiogenic effects of AIE have included only male subjects. However, the inclusion of female subjects in such studies is important, given human data regarding gender differences in prevalence of alcohol use disorders and in negative consequences of excessive alcohol use (Nolen-Hoeksema, 2004; SAMSHA, 2008; Schulte, Ramo, & Brown, 2009). In adulthood, women consume less alcohol and have fewer alcohol-related problems than men (SAMSHA, 2008), with 18.6% of men and 8.4% of women demonstrating a lifetime prevalence for alcohol dependence (Grant, 1997). However, the rate of alcohol use disorders is not different between boys and girls aged 12 to 17 (Schulte et al., 2009). Taken together, these findings suggest that adolescent males are at higher risk to become alcohol-dependent later in life than adolescent females. To the extent that our experimental findings are applicable to humans, the results of Experiment 1 confirm that adolescent males are more vulnerable to the harmful effects of ethanol exposure than their female counterparts, in that social anxiety-like behavioral alterations were evident only in males following e-AIE. Although demonstrating significant decreases of social investigation and social preference under basal, no injection conditions, the e-AIE males did not differ from their water-exposed and non-exposed counterparts in terms of CORT levels, suggesting that under certain experimental conditions CORT levels do not necessarily reflect anxiety-like behavioral alterations when tested under basal, no injection conditions.
One of the possible explanations of these differences in the consequences of e-AIE in males versus females is that females do not demonstrate social anxiety-related behavioral alterations in a way their male counterparts do. It is unlikely, however, given that adult females, similarly to their male counterparts, respond to repeated restraint by decreases in social investigation and social preference (Doremus-Fitzwater et al., 2009; Varlinskaya et al., 2010). An alternative possibility is that adolescent females are less sensitive to ethanol-associated alterations within the brain systems implicated in modulation of anxiety-like behavior. Indeed, prior work examining the effects of intraperitoneal exposure to ethanol in adolescence on gene expression of two critical regulators of stress and anxiety in the paraventricular nucleus likewise reported increased corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) gene expression in males, but not females (Przybycien-Szymanska, Rao, & Pak, 2010). Neuroactive steroids may play a role in the greater protection of the female than the male brain from the detrimental effects of ethanol during adolescence. Progesterone-derived neurosteroids are present at higher levels in females than males (Corpéchot et al., 1993; Torres, Ruiz, & Ortega, 2001) and have been shown to have anxiolytic effects in a number of behavioral paradigms (Bitran, Hilvers, & Kellogg, 1991; Brot, Koob, & Britton, 1995; Carboni, Wieland, Lan, & Gee, 1996; Eser, Baghai, Schüle, Nothdurfter, & Rupprecht, 2008). Therefore, higher levels of endogenous neurosteroid anxiolytics in adolescent females, relative to their male counterparts, may play a substantial role in protecting these females from ethanol-induced social anxiety-like behavioral alterations during AIE.
The observed differences in the social consequences of early versus late adolescent intermittent ethanol exposure suggest that neural systems affected by repeated ethanol may vary with age even within the adolescent developmental period. The decrease in social investigation and social preference among adult males with a history of e-AIE may be related, at least in part, to ethanol-associated disruption of neural substrates implicated in social behavior and anxiety. Although studies examining neural regions critical for peer-directed social interactions in rodents are limited, recent research has revealed that frontal cortical regions, the amygdala, and the hippocampus play important roles in peer-directed social interactions in juvenile and adolescent animals (Daenen, Wolterink, Gerrits, & Van Ree, 2002; Flores, Silva-Gómez, Ibáñez, Quirion, & Srivastava, 2005; Shah & Treit, 2003; van Kerkhof, Damsteegt, Trezza, Voorn, & Vanderschuren, 2013) as well as in modulation of anxiety-like behavior (Canteras, Resstel, Bertoglio, Carobrez Ade, & Guimarães, 2010). These brain regions undergo substantial remodeling during adolescence (Crews, He, & Hodge, 2007; Doremus-Fitzwater et al., 2009; Ernst & Fudge, 2009; Spear, 2000, 2004; Sturman & Moghaddam, 2011) and hence, it would not be surprising if some of these regions are among those vulnerable to disruption by repeated ethanol exposure at this time.
