Abstract
Cancer screening procedures have brought great benefit to the public’s health. However, the science of cancer screening and the evidence arising from research in this field as it is applied to policy is complex and has been difficult to communicate, especially on the national stage. We explore how epidemiologists have contributed to this evidence base and to its translation into policy. Our essay focuses on breast and lung cancer screening to identify commonalities of experience by epidemiologists across two different cancer sites and describe how epidemiologists interact with evolving scientific and policy environments. We describe the roles and challenges that epidemiologists encounter according to the maturity of the data, stakeholders, and the related political context. We also explore the unique position of cancer screening as influenced by the legislative landscape where, due to recent healthcare reform, cancer screening research plays directly into national policy. In the complex landscape for cancer screening policy, epidemiologists can increase their impact by learning from past experiences, being well prepared and communicating effectively.
MeSH Headings: Epidemiology, Public Policy, Health Policy, Cancer Screening
INTRODUCTION
Policies that promote screening the general public for detectable preclinical cancers have proven effective in reducing mortality and, next to reductions in tobacco use, are likely the main factor in the reduction in overall cancer mortality since the mid 1990s (1, 2). The evidence for early detection effectiveness has been derived primarily from epidemiology studies and the efforts of epidemiologists in study design, implementation and analysis. The issues and challenges of assessing population health benefits from cancer screening, and screening in general, have generated methodological insights that are now part of the epidemiologic armamentarium. For example, the concepts of lead-time bias, overdiagnosis and the importance of using the prevention of mortality as the screening outcome rather than the detection of early stage cancers or survival (3, 4). However, during the process of applying such research to guide recommendations and other policy changes, epidemiologists have faced challenges as they travel the road from translation of epidemiologic evidence to policy. Along this road to policy a multitude of other disciplinary and professional perspectives are encountered, including third-party payors with coverage and reimbursement interests, as well as, professional societies, politicians and cancer advocates.
Here we consider these and related experiences in cancer screening research and argue that epidemiologists play distinctly different roles depending on the maturity of the underlying science and level of the policy debate. While our focus is on cancer screening, our observations are applicable to other disease screening programs. The recent experience gleaned from controversies over breast cancer screening and the conclusion of the National Lung Screening Trial (NLST) provide a unique opportunity to examine the interplay between science and policy and the concomitant evolving role of epidemiologists. It is not our purpose to review the epidemiologic evidence or the details of the debates themselves (5–9), but rather to consider the role of epidemiologists in the process from research to dissemination and implementation across the spectrum of cancer screening procedures.
Cancer is a common disease in the United States. Approximately 1,529,560 new cases and 569,490 deaths from cancer occurred in the United States in 2010 (2). One in two men and one in three women will be diagnosed with cancer during their lifetime (10). Although cancer is the second leading cause of death in the U.S., approximately 11.7 million cancer survivors are alive today (11) and cancer screening has contributed to these extended years of life after cancer diagnosis and treatment. The Centers for Medicare and Medicaid Services (CMS) provides payment for more than 50% of all healthcare services in the United States. Prior to 2010 Medicare did not cover preventive services because the 1965 Medicare authorizing legislation stated that coverage was for therapeutic and diagnostic services. An exception was made for only 15 preventive services under very specific conditions. Screening mammography was one of the 15 covered preventive services, but was not covered until 1992 due to a specific provision in the Omnibus Budget Reconciliation Act of 1990 (OBRA ’90; Public Law 101–508).
There have been multiple occasions when policy decisions about cancer screening coverage and reimbursement by Medicare have used epidemiologic evidence. The Medicare Improvements for Patients and Providers Act of 2008 (MIPPA; Public Law 110–275) permitted (but did not require) coverage of preventive services recommended with a grade of A or B by the United States Preventive Service Task Force (USPSTF) using the national coverage determination process. The Patient Protection and Affordable Care Act of 2010 (PPACA) went further by eliminating co-insurance for preventive services covered by Medicare and by requiring group health plans and private insurers to provide coverage, without cost sharing, for services with a rating of A or B under the current recommendations of the USPSTF. At each review of its recommendations, the USPSTF incorporates new literature into its ratings, primarily from epidemiologic studies. Because of this legislation and its empowerment of the USPSTF review process, future cancer screening research has the potential to immediately enter the national policy arena, an arena few epidemiologists are trained to navigate.
