Abstract
Following injury to a peripheral nerve the denervated distal nerve segment undergoes remarkable changes including loss of the blood-nerve barrier, Schwann cell proliferation, macrophage invasion, and the production of many cytokines and neurotrophic factors. The aggregate consequence of such changes is that the denervated nerve becomes a permissive and even preferred target for regenerating axons from the proximal nerve segment. The possible role that an original end-organ target (e.g. muscle) may play in this phenomenon during the regeneration period is largely unexplored. We used the rat femoral nerve as an in vivo model to begin to address this question. We also examined the effects of disrupting communication with muscle in terms of the accuracy of regenerating motor neurons as judged by their ability to correctly project to their original terminal nerve branch. Our results demonstrate that the accuracy of regenerating motor neurons is dependent upon the denervated nerve segment remaining in uninterrupted continuity with muscle. We hypothesized that this influence of muscle on the denervated nerve might be via diffusion driven movement of biomolecules or the active axonal transport that continues in severed axons for several days in the rat, so we devised experiments to separate these two possibilities. Our data show that disrupting ongoing diffusion driven movement in a denervated nerve significantly reduces the accuracy of regenerating motor neurons.
Keywords: PNS regeneration, denervated nerve, diffusion, Band of Bungner
Introduction
Peripheral nerve injury is a major problem confronting modern medicine, with more than 360,000 procedures performed annually in the United States (Kreiger et al., 1981). Unfortunately, the success rate of such procedures has not markedly improved since the introduction of enhanced microsurgical techniques several decades ago: only ~ 10% of adults will recover normal nerve function using state-of-the-art techniques (Sunderland, 1991, Madison etal., 1992, Brushart, 1998).
Our laboratory has a long standing interest in the field of peripheral nerve repair and the underlying mechanisms that guide the regeneration accuracy of motor neurons at the level of a terminal nerve branch, often using the femoral nerve of rodents as an in vivo model system (Madison et al., 1996, Madison et al., 1999, Robinson and Madison, 2005, Madison et al., 2007, Uschold et al., 2007). Proximally in the leg, femoral nerve motor axons are dispersed across the entire mixed nerve. Distally, the nerve divides into two approximately equal terminal branches; a muscle branch to the quadriceps muscle (which contains all of the motor neuron projections) and a purely sensory branch which continues as the saphenous nerve (Brushart, 1988). Thus by using simple retrograde tracing techniques one can obtain information regarding both the total number of motor neurons that regenerate following parent nerve injury as well as the number that correctly project back to the terminal muscle branch versus inappropriately projecting to the terminal cutaneous branch.
A large body of work has led to the hypothesis that there are two main sources of trophic support for regenerating motor neurons in this model, Schwann cells in the distal nerve stump and signals that emanate from muscle. Some studies have shown overall molecular differences between the muscle and cutaneous terminal nerve branches in the femoral nerve model and have suggested that it is these inherent molecular differences that guide motor neuron reinnervation of the terminal muscle branch such as L2/HNK-1 (Martini et al., 1992, Löw et al., 1994, Martini et al., 1994), enhanced BDNF/TrkB signaling by the muscle pathway (Eberhardt et al., 2006), or specific motor and sensory Schwann cell identities that are recognized by regenerating motor axons (Brushart et al., 1998, Hoke et al., 2006). However, using molecular targeting techniques the respective roles of the end-organ influence of muscle versus terminal nerve Schwann cells were examined by selectively removing Schwann cells distal to a nerve lesion (Madison et al., 2009). This work demonstrated that the presence of the original population of Schwann cells in the terminal muscle nerve branch is neither sufficient nor necessary for the preferential projection of motor neurons to that terminal nerve branch. Rather, the influence of muscle appears to be the key factor. Motor neuron regeneration accuracy in that study was quantified eight weeks following parent femoral nerve repair, at a time point when regenerating motor neurons had clearly reinnervated the quadriceps muscle. This raises the question as to whether physical axonal contact with muscle by the regenerating motor neurons was necessary for this effect, or if perhaps the distal terminal nerve branch itself might be able to exert some effect prior to actual muscle contact.
We therefore undertook the following experiments to assess whether signals from muscle might influence motor neuron regeneration accuracy prior to muscle contact, and if so, how such signals come to reside within the denervated nerve. It is well known that with an intact innervation signals from muscle are continuously retrogradely transported back to motor neurons, and that motor neurons rapidly react to becoming disconnected from muscle (Vrbova et al., 1995). It has more recently been shown that such transport mechanisms continue in the denervated nerve segment for several days following a nerve lesion (Hassig et al., 1991, Smith, 1993, Watson et al., 1993). This time-limited ongoing active transport might be one mechanism by which muscle derived signals influence subsequent motor neuron regeneration. Another possible mechanism could be that muscle-derived signals gained access, perhaps by diffusion driven movement, to the denervated nerve that remains tightly attached to muscle. The present experiments were undertaken to separate these two mechanisms as much as possible in order to ascertain their respective influence on shaping motor neuron regeneration accuracy.
Experimental Procedures
All animal procedures were approved by the Veterans Affairs Medical Center animal use committee. Sprague Dawley rats (male and female, 150–200 grams, obtained from Charles River) were housed on a standard 12 hr light/dark cycle and fed ad libitum. Surgical procedures were carried out under deep anesthesia using intraperitoneal administration of ketamine, xylazine and acepromazine (as a combined solution containing 100, 6 and 1 mg/kg respectively) in normal (0.9%) saline. The fibrin sealant used in the present work for nerve repair was prepared according to the manufacturer’s instructions (Tisseel, Baxter Healthcare Products, Glendale, CA).
Femoral Nerve Surgeries
We utilized the intact-muscle:short-cutaneous (IM-SC, Fig. 1) preparation which has been previously described (Uschold et al., 2007, Madison et al., 2009). Briefly, the parent femoral nerve was exposed unilaterally and freed up from surrounding tissue using an inguinal approach which also involved transecting the nerve branches to the iliacus and pectineus muscles. The blunt tip of a forceps was then gently inserted under the distal femoral nerve branches to skin (saphenous nerve) and to muscle (quadriceps) such that the nerve branches were freed from surrounding tissue the entire length of the parent nerve down to the level of the quadriceps muscle. The parent femoral nerve was then transected with microscissors above the level of the iliacus branch, a region where axons to the two terminal nerve branches are randomly distributed (Brushart, 1990). A small amount of fibrin sealer (without activator) was then placed on the parent femoral nerve transection site, the nerve ends were reapposed, and then activator was applied to complete the sealant repair. Distally, the terminal cutaneous branch was transected at a point where its length was equal to that of the intact muscle branch, and then both the proximal and distal cutaneous nerve ends were ligated with 7-O silk suture (Ethicon, Somerville, NJ). The site was then closed and sutured with 4-0 Vicryl (Ethicon Inc, Somerville, NJ).
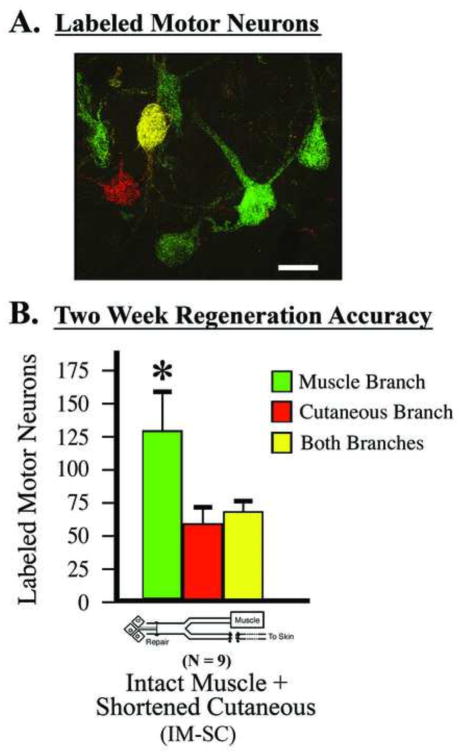
Figure 1.
Surgical model used to quantify motor neuron regeneration accuracy. A) Retrogradely labeled motor neurons in the Intact-muscle:shortened-cutaneous (IM-SC) surgical model. Single labeled motor neurons are quantified as projecting solely to either the terminal muscle branch or cutaneous branch (i.e., either green or red), while the double-labeled motor neurons are quantified as projecting to both branches (i.e., green and red, appearing as yellow). Size bar = 25 μm. B) Quantification in this model system two weeks after parent femoral nerve repair shows a significant preference for motor neurons to project to the terminal muscle branch compared to the cutaneous branch; mean ± SEM, paired t-test (t=2.67, p=.02).
