Abstract
The relationship between ligand-receptor affinity and antitumor potency of an oncolytic virus was investigated using a panel of six HER2/neu (HER2) targeted measles viruses (MV) displaying single-chain antibodies (scFv) that bind to the same epitope on HER2, but with affinities ranging from 10−6 to 10−11 M. All viruses were able to infect SKOV3ip.1 human ovarian cancer cells in vitro, but only the high affinity MV (Kd > 10−8 M) induced cytopathic effects of syncytia formation in the cell monolayers. In contrast, all six viruses were therapeutically active in vivo against orthotopic human ovarian SKOV3ip.1 tumor xenografts in athymic mice compared to saline treated controls. The oncolytic activities of MV displaying the high affinity scFv (Kd=10−9, 10−10, 10−11 M) were not significantly superior to MV displaying scFv with Kd of 10−8 M or less. Results from this study suggest that increasing the receptor affinity of the attachment protein of an oncolytic measles virus has minimal impact on its in vivo efficacy against a tumor that expresses the targeted receptor.
Keywords: Her2/neu targeted, oncolytic measles virus, single-chain antibody, receptor affinity, receptor density, antitumor potency
INTRODUCTION
Replication competent and tumor selective viruses are being developed as oncolytic agents for cancer therapy.1 Several phase II trials are in progress and a recently completed Phase III melanoma trial with intralesional injection of Talimogene laherparepvec (Oncovex, herpes simplex virus) showed promising survival data. Ideally, the oncolytic virus should infect and spread efficiently in cancer cells but minimally in normal cells. With some viruses, this can be achieved by transcription targeting through use of tissue specific promoters or transductional targeting through specific receptor-ligand interactions to achieve tumor selective entry.2 Adenoviruses which have poor infectivity in cancer cells due to downregulation of the coxsackie adenovirus receptor have been engineered to display binding peptides to enhance their infectivity and spread on cancer cells.3 Viruses such as Vesicular Stomatitis Virus (VSV) that have a broad tropism can be redirected to bind to tumor associated receptors by pseudotyping them with Sindbis or measles envelope glycoproteins displaying single-chain antibodies (scFv) with specificity for tumor associated receptors.4, 5
The attenuated Edmonston B strain of measles virus (MV) has potent oncolytic activity against various types of cancers.6, 7 It selectively causes extensive syncytia formation and cell death in tumor cells and shows minimal cytopathic effects in normal cells.8, 9 Fully retargeted MV that do not bind to their natural receptors, CD46 and SLAM (signaling lymphocyte activation molecule), but efficiently infect and fuse cells through alternative receptors have been generated.10 The virus tropism is now dependent on the displayed ligand at the C terminus of the measles virus hemagglutinin (H) attachment protein.11-16
HER2/neu (HER2), also known as ErbB-2, is a member of the epidermal growth factor receptor family.17 Aberrant HER2 expression, generally attributed to gene amplification and receptor overexpression, has been described in various types of cancer, especially breast and ovarian cancers. 18-20 Retargeted HER2 specific oncolytic viruses have been established from MV, herpes simplex virus, adenovirus serotype 5, simian adenovirus serotype 24 and vesicular stomatitis virus. 4, 21-26 Due to its importance as a cancer target, there has been intense interest in the development of HER2 targeted therapeutics.27 A panel of anti-human HER2 specific scFvs was generated by phage display and sequential mutagenesis.28-31 These scFvs target the same epitope but bind to HER2 with affinities (Kd) ranging from 10−6 to 10−11 M. We previously established a panel of six recombinant MV displaying this panel of HER2 scFvs.21 The scFv is displayed as a C-terminal extension on the measles H attachment protein that is ablated for binding to MV receptors, CD46 and SLAM (signaling lymphocyte molecule). As such, the HER2 targeted MV binds to and infects cells exclusively through the HER2/neu receptor. The panel of HER2 viruses selectively infected and caused cytopathic effect specifically in HER2 positive cells. In vitro, there was a threshold scFv affinity (Kd=10−8 M) required for efficient scFv-receptor interaction below which viral infectivity and intercellular fusion were severely compromised. Viruses displaying scFv with affinities above 10−8 M did not induce larger syncytia in the HER2 positive cells in vitro. In contrast to the lower affinity viruses, the higher affinity viruses (>10−9 M) were able to induce syncytia formation in low HER2 expressing cells.21
The role of ligand-receptor affinity in oncolytic activity of MV has not been previously evaluated in vivo. Here, we used multicellular spheroids composed entirely of HER2 positive tumor cells and human tumor xenografts grown in athymic mice as models. Virus infectivity, size of infectious centers, intratumoral spread and antitumor activity in an orthotopic intraperitoneal model of ovarian cancer was evaluated.