CONTEXT: Exploring Epidemiologists Role in Screening Research
Early during the development of screening procedures, epidemiologists have been called upon to perform their traditional role as researchers examining disease etiology, disease burden, risk estimation, and causality. Epidemiologists are key players in any such research program and are often sought by research teams to provide guidance regarding appropriate study design, methodology and analytic plans. Findings from this early stage of research are communicated primarily within the academic community. The epidemiologists play an active role in the research process by directing their research, participating with other research teams, and directly engaging the scientific community. Using the simplified but illuminating metaphor of a journey requiring various modes of transportation, this early stage of screening research is symbolized by the epidemiologist driving the car as one of a few passengers in the research and translational endeavor (Figure 1).
Figure 1.
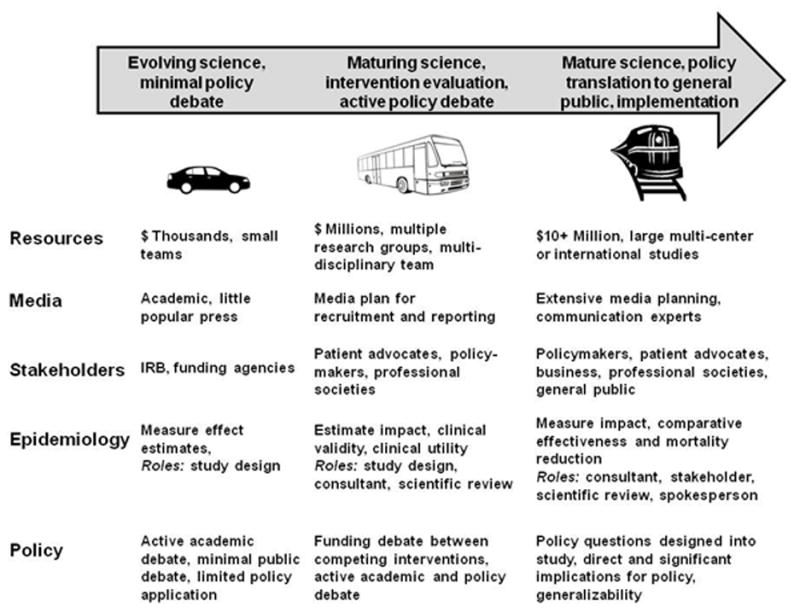
Evolution of evidence into policy for cancer screening programs
As the science regarding etiology matures and evidence for potential effectiveness of a screening modality accumulates, epidemiologists and funding agencies for research (e.g., NCI) come together to design and fund clinical trials. Research of promising screening modalities is coupled with clinical interventions to explore broader research questions. The roles epidemiologists take dramatically broaden at this transitional point when the scientific evidence may be in its adolescence and policy considerations in their infancy. Epidemiologists join participants from other disciplines and varied perspectives in the evolution of the science, technology and policy of cancer screening at this stage. They are, figuratively, navigating this portion of the scientific or policy debate as passengers on the bus (Figure 1) with many more passengers. At this stage of the journey, epidemiologists may be directly involved with conducting the research or be indirectly involved as consultants or content experts either for stakeholders or the research team itself. They may also be called upon to collate, codify and communicate research results and the strength of the evidence to either the public or engaged policymakers. The number of “passengers” increases compared to earlier evolving science. At this stage, more specialists are required to perform the research, measure its implications and communicate results to a wider audience. While many of these roles benefit from the epidemiology perspective, few epidemiologists have formal training in media communication, health policy, community involvement, comparative effectiveness, decision analysis or program evaluation.
The next stage of the journey translating from epidemiologic evidence to policy for cancer screening reflects a mature scientific and policy environment where incremental changes to the science require large studies and teams to manage them. A train symbolizes this stage of research requiring extensive infrastructure and investments of time and money by multiple parties to answer the scientific or policy questions. The epidemiologist is one among many disciplines in this environment while still playing a critical role. Answering questions regarding policy or programmatic implementation are integral to proposed studies. The outcome of this stage of screening research is instrumental in determining whether screening should be covered by insurance and recommended to the general public. Comparative effectiveness and program evaluation of screening modalities influence the policy debate, which is framed within the cultural and demographic characteristics of populations likely to follow screening recommendations. A single expensive controlled trial may be implemented to address the potential efficacy of a screening program. The cost of such research is high, in the tens or hundreds of millions of dollars.