Other regions and systems may be targeted as well. For instance, early adolescents demonstrate more pronounced ethanol-induced damage in anterior cortical regions than their older counterparts following 4 days of exposure to high ethanol daily doses of 9–10 g/kg (Crews, Braun, Hoplight, Switzer, & Knapp, 2000), and repeated (Ehlers, Liu, Wills, & Crews, 2013) as well as acute adolescent exposure to ethanol has been reported to effectively inhibit neurogenesis in these immature animals (Crews, Mdzinarishvili, Kim, He, & Nixon, 2006). Alterations are also evident in the glutamatergic (Guerri & Pascual, 2010; Pascual, Boix, Felipo, & Guerri, 2009) and gamma-aminobutyric acid systems (Falco, Bergstrom, Bachus, & Smith, 2009; Fleming, Acheson, Moore, Wilson, & Swartzwelder, 2012) – neural systems that play a substantial role in modulation of social behavior (Morales, Varlinskaya, & Spear, 2013a,b; Silvestre, Nadal, Pallarés, & Ferré, 1997; Siviy, Line, & Darcy, 1995) and anxiety-like behavioral alterations (Atack, 2005; Möhler, 2012; Trincavelli, Da Pozzo, Daniele, & Martini, 2012).
In Experiment 1, e-AIE resulted in substantial increases in anxiety-like behavior evident in adult males under social circumstances. Similarly, increased anxiety-like behavior in the light-dark box has been reported in adult males intermittently exposed to ethanol vapor during adolescence (Slawecki, Thorsell, & Ehlers, 2004). In contrast, some studies have reported decreases in anxiety-like behavior using assessments involving the cross-maze, elevated plus maze, and light-dark box following adolescent ethanol exposure via drinking (e.g., Gilpin, Karanikas, & Richardson, 2012; Hughes, 2011; Salimov, McBride, McKinzie, Lumeng, & Li, 1996). These discrepancies may be associated, to some extent, with differences in the mode of ethanol exposure and methods used for assessment of anxiety-like alterations. For instance, elevations of anxiety-like behavior were seen following either experimenter-administered ethanol (present study) or exposure to ethanol vapor (Slawecki et al., 2004), whereas decreased anxiety was reported following ethanol drinking (Gilpin et al., 2012; Hughes, 2011; Salimov et al., 1996). It is also possible that observed anxiogenic alterations may be relatively specific for social behavior and hence may better reflect social rather than generalized anxiety, although Slawecki et al. (2004) have reported increased anxiety-like behavior following AIE in the light/dark box. Clearly, more research is needed for better understanding of the exposure mode and test circumstances leading to long-lasting anxiogenic effects of AIE.
The consequences of e-AIE in males were not limited to alterations in social behavior, with these males demonstrating substantial changes in responsiveness to acute ethanol challenge as well. In contrast to the significantly lower levels of social investigation and play fighting seen in e-AIE males relative to non-exposed and water-exposed controls when challenged with saline, after ethanol challenge, e-AIE males displayed notable increases in social investigation and play fighting, whereas non-exposed and water-exposed controls showed only an age-typical inhibition of social behavior following acute ethanol challenge. Ethanol-induced social facilitation observed in adult males after e-AIE is reminiscent of that seen normally during adolescence (Varlinskaya & Spear, 2002, 2006), and hence is consistent with the prior suggestion that repeated exposure to ethanol during early adolescence may “lock-in” adolescent-like ethanol sensitivity in adulthood (Fleming et al., 2012). Surprisingly, the adolescent-typical sensitivity evident in the e-AIE males was limited to social investigation and play fighting, whereas social preference was transformed into social avoidance at 1.0 g/kg regardless of early adolescent exposure. In contrast, substantial ethanol-induced increases in social preference were evident in animals tested 48 h after adolescent exposure to 1.0 g/kg ethanol given i.p. for 7 days (Varlinskaya & Spear, 2007). Thus, social consequences of adolescent ethanol exposure evident in adulthood appear to differ from those observed in adolescents shortly after ethanol withdrawal.