MATERIALS AND METHODS
Cell lines and viruses
The human epithelial ovarian carcinoma cell line, SKOV3ip.1 (a kind gift from Ellen Vitetta, University of Texas Southwestern Medical Center) and SKOV3ip.1-Fluc cells which stably express firefly luciferase, were maintained in alpha-MEM supplemented with 20% FBS and 2 mM L-glutamine.16, 32 Human rhabdomyosarcoma TE671 and Vero-αHis cells, which stably express a membrane-anchored scFv that recognizes a six-histidine peptide, were maintained in DMEM supplemented with 10% or 5% FBS, respectively.10 The six fully retargeted HER2-specific MV (MV-αHER-6 to MV-αHER-11) were propagated and titered on Vero-αHis cells as previously described.21 These viruses were ablated for binding to their natural receptors, CD46 and SLAM, and encode an enhanced GFP reporter gene.10 A six histidine (His) peptide was inserted at the C-terminus of H to enable virus rescue and propagation on Vero-αHis cells.
Generation and infection of multicellular tumor spheroids
Multicellular spheroids of tumor cells were prepared by using the liquid overlay technique.33 Spheroids were incubated with viruses (MOI 0.5) at 37°C. To quantitate the extent of virus infection, spheroids were fixed with 4% paraformaldehyde and nuclei were stained with 20 ng/ml Hoechst 33342 (Molecular Probes, Eugene, OR). Virus infection and viral spread (size of GFP foci) were assessed using a Zeiss LSM 510 confocal system on an Axioplan-2 microscope (Carl Zeiss, Dublin, CA).
In vivo Experiments
All procedures involving animals were approved by and performed according to the guidelines of the Mayo Foundation Institutional Animal Care and Use Committee. Female 4-5 week-old NCR athymic mice (Harlan-Sprague-Dawley, Indianapolis, IN or Taconic Laboratories, Germantown, NY) were injected intraperitoneally (i.p.) with 2 × 106 SKOV3ip.1_Fluc cells in 250 μl DPBS. Five days later, when tumors were established in the omentum, mice (n = 10 per group) were treated i.p. with three doses, given every other day, of 2 × 106 TCID50 of each of the HER2 targeted viruses or saline as control. Mice were euthanized if they developed ascites, subcutaneous injection site tumors that were >10% of body weight, or if they lost >20% of body weight. All surviving mice were euthanized at the end of the experiment (day 90 after first virus treatment). Kaplan-Merier survival curves were plotted and compared by log-rank sum test. Tumor burden was monitored weekly by noninvasive bioluminescence imaging for firefly luciferase activity (Xenogen Corporation, Alameda, CA).
Immunohistochemistry
Tumor samples frozen in OCT were cryosectioned (5 μm thick) and fixed in −20°C prechilled acetone. Nonspecific binding was blocked with 5% normal horse serum in 0.01% Triton X-100/PBS. Sections were then incubated with biotinylated anti-measles nucleoprotein antibody (Chemicon, Temecula, CA) and subsequently with Vectastain ABC-AP kit (Vector Labs, Burlingame, CA). Signals were developed using a blue substrate kit (Vector Labs) and nuclei were counterstained with Nuclear Fast Red (Vector Labs).
