Abstract
The M3 and M2 muscarinic acetylcholine receptors (mAChRs) and beta-2-adrenoceptors (β2ARs) are important regulators of airway cell function, and drugs targeting these receptors are among the first line drugs in the treatment of the obstructive lung diseases asthma and chronic obstructive lung disease (COPD). Cross-regulation or crosstalk between mAChRs and β2ARs in airway smooth muscle (ASM) helps determine the contractile state of the muscle, thus airway diameter and resistance to airflow. In this review we will detail mAChR and β2AR-signaling and crosstalk, focusing on events in the ASM cell but also addressing the function of these receptors in other cell types that impact airway physiology. We conclude by discussing how recent advances in GPCR pharmacology offer a unique opportunity to fine tune mAChR and β2AR signaling and their crosstalk, and thereby produce superior therapeutics for obstructive lung and other diseases.
Introduction
The contractile state or “tone” of airway smooth muscle (ASM) is the principal determinant of airway diameter and thus resistance to airflow. Under physiological conditions ASM has relatively little tone; airways are patent and airway resistance does not limit breathing. Under conditions of lung inflammation that occur with obstructive lung diseases such as asthma, increased presentation of agents that promote contraction by activating various G protein-coupled receptors (GPCR) on ASM increase ASM tone and thus airway resistance. Although multiple agents and their cognate receptors can contribute to increased ASM contraction, the M3 muscarinic acetylcholine receptor (mAChR) is arguably the most important, being activated by acetylcholine (ACh) released from nerves of the parasympathetic nervous system. Conversely, the beta-2-adrenoceptor (β2AR) is arguably the most important GPCR capable of antagonizing ASM contraction. The importance of both the M3 mAChR and β2AR in regulating ASM contraction is underscored by the importance of mAChR antagonists and β2AR agonists as bronchodilators in airway diseases. The signaling crosstalk between mAChRs (both M3 and M2) and β2ARs plays a prominent role in determining ASM contractile state, and is critical to the both the efficacy and limitations of those asthma therapeutics targeting these receptors. In this review we will detail mAChR and β2AR signaling and crosstalk, focusing on events in the ASM cell but also addressing the function of these receptors in other cell types that impact airway physiology. We will conclude by discussing how recent advances in GPCR pharmacology offer a unique opportunity to fine tune mAChR and β2AR signaling and their crosstalk, and thereby produce superior therapeutics for obstructive lung and other diseases.
GPCR signaling and function in airway cells
M3 mAChR signaling and function
M3 muscarinic acetylcholine receptors are coupled to heterotrimeric Gq proteins. The binding of ACh to the receptor induces a conformation change in the receptor, which promotes association with and activation of the Gq protein by exchanging GTP for GDP on the Gα subunit. The subsequently released Gα subunit activates phospholipase C which hydrolyzes PIP2 into inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to IP3 receptors on the endoplasmatic reticulum, releasing Ca2+ from intracellular stores whereas DAG activates protein kinase C (PKC).
M3 mAChRs expressed on ASM cells are the principal mediators of ASM contraction or tone under physiological conditions, and the majority of studies posit them as significant in mediating the pathological contraction (bronchoconstriction) associated with asthma and chronic obstructive pulmonary disease (COPD) (for a comprehensive analysis of Gq-coupled receptor signaling that mediates ASM contraction, see [1;2] and references therein). Briefly, the initial ACh-induced increase in intracellular Ca2+ is followed by a more sustained increase in Ca2+ mediated by the Ca2+-sensitive ryanodine receptors (RyR) on the endoplasmic reticulum, and by Ca2+ influx from the extracellular space involving store-operated Ca2+ channels and further promoted by a PKC-dependent increase in the open probability of Ca2+ channels on the cell membrane (reviewed in [3]). Formation of the Ca2+/calmodulin complexes activates myosin light chain kinase (MLCK) which phosphorylates myosin light chain and allows activation of myosin ATPase resulting in generation of force through cross-bridge cycling. Concomitantly-activated PKC and Rho kinase (the downstream effector of RhoA), which are activated by not only the M3 mAChR but other Gq-coupled GCPRs in ASM, serve to augment this contractile signaling by inhibiting myosin light chain phosphatase (MLCP). Both PKC and Rho kinase activate CPI-17, an endogenous inhibitor of MLCP. MLCP serves as a brake on contraction by reversing MLCK-induced phosphorylation of MLC. MLCP inhibition results in increased MLCK phosphorylation/activity at any given level of intracellular calcium. MLCP inhibition is thus a key mechanism mediating “calcium sensitization,” enabling maintenance of ASM contraction as intracellular calcium levels wane, and increasing ASM responsiveness to contractile agents (a key mechanism mediating airway hyperreactivity in the asthmatic). PKC may also promote contraction by directly phosphorylating MLCK and MLC, however, the relevance of PKC-mediated MLCK phosphorylation needs to be confirmed in ASM whereas the contribution of PKC phosphorylation sites on MLC to contraction is uncertain. Although the above-described receptor-mediated “pharmaco-mechanical coupling” is viewed as the predominant pathway mediating contraction, recent studies have indicated an important role for ACh-activated RhoA in promoting ASM contraction through dynamic actin remodeling [4].
As alluded to above, the important role of M3 mAChRs in mediating increased airway resistance and airway hyperresponsiveness (AHR) in obstructive lung diseases is underscored by the use of mAChR antagonists in the treatment of both asthma and COPD [5;6]. In COPD, the increased cholinergic ASM tone is the major reversible component of airway resistance [7–9] and mAChR antagonists are used as first line treatments. Ipratropium, a nonselective (targeting M1, M2 and M3 mAChRs) mAChR antagonist, has been a COPD treatment for almost 40 years, and has recently been supplanted by the long-acting tiotropium, whose binding kinetics render it more selective for M3 mAChR antagonism [10;11].
In addition to their role in mediating aberrant ASM tone, M3 mAChRs have also been implicated in airway remodeling and inflammation. M3 mAChRs have been shown to augment growth factor-induced proliferation of ASM [12;13] as well as the release of IL-8 in response to TNF-α or cigarette smoke [14;15]. The M3 mAChR also stimulates the release of neutrophil-attracting chemokines from airway epithelial cells [16] and alveolar macrophages [17].
More recent studies also indicate cooperativity between M3 mAChRs and TGF-β signaling in increasing contractile protein expression in ASM cells. Using guinea pig precision cut lung slices, Oenema et al. [18] determined that M3 mAChR- (as well as histamine- and KCl-) -mediated bronchoconstriction results in the release of active TGF-β which promotes ASM remodeling. These findings suggest a mechanism explaining the findings of Grainge et al. [19] in which bronchoconstriction alone (in the absence of an inflammatory stimulus) was sufficient to induce airway remodeling in asthmatics.