Several experimental groups of animals received the basic IM-SC preparation and also received various interventions to the terminal nerve branch to the quadriceps muscle to alter retrograde transport and/or diffusion driven movement of biomolecules. To examine the effect of blocking retrograde transport colchicine was applied to the terminal muscle branch as follows. A small sheet of parafilm (Pechiney, Chicago, IL) was placed under the exposed terminal muscle branch, and then a gelfoam pledget (Pfizer, NY, NY) soaked in 25 mM colchicine (C9754, Sigma-Aldrich, St. Louis, MO dissolved in normal saline) was applied to the muscle branch for 15 minutes. The dose and time of colchicine application was based on previous studies in the literature for rat nerves (Colburn and DeLeo, 1999, Mader et al., 2004), and the effectiveness of this approach was verified with small pilot studies (data not shown). After removing the gelfoam, the application zone was rinsed with saline and the site closed. As a control for the colchicine application, some animals received gelfoam soaked in saline. To disrupt both diffusion driven movement and active axonal transport additional groups of animals received a crush of the muscle branch at various time points after the parent femoral nerve repair by applying pressure for 15 seconds using fine forceps, then rotating the forceps 90 degrees and crushing again for an additional 15 seconds.
Determination of Pathway Reservation Preference
Motor neuron reinnervation accuracy was determined two weeks after parent femoral nerve repair. The terminal branches were re-exposed and separated from each other by food-grade silicone grease dams, trimmed to ~3 mm distal to the normal femoral nerve bifurcation, and randomly assigned to receive crystals of diffusible dextrans (Fritzsch, 1993) labeled with either Alexafluor 488 (D-22910, Molecular Probes, Eugene, OR, USA) or Alexafluor 594 (D-22913, Molecular Probes). This anatomical location is proximal to the intervention zone shown in Figure 5A such that regenerated axons that had grown just past the normal femoral nerve bifurcation were exposed to the dextran labels. After crystal application, each branch was blotted and sealed with silicone grease. The surgical site was closed, sutured, and the rats allowed to recover. Three days later, rats received an overdose of anesthetic and were perfused through the heart with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in PBS. The lumbar spinal cord was removed, post-fixed for several hours, and sucrose protected overnight. The cord was frozen on dry ice and horizontal sections were cut with a cryostat. Serial 25-μm frozen sections were thawed in PBS, mounted onto glass slides, air dried and coverslipped using Prolong according to the manufacturer’s instructions (P-7481, Molecular Probes). All serial sections were examined and retrogradely labeled motor neurons containing a nucleus were identified using a composite filter set that allowed simultaneous visualization of both labels (#51006, Chroma Technology, Brattleboro, VT, USA) in a fluorescence-equipped Zeiss Axiophot microscope, at 250X magnification. Motor neuron counts were carried out by blinded independent observers and scored as either single-labeled (488 or 594 only) or double-labeled (both 488 and 594). Motor neurons labeled only from the muscle branch, only from the cutaneous branch or from both branches were tabulated and their counts corrected for split cells (Abercrombie, 1946). Six naïve animals were used to establish the baseline number of femoral motor neurons and to examine the parity between the two dextran labels. Spinal cords were rejected for further analysis if they contained less than a total of 100 labeled motor neurons (N=2). All numbers are expressed as mean ± SEM. Because our data involve quantifying labeled motor neurons we consistently refer to regeneration choices made by the motor neurons themselves rather than their projecting axons which we have not attempted to quantify.
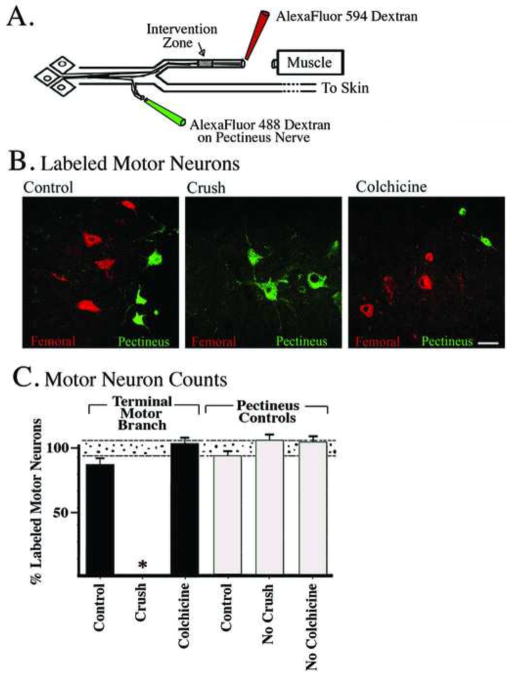
Figure 5.
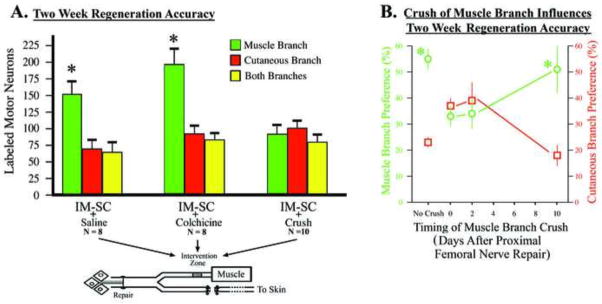
Motor neuron regeneration accuracy is lost by a nerve crush of the terminal muscle branch. A) Animals received the intact-muscle:short-cutaneous (IM-SC) surgical preparation and also received various interventions to the terminal muscle branch during the same surgery (“intervention zone”). Regeneration accuracy was quantified two weeks later as the number of femoral nerve motor neurons that correctly projected back to the original terminal muscle branch versus the inappropriate cutaneous branch. Animals that received colchicine application to the terminal muscle branch, to disrupt retrograde transport in the denervated nerve, displayed robust regeneration accuracy (t=3.45, p=.01) similar to control animals that received saline application (t=3.12, p=.0001). Regeneration accuracy was blocked when animals received a crush of the terminal muscle branch that disrupts both retrograde transport and diffusion in the denervated nerve (t=0.63, p=.56). B) To further investigate the effects of a crush to the terminal muscle branch additional animals received a crush of the terminal muscle branch at various days after the parent femoral nerve repair. The “pathway reinnervation preference” was quantified as described in Methods. When there was no crush of the terminal muscle branch there was a significant muscle branch preference for regenerating motor neurons (t=5.56, p=.001). If the terminal muscle branch was crushed at the same time or 2 days after the parent femoral nerve repair the muscle branch preference was blocked. However, if the terminal muscle branch was crushed 10 days after the femoral nerve repair there was a significant muscle branch preference (t=3.12, p=.02) as was seen with no crush.
A regeneration pathway preference index for each animal was determined by dividing the number of retrogradely labeled motor neurons reinnervating only one pathway by the total number of labeled motor neurons and expressing the quotient as a percentage. Thus, % muscle pathway preference was determined by dividing the number of motor neurons labeled from only the muscle pathway by the total number of labeled motor neurons; % cutaneous pathway preference was determined in an identical fashion using the number of motor neurons labeled from only the cutaneous pathway as the numerator. Combining these two percentages for each animal is always less than 100% because the number of double-labeled neurons was included in the denominator for each pathway preference (i.e., as part of the total number of labeled cells) but not in the numerator.
Statistics
Student t-tests for paired data were used within groups to compare the number of motor neurons labeled from the muscle and cutaneous branches. Differences were considered statistically significant when p < .05. Analysis of variance was used to determine differences among groups. Differences were considered statistically significant when F < .05 and p < .05.
Muscle Injections of GFP-labeled Non-toxic c-fragment of Tetanus Toxin (GFP-TTC)
To examine the possibility of muscle derived signals accumulating at a more proximal nerve site we injected GFP-TTC into quadriceps muscle following the ligation of the parent femoral nerve (Fig. 2). The parent femoral nerve was isolated as described above and ligated with 7-O silk suture (Ethicon, Somerville, NJ). During the same surgery GFP-TTC (a kind gift from Dr. G. Schiavo) was injected into the quadriceps muscle using a custom made glass pipette (12 micrograms/25 microliters) and the surgical site was closed. Eighteen hours later TRITC-dextran (3,000 MW lysine fixable, Molecular Probes, D3308) was applied as described above to the distal terminal muscle branch to label motor neuron axons. After an additional 4 hours the animal was perfused as described below, and the femoral nerve ligation site was sectioned and analyzed for the presence of GFP-TTC and TRITC-dextran.