Statistical analysis
In the spheroids experiment, comparison of successful infections between all viruses was tested by chi-square test. Differences in tumor burden were analyzed by two-way ANOVA of the photon counts from the imaging data. Survival curves were represented using the Kaplan-Meier method. The log-rank test was used to examine the significance of differences in the survival between groups using GraphPad Prism (GraphPad Software, San Diego, CA). A p-value of <0.05 was considered to be significant.
RESULTS
Infection and spread of MV-αHer2 viruses in multicellular spheroids
We previously showed that a scFv Kd of 10−8 M is required for mediating efficient virus infection (40-60% at MOI 1) and syncytial formation (intercellular fusion) in SKOV3ip.1 cell monolayers.21 The low affinity viruses did not induce syncytium and only low levels of infection (<10%) were observed. Here, we exposed spheroids composed entirely of tumor cells to media containing MV. A chi-square or Fisher's exact test was used to test the null hypothesis of equal proportion of successful infection between all viruses. Experiments were undertaken in three to four independent replicates, each time with 12-36 spheroids per virus. A spheroid with one or more GFP positive foci (independent of size) is classified as ‘infected’. In Vero-αHis spheroids, all viruses had equally high successful infection rates (80.4% to 100%), confirming that equivalent amounts of virus were used in the assay. In SKOV3ip.1 spheroids, the infection rates were: MV-αHER-9 (63.9%), MV-αHER-7 (68.8%), MV-αHER-10 (79.0%), MV-αHER-8 (83.9%), MV-αHER-11 (88.3%) and MV-αHER-6 (94.4%). There was no apparent correlation between scFv affinity and infection rates in SKOV3ip.1 spheroids. We also evaluated the size of infected areas. Spheroids of similar sizes with successful infection events were analyzed. The extent of virus infection throughout the spheroid, as reflected by GFP expression, was captured using confocal microscopy through the Z-axis and analyzed using the NIH ImageJ program. There was no correlation between the size of infected area and scFv affinity (data not shown). As expected, receptor abundance plays a predominant role in determining the size of infected areas. In the low HER2 TE671 human rbadomyosarcoma spheroids (4.3×103 molecules per cell), the size of infected area was significantly lower (about 10-times) than in SKOV3ip.1 spheroids (1.5×105 molecules per cell).
MV-αHER2 infection of tumors in vivo
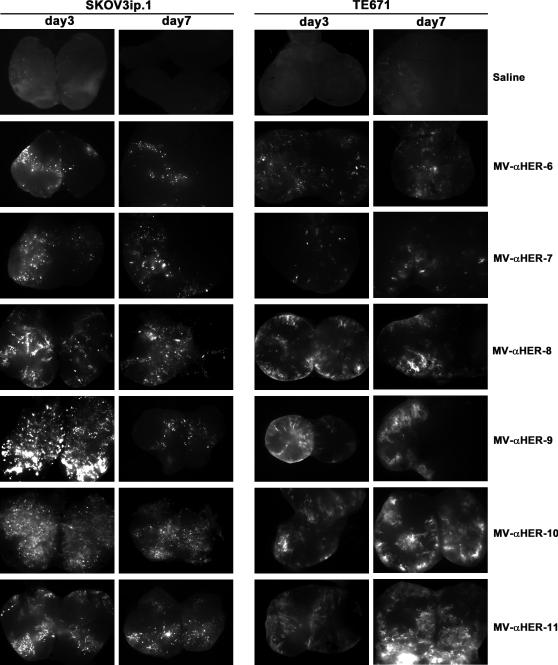
Subcutaneous SKOV3ip.1 and TE671 flank tumors grown in athymic mice were injected directly with 106 TCID50 viruses. Three days or seven days post virus administration, tumors were harvested, cut into half and observed under a fluorescence microscope for MV infected areas. GFP positive cells or areas were seen in SKOV3ip.1 tumors injected with all members of the HER2 targeted MV (Figure 1). While negligible infection was observed in vitro in TE671 spheroids, injection of MV-αHER2 viruses into TE671 tumors resulted in detectable levels of GFP expression by the high affinity viruses (Figure 1).
Figure 1.