M3 mAChRs also promote mucus secretion from submucosal glands in the conducting airways, which are innervated by the vagus nerve. M3 mAChR stimulation under pathophysiological conditions in diseased airways, by either neuronal or non-neuronal sources of ACh, leads to mucus hypersecretion as well as goblet cell hyperplasia which contribute to increased airway resistance [8;20]
Animal models employing pharmacological or genetic inhibitory strategies provide the most compelling evidence to date implicating the M3 mAChR in airway remodeling and inflammation. The mAChR antagonist tiotropium prevents ASM (and vascular smooth muscle) hypertrophy, goblet cell hyperplasia and eosinophilic inflammation a guinea pig model of chronic allergic asthma [21;22]. Similarly, in a guinea pig model of COPD, tiotropium inhibits neutrophilic inflammation, goblet cell hyperplasia and airway fibrosis [23]. A recent study from the Gosens lab [24] provides conclusive evidence for the role of M3 mAChRs as allergen-induced ASM (and vascular smooth muscle) remodeling, goblet cell metaplasia and airway fibrosis were all attenuated in M3 mAChR knockout mice relative to wild type and M2 mAChR knockout mice. Importantly, M3 mAChR knockout did not affect the development of eosinophilic inflammation exhibited in wild type mice, again supporting the findings from Grainge et al. [25] suggesting that inflammation is not required for the development of airway remodeling.
M2 mAChR signaling and function
M2 mAChRs are expressed on various cell types in the airways, although their function in ASM and parasympathetic pre-synaptic nerves appears to have the greatest impact on airway function. M2 mAChRs are coupled to the Gi heterotrimeric protein, and early studies in S49 lymphoma cell membranes demonstrated the ability of activated Giα subunit to bind and inhibit adenylyl cyclase activated by the Gsα [26]. Thus, as will be discuss below, M2 mAChR activation constrains the signaling and bronchorelaxant effect of β2ARs by antagonizing β2AR/Gs activation of adenylyl cyclase. However, M2 mAChRs expressed pre-synaptically on parasympathetic nerve endings, when activated exert negative feedback on neuronal ACh release, thereby limiting bronchoconstriction. The dysfunction of the presynaptic M2 mAChR receptors has been proposed as a pathophysiological mechanism of AHR in asthma. Several animal model studies have shown that allergen challenge as well as viral infections or noxious insult to the lung may result in the dysfunction of the presynaptic M2 mAChR receptor, likely mediated by eosinophilic inflammation, and contribute to AHR [27].
The capacity of M2 mAChRs expressed on ASM cells to promote contraction through pharmacomechanical coupling is not apparent based on studies comparing mice in which each of the (M1-M5) mAChR knockout mice have been compared [28;29]. However, a role for M2 mAChRs in Gαi-dependent activation of RhoA and subsequent stress fiber formation through actin polymerization has been proposed in ASM [30;31] and may contribute to tension development.
A role for M2 mAChRs in regulating airway remodeling has also been suggested. M2 mAChR stimulation in fibroblasts results in increased proliferation and collagen synthesis, a finding which suggests potential for contributing to airway fibrosis [32;33]. A recent study also shows that ASM cell proliferation induced by prolonged TGF-β1 stimulation is augmented by M2 mAChRs [34].
Collectively, several studies suggest that the M2 mAChR may have a role in mediating increased ASM contraction through both direct and indirect actions on ASM, and also mediate other features of obstructive airway disease. However, the relative importance of these mechanisms is unclear.
β2AR signaling and function
β2ARs are coupled to Gs heterotrimeric G proteins. Binding of an agonist (e.g., endogenous epinephrine or exogenous beta-agonist) to the receptor promotes Gαs activation which in turn activates adenylyl cyclase which hydrolyzes adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP). The binding of cAMP to the regulatory subunits of cAMP-dependent protein kinase (protein kinase A (PKA)) releases the catalytic subunit of the PKA, and activated PKA exerts myriad effects in ASM by phosphorylating numerous intracellular targets. cAMP-/PKA- dependent signaling promoted by the β2AR inhibits M3 mAChR (and other pro-contractile Gq-coupled receptors in ASM) signaling at multiple steps and constitutes the “crosstalk” that antagonizes ASM contraction (see below).
In addition to the pro-relaxant capability of β2ARs, immunomodulatory functions of the β2AR have been described, including the ability to induce IL-6 and TSLP expression, and inhibit cytokine-induced GM-CSF, RANTES and eotaxin release from ASM cells [35–37]. The immunomodulatory role is also evident in other cell types including bronchial epithelial and mast cells in which beta-agonists increase release of TSLP and inhibit histamine release, respectively [38;39]. In type 2 T cells, beta-agonists augment cytokine-induced cell accumulation by prolonging T-cell survival [40]. PKA-mediated regulation of gene expression occurs through phosphorylation of the transcription factor CREB and phosphorylation of GPCRs and receptor tyrosine kinases [1]. The anti-mitogenic and anti-migration properties of beta-agonists in ASM cells [41;42] have also been shown to be mediated by PKA, with PKA being directly implicated via heterologous expression of PKA-inhibitory peptides.
In addition to the well characterized PKA-mediated β2AR signaling, it is now appreciated that the regulatory proteins arrestins can promote β2AR signaling events distinct from those of PKA and independent of β2AR-Gs coupling [43]. Arrestins were originally identified as important in regulating β2AR both desensitization and resensitization by promoting β2AR internalization to either recycling or degradative pathways. Subsequent studies determined that arrestins also serve as scaffolds and initiators of signaling to numerous pathways including p42/p44, JNK, and NF-κB (see below). Arrestin-dependent signaling in ASM or any airway cell remains poorly defined, but in mice with beta-arrestin-2 knockout, both lung inflammation and AHR due to allergen sensitization and challenge is significantly attenuated [44]. As will be discussed below, future studies distinguishing between G protein- and arrestin- dependent signaling events and consequences in airway cells should ultimately greatly increase our understanding of GPCR control of airway function in health and disease, and likely lead to a new generation of drugs capable of exploiting this diverse signaling capacity.