Given the differences seen in the stimulatory effects of ethanol on social behavior and social preference after e-AIE, it is likely that these behaviors may reflect different underlying mechanisms: ethanol-associated increases in social investigation and play fighting evident after low doses in adolescent animals regardless of prior stress history may reflect ethanol social facilitation (Varlinskaya & Spear, 2002, 2006), whereas increases in social preference induced by low to moderate doses of ethanol in previously stressed (Varlinskaya et al., 2010) or acutely ethanol withdrawn adolescents (Varlinskaya & Spear, 2007), but not in their non-manipulated counterparts, may reflect socially anxiolytic effects of ethanol. Taken together with the previous findings, the data presented suggest that in adult males, prior e-AIE resulted in the enhancement of sensitivity to the socially facilitating, rather than socially anxiolytic effects of ethanol. Indeed, socially facilitating effects of ethanol have been found to be related to ethanol-induced activation of the endogenous mu opioid receptor (MOR) system (Trezza, Baarendse, & Vanderschuren, 2009; Varlinskaya & Spear, 2009) as well as N-methyl-D-aspartate (NMDA) receptor antagonism (Morales et al., 2013b), whereas ethanol anxiolysis is generally thought to reflect interactions with the GABAA receptor system (Eckardt et al., 1998; Morris, Dawson, Reynolds, Atack, & Stephens, 2006).
In Experiment 2, l-AIE males were found to be notably insensitive to the socially suppressing effects of acute ethanol challenge that were evident after doses of 0.75 and 1.0 g/kg ethanol in both control groups. This insensitivity to ethanol-induced social inhibition suggests that the l-AIE males developed chronic tolerance to the social consequences of ethanol, with this tolerance still evident 25 days after repeated exposure to ethanol. This tolerance appears functional rather than metabolic in nature, given that post-test BECs were comparable among the three adolescent exposure conditions. These findings are reminiscent of those reported by Sherill, Berthold, Koss, Juraska, & Gulley (2011), who found adolescent ethanol exposure to attenuate later sensitivity to aversive effects of ethanol in males but not females tested approximately 9 weeks following adolescent exposure. To the extent that adolescent ethanol exposure in males, particularly late in adolescence, induces insensitivity to the adverse effects of ethanol, this exposure could permit the ingestion of relatively large amounts of ethanol with limited negative consequences.
In contrast to their male counterparts, females showed little evidence for alterations in sensitivity to acute ethanol challenge following either early or late AIE. These sex differences may be related to lower sensitivity of adult females to ethanol-induced social inhibition relative to adult males. Indeed, in non-exposed and water-exposed control males, social investigation and play fighting were significantly suppressed by 1.0 g/kg ethanol when tested at P70, with this social inhibition in P90 control males evident at the doses of 0.75 and 1.0 g/kg. In contrast, females tested at P70 were insensitive to ethanol-induced inhibition of social investigation and play, whereas only non-exposed P90 females showed social inhibition at 1.0 g/kg. These observations are consistent with our previously reported results (Varlinskaya et al., 2010; Varlinskaya & Spear, 2012) as well as findings of other studies showing greater sensitivity in males than females to ethanol-induced sedation (Webb, Burnett, & Walker, 2002) and conditioned taste aversions (Sherill et al., 2011). Surprisingly, P70 females demonstrated ethanol-induced facilitation of social investigation and play fighting following the dose of 0.5 g/kg, but only when collapsed across adolescent exposure condition. This finding was unexpected, since in our prior work non-manipulated females tested at P70 did not exhibit social facilitation at any ethanol dose (Varlinskaya & Spear, 2002). It is possible that this earlier study was not powered sufficiently to reveal subtle sex differences in the socially facilitating effects of ethanol during early adulthood. An alternative explanation is that the stimulatory effects of ethanol on play fighting seen in P70 females were driven by animals exposed to water or ethanol during early adolescence (see Fig. 4), suggesting some mild effects of early adolescent experimental manipulations on sensitivity to the social consequences of ethanol later in life. In several instances, effects of the experimental manipulations were evident in females following late adolescent exposure. For instance, no inhibitory effects of acute ethanol on social investigation and play fighting were evident in either l-AIE females or their water-exposed counterparts, whereas significant ethanol-associated decreases were seen in these forms of social interactions following 1.0 g/kg in non-exposed females. Such enhanced sensitivity to effects of repeated experimental manipulations per se could also contribute to the relative ineffectiveness of l-AIE for altering responsiveness to acute ethanol challenge in females. In contrast to their female counterparts, non-exposed and water-exposed adult males demonstrated similar patterns of sensitivity to the social consequences of acute ethanol challenge, with very few differences evident between the two control conditions. This finding provides some evidence for the adolescent gavage procedure itself not having long-lasting consequences on social behavior and ethanol sensitivity in males tested during adulthood.