Epidemiologists continue to fill their previous roles but with the additional task of acting as a spokesperson for various policy or scientific positions. Epidemiologists can be team members of expert panels that either communicate directly with Congress or make recommendations that may influence an entire healthcare market. An expert panel’s recommendation to screen for a particular cancer creates a taxpayer obligation in the billions of dollars through CMS and generates concomitant billions of revenue dollars for the healthcare industry. Industries, professional societies and patient advocacy groups enter the policy debate and defend their constituents or positions. Communication specialists and media experts pervade the policy debate as each stakeholder tries to communicate its message in a complex media landscape. Epidemiologists have made important contributions to cancer screening policy for cervical (12), colorectal (13, 14) and prostate (15) cancers among others, but we focus here on the instructive experience and contrasts between breast and lung cancer screening.
Maturity of Breast Cancer Screening Science and Policy
Recognition of the potentially enormous expense and infrastructure of implementing population screening for breast cancer led to early decisions to mount randomized trials to assess the effectiveness of screening for breast cancer. These large trials assessed the effectiveness of various screening modalities including mammography and clinical breast examination to reduce cancer mortality. By the mid-1970’s, results of trials established the effectiveness of mammography screening in reducing breast cancer mortality among women between the ages of 50 and 69 at first screening (16). In 1989, the USPSTF first addressed the topic of screening mammography and recommended screening for women age 50–75 every 1–2 years. The USPSTF stated “it may be prudent to begin mammography at an earlier age for women at high risk of breast cancer.” In its 1996 Guide to Clinical Preventive Services, the USPSTF again recommended in favor of screening women 50–69 every 1–2 years. Mammography screening for women age 40–49 was given a C grade, which meant insufficient evidence existed to make a recommendation for or against screening at that time. In 2002, the USPSTF revised their recommendation for screening mammography to screening every 1–2 years for women age 40 or more years, a B grade recommendation, indicating that the net benefit of screening was considered moderate.
In November 2009 after systematic review of additional published evidence, USPSTF revised their screening mammography recommendations for asymptomatic women with average risk. The discovery of significant overdiagnosis, especially among younger individuals with breast cancer, as well as the determination that biennial screens are as efficacious as annual screening drove the resulting recommendation changes (17). While maintaining their recommendation for women age 50 to 74, the USPSTF stated that, “the decision to start regular screening before the age of 50 should be an individual one and take into account patient context, including values regarding specific benefits and harms.” This was a C grade recommendation (18), but this relatively minor change from the 2002 recommendations was met with a firestorm of criticism when first published. The chair of the Society for Breast Imaging described them as a step backward. Patient advocates expressed disappointment and worried that the recommendations would undermine any future screening efforts (19). The task force had voiced its recommendations two weeks prior to the opening of the Senate debate on healthcare reform legislation. The 2009 experience of questioning the size of the benefit of mammography for breast cancer screening based on the evidence was not the first time that evidence-based recommendations about mammography were the topic of intense media attention (20). In fact, mammograms among women aged 40 to 49 were controversial a decade earlier, and Congress had acted to require payment for screening mammography after the Task Force recommended against screening for that age group (21).
The recent criticism was politically fueled by proposed, and later ratified, PPACA legislation that required recommendations by the USPSTF to be covered by health insurers with one glaring exception. The PPACA stated:
“for the purposes of this Act, and for the purposes of any other provision of law, the current recommendations of the United States Preventive Service Task Force regarding breast cancer screening, mammography, and prevention shall be considered the most current other than those issued in or around November 2009.” (22)
Thus, the new PPACA legislation acknowledged the Task Force recommendations but legislated a specific exception for breast cancer screening, much like the 1997 legislative response. For breast cancer screening the law reverted to the USPSTF 2002 recommendation. Thus it maintained insurance coverage of mammograms for women under age 50. Communication of the mammography recommendation of 2009 was not done effectively and mammography recommendations were a lightning rod for heated discussion in the policy arena.
Few debate the benefit of the current recommendation for breast cancer screening among women age 50 to 74. Remaining questions that surround breast screening include screening in other age groups or the persistent racial disparities in mortality given similar mammogram screening rates (23–25) and issues of possible overdiagnosis (26). Referring to our evolving evidence and policy metaphor, mammogram screening for breast cancer is to the far right of the Figure. The remaining scientific issues are nuanced and focus on improving screening through incorporating new knowledge from genetics and health services research. Policy considerations are heavily contested by entrenched stakeholders. Conduct of another clinical trial to determine the amount of reduction in mortality for breast screening among women less than 50 is unlikely due to the cost of the research and the ethics of withholding screening. Throughout this latter part of the journey for mammography screening, epidemiologists have played key roles as interpreters of evidence and educators about what policy actions are justified based on this evidence.