Crosstalk among GPCRs in the airway
β2ARs regulating M3 mACHRs
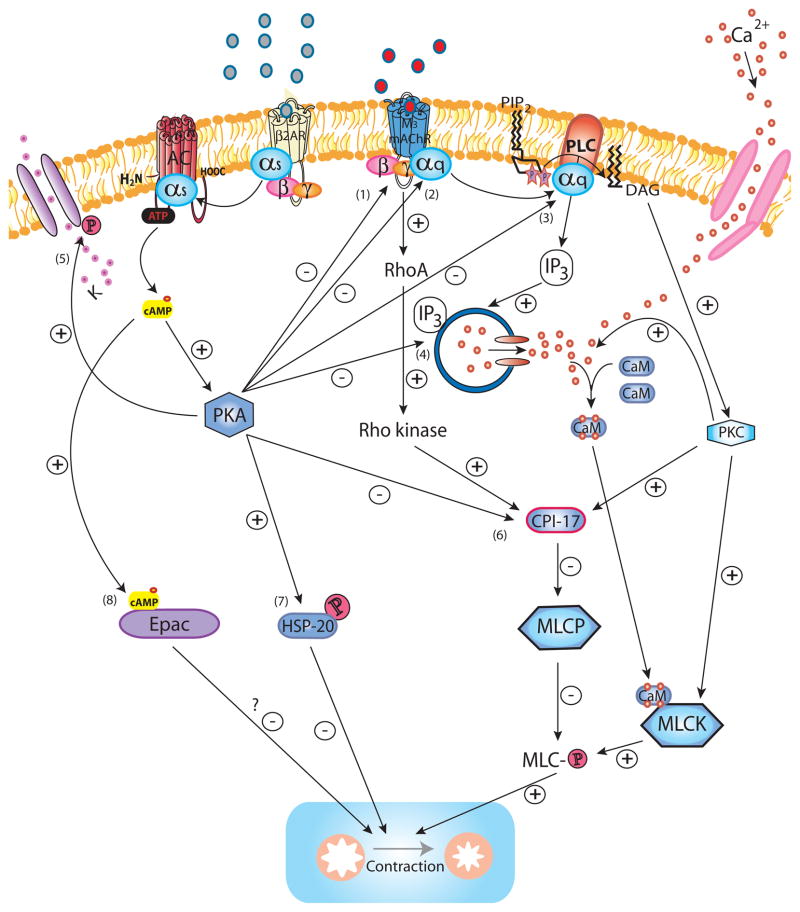
The physiological consequence of crosstalk between β2ARs and M3 mAChRs in ASM is described as functional antagonism. Although certain pro-relaxant mechanisms promoted by β2AR stimulation (phosphorylation of heat shock protein 20 (HSP20) [45;46] and KCa2+ channels [47]) do not involve inhibiting M3 mAChR or Gq–coupled receptor signaling per se, β2AR signaling effects bronchorelaxation by targeting M3 mAChR signaling at the plasma membrane and at numerous downstream intracellular junctures (Figure 1).
Figure 1. β2AR regulation of M3 mAChR signaling.
Upon activation of M3 mAChR by ligand binding, the Gαq subunit is released to bind and activate PLC, which hydrolyzes PIP2 into IP3 and DAG. IP3 induces Ca2+ release from intracellular stores via the IP3R whereas DAG activates PKC. The initial IP3-induced release of Ca2+, as well as cADP-ribose (not shown), both acting via RyR (not shown) contribute to a more sustained increase of intracellular Ca2+, also promoted by additional influx of Ca2+ from the extracellular space through Ca2+ channels on the cell membrane. Ca2+ binds calmodulin to form the Ca2+/calmodulin complex which activates MLCK to subsequently phosphorylate MLC allowing activation of myosin ATPase (not shown) which effectively leads to force generation by cross-bridge cycling (contraction). PKC promotes the influx of Ca2+ from the extracellular compartment by increasing the open probability of Ca2+ channels. PKC phosphorylation of the MLCP inhibitor CPI-17 mediates Ca2+ -sensitization as MLCP limits contraction by dephosphorylating MLC. Similarly, M3 mAChR activates RhoA and its downstream effector Rho kinase which also phosphorylates CPI-17 and thereby mediates Ca2+ -sensitization. PKC-mediated phosphorylation of MLCK may constitute an additional mechanism for Ca2+ -sensitization. Activation of the β2AR counteracts M3 mAChR-induced signaling and contraction at several levels. Ligand-induced conformation change of the β2AR results in the release of the Gαs subunit which subsequently activates AC to hydrolyze ATP to cAMP. cAMP binds to the PKA regulatory units (not shown) which then release the catalytically active PKA. PKA phosphorylates the M3 mAChR (1) (although relevance of this for desensitization in ASM is not certain), the Gαq subunit (2) and PLC (3) diminishing M3 mAChR activation and IP3 production (and possibly Rho kinase activation). The release of intracellular Ca2+ is inhibited through PKA-mediated phosphorylation of IP3R (4) and RyR (not shown), whereas phosphorylation of the KCa2+ (5) channels results in K+ efflux and hyperpolarization of the cell. PKA abrogates Ca2+ -sensitization by phosphorylating CPI-17 (6). PKA phosphorylation of HSP20 (7) renders it capable of inhibiting contraction through ill-defined mechanisms possibly involving regulation of actin dynamics and actin-myosin binding. Another potential effector downstream of cAMP, Epac (8), has been suggested as an inhibitor of contraction, however its role in ASM as an effector downstream of β2AR remains to be determined.
β2AR signaling limits M3 mAChR-mediated IP3 production by several distinct mechanisms, most presumed to involve PKA. Activated PKA can phosphorylate the M3 mAChR (1), Gq subunit (2) or PLC (3); promoting receptor desensitization (1 & 2) and decreased IP3 production (1–3). Although M3 mAChR has a PKA phosphorylation site [48], a study using overexpressed M3 mAChR in CHO cells did not show a role for PKA in M3 mAChR phosphorylation [49].
Downstream of PLC, PKA inhibits Ca2+ release from the intracellular Ca2+ stores by phosphorylating the IP3R (4) and RyR (not shown), while hyperpolarizing the cell through phosphorylation of KCa2+ channels (5). Moreover, PKA limits contraction by inhibiting the activation of CPI-17 (6) which otherwise promotes PKC- and Rho kinase-mediated Ca2+ sensitization. PKA also inhibits RhoA-mediated actin polymerization (loss of stress fibers) via an unknown mechanism (not shown). In addition, PKA phosphorylates HSP20 (7) which promotes relaxation, possibly by regulating actin dynamics and actin-myosin binding.
Although PKA has long been presumed the key effector of the β2AR mediating the functional antagonism of pro-contractile signaling, it is important to remember that little if any direct evidence implicating PKA in this regard exists. We simply know that the β2AR activates PKA, and that multiple agents capable of activating PKA (e.g., EP2/4 receptors, forskolin, and phosphodiesterases) can also relax contracted ASM. Recently, another cAMP effector, Exchange protein directly activated by cAMP (Epac) (8), has recently been proposed as sufficient to inhibit ASM contraction, and perhaps required in the effect of known bronchorelaxants such as beta-agonists. Two different groups have shown the capacity of Epac to induce relaxation of pre-contracted smooth muscle tissues [50;51]. However, it remains difficult to ascertain conclusively to what extent Epac contributes to relaxation mediated by β2ARs or other physiological inducers of cAMP, as pharmacological Epac inhibitors are not available and most studies simply demonstrate the sufficiency of Epac to promote signaling and functional consequences through the use of Epac-selective cAMP analogs.