It has been shown recently that adolescent ethanol exposure through ethanol inhalation blunts the HPA axis response to an acute ethanol challenge for at least several weeks (Allen, Lee, Koob, & Rivier, 2011; Logrip et al., 2013). In the present experiments, however, no significant interactions between early or late adolescent exposure and ethanol challenge dose on the CORT response to ethanol challenge were observed in either males or females, suggesting that sensitivity of the HPA axis to acute ethanol challenge was not altered by e-AIE and l-AIE. However, adult males tested at P70 showed decreased CORT response following e-AIE relative to their non-exposed counterparts when data were collapsed across ethanol challenge dose; this effect was specific to e-AIE and was not evident in males tested at P90 following late adolescent exposure. These findings suggest some habituation of the CORT response in males as a result of early adolescent exposure to ethanol. In contrast, females tested at P70 demonstrated significantly blunted CORT response following early adolescent exposure to water relative to non-exposed females, whereas this blunted CORT response was seen in P90 females exposed to either water or ethanol as late adolescents. Taken together with the behavioral findings, these results suggest that adolescent females, but not their male counterparts, could be more vulnerable to experimental manipulations per se, rather than repeated ethanol, with these manipulations producing long-lasting alterations in sensitivity to acute ethanol challenge and the CORT response. Obviously, more studies are needed in order to assess the level of stress associated with the intragastric route of administration during adolescence in females.
In summary, the results of the present study demonstrate a particular vulnerability of young adolescent males to long-lasting detrimental effects of repeated ethanol. Such adolescent ethanol exposure results in the emergence of social anxiety and preservation of adolescent-like ethanol sensitivity when tested later in adulthood. Retention of adolescent-typical sensitivity to socially facilitating properties of ethanol could make ethanol especially appealing to these males, therefore, promoting relatively high levels of ethanol intake later in life. Late adolescent males may be at high risk for the development of alcohol-related disorders later in life as well, given their enhanced ability to develop long-lasting chronic tolerance to the socially inhibiting effects of ethanol – tolerance that could permit ingestion of relatively large amounts of ethanol with limited negative consequences. In contrast to their male counterparts, females showed no signs of social anxiety and little evidence for alterations in sensitivity to acute ethanol challenge following either early or late AIE. Given the lower sensitivity to the social consequences of ethanol in females relative to males, higher ethanol exposure as well as challenge doses may be needed for females to demonstrate behavioral alterations and changes in ethanol sensitivity similar to those found for males in the present study.