Lung Cancer Screening and Learning From the Past
No lung cancer screening program currently exists for populations at high-risk for lung cancer. So our second example takes us back to the left hand side of the Figure. Randomized trials for screening for lung cancer were initiated in the early 1970s (27). Early trials assessed the effect of chest x-ray alone and in combination with sputum cytology. Trials showed longer survival times with no improvements in mortality. However, these trials were limited by low statistical power, low adherence to the screening intervention, crossover between treatment groups, and a lack of a true control group. In 1996, the USPSTF recommended against screening for lung cancer using chest x-ray with or without sputum cytology.
The advent of low dose computed tomography (CT) raised hopes that detection of treatable lung lesions might lead to significant decreases in lung cancer mortality. But the most recent USPSTF 2004 recommendation concluded that the evidence was still insufficient to recommend for or against screening high risk, asymptomatic persons for lung cancer of low dose CT, chest x-ray, sputum cytology, or any combination of tests. The American College of Chest Physicians, the Society of Thoracic Radiology, the National Cancer Institute and advocacy groups such as the American Cancer Society came to similar conclusions (28). This unity of recommendation regarding low dose CT for lung cancer screening is in stark contrast to the rhetoric over the Task Force’s changing the breast cancer mammography screening recommendation for women age 40 to 49 to a discussion of risks and benefits with one’s physician and from annual to biennial mammography.
Low dose CT showed promise in a number of non-randomized and single arm studies. These studies found increased survival and more early stage disease (29, 30). The assumption was that decreased mortality would accompany early detection. Patient advocates called for implementation of CT screening. However, other non-randomized studies found no reduction in pathological stage or in mortality after CT screening (31, 32). Unlike breast cancer, diagnosis of even early stage lung cancer requires invasive surgery which has a greater operative mortality rate than breast cancer (1–3% versus 0–0.24%, respectively) (33, 34). The possible harm from screening drove the policy need to accurately measure the mortality benefits and harms from lung cancer screening, diagnosis and treatment. As a result, medical societies, advocates and policy makers all supported waiting for the outcome of two large randomized trials, the Dutch-Belgian NELSON trial and NLST. The NLST, initiated in 2002, became the first randomized clinical trial of low-dose CT for early detection of lung cancer designed with sufficient statistical power to detect an important reduction in lung cancer mortality. The study was halted in November 2010 after investigators reported a reduction of lung cancer related mortality of 20% in the low dose CT group when compared to the chest x-ray arm of the study (3).
The NLST was designed specifically to answer questions regarding efficacy in mortality reduction, comparative effectiveness and cost of low dose CT in a clinical environment for lung cancer screening. The large team with expertise from previous large clinical trials thoroughly examined the necessary population and protocols. The initial results of the NLST have only recently been published (3, 4). However, the ramifications of these findings as to who should be screened, the cost of that screening to insurers and the burden of over-diagnosis are not yet determined (4, 35). Moreover, the costs and consequences of false positive lung cancer screens resulting in expensive follow up, stress to the patient and burden upon medical care system remain unknown. The debate over the science as well as the policy arising from this and other lung cancer clinical trials began well before any publication of results (36–38). The important questions of cost, benefit and overdiagnosis remain to be answered for lung cancer screening.
LESSONS LEARNED
Epidemiologists can benefit from the lessons learned from the road traveled by their colleagues before them. Several specific lessons deserve emphasis:
1) Any screening study can have policy implications
Healthcare legislation requires insurance coverage for screening recommendations made by the USPSTF (Patient Protection and Affordable Care Act of 2010 legislation; Public Law 111–148). Since the USPSTF periodically reviews the epidemiologic and other scientific literature and its application to screening, a study may unintentionally be thrust into the national spotlight.