Cross-talk in the other direction: M3 and M2 mAChR regulation of the β2AR
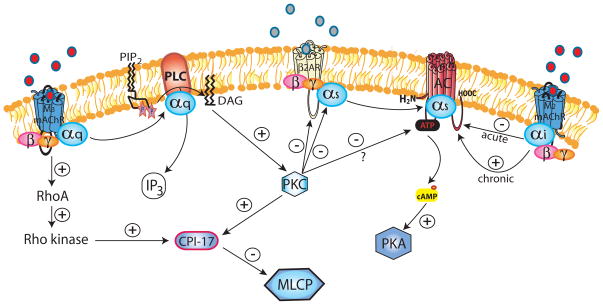
In addition to the induction of Ca2+-sensitization (detailed above), several studies have indicated that M3 mAChR activation promotes desensitization of the β2AR in a PKC-dependent manner (Figure 2). Using bovine ASM tissue strips, Boterman et al. have shown that M3 mAChR stimulation promotes heterologous (i.e. independent of beta-agonist-induced change in β2AR conformation) as well as homologous (i.e. beta-agonist-induced) desensitization of the β2AR resulting in diminished capacity to relax muscle [52;53]. A role for M3 mAChR-induced PKC-mediated heterologous β2AR desensitization has also been reported earlier in a study using co-expression of M3 mAChR and β2AR in CHO cells [54]. Direct activation of PKC by the DAG analog phorbol-12-myristate-13-acetate has been shown to inhibit AC, however it is still unknown whether this occurs in response to M3 mAChR activation [55].
Figure 2. M3/M2 mAChR regulation of the β2AR.
PKC is the major effector of M3 mAChR-mediated regulation of the β2AR receptor. In addition to the PKC and Rho kinase-mediated Ca2+ -sensitization as detailed above, PKC phosphorylates both the β2AR and the Gαs subunit, thereby promoting β2AR desensitization; both directly (heterologous) and by increasing sensitivity to beta-agonist-induced (homologous) desensitization. The activated M2 mAChR releases its Gαi subunit which inhibits AC, effectively limiting Gαs-induced cAMP production. However, prolonged activation of M2 mAChR can paradoxically sensitize AC and increase cAMP production, via an unknown mechanism. PMA-induced (receptor-independent) activation of PKC has been shown to inhibit AC, however, whether this holds true for M3 mAChR-stimulated PKC still requires confirmation.
Incubation with an M2 mAChR-selective muscarinic receptor agonist potentiates β2AR-mediated relaxation of tissues pre-contracted with MCh (but not those pre-contracted with histamine) [56;57], although some ex vivo studies have also reported that decreased tone post-M2 mAChR antagonist may also contribute to this effect and have questioned the role of M2 mAChR in functional antagonism [58]. The discrepancy may be a consequence of variation in pre-contracted tissue tone, mode of tension measurement (isometric versus isotonic) and species differences.
Functional antagonism mediated by M2 mAChR regulation of the β2AR has also been demonstrated in mice and is further exacerbated in GRK5−/− mice. β2AR treatment is less effective in relaxing either carbachol- or KCl-induced pre-contracted tracheal tissue of GRK5−/− mice [59]. This is presumably due to increased M2 mAChR Gi signaling inhibiting AC activity (potentially via GRK5 knockout alleviating M2 mAChR desensitization), as treatment with the M2 mAChR selective muscarinic receptor antagonist methocramine normalizes β2AR effectiveness to levels observed in WT mouse tissues. A role for GRK3 has similarly been suggested in the regulation of muscarinic receptor responses in the murine lung, as GRK3 ablation results in enhanced MCh constrictor responses [60]. Although the muscarinic receptor subtype responsible for the increased bronchoconstrictor response in this study has not been identified, the GRK3 locus has been implicated as a potential regulator of functional antagonism. Interestingly, although acute M2 mAChR stimulation inhibits AC activity, prolonged (18 h) treatment of human ASM cell cultures paradoxically results in increased basal and stimulated AC activity [55]. Opposing actions of M2 mAChR (stimulatory) and β2AR (inhibitory) activation on lung fibroblast proliferation and collagen synthesis implies a role for this antagonism in lung fibrosis [32;33;61].
Relevance to disease and therapy
Obstructive airway diseases are characterized and perhaps driven by dysfunction of GPCR-mediated signaling in the lung. Overpresentation of ACh drives a pathogenic increase in muscarinic (pro-contractile, pro-remodeling) signaling. It is unclear to what extent changes in receptor crosstalk per se contribute to the pathogenesis, but given the signaling between mAChRs and β2AR is competitive, an increase in mAChR signaling coupled with no change or a decrease in β2AR signaling (as a consequence of desensitization) alters the balance of signaling and results in greater ASM tone. There is evidence that approaches focusing on restoring the balance by regulating signaling to affect mAChR- β2AR competition are effective [62;63].
Beneficial effects of combined mAChR antagonists and beta-agonists relative to those of a (monotherapy) bronchodilator in COPD have been determined for SAMA/SABA [64] and more recently for LAMA/LABA [65;66]. Recent clinical trials provide evidence to support the longstanding off-label use of tiotropium as add-on therapy to LABA/inhaled corticosteroid (ICS) in asthma [67–69]. In patients with poorly controlled asthma, tiotropium improved lung function [67;68] and reduced exacerbations when added on to LABA/ICS [68]. Similarly, add-on of tiotropium in patients with COPD and concomitant asthma results in improved bronchodilatation and lung function [70].
A number of combination therapy inhalers are currently under development [5]. There is now ample clinical/scientific evidence of the benefits LAMA/LABA combination therapy. However, the nature of the cooperativity, in what cell types, contributing to this effect remains unclear; effects on ASM contractility are probably significant. An increasing number of studies in cell-based and animal models of lung disease combining LAMA and LABA are currently being performed and should be published in the near future. The data at the functional level are encouraging and the molecular mechanisms require further exploration [71;72].
In addition, the current combination therapies of LABA plus inhaled corticosteroids (Advair, Symbicort) also affect the balance of mAChR- β2AR signaling by activating β2ARs while reducing inflammation-induced cholinergic discharge [8].
The idea of mAChR- β2AR crosstalk promoting functional antagonism and controlling ASM contractile state provides a convenient paradigm for explaining the roles of these receptors in disease pathogenesis and therapy. However, recent studies in GPCR biology suggest that mAChR and β2AR (and probably all GPCR) signaling is more complex and extends beyond the canonical G protein signaling known for each receptor. If true, we are likely unappreciative of the full impact of GPCRs on various diseases, including effects on pathobiology and in their capacity to function therapeutically.