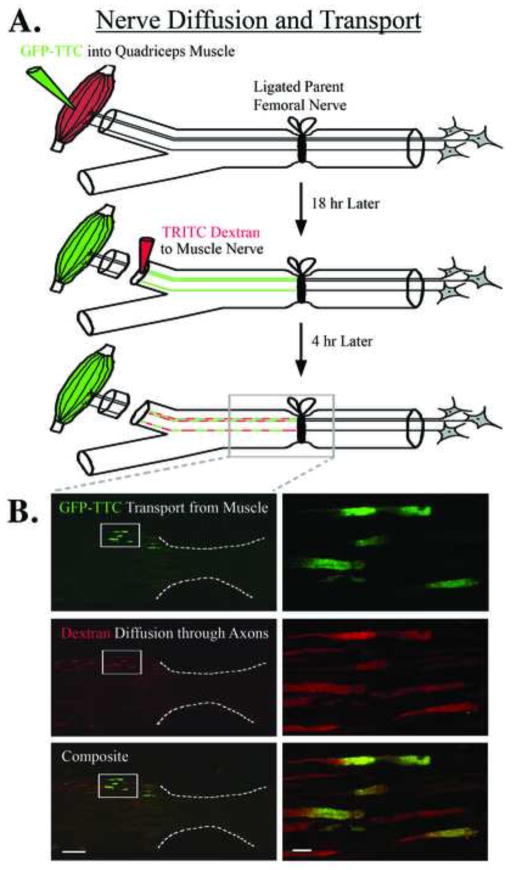
Figure 2.
Muscle-derived signals accumulate at a proximal nerve repair site. A) GFP-labeled tetanus toxin c-fragment (GFP-TTC) was injected into quadriceps muscle during the same surgery that ligated the parent femoral nerve. Eighteen hours later TRITC-labeled dextran was applied to the distal terminal muscle branch, and 4 hours after that the nerve was processed for histology. B) Upper panels show accumulation of the green GFP-labeled tetanus toxin c-fragment at the ligation site indicating that retrograde transport has continued in the denervated nerve over this time period; white box shown at higher magnification on the right for all panels, white dotted line indicates ligation site of the parent femoral nerve. Middle panels show that TRITC-labeled dextrans, which travel via diffusion driven movement, also accumulate at the repair site and are confined to specific axonal compartments and subsequent bands of Bungner which indicate that these compartments act as diffusional barriers. The lower panels are an overlay showing both the TRITC-dextran and the GFP-TTC labels. Because the TRITC-dextran was applied to the entire muscle branch it would be expected to label most of the axons within that branch, whereas the GFP-TTC would be expected to be restricted to fewer axons and only represent those motor neuron axons that were exposed to GFP-TTC due to the muscle injection. Size bar on the left = 100 μm, and on the right = 10 μm.
Manipulating retrograde transport and diffusion (Fig. 3)
Figure 3.
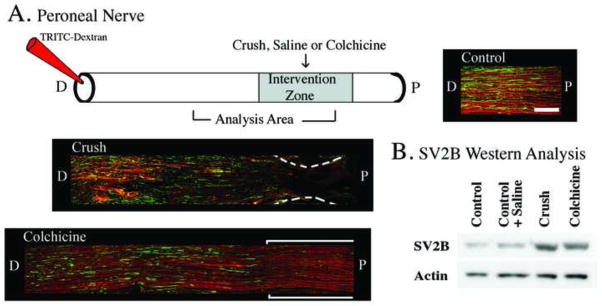
Methods to disrupt diffusion driven movement and/or active axonal transport. These experiments utilized histological and Western blot analysis using SV2B (synaptic vesicle protein 2B) as an example of a protein that moves via active axonal transport, and labeled dextrans as an example of a diffusion driven molecule. A) The upper panel depicts the application of saline (control), nerve crush, or colchicine to the peroneal nerve. Eighteen hours later TRITC-dextrans were applied distal to that intervention zone to assess diffusion driven movement, and four hours later the nerve was harvested for histological and Western analysis. Longitudinal histological sections of peroneal nerve are shown and illustrate that both TRITC-dextran and SV2B are present throughout the nerve after saline application (Control, right upper panel). However, when a nerve crush was applied (middle panel) both TRITC-dextran and SV2B stopped abruptly at the crush zone, indicating disruption of both diffusion driven movement and active axonal transport. When colchicine was applied to the nerve (lower left panel) SV2B transport was disrupted at the application zone but diffusion driven movement of the TRITC-dextran was not affected. Size bar in upper right panel (Control) represents 200 μm and applies to all panels. B) Western analysis verifying the immunohistochemistry data that both a nerve crush or the application of colchicine resulted in the accumulation of SV2B at the intervention zone. N=2 animals in each group; these experiments were replicated a minimum of three times using different animals (i.e. biological replicates).
We used the rat peroneal nerve for pilot studies to examine active transport versus diffusion driven movement because its surgical accessibility, longer length, and lack of branching made it ideal for anatomical and Western analysis studies (Fig. 3A and B). For immunohistochemical studies cohorts of N = 2 for each group were carried out after application of colchicine, saline or nerve crush of the peroneal nerve as described above for the femoral nerve; these experiments were replicated a minimum of three times using different animals (i.e. biological replicates). Eighteen hours later TRITC-dextran (3,000 MW lysine fixable, Molecular Probes, D3308) was applied distal to the intervention zone that had received colchicine, saline or crush using the same procedure as described above for retrograde labeling to determine the pathway reinnervation preference. The surgical site was then closed and the animal allowed to recover for four additional hours at which point the animal was sacrificed and the entire nerve site was then harvested and fixed in 4% paraformaldehyde for 30 minutes, washed extensively in PBS, then sucrose protected. Cryostat sections (30 μm) were mounted onto slides and processed for immunohistochemistry using an antibody recognizing synaptic vesicle glycoprotein 2B (SV2B, sc-28956, Santa Cruz Biotechnology, Santa Cruz, CA) with an Alexafluor 488 labeled secondary antibody (A-21430, Santa Cruz Biotechnology). The purpose of these experiments was to use the dextran as a marker for diffusion driven movement of biomolecules (Fritzsch, 1993) and the SV2B as a marker of proteins that move via active axonal transport.
Similar surgical techniques were used to generate tissue for Western blots. Cohorts of animals (N=2 for each group) received application of colchicine, saline or nerve crush of the peroneal nerve as described immediately above. Twenty four hours later a 1 cm nerve segment that included the intervention zone was harvested and flash frozen in liquid nitrogen. Nerve samples for each group were pooled and homogenized in lysis buffer (Complete Lysis-M, Roche Diagnostics, Mannheim, Germany), the proteins separated using acrylamide electrophoresis, transferred to PVDF membrane, and probed with the same antibody that was used for the immunohistochemistry described above that recognizes SV2B, and visualized using chemiluminescence (Supersignal West Pico, Thermoscientific, Rockford, IL); Fig. 3B. The membrane was then stripped and reprobed with an antibody against beta-actin (sc-130657, Santa Cruz) as a loading control. For each sample blot SV2B signal intensity was normalized to its beta-actin control for comparison across samples; these experiments were replicated a minimum of three times using different animals (i.e. biological replicates).
Following the above pilot studies and in order to further examine various aspects of diffusion driven movement we again exploited the diffusional properties of dextrans and their ability to retrogradely label motor neurons using the femoral nerve (Fig. 4). Cohorts of animals (N=2 for each group) received application of colchicine, saline or nerve crush to the terminal muscle branch of the femoral nerve as described above for the peroneal nerve; these experiments were replicated a minimum of three times using different animals (i.e. biological replicates). After rinsing the nerve with saline, Alexafluor 594 dextran (10,000 MW, D-22913, Molecular Probes) was then applied to the cut muscle branch distal to the intervention zone. For all animals we also applied Alexafluor 488 dextran (10,000 MW, D-22910, Molecular Probes, Eugene, OR, USA) to the naïve pectineus nerve. Three days later the rats were perfused and their spinal cords processed for retrogradely labeled motor neurons as described above for determining the pathway reinnervation preference. The distribution of pectineus motor neurons in the spinal cord overlaps that of the femoral nerve motor neuron pool and thus if the application of saline, crush, or colchicine to the terminal muscle branch of the femoral nerve blocked labeling of the femoral motor neuron pool we reasoned that we would still know the approximate location of the femoral nerve motor neuron pool because of its overlap with the labeled pectineus motor neurons. This allowed us to carefully screen those spinal cord levels for labeled femoral nerve motor neurons.