Assessment of intratumoral MV infection and spread post direct intratumoral injection. SKOV3ip.1 or TE671 tumor xenografts were injected directly with the panel of HER2 targeted MVs (106 TCID50 per dose) or saline. Three or seven days later tumors were harvested and cut into halves (butterflied). Images show GFP expression in infected cells or areas.
Antitumor activity of MV-αHER2 in an orthotopic model of ovarian cancer
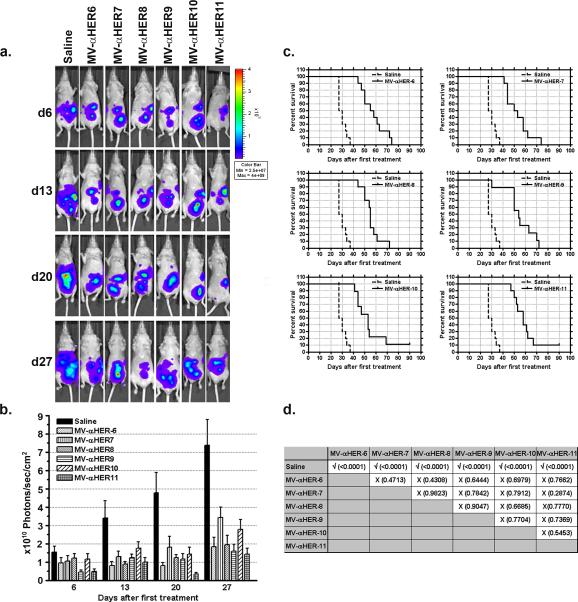
The antitumor potential of MV-αHER2 was examined in athymic mice bearing disseminated SKOV3ip.1 tumors expressing the firefly luciferase gene (SKOV3ip.1-FLuc). Mice received three IP doses of MV (2×106 TCID50 per dose) or saline (n=10 mice per group) every other day. All HER2 targeted viruses significantly inhibited tumor growth compared to saline treated controls (Figure 2). Tumor burden was quantitated by measuring whole abdominal photon counts from the bioluminescent imaging studies (Figure 2a). By day 28 after the first treatment, 50% of mice in the saline treated group had to be euthanized due to tumor burden. The average tumor burden in the MV treated groups was significantly lower than saline controls (Figure 2b). Survival curves of mice were compared (Figure 2c). Median survival of saline treated mice was 28.5 days. The median survivals of MV-αHER-6, MV-αHER-7, MV-αHER-8, MV-αHER-9, MV-αHER-10, MV-αHER-11 treated mice were 56.5, 51.5, 55, 54, 53 and 58 days, respectively. MV treatment significantly increased median survival by a factor of 1.98, 1.8, 1.93, 1.9, 1.86 and 2.04 respectively (p<0.0001). Importantly, all viruses were therapeutically active and the higher affinity viruses did not perform better than the lower affinity viruses (p>0.05). Tumors were harvested on day 4, 10 or 14 after the first treatment and immunohistochemical staining for measles N protein was performed on omental tumors. There was no apparent difference in the numbers or size of infectious foci between the high and low affinity viruses. None of the viruses were able to efficiently penetrate into the center of the omental tumors even at later time points (data not shown).
Figure 2.
In vivo anti-tumor activity of MV-αHER2. Mice were implanted with SKOV3ip.1_Fluc cells. Five days later mice were injected intraperitoneally with three doses of 2×106 TCID50 MV-αHER-6 to MV-αHER-11 or saline, given every other day (a) Bioluminescence images showing tumor burden in treatment groups. (b) Quantitation of tumor burden from the bioluminescence imaging study. 10 mice per group. (c) Kaplan-Meier survival curves of mice in each treatment group compared to saline control group. (d) Statistical difference between survival curves of mice in respective treatment groups was compared. The P-values were calculated using the logrank sum test.