The β2AR has been shown to promote diverse and qualitatively distinct signaling events. In additional to canonical Gs/cAMP/PKA signaling, the β2AR has been shown to coupleto Gi via a “specificity switch” that occurs upon PKA-mediated phosphorylation of the β2AR [73]. G protein-independent signaling has also been demonstrated, occurring through an arrestin-dependent mechanism. Arrestin molecules, originally demonstrated to play a critical role in β2AR desensitization (and resensitization), are now known to function as scaffold proteins capable of initiating signaling to multiple pathways distinct from those involving heterotrimeric G proteins. Arrestins have been shown to mediate β2AR signaling to the p42/p44 [74], and p38 [75] MAPK pathways, to NF-κB [76], and to RhoA [77]. Although arrestins have been shown to mediate β2AR desensitization in ASM in vitro, ex vivo, and in vivo [62;78], arrestin-dependent signaling in airway cells is poorly understood, However, several studies suggest that arrestin is critical to the development of allergic lung inflammation and the asthma phenotype. Walker et al. [44] originally reported that mice lacking the beta-arrestin-2 gene fail to develop significant allergic inflammation and associated AHR after allergen sensitization and challenge.
The obvious question raised by Walker et al. involves the receptors and cell types in which arrestin function plays a pro-inflammatory and pro-asthmatic role. A recent study by Nichols et al. [79], utilizing protease-activated receptor 2 (PAR2) knockout and beta-arrestin-2 knockout mice, implicates PAR2-dependent arrestin signaling as important in the development of allergic lung inflammation. While it is conceivable that numerous GPCRs on numerous cell types promote inflammation in an arrestin-dependent manner, a series of studies by Bond and colleagues has led us to consider the β2AR as a critical mediator of arrestin-dependent pathology in allergic lung inflammation. Original studies from the Bond lab implicated β2AR agonism as required for the development of both lung inflammation and AHR in ovalbumin sensitized and challenged mice. β2AR knockout [80], or treatment of mice with the inverse agonist nadolol (but not the antagonist alprenolol) [81], inhibited mice from developing lung inflammation and AHR, leading the authors to conclude that constitutive β2AR agonism (receptor activity in the absence of ligand; only inverse agonists inhibit this activity) was permissive to pathology. Yet that interpretation was abandoned when it was demonstrated that depletion of systemic epinephrine had the same effect as β2AR knockout or chronic nadolol. But why did some “beta-blockers” work (e.g., nadolol, ICI 118,551) whereas others did not (alprenolol, carvedilol)? Moreover, results from clinical trials suggested one beta-blocker (nadolol) was effective in reducing AHR in asthmatics [82;83], while another (propranolol) was not [84]. The differential effects of these beta-blockers could be explained by their differing capacities to stimulate arrestin-dependent signaling. It is important to keep in mind that to date, β2AR ligands have been defined as agonists or antagonists based on their ability to stimulate, and block agonist-induced stimulation of, the Gs-adenylyl cyclase-cAMP pathway. However, multiple recent studies (reviewed in [85]) have demonstrated that β2AR (and other GPCR) ligands can exhibit different abilities to stimulate G protein- and arrestin- dependent signaling. Interestingly, nadolol and ICI 118,551 cannot stimulate (but can block) both G protein- and arrestin- dependent signaling, whereas the “beta-blockers” carvedilol and propranolol antagonize G protein but stimulate arrestin-dependent signaling [86;87]. Thus, the therapeutic efficacy of β2AR ligands for asthma may rely on their capacity to not activate/antagonize arrestin signaling, while perhaps stimulating the Gs signaling known to have a pro-relaxant effect on ASM.
With respect to the M3 mAChR, signaling via G protein and arrestins has been shown to differentially affect cellular functions, raising the possibility that biased ligands for the M3 mAChR may be therapeutically superior to existing drugs. Kong et al. [88] demonstrated that the early phase of glucose-induced insulin secretion in pancreatic islets cells was stimulated by G protein-dependent signaling, whereas the late phase was dependent G protein-independent/arrestin-dependent. Insight into the utility of biased M3 mAChR ligands in the treatment of obstructive lung disease would first require an understanding of the role of M3 mAChR-mediated arrestin signaling in airway and inflammatory cells, of which nothing is currently known.
In conclusion, crosstalk between mAChRs and β2AR in the airway, and specifically in ASM, plays a profound role in determining lung function in health and disease. Future studies further delineating the function of these receptors, particularly with respect to the consequences of G protein- and arrestin- dependent signaling, in the numerous cell types whose function/dysfunction contributes to airway diseases, will undoubtedly facilitate the development of new and better therapies.
Highlights (for review).
Crosstalk between mAChRs and β2ARs dictates ASM contractile state and thus airway resistance and lung function.
mAChRs and β2ARs regulate multiple airway cell functions affecting airway inflammation and remodeling.
Biased ligands of mAChRs and β2ARs may promote “therapeutic” while avoiding “pathogenic” signaling.
Acknowledgments
Work in the Penn lab is funded by National Institutes of Health grants HL58506, HL114471, and HL93013.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Billington C, Penn R. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- 2.Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal. 2006;18:2105–2120. doi: 10.1016/j.cellsig.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Webb BL, Hirst SJ, Giembycz MA. Protein kinase C isoenzymes: a review of their structure, regulation and role in regulating airways smooth muscle tone and mitogenesis. Br J Pharmacol. 2000;130:1433–1452. doi: 10.1038/sj.bjp.0703452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Huang Y, Gunst SJ. The small GTPase RhoA regulates the contraction of smooth muscle tissues by catalyzing the assembly of cytoskeletal signaling complexes at membrane adhesion sites. J Biol Chem. 2012;287:33996–34008. doi: 10.1074/jbc.M112.369603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PJ. Triple inhalers for obstructive airways disease: will they be useful? Expert Rev Respir Med. 2011;5:297–300. doi: 10.1586/ers.11.26. [DOI] [PubMed] [Google Scholar]