2) Communicate results and inform policy
The debate surrounding the 2009 Task Force recommendation for breast cancer screening highlights the importance of effective communication. The immediacy and accessibility of the media allows epidemiologists and policymakers only one opportunity to deliver their message effectively. Planning for effective communication of the results of research on screening and recommendations about screening is of paramount importance. Communication planning must include review of research results, the basis for recommendations, and the implications of the research or recommendation by funding agencies, coalition members, and other stakeholder groups so that the communicators are prepared to address concerns or questions from politicians, policymakers and other stakeholders. When research may have direct policy implications, which is virtually always the case with cancer screening, the communication plans must be prepared for the public potentially affected by any policy change that may arise from the research or recommendation.
3) Map the scientific, cultural, political and policy terrain
The first step, once the basic message is decided upon, is to map the environment in which the message will be distributed. Such a mapping should define the methods for conveying the message. Stakeholder analysis helps define various audiences, their level of sophistication and willingness to hear the message communicated. The risks and benefits of a potential screening program must be tailored to the intended audience. Cultural norms play a role as well. For example, many in the public see lung cancer as an avoidable disease whose self-inflicted cause is smoking. Research money for lung cancer is far less than for other less common or deadly cancers in part due to this perception. Policy makers or the public may not be as willing to commit resources to lung cancer when compared to breast cancer or other public health issues. Epidemiologic researchers may also unwittingly enter an environment with a rich and complex history of coalition or conflict among stakeholders.
4) Know when to get help communicating results or promoting policy
While mapping the terrain for communication and policy debate, experts in media communication and health policy should be enlisted. The epidemiologist may have the content and population knowledge, but a successful marketing or policy agenda is often not in the epidemiologist’s toolbox. Media specialists or those with prior experience communicating to the media, to a specific audience or to Congress are invaluable.
5) Create coalitions and partnerships
Creating coalitions and orchestrating campaigns among numerous stakeholders with similar objectives makes the desired outcome or public health intervention much more likely to be successful. Other investigators have discussed the importance of and specific methods for coalition building (39–41).
6) Be prepared for changes in political environment or public opinion
New research may pique the public’s interest or a policymaker open to changing the status quo may enter public office. The epidemiologist must be ready, with data and recommendations, to adapt to changing environments. Coalition members are often the first to know of the shift in sentiment or policy, thus reinforcing the need for the type of coalitions with stakeholders mentioned above. Planning ahead and having the ability to be nimble by providing data, compelling arguments or ready intervention plans can be just the right lever at the right moment to move a policy agenda forward or gain a policymaker’s trust.
7) Stay on message, know your role
Finally, one caution unique to communicating in the public eye or participating in policy forums is the desire by stakeholders to draw the epidemiologist outside their scope or role. A policymaker may ask a spokesperson’s opinion regarding a topic tangential to that being presented. An epidemiologist speaking about cancer screening policy should not opine about global warming simply because their opinion is asked. Unless the epidemiologist has training in economic analysis, an economic analysis should not be offered. An opponent may seek to draw the researcher into a debate outside one’s area of expertise. One’s desire to be accurate, and honest and to contribute to a broader debate can be used to derail or distract from the message or primary result. Similarly, one shouldn’t speculate, make comparisons without data, accept dichotomization of choice in complex environments, or speak outside one’s purview. Simplicity and singularity of focus, when combined with strategies of coalition building and orchestrated effort ease the application of epidemiology into policy.
DISCUSSION
The historical perspective of breast and lung cancer screening research reveals a pattern of greater generality -- the roles that epidemiologists play and the challenges they encounter vary enormously according to the stakeholders, maturity of the data, and related political events. Traditional roles of directing and guiding research dominate the early aspects of cancer screening. As the screening research matures the occasion for basic research diminishes. Other roles open, such as consultant, content expert, evidence reviewer, evaluator, spokesperson, advocate and educator. Ultimately, screening programs and policies benefit from the perspective unique to epidemiologists all along this road.
Epidemiologists must continue to refine cancer screening research with an eye toward improving policies derived from the research. The arena of cancer screening offers several important lessons for epidemiologists and the policy makers with whom they collaborate. The importance of communicating and crafting the message; creating coalitions to gain trust, legitimacy and implementation of results; planning every aspect of research, publication and public communication; enlisting outside expertise; and preparing to adapt to a changing scientific or political landscape should not be underestimated. Finally, it is important to stay on message and within the epidemiologist’s expertise to communicate and translate epidemiology into screening policy. The complex and crowded national policy arena requires the epidemiologist to have a singular focus in order to successfully communicate a cancer screening message and inform policy.