Discussion
The panel of scFv displayed on the HER2 retargeted MV is composed of affinity mutants of the parental C6.5 scFv (Kd=10−8 M) and all scFv bind to the same epitope on HER2 but with affinities ranging from 10−6 to 10−11 M.28, 29 The virus hemagglutinin attachment protein is ablated for binding to two of the three measles virus cellular receptors; CD46 which is ubiquitously expressed on nucleated cells, and SLAM which is expressed on activated immune cells.34, 35 These HER2 viruses are not ablated for binding to Nectin-4, the recently identified third receptor of measles virus.36 Nectin-4 is overexpressed on lung, ovarian and breast cancers and may enhance infectivity of the HER2 targeted viruses on the ovarian cancer cells.36 However, SKOV3ip.1 tumor cells do not express detectable levels of nectin-4 as determined by antibody staining and analysis by flow cytometry (Peng, unpublished data). Thus, MV-αHER2 entry and infection of SKOV3ip.1 cells and tumors is mediated through scFv binding to HER2 receptor.
Our goal was to evaluate the importance of ligand-receptor affinity on the antitumor activity of oncolytic measles viruses. Results indicate that the antitumor activity of the low and high affinity viruses were comparable against intraperitoneal SKOV3ip1. ovarian tumors. The “seemingly negative” result that we report is the first study to demonstrate that receptor affinity of the attachment protein of an oncolytic virus has minimal impact on its in vivo efficacy against a tumor that expresses the targeted receptor. The study therefore challenges a fundamental assumption about the in vivo behavior of viruses, that is, high affinity interactions would be desirable for therapeutic activity of an oncolytic virus. This finding has not yet been generalized to all virus families and currently, on the basis of the presented work, applies only to retargeted MV.
The HER2 receptor is overexpressed on 15–20% of breast cancers and its expression is correlated with more aggressive tumors.37 HER2 is overexpressed in 20 of 20 ovarian tumor cell lines derived from advanced stage III and stage IV tumors.38 The monoclonal antibody trastuzumab is currently the only approved adjuvant treatment specifically for patients with HER2-positive early stage breast cancer.37 A limitation of trastuzumab monoclonal antibody therapy is that its activity is largely restricted to cancers with the highest level of HER2 overexpression or HER2 gene amplification. To test suitability for trastuzumab therapy, patient tumor samples are stained with an anti-Her2 antibody and need to be graded as +3 or +4 for immunopositivity. However, there is a large population of breast or ovarian cancers that have low or moderate HER2 expression. We propose that these low HER2 positive tumors can potentially benefit from oncolytic MV targeted therapy using the high affinity MV which efficiently infects low HER2 expressing tumor cells.
The panel of HER2 scFv affinity mutants that was developed by Jim Marks and colleagues has been very useful for investigations in understanding the role of receptor-ligand affinity in modulating the biodistribution, tumor penetration and the antitumor activity of various therapeutic agents.30, 31, 39-41 In nephrectomized SCID mice bearing SKOV3 tumors, Adams et al. showed scFv need to have sufficiently high affinity to achieve good tumor localization. As such, the 10−7 Kd scFv failed to accumulate in significant amounts in the tumors compared to the higher affinity scFvs (10−8 and 10−9M).31 Accumulation in the tumor ceased to increase with affinity and was nearly the same for scFv with Kd of 10−9, 10−10 and 10−11M.41 However, undesirable side effects could arise with high affinity binders. Immunohistochemical analysis of well-vascularized tumors showed the highest affinity scFv limited to tumor space adjacent to the blood vessel while the low-affinity scFv diffused uniformly throughout the tumor interior.41
In contrast to the studies above, we did not observe a significant difference in the in vivo performance of low and high affinity HER2 targeted MV after intraperitoneal administration into mice with orthotopic ovarian disease. Immunohistochemical staining for MV-N protein indicate sites of virus infection in the tumors, but there were no significant areas of intercellular fusion or differences in size of infected areas by the low and high affinity viruses. In contrast to the in vitro study where extensive intercellular fusion was seen in infected cell monolayers, intercellular fusion may not be a major factor involved in oncolytic activity of these fusogenic measles viruses in the tumors. Importantly, the in vitro results were not predictive of the antitumor activity of the retargeted MV in vivo. Unlike scFv or antibodies, a virus has several hundred copies of the scFv displayed on the viral coat, thereby significantly increasing the avidity of the agent. Results from this study indicate that increasing the affinity of attachment protein-receptor interaction does not enhance virus delivery or therapeutic activity in vivo, and that future studies could focus on improving delivery of therapeutic viruses by other strategies other than increasing affinity.