Figure 4.
Disruption of diffusion driven movement and/or active axonal transport in the femoral nerve. A) Depiction of model system. B) Representative spinal cord sections from animals whose terminal muscle branch of the femoral nerve received either application of saline (control), a nerve crush, or the application of colchicine. After an additional 15 minutes the terminal muscle branch was transected just distal to the intervention zone (see A) and Alexa-594-labeled dextran was applied to the terminal muscle branch. The rational was that if the more proximal nerve intervention (saline, crush, colchicine) blocked the diffusion driven movement of the labeled dextran that was applied distally than no motor neurons would be labeled in the spinal cord. Conversely if the more proximal nerve intervention did not block the diffusion driven movement of the labeled dextran than labeling of femoral nerve motor neurons should take place. During this same labeling procedure the pectineus nerve received application of Alexa-488-labeled dextran as a control. Because of the overlap of the distribution of pectineus and femoral motor neuron pools in the spinal cord this allowed us to carefully screen those spinal cord levels for labeled femoral nerve motor neurons. C) Motor neuron counts showed that the application of saline (Control) or colchicine resulted in complete retrograde labeling of the femoral nerve motor pool and verified that the dextrans are effective even when active axonal transport is blocked by colchicine, whereas animals that received a nerve crush demonstrated no retrograde labeling of femoral nerve motor neurons. The pectineus motor neuron counts were similar for all three groups and indicated the integrity of the general labeling procedures. N=2 animals in each group; these experiments were replicated a minimum of three times using different animals (i.e. biological replicates).
We were also interested in the possibility that a nerve crush might prevent diffusion driven movement of biomolecules within the nerve for several days. To model this possibility we carried out a time course of assessing diffusion driven movement of dextrans across a crush zone of the terminal muscle branch of the femoral nerve (Fig. 6). Cohorts of animals (N = 2 in each group) received a ligation of the parent femoral nerve at the same anatomical level that we use for nerve repair in the IM-SC femoral nerve repair model. During the same surgery the distal terminal muscle branch was crushed as described above; a control group received a sham crush. At 0, 5 or 10 days after this initial surgery Alexafluor 647 dextran (10,000 MW, D22914, Invitrogen) was applied distal to the original muscle branch crush site using the same procedure as described above for determining the pathway reinnervation preference. Eighteen hours after dextran application the nerves were harvested, fixed in 4% paraformaldehyde for 30 minutes, washed, and processed as flattened whole mounts using Prolong as a mounting medium; these experiments were replicated a minimum of three times using different animals (i.e. biological replicates).
Figure 6.
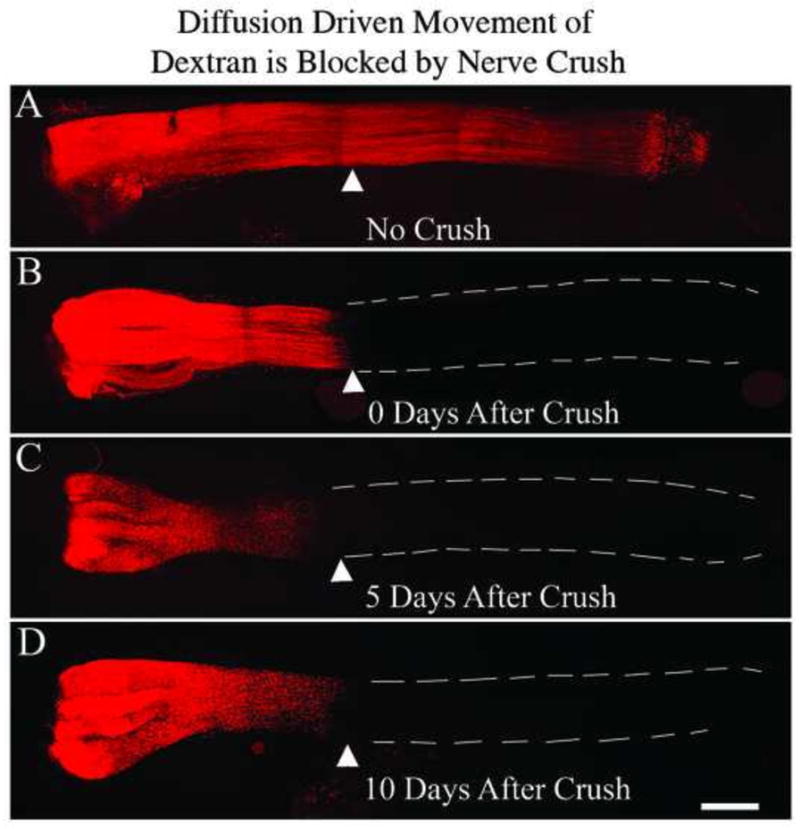
Crush of terminal muscle branch results in continued disruption of nerve diffusion. Cohorts of N=2 animals in each group received a ligation of the parent femoral nerve and during the same surgery the terminal muscle branch was crushed; a control group received a sham crush. At 0, 2, or 10 days after this initial surgery Alexa-647-labeled dextran was applied distal to the nerve crush site. Eighteen hours after dextran application the nerves were harvested and processed as flattened whole mounts. The white triangle represents the nerve crush site. A nerve crush applied even 10 days earlier still blocks diffusion of the labeled dextran.
Results
Control studies established the number of motor neurons normally supplying the quadriceps muscle (398±12, N=6) and the parity of labeling by the two diffusible dextrans (± 2%). This baseline number of femoral motor neurons in the adult rat is consistent with previous studies from other laboratories (Brushart, 1988).
For the regeneration accuracy experiments (Fig. 1A), the motor neurons were brightly labeled with the diffusible dextrans such that quantification of single and double labeled neurons was straightforward. Significantly more femoral motor neurons projected to the terminal muscle branch two weeks after the intact-muscle:short-cutaneous (IM-SC) surgery (t=6.69, p=.0001). To examine the possibility that muscle-derived signals might accumulate at the more proximal parent femoral nerve repair site, we injected the target quadriceps muscle with GFP-TTC at the same time as ligating the parent femoral nerve, and 18 hours later applied TRITC-dextran to the terminal muscle branch. Four hours later the femoral nerve ligation site was examined, and GFP-TTC was evident in axons from the muscle branch labeled with the TRITC-dextran (Fig. 2).
The effects of blocking active axonal transport mechanisms and/or diffusion driven movement within peripheral nerves is shown in Figures 3 and 4. When TRITC-dextran was applied to the peroneal nerve and the nerve was then harvested four hours later and processed for SV2B immunohistochemistry and both SV2B and the dextran were shown to be visible in more proximal areas of the nerve (Fig. 3A, Control). However, when a crush was applied to the peroneal nerve proximal to the application site of the labeled dextran, both diffusion driven movement of TRITC-dextran, and active axonal transport as judged by accumulation of SV2B were disrupted at the crush site (Fig. 3A, Crush). When colchicine was applied to peroneal nerve proximal to the application site of the labeled dextran, active axonal transport was disrupted at the application site as judged by immunohistochemistry, but diffusion driven movement of TRITC-dextran was not affected (Fig. 3A, Colchicine). Western blot analysis (Fig. 3B) verified that both crush and colchicine application resulted in significant accumulation of SV2B at the intervention zone. To quantify this, the ratio of SV2B/actin for control nerves was set to 100% and then the other samples were expressed as a % of this control ratio; control+saline = 101%, crush = 273%, and colchicine = 199%.
Likewise, a nerve crush of the terminal muscle branch of the femoral nerve applied proximal to a diffusible dextran completely blocked retrograde labeling of the femoral motor neuron pool (Fig. 4C, Crush; zero labeled motor neurons compared to Control of 335±21); further indicating that a nerve crush blocks both active axonal transport as well as diffusion driven movement of dextrans. On the other hand, colchicine application to block active axonal transport proximal to a diffusible dextran did not block the retrograde labeling of motor neurons (Fig. 4C, Colchicine, 392±19). These results corroborate earlier studies that suggested that the dextrans used in this study travel primarily, if not entirely, by diffusion driven movement as opposed to active axonal transport (Fritzsch, 1993).