- 6.Kistemaker LEM, Oenema TA, Meurs H, Gosens R. Regulation of airway inflammation and remodeling by muscarinic receptors: Perspectives on anticholinergic therapy in asthma and COPD. Life Sci. 2012;91:1126–1133. doi: 10.1016/j.lfs.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311:421–425. doi: 10.1056/NEJM198408163110701. [DOI] [PubMed] [Google Scholar]
- 8.Gosens R, Zaagsma J, Meurs H, Halayko A. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meurs H, Oenema TA, Kistemaker LE, Gosens R. A new perspective on muscarinic receptor antagonism in obstructive airways diseases. Curr Opin Pharmacol. 2013;13:316–323. doi: 10.1016/j.coph.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Haddad EB, Mak JC, Barnes PJ. Characterization of [3H]Ba 679 BR, a slowly dissociating muscarinic antagonist, in human lung: radioligand binding and autoradiographic mapping. Mol Pharmacol. 1994;45:899–907. [PubMed] [Google Scholar]
- 11.Disse B, Speck GA, Rominger KL, Witek TJ, Jr, Hammer R. Tiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci. 1999;64:457–464. doi: 10.1016/s0024-3205(98)00588-8. [DOI] [PubMed] [Google Scholar]
- 12.Gosens R, Nelemans SA, Grootte Bromhaar MM, McKay S, Zaagsma J, Meurs H. Muscarinic M3-receptors mediate cholinergic synergism of mitogenesis in airway smooth muscle. Am J Respir Cell Mol Biol. 2003;28:257–262. doi: 10.1165/rcmb.2002-0128OC. [DOI] [PubMed] [Google Scholar]
- 13.Billington CK, Kong KC, Bhattacharyya R, Wedegaertner PB, Panettieri RA, Jr, Chan TO, Penn RB. Cooperative regulation of p70S6 kinase by receptor tyrosine kinases and G protein-coupled receptors augments airway smooth muscle growth. Biochemistry. 2005;44:14595–14605. doi: 10.1021/bi0510734. [DOI] [PubMed] [Google Scholar]
- 14.Gosens R, Rieks D, Meurs H, Ninaber DK, Rabe KF, Nanninga J, Kolahian S, Halayko AJ, Hiemstra PS, Zuyderduyn S. Muscarinic M3 receptor stimulation increases cigarette smoke-induced IL-8 secretion by human airway smooth muscle cells. Eur Respir J. 2009;34:1436–1443. doi: 10.1183/09031936.00045209. [DOI] [PubMed] [Google Scholar]
- 15.Oenema T, Kolahian S, Nanninga J, Rieks D, Hiemstra P, Zuyderduyn S, Halayko A, Meurs H, Gosens R. Pro-inflammatory mechanisms of muscarinic receptor stimulation in airway smooth muscle. Respir Res. 2010;11:130. doi: 10.1186/1465-9921-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Profita M, Bonanno A, Siena L, Ferraro M, Montalbano AM, Pompeo F, Riccobono L, Pieper MP, Gjomarkaj M. Acetylcholine mediates the release of IL-8 in human bronchial epithelial cells by a NFkB/ERK-dependent mechanism. Eur J Pharmacol. 2008;582:145–153. doi: 10.1016/j.ejphar.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Sato E, Koyama S, Okubo Y, Kubo K, Sekiguchi M. Acetylcholine stimulates alveolar macrophages to release inflammatory cell chemotactic activity. Am J Physiol Lung Cell Mol Physiol. 1998;274:L970–L979. doi: 10.1152/ajplung.1998.274.6.L970. [DOI] [PubMed] [Google Scholar]
- 18.Oenema TA, Maarsingh H, Smit M, Groothuis GMM, Meurs H, Gosens R. Bronchoconstriction induces TGF-β1 release and airway remodelling in guinea pig lung slices. PLoS One. 2013;8:e65580. doi: 10.1371/journal.pone.0065580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grainge CL, Lau LCK, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of Bronchoconstriction on Airway Remodeling in Asthma. N Engl J Med. 2011;364:2006–2015. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 20.Ramnarine SI, Haddad EB, Khawaja AM, Mak JC, Rogers DF. On muscarinic control of neurogenic mucus secretion in ferret trachea. J Physiol. 1996;494:577–586. doi: 10.1113/jphysiol.1996.sp021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosens R, Bos IS, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005;171:1096–1102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]
- 22.Bos IST, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H, Zaagsma J. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007;30:653–661. doi: 10.1183/09031936.00004907. [DOI] [PubMed] [Google Scholar]
- 23.Pera T, Zuidhof A, Valadas J, Smit M, Schoemaker RG, Gosens R, Maarsingh H, Zaagsma J, Meurs H. Tiotropium inhibits pulmonary inflammation and remodelling in a guinea pig model of COPD. Eur Respir J. 2011;38:789–796. doi: 10.1183/09031936.00146610. [DOI] [PubMed] [Google Scholar]
- 24.Kistemaker LEM, Bos ST, Mudde WM, Hylkema MN, Hiemstra PS, Wess J, Meurs H, Kerstjens HAM, Gosens R. Muscarinic M3 receptors contribute to allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2013-0220OC. In press. This study shows conclusively for the first time that m3 mAChRs contribute to allergen-induced remodeling independent of inflammation. [DOI] [PubMed] [Google Scholar]
- 25.Grainge CL, Lau LCK, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of Bronchoconstriction on Airway Remodeling in Asthma. N Engl J Med. 2011;364:2006–2015. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 26.Katada T, Northup JK, Bokoch GM, Ui M, Gilman AG. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984;259:3578–3585. [PubMed] [Google Scholar]
- 27.Costello RW, Jacoby DB, Fryer AD. Pulmonary neuronal M2 muscarinic receptor function in asthma and animal models of hyperreactivity. Thorax. 1998;53:613–618. doi: 10.1136/thx.53.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, Wess J, Haberberger RV. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol. 2003;64:1444–1451. doi: 10.1124/mol.64.6.1444. [DOI] [PubMed] [Google Scholar]
- 29.Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004;18:711–713. doi: 10.1096/fj.03-0648fje. [DOI] [PubMed] [Google Scholar]
- 30.Hirshman CA, Emala CW. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am J Physiol Lung Cell Mol Physiol. 1999;277:L653–L661. doi: 10.1152/ajplung.1999.277.3.L653. [DOI] [PubMed] [Google Scholar]
- 31.Togashi H, Emala CW, Hall IP, Hirshman CA. Carbachol-induced actin reorganization involves Gi activation of Rho in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 1998;274:L803–L809. doi: 10.1152/ajplung.1998.274.5.L803. [DOI] [PubMed] [Google Scholar]
- 32.Matthiesen S, Bahulayan A, Kempkens S, Haag S, Fuhrmann M, Stichnote C, Juergens UR, Racke K. Muscarinic receptors mediate stimulation of human lung fibroblast proliferation. Am J Respir Cell Mol Biol. 2006;35:621–627. doi: 10.1165/rcmb.2005-0343RC. [DOI] [PubMed] [Google Scholar]
- 33.Haag S, Matthiesen S, Juergens UR, Racke K. Muscarinic receptors mediate stimulation of collagen synthesis in human lung fibroblasts. Eur Respir J. 2008;32:555–562. doi: 10.1183/09031936.00129307. [DOI] [PubMed] [Google Scholar]
- 34.Oenema TA, Mensink G, Smedinga L, Halayko AJ, Zaagsma J, Meurs H, Gosens R, Dekkers BG. Cross-talk between transforming growth factor-beta(1) and muscarinic M(2) receptors augments airway smooth muscle proliferation. Am J Respir Cell Mol Biol. 2013;49:18–27. doi: 10.1165/rcmb.2012-0261OC. [DOI] [PubMed] [Google Scholar]