Acknowledgements
This work is supported by grants from the National Institutes of Health/National Cancer Institutes (R01CA118488, R01CA129193).
Financial Support: National Institutes of Health/National Cancer Institute (CA118488, CA129193 and CA129966)
Footnotes
Conflicts of Interest
Drs. Russell and Peng and Mayo Clinic have financial interests in the technology used in this research.
References
- 1.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28(7):326–33. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty MS, Curiel DT. Chapter two--Adenovirus strategies for tissue-specific targeting. Adv Cancer Res. 2012;115:39–67. doi: 10.1016/B978-0-12-398342-8.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman I, Whitaker-Dowling P, Gao Y, Griffin JA, Watkins SC. Vesicular stomatitis virus expressing a chimeric Sindbis glycoprotein containing an Fc antibody binding domain targets to Her2/neu overexpressing breast cancer cells. Virology. 2003;316(2):337–47. doi: 10.1016/j.virol.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Ayala-Breton C, Barber GN, Russell SJ, Peng KW. Retargeting vesicular stomatitis virus using measles virus envelope glycoproteins. Hum Gene Ther. 2012;23(5):484–91. doi: 10.1089/hum.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Msaouel P, Iankov ID, Dispenzieri A, Galanis E. Attenuated oncolytic measles virus strains as cancer therapeutics. Curr Pharm Biotechnol. 2012;13(9):1732–41. doi: 10.2174/138920112800958896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–41. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong HT, Timm MM, Greipp PR, Witzig TE, Dispenzieri A, Russell SJ, et al. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp Hematol. 2006;34(6):713–20. doi: 10.1016/j.exphem.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64(14):4919–26. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23(2):209–14. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 11.Bach P, Abel T, Hoffmann C, Gal Z, Braun G, Voelker I, et al. Specific elimination of CD133+ tumor cells with targeted oncolytic measles virus. Cancer research. 2013;73(2):865–74. doi: 10.1158/0008-5472.CAN-12-2221. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich K, Hanauer JR, Prufer S, Munch RC, Volker I, Filippis C, et al. DARPin-targeting of measles virus: unique bispecificity, effective oncolysis, and enhanced safety. Mol Ther. 2013;21(4):849–59. doi: 10.1038/mt.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaiw KC, Miest TS, Frenzke M, Timm M, Johnston PB, Cattaneo R. CD20-targeted measles virus shows high oncolytic specificity in clinical samples from lymphoma patients independent of prior rituximab therapy. Gene therapy. 2011;18(3):313–7. doi: 10.1038/gt.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossow S, Grossardt C, Temme A, Leber MF, Sawall S, Rieber EP, et al. Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer gene therapy. 2011;18(8):598–608. doi: 10.1038/cgt.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Hasegawa K, Russell SJ, Sadelain M, Peng KW. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate. 2009;69(10):1128–41. doi: 10.1002/pros.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, Figini M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6170–8. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135(1):55–62. doi: 10.1043/2010-0454-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raspollini MR, Amunni G, Villanucci A, Castiglione F, Degl'Innocenti DR, Baroni G, et al. HER-2/neu and bcl-2 in ovarian carcinoma: clinicopathologic, immunohistochemical, and molecular study in patients with shorter and longer survival. Appl Immunohistochem Mol Morphol. 2006;14(2):181–6. doi: 10.1097/01.pai.0000155192.94214.f9. [DOI] [PubMed] [Google Scholar]