The effect on motor neuron regeneration accuracy of various interventions to the terminal muscle branch of the femoral nerve is shown in Figure 5. Groups of animals received the basic IM-SC surgery and at the same time application of a crush, saline, or colchicine to the terminal muscle branch. Two weeks later the two terminal femoral nerve branches received application of two different colored dextrans and the spinal cords were processed as described in methods to determine the pathway reinnervation preference. Femoral nerve motor neurons were scored by a blinded observer as projecting only to one or the other terminal nerve branch, or to both branches.
Neither saline nor colchicine changed the increase in preference for the muscle branch normally seen in this model (compare Fig. 5A to Fig. 1): significantly more motor neurons projected to the terminal muscle branch versus the cutaneous branch (Fig. 5A, colchicine, t=3.45, p=.01, saline, t=3.12, p=.0001). In contrast, a crush of the terminal muscle branch at the time of parent femoral nerve repair blocked the regeneration preference of motor neurons, with approximately equal numbers projecting to either one of the terminal branches, or maintaining projections to both branches simultaneously (Fig. 5A, IM-SC +Crush).
In order to further examine the effects of a crush of the terminal muscle branch on the regeneration preference of femoral motor neurons we carried out a time course of when the terminal muscle branch was crushed in relation to the parent femoral nerve repair (Fig. 5B). Femoral nerve motor neurons failed to display a preference to reinnervate the terminal muscle branch when the terminal muscle branch was crushed at the same time as the IM-SC surgery (Fig. 5B, Day 0) or when it was crushed two days after the initial surgery (Fig. 5B, Day 2). However, when the terminal muscle branch crush was carried out 10 days after the parent femoral nerve repair, there was a significant preference for regenerating femoral nerve motor neurons to correctly project back to the terminal muscle branch (Fig. 5B, Day 10); similar to that seen when there is no crush of terminal muscle branch.
The time course of a nerve crush disrupting diffusion driven movement of dextrans is shown in Figure 6. Diffusion driven movement of dextrans across a nerve crush site is blocked for at least 10 days, the latest time point studied. This is an important observation because it suggests that diffusion driven movement within a denervated nerve is severely compromised for many days by a concomitant crush of the already denervated nerve, which might be common in clinical cases such as polytrauma to the extremities.
Discussion
We have examined the influence of ongoing active axonal transport and/or diffusion driven movement of molecules in a denervated nerve in terms of influencing motor neuron regeneration accuracy at the terminal nerve branch level. Several lines of evidence are presented that ongoing diffusion driven movement within the denervated nerve is essential in promoting regeneration accuracy. Although active axonal transport continues in a denervated nerve for a few days, it does not appear to play a defining role in shaping subsequent motor neuron regeneration accuracy.
In this nerve injury model it is important to keep in mind that manipulations to the terminal muscle branch occur after a more proximal femoral nerve repair, or in other words, to a nerve segment that has already had its axons severed more proximally. The two week time point that we utilized for quantifying regeneration accuracy is important because we wanted to minimize the possible influence of regenerating axons physically contacting muscle. In the rat, two weeks following parent femoral nerve repair, motor axons are just beginning to reach the quadriceps muscle and motor endplate reinnervation is very rare (Brushart, 1993). Our hypothesis was that muscle-derived signals accumulate within a lesioned nerve, and that these signals may impact motor neuron regeneration accuracy. We reasoned that such muscle-derived signals might enter the denervated nerve by either ongoing active axonal transport or by diffusion driven movement of biomolecules. Previous work from different laboratories has offered support for both of these possibilities, but has not ruled out either. The present experiments were designed to separate as much as possible the role of active transport versus diffusion.
Continued Active Axonal Transport in Denervated Nerve
Using high-powered video-microscopy Smith and Bisby (Smith, 1993) were able to show normal amounts of organelle transport 24 hours after axotomy, with organelles cycling back and forth within the isolated sealed axon [also see (Hassig et al., 1991, Watson et al., 1993)]. Several studies using a double-ligation model of the sciatic nerve are also consistent with continued retrograde transport in acutely damaged axons, and have shown accumulation within the distal nerve segment of several molecules known to be involved in axonal guidance [e.g., (Ma et al., 1994, Jiang et al., 2000)]. The progressive time course of Wallerian degeneration has recently been elucidated in transgenic mice with fluorescent axons and shows a latent period of approximately 40 hours during which axons remain intact, followed by rapid fragmentation within a few additional hours (Beirowski et al., 2004, Beirowski et al., 2005, Kerschensteiner et al., 2005). Most pertinent to our studies is the finding in rats that, for up to 20 hours after transection, distal motor axons can still transmit impulses to muscle (Miledi and Slater, 1968, 1970, Stanley and Drachman, 1980, Beirowski et al., 2004, Beirowski et al., 2005, Kerschensteiner et al., 2005, English et al., 2007, Moldovan et al., 2009), demonstrating an ongoing functional neuromuscular junction.
Muscle-Derived Signals Accumulate at a Proximal Nerve Injury Site
To demonstrate that muscle-derived signals may be rapidly transported from muscle to the proximal femoral nerve repair site we utilized the non-toxic c-fragment of tetanus toxin (TTC), which is an effective tool for analyzing endocytotic function at the neuromuscular junction. After injection into muscle TTC is concentrated at the neuromuscular junction, released to motor axon terminals, and is taken up into “lipid rafts” and transported back to the motor neuron in the spinal cord (Herreros, 2001, 2002, Lalli, 2002). TTC is thus a convenient marker for lipid rafts and signaling endosomes which in turn have been shown to contain many neurotrophic factors for motor neurons (Wu, 1997, Bruckner, 1999, Tansey, 2000, Frenzel, 2001, Paratcha, 2001). There are two main findings from this particular experiment (Fig. 2). First, the data strongly suggest that for some time after a proximal parent nerve lesion, muscle-derived signals could originate from muscle and be taken up by the axon terminal, packaged along with lipid rafts (to comprise a signaling endosome), and be retrogradely transported to the proximal nerve injury site where they could accumulate on the distal side of the parent nerve lesion. Secondly, the restricted localization of the diffusible dextran to axonal compartments or bands of Bungner shows that these compartments can act as diffusional barriers. Both of these mechanisms could thus result in muscle-derived signals accumulating in specific Schwann cell tubes (Bands of Bungner) at the parent nerve repair site. The eventual distal destination of regenerating axons is largely determined by the Schwann cell tubes that they enter at the nerve transection site (Brown and Hopkins, 1981, Brown and Hardman, 1987, Lee and Farel, 1988, Nguyen et al., 2002). Functional recovery will therefore largely depend upon the accuracy of these initial choices.
Diffusion of Neurotropic/Neurotrophic Signals from Denervated Muscle
Classical experiments carried out almost 40 years ago demonstrated that if a foreign nerve was sutured to an intact muscle, nerve fibers would grow over the surface of the muscle, but they would not form neuromuscular junctions. However, shortly after cutting the original nerve the muscle would become functionally innervated by the foreign nerve fibers [e.g. (Frank et al., 1975, Sanes and Lichtman, 1999)], suggesting that the acutely denervated muscle was secreting signals that acted over significant distances and that successfully directed the foreign nerve fibers to neuromuscular junctions. More recently it has been shown that neurotrophic factors such as hepatocyte growth factor, fibroblast growth factors, and brain-derived neurotrophic factor are indeed increased in muscle due to denervation (Jennische et al., 1993, Lie and Weis, 1998, Tonra et al., 1998, Wallenius et al., 2000, Wehrwein et al., 2002, Yamaguchi et al., 2004, Zhao et al., 2004), and that the increased endocytotic/exocytotic activity that has been observed occurs predominantly at the endplate region of the muscle (Lawoko and Tagerud, 1995). Data also suggests that conditioned media from muscle can act as a trophic factor for motor neurons (e.g. (Taylor et al., 2007)). These observations have led to the suggestion that the high exocytotic activity of denervated muscle is due to the increased secretion of factors by the muscle in an attempt to stimulate reinnervation by motor neuron axons (Libelius, 1989, Thesleff, 1989, Henderson et al., 1993, deLapeyriere and Henderson, 1997, Magnusson et al., 2005).