- 35.Hallsworth MP, Twort CHC, Lee TH, Hirst SJ. β2-Adrenoceptor agonists inhibit release of eosinophil-activating cytokines from human airway smooth muscle cells. Br J Pharmacol. 2001;132:729–741. doi: 10.1038/sj.bjp.0703866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazzeri N, Belvisi MG, Patel HJ, Yacoub MH, Fan Chung K, Mitchell JA. Effects of Prostaglandin E2 and cAMP Elevating Drugs on GM-CSF Release by Cultured Human Airway Smooth Muscle Cells. Am J Respir Cell Mol Biol. 2001;24:44–48. doi: 10.1165/ajrcmb.24.1.4027. [DOI] [PubMed] [Google Scholar]
- 37.Ammit AJ, Lazaar AL, Irani C, O’Neill GM, Gordon ND, Amrani Y, Penn RB, Panettieri RA., Jr Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth muscle cells: modulation by glucocorticoids and beta-agonists. Am J Respir Cell Mol Biol. 2002;26:465–474. doi: 10.1165/ajrcmb.26.4.4681. [DOI] [PubMed] [Google Scholar]
- 38.Futamura K, Orihara K, Hashimoto N, Morita H, Fukuda S, Sagara H, Matsumoto K, Tomita Y, Saito H, Matsuda A. beta2-Adrenoceptor agonists enhance cytokine-induced release of thymic stromal lymphopoietin by lung tissue cells. Int Arch Allergy Immunol. 2010;152:353–361. doi: 10.1159/000288288. [DOI] [PubMed] [Google Scholar]
- 39.Marone G, Ambrosio G, Bonaduce D, Genovese A, Triggiani M, Condorelli M. Inhibition of IgE-mediated histamine release from human basophils and mast cells by fenoterol. Int Arch Allergy Appl Immunol. 1984;74:356–361. doi: 10.1159/000233573. [DOI] [PubMed] [Google Scholar]
- 40.Loza MJ, Peters SP, Foster S, Khan IU, Penn RB. β-Agonist enhances type 2 T-cell survival and accumulation. J Allergy Clin Immunol. 2007;119:235–244. doi: 10.1016/j.jaci.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Goncharova EA, Goncharov DA, Zhao H, Penn RB, Krymskaya VP, Panettieri RA., Jr beta2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein. Am J Respir Cell Mol Biol. 2012;46:48–54. doi: 10.1165/rcmb.2011-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, Pascual RM, Panettieri RA, Penn RB. Anti-mitogenic effects of β-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J. 2011;25:389–397. doi: 10.1096/fj.10-164798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenoy SK, Lefkowitz RJ. β-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komalavilas P, Penn RB, Flynn CR, Thresher J, Lopes LB, Furnish EJ, Guo M, Pallero MA, Murphy-Ullrich JE, Brophy CM. The small heat shock-related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L69–L78. doi: 10.1152/ajplung.00235.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ba M, Singer CA, Tyagi M, Brophy C, Baker JE, Cremo C, Halayko A, Gerthoffer WT. HSP20 phosphorylation and airway smooth muscle relaxation. Cell Health Cytoskelet. 2009;2009:27–42. doi: 10.2147/chc.s5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. Beta-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willets JM, Mistry R, Nahorski SR, Challiss RA. Specificity of g protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell m3 muscarinic acetylcholine receptor signaling. Mol Pharmacol. 2003;64:1059–1068. doi: 10.1124/mol.64.5.1059. [DOI] [PubMed] [Google Scholar]
- 49.Tobin AB, Nahorski SR. Rapid agonist-mediated phosphorylation of m3- muscarinic receptors revealed by immunoprecipitation. J Biol Chem. 1993;268:9817–9823. [PubMed] [Google Scholar]
- 50.Roscioni SS, Maarsingh H, Elzinga CRS, Schuur J, Menzen M, Halayko AJ, Meurs H, Schmidt M. Epac as a novel effector of airway smooth muscle relaxation. J Cell Mol Med. 2011;15:1551–1563. doi: 10.1111/j.1582-4934.2010.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, Neppl RL, Stevenson AS, Khromov AS, Chrzanowska-Wodnicka M, et al. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J Biol Chem. 2011;286:16681–16692. doi: 10.1074/jbc.M110.205062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boterman M, Elzinga CRS, Wagemakers D, Eppens PB, Zaagsma J, Meurs H. Potentiation of β-adrenoceptor function in bovine tracheal smooth muscle by inhibition of protein kinase C. Eur J Pharmacol. 2005;516:85–92. doi: 10.1016/j.ejphar.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 53.Boterman M, Smits SRJG, Meurs H, Zaagsma J. Protein kinase C potentiates homologous desensitization of the β2-adrenoceptor in bovine tracheal smooth muscle. Eur J Pharmacol. 2006;529:151–156. doi: 10.1016/j.ejphar.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 54.Budd DC, Challiss RA, Young KW, Tobin AB. Cross talk between m3-muscarinic and beta(2)-adrenergic receptors at the level of receptor phosphorylation and desensitization. Mol Pharmacol. 1999;56:813–823. [PubMed] [Google Scholar]
- 55.Billington CK, Hall IP, Mundell SJ, Parent JL, Panettieri RA, Jr, Benovic JL, Penn RB. Inflammatory and contractile agents sensitize specific adenylyl cyclase isoforms in human airway smooth muscle. Am J Respir Cell Mol Biol. 1999;21:597–606. doi: 10.1165/ajrcmb.21.5.3759. [DOI] [PubMed] [Google Scholar]
- 56.Fernandes LB, Fryer AD, Hirshman CA. M2 muscarinic receptors inhibit isoproterenol-induced relaxation of canine airway smooth muscle. J Pharmacol Exp Ther. 1992;262:119–126. [PubMed] [Google Scholar]
- 57.Mitchell RW, Koenig SM, Popovich KJ, Kelly E, Tallet J, Leff AR. Pertussis toxin augments beta-adrenergic relaxation of muscarinic contraction in canine trachealis. Am Rev Respir Dis. 1993;147:327–331. doi: 10.1164/ajrccm/147.2.327. [DOI] [PubMed] [Google Scholar]
- 58.Roffel AF, Meurs H, Elzinga CRS, Zaagsma J. Muscarinic M2 receptors do not participate in the functional antagonism between methacholine and isoprenaline in guinea pig tracheal smooth muscle. Eur J Pharmacol. 1993;249:235–238. doi: 10.1016/0014-2999(93)90438-n. [DOI] [PubMed] [Google Scholar]
- 59.Walker JKL, Gainetdinov RR, Feldman DS, McFawn PK, Caron MG, Lefkowitz RJ, Premont RT, Fisher JT. G protein-coupled receptor kinase 5 regulates airway responses induced by muscarinic receptor activation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L312–L319. doi: 10.1152/ajplung.00255.2003. [DOI] [PubMed] [Google Scholar]
- 60.Walker JKL, Peppel K, Lefkowitz RJ, Caron MG, Fisher JT. Altered airway and cardiac responses in mice lacking G protein-coupled receptor kinase 3. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1214–R1221. doi: 10.1152/ajpregu.1999.276.4.R1214. [DOI] [PubMed] [Google Scholar]
- 61.Lamyel F, Warnken-Uhlich M, Seemann WK, Mohr K, Kostenis E, Ahmedat AS, Smit M, Gosens R, Meurs H, Miller-Larsson A, et al. The β2-subtype of adrenoceptors mediates inhibition of pro-fibrotic events in human lung fibroblasts. Naunyn-Schmiedeberg’s Arch Pharmacol. 2011;384:133–145. doi: 10.1007/s00210-011-0655-5. [DOI] [PubMed] [Google Scholar]