Acknowledgments
1U01ES019457-01 NIEHS/NCI, American College of Epidemiology, Washington University, and Ross Brownson for hosting the April 21-22, 2011 ACE Policy Committee workshop.
Select Abbreviations and Acronyms
- CT
Computed tomography
- NLST
National Lung Screening trial
- PPACA
Patient Protection and Affordable Care Act of 2010 legislation
- USPSTF
United States Preventive Service Task Force
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Annals of Oncology. 2007;18(3):581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Aberle DR, Adams AM, Berg C, Black WC, Clapp JD, et al. National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. New England Journal of Medicine. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sox HC. Better Evidence about Screening for Lung Cancer. New England Journal of Medicine. 2011;365(5):455–7. doi: 10.1056/NEJMe1103776. [DOI] [PubMed] [Google Scholar]
- 5.Welch HG. Screening Mammography — A Long Run for a Short Slide? New England Journal of Medicine. 2010;363(13):1276–8. doi: 10.1056/NEJMe1008369. [DOI] [PubMed] [Google Scholar]
- 6.Quanstrum KH, Hayward RA. Lessons from the Mammography Wars. New England Journal of Medicine. 2010;363(11):1076–9. doi: 10.1056/NEJMsb1002538. [DOI] [PubMed] [Google Scholar]
- 7.Silvestri GA. Screening for Lung Cancer: It Works, but Does It Really Work? Annals of Internal Medicine. 2011:E–364. doi: 10.7326/0003-4819-155-8-201110180-00364. [DOI] [PubMed] [Google Scholar]
- 8.Jett JR, Midthun DE. Screening for Lung Cancer: For Patients at Increased Risk for Lung Cancer, It Works. Annals of Internal Medicine. 2011:E–367. doi: 10.7326/0003-4819-155-8-201110180-00367. [DOI] [PubMed] [Google Scholar]
- 9.Harris R. Overview of Screening: Where We Are and Where We May Be Headed. Epidemiologic Reviews. 2011;33(1):1–6. doi: 10.1093/epirev/mxr006. [DOI] [PubMed] [Google Scholar]
- 10.U.S. National Institutes of Health NCI. SEER Training Modules, Cancer Facts & the War on Cancer. National Cancer Institute; 2010. [Accessed 07/20/2011]. ( http://training.seer.cancer.gov/disease/war/) [Google Scholar]
- 11.CDC. Cancer survivors—United States, 2007 [electronic article] MMRW. 2011;60:269–72. [PubMed] [Google Scholar]
- 12.Hartman K, Hall S, Nanda K, Boggess J, Zolnoun D. Screening for Cervical Cancer: Systematic Evidence Review No. 25. Rockville, MD: AHRQ; 2002. [PubMed] [Google Scholar]
- 13.Phillips KA, Liang S-Y, Ladabaum U, Haas J, Kerlikowske K, Lieberman D, et al. Trends in Colonoscopy for Colorectal Cancer Screening. Medical Care. 2007;45(2):160–7. doi: 10.1097/01.mlr.0000246612.35245.21. [DOI] [PubMed] [Google Scholar]
- 14.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized Colorectal Cancer Screening in Integrated Health Care Systems. Epidemiologic Reviews. 2011;33(1):101–10. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 15.Potosky AL, Feuer EJ, Levin DL. Impact of Screening on Incidence and Mortality of Prostate Cancer in the United States. Epidemiologic Reviews. 2001;23(1):181–6. doi: 10.1093/oxfordjournals.epirev.a000787. [DOI] [PubMed] [Google Scholar]
- 16.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan B, Nygren P, et al. AHRQ Publication No 10-05142-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Screening for Breast Cancer: Systematic Evidence Review Update for the U.S. Preventive Services Task Force. Evidence Review Update No. 74. [PubMed] [Google Scholar]
- 17.Kolata G. Behind Cancer Guidelines, Quest for Data. New York Times. 2009 Nov 23;2009:A19. [Google Scholar]
- 18.U.S. Preventive Services Task Force. [Accessed 07/20/2011 2011];Screening for Breast Cancer. 2009 ( http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm#related)
- 19.Rabin RC. New York Times. 2009. New Guidelines on Breast Cancer Draw Opposition; p. D5. [Google Scholar]
- 20.Woolf SH, Lawrence RS. Preserving Scientific Debate and Patient Choice. JAMA: The Journal of the American Medical Association. 1997;278(23):2105–8. doi: 10.1001/jama.278.23.2105. [DOI] [PubMed] [Google Scholar]