Separating the Influence of Active Axonal Transport versus Diffusion Driven Movement on Motor Neuron Regeneration Accuracy
The application of colchicine to the terminal muscle branch of the femoral nerve did not block the labeling of motor neurons via diffusion of labeled dextrans even though it blocks active axonal transport mechanisms (Fig. 4C). Similarly, colchicine application to the terminal muscle branch coincident with parent femoral nerve repair did not reduce motor neuron regeneration accuracy when measured two weeks later (Fig. 5A). Conversely, a nerve crush of the muscle branch coincident with parent femoral nerve repair blocked both the labeling of motor neurons via diffusion of labeled dextrans (Fig. 4C) as well as motor neuron regeneration accuracy when measured two weeks later (Fig. 5A).
There is no known intervention to a nerve that will block diffusion driven movement of molecules without also interrupting active axonal transport. We therefore used the timing of a crush on the terminal muscle branch in relation to the parent femoral nerve repair to attempt to separate the influence of these two mechanisms. Consistent with previous work (Ratliff and Oldfield, 2001) we found that a nerve crush blocked diffusion driven movement of molecules for at least 10 days, the latest time point studied (Fig. 6). Our reasoning was that a crush of the distal muscle branch made coincident with parent femoral nerve repair would block any active axonal transport or diffusion driven movement from muscle from reaching the proximal repair site during the regeneration period. However, if the crush was delayed by two days it would have been possible for active axonal transport or diffusion driven movement from muscle to reach the proximal repair site. As shown in Fig. 5B, muscle branch crush coincident with, or two days after, parent femoral nerve repair blocked motor neuron regeneration accuracy. However, if the crush of the muscle branch was delayed until 10 days after the parent femoral nerve repair there was a significant preference for motor neurons to correctly project back to the terminal muscle branch (Fig. 5B).
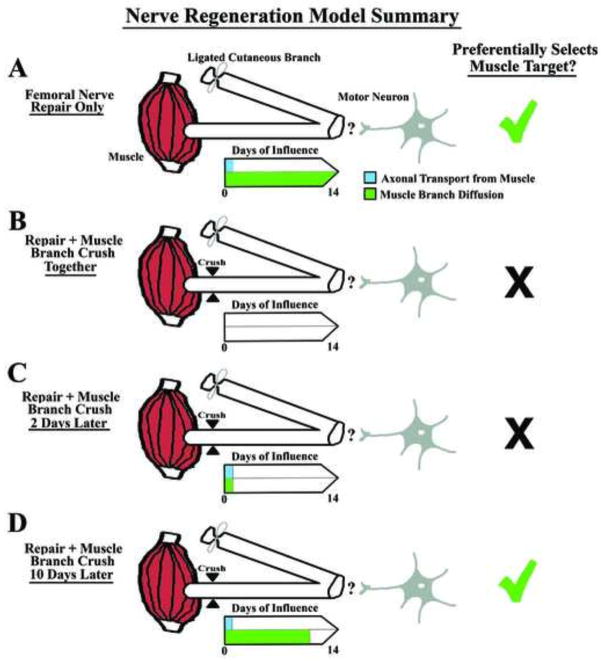
Our overall interpretation of these experiments is shown as a model (Fig. 7) focusing on the “days of influence” of either retrograde transport or diffusion. When both of these occur during the two week survival period there is a significant preference for regenerating motor neurons to preferentially project to the muscle branch (Fig. 7A), as indicated by the green check mark. However, when both axonal transport and diffusion are disrupted on day 0 by a crush of the distal muscle branch, this regeneration accuracy is blocked (Fig. 7B) as indicated by the black X. This blockage also occurs if the distal muscle branch is crushed 2 days after the parent femoral nerve repair (Fig. 7C), indicating that the two days of ongoing axonal transport in a distal lesioned nerve is not sufficient by itself to generate motor neuron regeneration accuracy in this model or even when it is combined with ongoing diffusion driven movement for two days. However when the distal muscle branch crush is carried out 10 days after the parent femoral nerve repair (allowing the influence of diffusion to proceed for 10 days), the preference for regenerating motor neurons to project to the terminal muscle branch is similar to that seen in the control group with no manipulation of the terminal muscle branch (Fig. 7D).
Figure 7.
Model Summary. This drawing highlights the “days of influence” of axonal transport (that continues in a denervated nerve for approximately two days) versus possible diffusion driven movement of molecules from muscle. (A) When both axonal transport and diffusion driven movement are allowed to take place during the two week survival period, regenerating motor neurons preferentially project to the terminal muscle branch, showing appropriate regeneration accuracy as indicated by the green check mark. (B) A distal muscle branch crush applied coincident with parent femoral nerve repair blocks both axonal transport and diffusion driven movement, and blocks regeneration accuracy. This suggests the requirement for one or both of these ongoing mechanisms for regeneration accuracy. (C) A distal muscle branch crush applied two days after parent femoral nerve repair also blocks regeneration accuracy and suggests that the initial two days of axonal transport and/or diffusion driven movement are not sufficient to result in regeneration accuracy. (D) A distal muscle branch crush applied 10 days after parent femoral nerve repair results in regeneration accuracy being restored and suggests that ongoing diffusion driven movement in the denervated nerve is critical for this process.
Diffusion driven movement of biomolecules is a quite complex process with multiple variables affecting both the distance and size of the molecule that can be moved (for review, see (Muller and Schier, 2011)). Fritzsch (Fritzsch, 1993) showed empirically quite some time ago that low molecular weight dextrans diffused quite rapidly within cellular cytoplasm, and Popov and Poo (Popov and Poo, 1992) showed similar movement in neurites in vitro. Likewise, we have shown that such dextrans effectively retrogradely label motor neurons in the spinal cord of the mouse and rat within a few days of being applied to a distal nerve. The present work shows that such motor neuron labeling takes place even when colchicine has been applied to the nerve to block active axonal transport (Fig. 4B and C). Mathematical models predict that a 40–50 kDa protein could move several mm within a few hours and a cm within a few days (Muller and Schier, 2011). Interestingly, there is precedent for similar sized molecules moving within denervated peripheral nerve. Deshpande et al. (Deshpande et al., 2006) transplanted embryonic stem cells into the spinal cord of paralyzed rats and at the same time injected GDNF secreting cells into denervated sciatic nerve in an attempt to attract neurites from the transplanted stem cells. They presented evidence of increased GDNF protein for several centimeters from the site of injection of the GDNF-secreting cells, and were even able to verify actual cell migration within the denervated nerve. The transplanted GDNF-secreting cells were only one part of a complex intervention regimen, however only when such cells were injected into the sciatic nerve were the authors able to show effective outgrowth from the spinal cord stem cells all the way to distal muscle. This work demonstrates the possible movement of biomolecules and even structures as large as a cell within the microenvironment of a denervated nerve.
Clinicians have recognized for some time now that human peripheral nerves that sustain multiple crush injuries are more susceptible to pathological syndromes, e.g. the double crush syndrome (Dellon and Mackinnon, 1991, Mackinnon, 1992, Smith et al., 2008). It is interesting to speculate that such multiple crush sites might affect diffusion driven movement of biomolecules within the nerve. However, in terms of the applicability of our current animal work to human nerve regeneration it is important to keep in mind the much longer distances involved with human nerves compared to rat nerves. For instance, the distance from the parent femoral nerve in the rat to the terminal femoral nerve branches may be approximately 2–3 cm whereas in the human it would be approximately 10 times that distance. Because diffusion rates decrease according to the square of the distance (Muller and Schier, 2011), one can quickly ascertain that simple diffusion over the length of a human peripheral nerve would take a very long time. Thus, to play a role in human nerve regeneration one might suspect that there would have to be various mechanisms that would “facilitate” diffusion driven movement of biomolecules. Alternatively, one could imagine that such diffusion driven biomolecules might set up a type of chain-reaction in cells in the denervated nerve (e.g. Schwann cells) by influencing their molecular secretome (Drago et al., 2013) beginning with those cells closest to muscle.
The work we have presented in this manuscript is focused on the accuracy of motor neuron regeneration at the level of a terminal nerve branch. We believe that in terms of functional recovery the final accuracy of regeneration may be much more important than simply trying to increase the amount of axonal regeneration, and that this will apply both to the central nervous system as well as the peripheral nervous system. For instance, recent elegant work has shown that by using a complex combinatorial approach it is possible to significantly increase the extent of axonal regeneration after partial and complete spinal cord injury (Lu et al., 2012). Although the authors were able to definitely show increased motor axonal regeneration beyond the spinal cord lesion site and even the formation of synaptic connections, the motor outcomes worsened after partial cervical level injuries and spasticity worsened after complete spinal cord transection. These findings in the CNS highlight the importance of the accuracy of regeneration in terms of final functional recovery.