Acknowledgments
The research presented in this paper was supported by NIH grants R01 AA017355, R01 AA018026, and U01 AA019972 to Linda P. Spear.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams J, Barone S, Jr, LaMantia A, Philen R, Rice DC, Spear L, et al. Workshop to identify critical windows of exposure for children’s health: neurobehavioral work group summary. Environmental Health Perspectives. 2000;108(Suppl 3):535–544. doi: 10.1289/ehp.00108s3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain, Behavior, and Immunity. 2011;25(Suppl 1):S50–60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opinion on Investigational Drugs. 2005;14:601–618. doi: 10.1517/13543784.14.5.601. [DOI] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Review. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Research. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Development of the social brain in adolescence. Journal of the Royal Society of Medicine. 2012;105:111–116. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction. 2004;99:1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Brot MD, Koob GF, Britton KT. Anxiolytic effects of steroid hormones during the estrous cycle. Interactions with ethanol. Recent Developments in Alcoholism. 1995;12:243–259. doi: 10.1007/0-306-47138-8_16. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Eggleston AM, Schmidt NB. Social anxiety and problematic alcohol consumption: the mediating role of drinking motives and situations. Behavior Therapy. 2006;37:381–391. doi: 10.1016/j.beth.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. Journal of Psychiatric Research. 2008;42:230–239. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Resstel LB, Bertoglio LJ, de Carobrez AP, Guimarães FS. Neuroanatomy of anxiety. Current Topics in Behavioral Neurosciences. 2010;2:77–96. doi: 10.1007/7854_2009_7. [DOI] [PubMed] [Google Scholar]
- Carboni E, Wieland S, Lan NC, Gee KW. Anxiolytic properties of endogenously occurring pregnanediols in two rodent models of anxiety. Psychopharmacology. 1996;126:173–178. doi: 10.1007/BF02246353. [DOI] [PubMed] [Google Scholar]
- Carmer SG, Swanson MR. Evaluation of ten pairwise multiple comparison procedures by Monte Carlo methods. Journal of the American Statistical Association. 1973;68:66–74. [Google Scholar]
- Carrigan MH, Randall CL. Self-medication in social phobia: a review of the alcohol literature. Addictive Behaviors. 2003;28:269–284. doi: 10.1016/s0306-4603(01)00235-0. [DOI] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. British Journal of Addiction. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Corpéchot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, et al. Neurosteroids: 3 alpha-hydroxy-5 alpha-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clinical and Experimental Research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, Biochemistry, and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behavioural Brain Research. 2002;136:571–582. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. The American Journal of Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & Behavior. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcoholism: Clinical and Experimental Research. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013;244:1–15. doi: 10.1016/j.neuroscience.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcoholism: Clinical and Experimental Research. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser D, Baghai TC, Schüle C, Nothdurfter C, Rupprecht R. Neuroactive steroids as endogenous modulators of anxiety. Current Pharmaceutical Design. 2008;14:3525–3533. doi: 10.2174/138161208786848838. [DOI] [PubMed] [Google Scholar]
- Faden VB. Trends in initiation of alcohol use in the United States 1975 to 2003. Alcoholism: Clinical and Experimental Research. 2006;30:1011–1022. doi: 10.1111/j.1530-0277.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Falco AM, Bergstrom HC, Bachus SE, Smith RF. Persisting changes in basolateral amygdala mRNAs after chronic ethanol consumption. Physiology & Behavior. 2009;96:169–173. doi: 10.1016/j.physbeh.2008.09.019. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. Journal of Neuroscience Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? British Journal of Pharmacology. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. European Journal of Pharmacology. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcoholism: Clinical and Experimental Research. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Silva-Gómez AB, Ibáñez O, Quirion R, Srivastava LK. Comparative behavioral changes in postpubertal rats after neonatal excitotoxic lesions of the ventral hippocampus and the prefrontal cortex. Synapse. 2005;56:147–153. doi: 10.1002/syn.20140. [DOI] [PubMed] [Google Scholar]
- Gilles DM, Turk CL, Fresco DM. Social anxiety, alcohol expectancies, and self-efficacy as predictors of heavy drinking in college students. Addictive Behaviors. 2006;31:388–398. doi: 10.1016/j.addbeh.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PloS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of Studies on Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. Journal of Substance Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44:15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones and Behavior. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hill KG, White HR, Chung IJ, Hawkins JD, Catalano RF. Early adult outcomes of adolescent binge drinking: person- and variable-centered analyses of binge drinking trajectories. Alcoholism: Clinical and Experimental Research. 2000;24:892–901. [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Archives of Pediatrics & Adolescent Medicine. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology and Teratology. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hughes RN. Adult anxiety-related behavior of rats following consumption during late adolescence of alcohol alone and in combination with caffeine. Alcohol. 2011;45:365–372. doi: 10.1016/j.alcohol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiology & Behavior. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. 2012 Overview; Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. Monitoring the Future: National survey results on drug use, 1975–2012. [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology. 1979;64:61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Rivier C, Lau C, Im S, Vaughan J, Lee S. Adolescent alcohol exposure alters the rat adult hypothalamic-pituitary-adrenal axis responsiveness in a sex-specific manner. Neuroscience. 2013;235:174–186. doi: 10.1016/j.neuroscience.2012.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Lynch KG, Pollock NK, Clark DB. Gender differences and similarities in the personality correlates of adolescent alcohol problems. Psychology of Addictive Behaviors. 2000;14:121–133. doi: 10.1037//0893-164x.14.2.121. [DOI] [PubMed] [Google Scholar]
- Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Anxiolytic effects of the GABA(A) receptor partial agonist, L-838,417: impact of age, test context familiarity, and stress. Pharmacology, Biochemistry, and Behavior. 2013a;109:31–37. doi: 10.1016/j.pbb.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Low doses of the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, induces social facilitation in adolescent male rats. Behavioural Brain Research. 2013b;250:18–22. doi: 10.1016/j.bbr.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EP, Stewart SH, Ham LS. The relationship between social anxiety disorder and alcohol use disorders: a critical review. Clinical Psychology Review. 2005;25:734–760. doi: 10.1016/j.cpr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN. Both alpha2 and alpha3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. The European Journal of Neuroscience. 2006;23:2495–2504. doi: 10.1111/j.1460-9568.2006.04775.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clinical Psychology Review. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Developmental Psychobiology. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of Neurochemistry. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus-Norvegicus. Aggressive Behavior. 1987;13:227–242. [Google Scholar]
- Przybycien-Szymanska MM, Rao YS, Pak TR. Binge-pattern alcohol exposure during puberty induces sexually dimorphic changes in genes regulating the HPA axis. American Journal of Physiology Endocrinology and Metabolism. 2010;298:E320–328. doi: 10.1152/ajpendo.00615.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimov RM, McBride WJ, McKinzie DL, Lumeng L, Li TK. Effects of ethanol consumption by adolescent alcohol-preferring P rats on subsequent behavioral performance in the cross-maze and slip funnel tests. Alcohol. 1996;13:297–300. doi: 10.1016/0741-8329(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clinical Psychology Review. 2009;29:535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Research. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behavioural Brain Research. 2011;225:104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM. Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harvard Review of Psychiatry. 2012;20:189–200. doi: 10.3109/10673229.2012.714642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Nadal R, Pallarés M, Ferré N. Acute effects of ketamine in the holeboard, the elevated-plus maze, and the social interaction test in Wistar rats. Depression and Anxiety. 1997;5:29–33. [PubMed] [Google Scholar]
- Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiology & Behavior. 1995;57:843–847. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Annals of the New York Academy of Sciences. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Annals of the New York Academy of Sciences. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Development Perspectives. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Developmental Psychobiology. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2010;35:570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neuroscience and Biobehavioral Reviews. 2011;35:1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2008. NSDUH Series H-34, DHHS Publication No. SMA 08-4343. [Google Scholar]
- Torres JM, Ruiz E, Ortega E. Effects of CRH and ACTH administration on plasma and brain neurosteroid levels. Neurochemical Research. 2001;26:555–558. doi: 10.1023/a:1010925331768. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology. 2009;34:2560–2573. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trincavelli ML, Da Pozzo E, Daniele S, Martini C. The GABAA-BZR complex as target for the development of anxiolytic drugs. Current Topics in Medicinal Chemistry. 2012;12:254–269. doi: 10.2174/1568026799078787. [DOI] [PubMed] [Google Scholar]
- van Kerkhof LW, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJ. Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology. 2013;38:1899–1909. doi: 10.1038/npp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior. 2010;96:228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Developmental Psychobiology. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicology and Teratology. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behavioural Brain Research. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcoholism: Clinical and Experimental Research. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacology, Biochemistry, and Behavior. 2012;100:440–450. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiology & Behavior. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcoholism: Clinical and Experimental Research. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Webb B, Burnett PW, Walker DW. Sex differences in ethanol-induced hypnosis and hypothermia in young Long-Evans rats. Alcoholism: Clinical and Experimental Research. 2002;26:695–704. [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, Fergusson DM. Drinking patterns in mid-adolescence and psychosocial outcomes in late adolescence and early adulthood. Addiction. 2004;99:1529–1541. doi: 10.1111/j.1360-0443.2004.00918.x. [DOI] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, et al. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Suppl 4):S273–289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]