- 19.Zhou BP, Hung MC. Dysregulation of cellular signaling by HER2/neu in breast cancer. Semin Oncol. 2003;30(5 Suppl 16):38–48. doi: 10.1053/j.seminoncol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto H, Fukasawa H, Honda T, Hirata S, Hoshi K. HER-2/neu expression in ovarian clear cell carcinomas. Int J Gynecol Cancer. 2003;13(1):28–31. doi: 10.1046/j.1525-1438.2003.13028.x. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa K, Hu C, Nakamura T, Marks JD, Russell SJ, Peng KW. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J Virol. 2007;81(23):13149–57. doi: 10.1128/JVI.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reisoli E, Gambini E, Appolloni I, Gatta V, Barilari M, Menotti L, et al. Efficacy of HER2 retargeted herpes simplex virus as therapy for high-grade glioma in immunocompetent mice. Cancer gene therapy. 2012;19(11):788–95. doi: 10.1038/cgt.2012.62. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson MK, Kraaij R, Leadley RM, De Ridder CM, van Weerden WM, Van Schie KA, et al. A transductionally retargeted adenoviral vector for virotherapy of Her2/neu-expressing prostate cancer. Hum Gene Ther. 2012;23(1):70–82. doi: 10.1089/hum.2011.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belousova N, Mikheeva G, Xiong C, Soghomonian S, Young D, Le Roux L, et al. Development of a targeted gene vector platform based on simian adenovirus serotype 24. Journal of virology. 2010;84(19):10087–101. doi: 10.1128/JVI.02425-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menotti L, Cerretani A, Hengel H, Campadelli-Fiume G. Construction of a fully retargeted herpes simplex virus 1 recombinant capable of entering cells solely via human epidermal growth factor receptor 2. J Virol. 2008;82(20):10153–61. doi: 10.1128/JVI.01133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergman I, Griffin JA, Gao Y, Whitaker-Dowling P. Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu. Int J Cancer. 2007;121(2):425–30. doi: 10.1002/ijc.22680. [DOI] [PubMed] [Google Scholar]
- 27.Jelovac D, Emens LA. HER2-directed therapy for metastatic breast cancer. Oncology (Williston Park) 2013;27(3):166–75. [PubMed] [Google Scholar]
- 28.Schier R, McCall A, Adams GP, Marshall KW, Merritt H, Yim M, et al. Isolation of picomolar affinity anti-c-erbB-2 single-chain Fv by molecular evolution of the complementarity determining regions in the center of the antibody binding site. J Mol Biol. 1996;263(4):551–67. doi: 10.1006/jmbi.1996.0598. [DOI] [PubMed] [Google Scholar]
- 29.Schier R, Bye J, Apell G, McCall A, Adams GP, Malmqvist M, et al. Isolation of high-affinity monomeric human anti-c-erbB-2 single chain Fv using affinity-driven selection. J Mol Biol. 1996;255(1):28–43. doi: 10.1006/jmbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 30.Adams GP, Schier R. Generating improved single-chain Fv molecules for tumor targeting. J Immunol Methods. 1999;231(1-2):249–60. doi: 10.1016/s0022-1759(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 31.Adams GP, Schier R, Marshall K, Wolf EJ, McCall AM, Marks JD, et al. Increased affinity leads to improved selective tumor delivery of single-chain Fv antibodies. Cancer Res. 1998;58(3):485–90. [PubMed] [Google Scholar]
- 32.Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62(16):4656–62. [PubMed] [Google Scholar]
- 33.Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. Journal of biomolecular screening. 2006;11(8):922–32. doi: 10.1177/1087057106292763. [DOI] [PubMed] [Google Scholar]
- 34.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 35.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406(6798):893–7. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 36.Noyce RS, Richardson CD. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012;20(9):429–39. doi: 10.1016/j.tim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9(1):16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 38.Hellstrom I, Goodman G, Pullman J, Yang Y, Hellstrom KE. Overexpression of HER-2 in ovarian carcinomas. Cancer research. 2001;61(6):2420–3. [PubMed] [Google Scholar]
- 39.Rudnick SI, Adams GP. Affinity and avidity in antibody-based tumor targeting. Cancer biotherapy & radiopharmaceuticals. 2009;24(2):155–61. doi: 10.1089/cbr.2009.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams GP, Schier R, McCall AM, Crawford RS, Wolf EJ, Weiner LM, et al. Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu. Br J Cancer. 1998;77(9):1405–12. doi: 10.1038/bjc.1998.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61(12):4750–5. [PubMed] [Google Scholar]