It has been 100 years since Ramon Y Cajal wrote his original Spanish treatise on Degeneration and Regeneration of the Nervous System [English edition (Cajal, 1928)]. Cajal posited both “generic and specific” stimuli encountered by regenerating peripheral nerve axons and suggested that “the cells of Schwann .... have a generic character, acting without distinction on all sprouts” while specific influences arose from end-organ targets such as “spindles of Kuhne, motor plates,… etc.” and that these acted “only on certain functional categories of regenerated axons” (pg. 371). Within this last century there has been much appreciation of the general influence of a distal denervated nerve and its associated Schwann cells on nerve regeneration, while much less attention has been given to the possibility of specific signals within a lesioned peripheral nerve. The data from our experiments support the concept that muscle, as an end-organ, elaborates signals that regenerating motor neurons respond to at a more proximal repair site and we further suggest that such signals travel within the distal denervated nerve by diffusion driven movement.
Highlights.
Ongoing diffusion driven movement in denervated nerve affects regeneration accuracy
If a denervated nerve remains in contact with the end organ it increases regeneration
Additional manipulations to a denervated nerve influences axon regeneration
Acknowledgments
We thank Christopher McGee and Richelle Bangi for excellent technical assistance. Supported by NIH (NS069597 to RDM) and the Office of Research and Development, Department of Veterans Affairs (1386-015 to RDM). RDM is a Research Career Scientist for the Biological Laboratory Research and Development Service, Department of Veterans Affairs.
Footnotes
Author Disclosure Statement: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. AnatRec. 1946;4:239–246. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC neuroscience. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Berek L, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice. J Neurosci Methods. 2004;134:23–35. doi: 10.1016/j.jneumeth.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Brown MC, Hardman VJ. A reassessment of the accuracy of reinnervation by motoneurons following crushing or freezing of the sciatic or lumbar spinal nerves of rats. Brain. 1987;110 (Pt 3):695–705. doi: 10.1093/brain/110.3.695. [DOI] [PubMed] [Google Scholar]
- Brown MC, Hopkins WG. Role of degenerating axon pathways in regeneration of mouse soleus motor axons. J Physiol. 1981;318:365–373. doi: 10.1113/jphysiol.1981.sp013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner K, Labrador JP, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Preferential motor reinnervation: a sequential double-labeling study. Restorative neurology and neuroscience. 1990;1:281–287. doi: 10.3233/RNN-1990-13416. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair and Grafting. In: Green D, Hotchkiss R, Pederson R, editors. Green’s Operative Hand Surgery. New York: Churchill Livingston; 1998. pp. 1381–1403. [Google Scholar]
- Brushart TM, Gerber J, Kessens P, Chen Y-G, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci. 1998;18(21):8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TME. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TME. Motor axons preferentially reinnervate motor pathways. J of Neurosci. 1993;13(6):2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal RS. Degeneration and regeneration of the nervous system (English Translation, 1968) London/New York: Hafner Publishing Company; 1928. [Google Scholar]
- Colburn RW, DeLeo JA. The effect of perineural colchicine on nerve injury-induced spinal glial activation and neuropathic pain behavior. Brain research bulletin. 1999;49:419–427. doi: 10.1016/s0361-9230(99)00075-1. [DOI] [PubMed] [Google Scholar]
- deLapeyriere O, Henderson CE. Motoneuron differentiation, survival and synaptogenesis. Current opinion in genetics & development. 1997;7:642–650. doi: 10.1016/s0959-437x(97)80012-3. [DOI] [PubMed] [Google Scholar]
- Dellon AL, Mackinnon SE. Chronic nerve compression model for the double crush hypothesis. Annals of plastic surgery. 1991;26:259–264. doi: 10.1097/00000637-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Deshpande DM, Kim YS, Martinez T, Carmen J, Dike S, Shats I, Rubin LL, Drummond J, Krishnan C, Hoke A, Maragakis N, Shefner J, Rothstein JD, Kerr DA. Recovery from paralysis in adult rats using embryonic stem cells. Annals of neurology. 2006;60:32–44. doi: 10.1002/ana.20901. [DOI] [PubMed] [Google Scholar]
- Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271–2285. doi: 10.1016/j.biochi.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt KA, Irintchev A, Al-Majed AA, Simova O, Brushart TM, Gordon T, Schachner M. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp Neurol. 2006;198:500–510. doi: 10.1016/j.expneurol.2005.12.018. [DOI] [PubMed] [Google Scholar]
- English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J Neurophysiol. 2007;97:1127–1134. doi: 10.1152/jn.01035.2006. [DOI] [PubMed] [Google Scholar]
- Frank E, Jansen JK, Lomo T, Westgaard RH. The interaction between foreign and original motor nerves innervating the soleus muscle of rats. J Physiol. 1975;247:725–743. doi: 10.1113/jphysiol.1975.sp010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel KE, Falls DL. Neuregulin-1 proteins in rat brain and transfected cells are localized to lipid rafts. J Neurochem. 2001;77:1–12. doi: 10.1046/j.1471-4159.2001.t01-1-00132.x. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Fast axonal diffusion of 3000 molecular weight dextran amines. J Neurosci Methods. 1993;50:95–103. doi: 10.1016/0165-0270(93)90060-5. [DOI] [PubMed] [Google Scholar]
- Hassig R, Tavitian B, Pappalardo F, Di Giamberardino L. Axonal transport reversal of acetylcholinesterase molecular forms in transected nerve. J Neurochem. 1991;57:1913–1920. doi: 10.1111/j.1471-4159.1991.tb06403.x. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Bloch-Gallego E, Camu W, Gouin A, Lemeulle C, Mettling C. Motoneuron survival factors: biological roles and therapeutic potential. Neuromuscular disorders : NMD. 1993;3:455–458. doi: 10.1016/0960-8966(93)90096-3. [DOI] [PubMed] [Google Scholar]
- Herreros J, Ng T, Schiavo G. Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. Mol Biol Cell. 2001;12:2947–2960. doi: 10.1091/mbc.12.10.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros J, Schiavo G. Lipid microdomains are involved in neurospecific binding and internalization of clostridial neurotoxins. Int J Med Microbiol. 2002;291:447–453. doi: 10.1078/1438-4221-00152. [DOI] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennische E, Ekberg S, Matejka GL. Expression of hepatocyte growth factor in growing and regenerating rat skeletal muscle. The American journal of physiology. 1993;265:C122–128. doi: 10.1152/ajpcell.1993.265.1.C122. [DOI] [PubMed] [Google Scholar]
- Jiang Y, McLennan IS, Koishi K, Hendry IA. Transforming growth factor-beta 2 is anterogradely and retrogradely transported in motoneurons and up-regulated after nerve injury. Neuroscience. 2000;97:735–742. doi: 10.1016/s0306-4522(00)00084-1. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature medicine. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Kreiger N, Kelsey JL, Harris C, Pastides H. Injuries to the upper extremity: patterns of occurrence. Clinics in plastic surgery. 1981;8:13–19. [PubMed] [Google Scholar]
- Lalli G, Schiavo G. Analysis of retrograde transport in motor neurons reveals common endocytic carriers for tetanus toxin and neurotrophin p75NTR. J Cell Biol. 2002;156:233–239. doi: 10.1083/jcb.200106142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawoko G, Tagerud S. High endocytotic activity occurs periodically in the endplate region of denervated mouse striated muscle fibers. Exp Cell Res. 1995;219:598–603. doi: 10.1006/excr.1995.1269. [DOI] [PubMed] [Google Scholar]
- Lee MT, Farel PB. Guidance of regenerating motor axons in larval and juvenile bullfrogs. J Neurosci. 1988;8:2430–2437. doi: 10.1523/JNEUROSCI.08-07-02430.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libelius R, Tagerud S. Lysosomes in sleletal muscle. In: Libelius R, Thesleff S, editors. Neuromuscular Junction. Amsterdam: Elsevier; 1989. pp. 481–485. [Google Scholar]
- Lie DC, Weis J. GDNF expression is increased in denervated human skeletal muscle. Neurosci Lett. 1998;250:87–90. doi: 10.1016/s0304-3940(98)00434-0. [DOI] [PubMed] [Google Scholar]