- 62.Deshpande DA, Theriot BS, Penn RB, Walker JKL. β-Arrestins specifically constrain β2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22:2134–2141. doi: 10.1096/fj.07-102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deshpande DA, Yan H, Kong KC, Tiegs BC, Morgan SJ, Pera T, Panettieri RA, Eckhart AD, Penn RB. Exploiting functional domains of GRK2/3 to alter the competitive balance of pro- and anticontractile signaling in airway smooth muscle. FASEB J. 2013;28:956–65. doi: 10.1096/fj.13-240226. This study reveals the potential for using functional domains of GRK2/3 to selectively inhibit pro-contractile and enhance relaxant signaling in ASM. Beta-2 adrenoceptor (but not m3 mAChR) signaling and function is augmented by peptides that inhibit GRK2/3, while peptides containing the N-terminus of GRK2 inhibit m3 mAChR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.COMBIVENT Inhalation Aerosol Study Group. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. an 85-day multicenter trial combivent inhalation aerosol study group. Chest. 1994;105:1411–1419. doi: 10.1378/chest.105.5.1411. [DOI] [PubMed] [Google Scholar]
- 65.van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, Cornelissen PJ. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129:509–517. doi: 10.1378/chest.129.3.509. [DOI] [PubMed] [Google Scholar]
- 66.Magnussen H, Paggiaro P, Schmidt H, Kesten S, Metzdorf N, Maltais F. Effect of combination treatment on lung volumes and exercise endurance time in COPD. Respir Med. 2012;106:1413–1420. doi: 10.1016/j.rmed.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 67.Kerstjens HA, Disse B, Schroder-Babo W, Bantje TA, Gahlemann M, Sigmund R, Engel M, van Noord JA. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128:308–314. doi: 10.1016/j.jaci.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 68.Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, Bateman ED. Tiotropium in Asthma Poorly Controlled with Standard Combination Therapy. N Engl J Med. 2012;367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 69.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, Boushey HA, Calhoun WJ, Castro M, Cherniack RM, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–1726. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magnussen H, Bugnas B, van Noord J, Schmidt P, Gerken F, Kesten S. Improvements with tiotropium in COPD patients with concomitant asthma. Respir Med. 2008;102:50–56. doi: 10.1016/j.rmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Costa L, Roth M, Miglino N, Keglowich L, Zhong J, Lardinois D, Tamm M, Borger P. Tiotropium sustains the anti-inflammatory action of olodaterol via the cyclic AMP pathway. Pulm Pharmacol Ther. 2013 doi: 10.1016/j.pupt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Smit M, Zuidhof AB, Bos SIT, Maarsingh H, Gosens R, Zaagsma J, Meurs H. Bronchoprotection by olodaterol is synergistically enhanced by tiotropium in a guinea pig model of allergic asthma. J Pharmacol Exp Ther. 2013;348:303–10. doi: 10.1124/jpet.113.208439. [DOI] [PubMed] [Google Scholar]
- 73.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 74.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. beta- arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 75.Gong K, Li Z, Xu M, Du J, Lv Z, Zhang Y. A novel protein kinase A-independent, beta-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by beta2-adrenergic receptors. J Biol Chem. 2008;283:29028–29036. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 77.Ma X, Zhao Y, Daaka Y, Nie Z. Acute activation of beta2-adrenergic receptor regulates focal adhesions through betaArrestin2- and p115RhoGEF protein-mediated activation of RhoA. J Biol Chem. 2012;287:18925–18936. doi: 10.1074/jbc.M112.352260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penn RB, Pascual RM, Kim YM, Mundell SJ, Krymskaya VP, Panettieri RA, Jr, Benovic JL. Arrestin specificity for G protein-coupled receptors in human airway smooth muscle. J Biol Chem. 2001;276:32648–32656. doi: 10.1074/jbc.M104143200. [DOI] [PubMed] [Google Scholar]
- 79.Nichols HL, Saffeddine M, Theriot BS, Hegde A, Polley D, El Mays T, Vliagoftis H, Hollenberg MD, Wilson EH, Walker JK, et al. beta-Arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway. Proc Natl Acad Sci USA. 2012;109:16660–16665. doi: 10.1073/pnas.1208881109. This study indicates that PAR2 promotes beneficial G protein-mediated signaling as well as pathogenic beta-arrestin signaling, suggesting potential for biased PAR2 ligands in the treatment of airway disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thanawala VJ, Forkuo GS, Al Sawalha N, Azzegagh Z, Nguyen LP, Eriksen JL, Tuvim MJ, Lowder TW, Dickey BF, Knoll BJ, et al. β2-Adrenoceptor Agonists Are Required for Development of the Asthma Phenotype in a Murine Model. Am J Respir Cell Mol Biol. 2013;48:220–229. doi: 10.1165/rcmb.2012-0364OC. This study suggests that β2AR agonists (including endogenous epinephrine), rather than β2AR constitutive receptor activity, are required for the development of asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, Knoll BJ, Dickey BF, Bond RA. β2-Adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanania NA, Singh S, El Wali R, Flashner M, Franklin AE, Garner WJ, Dickey BF, Parra S, Ruoss S, Shardonofsky F, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: an open-label pilot study. Pulm Pharmacol Ther. 2008;21:134–141. doi: 10.1016/j.pupt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanania NA, Mannava B, Franklin AE, Lipworth BJ, Williamson PA, Garner WJ, Dickey BF, Bond RA. Response to salbutamol in patients with mild asthma treated with nadolol. Eur Respir J. 2010;36:963–965. doi: 10.1183/09031936.00003210. [DOI] [PubMed] [Google Scholar]
- 84.Short PM, Williamson PA, Anderson WJ, Lipworth BJ. Randomized placebo-controlled trial to evaluate chronic dosing effects of propranolol in asthma. Am J Respir Crit Care Med. 2013;187:1308–1314. doi: 10.1164/rccm.201212-2206OC. [DOI] [PubMed] [Google Scholar]
- 85.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stallaert W, Dorn JF, van der WE, Audet M, Bouvier M. Impedance responses reveal beta(2)-adrenergic receptor signaling pluridimensionality and allow classification of ligands with distinct signaling profiles. PLoS One. 2012;7:e29420. doi: 10.1371/journal.pone.0029420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kong KC, Butcher AJ, McWilliams P, Jones D, Wess J, Hamdan FF, Werry T, Rosethorne EM, Charlton SJ, Munson SE, et al. M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc Natl Acad Sci USA. 2010;107:21181–21186. doi: 10.1073/pnas.1011651107. This study demonstrates the m3 mAChR transduces 2 distinct signaling events in islet cells: one G protein-dependent promoting acute insulin secretion, another G protein-independent and arrestin-dependent mediating a late phase insulin secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]