- Löw K, Orberger G, Schmitz B, Martini R, Schachner M. The L2/HNK-1 carbohydrate is carried by the myelin associated glycoprotein and sulphated glucuronyl glycolipids in muscle but not cutaneous nerves of adult mice. Eur J Neurosci. 1994;6:1773–1781. doi: 10.1111/j.1460-9568.1994.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Lu P, Blesch A, Graham L, Wang Y, Samara R, Banos K, Haringer V, Havton L, Weishaupt N, Bennett D, Fouad K, Tuszynski MH. Motor axonal regeneration after partial and complete spinal cord transection. J Neurosci. 2012;32:8208–8218. doi: 10.1523/JNEUROSCI.0308-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Yang SX, Ho GJ, Festoff BW. Insulin-like growth factor binding protein-1 is pre-synaptic at mouse neuromuscular synapses and is transported in nerve. Neurochemical research. 1994;19:1363–1368. doi: 10.1007/BF00972464. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE. Double and multiple “crush” syndromes. Double and multiple entrapment neuropathies. Hand clinics. 1992;8:369–390. [PubMed] [Google Scholar]
- Mader K, Andermahr J, Angelov DN, Neiss WF. Dual mode of signalling of the axotomy reaction: retrograde electric stimulation or block of retrograde transport differently mimic the reaction of motoneurons to nerve transection in the rat brainstem. J Neurotrauma. 2004;21:956–968. doi: 10.1089/0897715041526113. [DOI] [PubMed] [Google Scholar]
- Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J Neurosci. 1996;16:5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison RD, Archibald SJ, Krarup C. Peripheral nerve injury. In: Cohen IK, et al., editors. Wound Healing: Biochemical and Clinical Aspects. Philadelphia: W.B. Saunders Co; 1992. pp. 450–480. [Google Scholar]
- Madison RD, Archibald SJ, Lacin R, Krarup C. Factors contributing to preferential motor reinnervation in the primate peripheral nervous system. J Neurosci. 1999;19:11007–11016. doi: 10.1523/JNEUROSCI.19-24-11007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison RD, Robinson GA, Chadaram SR. The specificity of motor neurone regeneration (preferential reinnervation) Acta Physiol (Oxf) 2007;189:201–206. doi: 10.1111/j.1748-1716.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Madison RD, Sofroniew MV, Robinson GA. Schwann cell influence on motor neuron regeneration accuracy. Neuroscience. 2009;163:213–221. doi: 10.1016/j.neuroscience.2009.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson C, Svensson A, Christerson U, Tagerud S. Denervation-induced alterations in gene expression in mouse skeletal muscle. Eur J Neurosci. 2005;21:577–580. doi: 10.1111/j.1460-9568.2005.03855.x. [DOI] [PubMed] [Google Scholar]
- Martini R, Schachner M, Brushart TM. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J Neurosci. 1994;14:7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schmitz B, Schachner M. The L2/HNK-1 carbohydrate epitope is involved in the preferential outgrowth of motor neurons on ventral roots and motor nerves. Eur J Neurosci. 1992;4:628–639. doi: 10.1111/j.1460-9568.1992.tb00171.x. [DOI] [PubMed] [Google Scholar]
- Miledi R, Slater CR. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proceedings of the Royal Society of London Series B, Containing papers of a Biological character Royal Society. 1968;169:289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Miledi R, Slater CR. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970;207:507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan M, Alvarez S, Krarup C. Motor axon excitability during Wallerian degeneration. Brain. 2009;132:511–523. doi: 10.1093/brain/awn332. [DOI] [PubMed] [Google Scholar]
- Muller P, Schier AF. Extracellular movement of signaling molecules. Developmental cell. 2011;21:145–158. doi: 10.1016/j.devcel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nature neuroscience. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29:171–184. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- Popov S, Poo MM. Diffusional transport of macromolecules in developing nerve processes. J Neurosci. 1992;12:77–85. doi: 10.1523/JNEUROSCI.12-01-00077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff JK, Oldfield EH. Convection-enhanced delivery in intact and lesioned peripheral nerve. J Neurosurg. 2001;95:1001–1011. doi: 10.3171/jns.2001.95.6.1001. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Manipulations of the mouse femoral nerve influence the accuracy of pathway reinnervation by motor neurons. Exp Neurol. 2005;192:39–45. doi: 10.1016/j.expneurol.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annual review of neuroscience. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Smith RS, Bisby MA. Persistence of axonal transport in isolated axons of the mouse. Eur J Neurosci. 1993;5:1127–1135. doi: 10.1111/j.1460-9568.1993.tb00967.x. [DOI] [PubMed] [Google Scholar]
- Smith TM, Sawyer SF, Sizer PS, Brismee JM. The double crush syndrome: a common occurrence in cyclists with ulnar nerve neuropathy-a case-control study. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2008;18:55–61. doi: 10.1097/JSM.0b013e31815c1d7a. [DOI] [PubMed] [Google Scholar]
- Stanley EF, Drachman DB. Denervation and the time course of resting membrane potential changes in skeletal muscle in vivo. Exp Neurol. 1980;69:253–259. doi: 10.1016/0014-4886(80)90209-5. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Nerve injuries and their repair: A critical Appraisal. New York: Churchill Livingstone; 1991. [Google Scholar]
- Tansey MG, Baloh RH, Milbrandt J, Johnson EM. GFRalpha mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation and neuronal survival. Neuron. 2000;25:611–623. doi: 10.1016/s0896-6273(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Taylor AR, Gifondorwa DJ, Newbern JM, Robinson MB, Strupe JL, Prevette D, Oppenheim RW, Milligan CE. Astrocyte and muscle-derived secreted factors differentially regulate motoneuron survival. J Neurosci. 2007;27:634–644. doi: 10.1523/JNEUROSCI.4947-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff S. Botulinal neurotoxins as tools in studies of synaptic mechanisms. Quarterly journal of experimental physiology. 1989;74:1003–1017. doi: 10.1113/expphysiol.1989.sp003329. [DOI] [PubMed] [Google Scholar]
- Tonra JR, Curtis R, Wong V, Cliffer KD, Park JS, Timmes A, Nguyen T, Lindsay RM, Acheson A, DiStefano PS. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uschold T, Robinson GA, Madison RD. Motor neuron regeneration accuracy: balancing trophic influences between pathways and end-organs. Exp Neurol. 2007;205:250–256. doi: 10.1016/j.expneurol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Vrbova G, Gordon T, Jones R. Nerve regeneration and muscle reinnervation. In: Vrbova G, et al., editors. Nerve-Muscle Interaction. London: Chapman and Hall; 1995. pp. 197–235. [Google Scholar]
- Wallenius V, Hisaoka M, Helou K, Levan G, Mandahl N, Meis-Kindblom JM, Kindblom LG, Jansson JO. Overexpression of the hepatocyte growth factor (HGF) receptor (Met) and presence of a truncated and activated intracellular HGF receptor fragment in locally aggressive/malignant human musculoskeletal tumors. The American journal of pathology. 2000;156:821–829. doi: 10.1016/S0002-9440(10)64950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DF, Glass JD, Griffin JW. Redistribution of cytoskeletal proteins in mammalian axons disconnected from their cell bodies. J Neurosci. 1993;13:4354–4360. doi: 10.1523/JNEUROSCI.13-10-04354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Roskelley EM, Spitsbergen JM. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve. 2002;26:206–211. doi: 10.1002/mus.10179. [DOI] [PubMed] [Google Scholar]
- Wu C, Butz S, Ying Y, Anderson RG. Tyrosine kinase receptors concentrated in caveloae-like domains from neuronal plasma membrane. J Biol Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Ishii H, Morita I, Oota I, Takeda H. mRNA expression of fibroblast growth factors and hepatocyte growth factor in rat plantaris muscle following denervation and compensatory overload. Pflugers Archiv : European journal of physiology. 2004;448:539–546. doi: 10.1007/s00424-004-1282-5. [DOI] [PubMed] [Google Scholar]
- Zhao C, Veltri K, Li S, Bain JR, Fahnestock M. NGF, BDNF, NT-3, and GDNF mRNA expression in rat skeletal muscle following denervation and sensory protection. J Neurotrauma. 2004;21:1468–1478. doi: 10.1089/neu.2004.21.1468. [DOI] [PubMed] [Google Scholar]