- 21.Lerner BH. Fighting the War on Breast Cancer: Debates over Early Detection, 1945 to the Present. Annals of Internal Medicine. 1998;129(1):74–8. doi: 10.7326/0003-4819-129-1-199807010-00028. [DOI] [PubMed] [Google Scholar]
- 22.Petitti D. Prevention and the science and politics of evidence. In: Menzel PaFH., editor. Prevention vs Treatment: Philosophical, Empirical and Cultural Reflections. Oxford University Press; 2011. [Google Scholar]
- 23.Fletcher SW. Breast Cancer Screening: A 35-Year Perspective. Epidemiologic Reviews. 2011;33(1):165–75. doi: 10.1093/epirev/mxr003. [DOI] [PubMed] [Google Scholar]
- 24.Murphy AM. Mammography Screening for Breast Cancer. JAMA: The Journal of the American Medical Association. 2010;303(2):166–7. doi: 10.1001/jama.2009.1991. [DOI] [PubMed] [Google Scholar]
- 25.Njai R, Siegel P, Miller J, Liao Y. Misclassification of survey responses and black-white disparity in mammography use, Behavioral Risk Factor Surveillance System, 1995–2006 [electronic article] Preventing Chronic Disease. 20118:A59. [PMC free article] [PubMed] [Google Scholar]
- 26.de Gelder R, Heijnsdijk EAM, van Ravesteyn NT, Fracheboud J, Draisma G, de Koning HJ. Interpreting Overdiagnosis Estimates in Population-based Mammography Screening. Epidemiologic Reviews. 2011;33(1):111–21. doi: 10.1093/epirev/mxr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the U.S Preventive Services Task Force. Ann Intern Med. 2004;140(9):740–53. doi: 10.7326/0003-4819-140-9-200405040-00015. [DOI] [PubMed] [Google Scholar]
- 28.Bach PB, Silvestri GA, Hanger M, Jett JR. Screening for Lung Cancer ACCP guidelines. Chest. 2007;132:69S–77S. doi: 10.1378/chest.07-1349. [DOI] [PubMed] [Google Scholar]
- 29.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–71. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 30.Yankelevitz D. CT screening for lung cancer. Am J Roentgenol. 2003;180(6):1736–7. doi: 10.2214/ajr.180.6.1801736a. author reply 7. [DOI] [PubMed] [Google Scholar]
- 31.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235(1):259–65. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 32.Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and lung cancer outcomes. JAMA: The Journal of the American Medical Association. 2007;297(9):953–61. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- 33.Grogan EL, Jones DR. VATS lobectomy is better than open thoracotomy: what is the evidence for short-term outcomes? Thorac Surg Clin. 2008;18(3):249–58. doi: 10.1016/j.thorsurg.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 34.El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W. Morbidity and Mortality Following Breast Cancer Surgery in Women: National Benchmarks for Standards of Care. Annals of Surgery. 2007;245(5):665–71. doi: 10.1097/01.sla.0000245833.48399.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall E. The Promise and Pitfalls of a Cancer Breakthrough. Science. 2010;330(6006):900–1. doi: 10.1126/science.330.6006.900-b. [DOI] [PubMed] [Google Scholar]
- 36.Marshall E. A Bruising Battle Over Lung Scans. Science. 2008;320(5876):600–3. doi: 10.1126/science.320.5876.600. [DOI] [PubMed] [Google Scholar]
- 37.Black C, de Verteuil R, Walker S, Ayres J, Boland A, Bagust A, et al. Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature. Thorax. 2007;62(2):131–8. doi: 10.1136/thx.2006.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bach PB. Inconsistencies in Findings From the Early Lung Cancer Action Project Studies of Lung Cancer Screening. Journal of the National Cancer Institute. 2011;103(13):1002–6. doi: 10.1093/jnci/djr202. [DOI] [PubMed] [Google Scholar]
- 39.Mercer SL, Sleet DA, Elder RW, Cole KH, Shults RA, Nichols JL. Translating Evidence into Policy: Lessons Learned from the Case of Lowering the Legal Blood Alcohol Limit for Drivers. Annals of Epidemiology. 2010;20(6):412–20. doi: 10.1016/j.annepidem.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Widome R, Samet JM, Hiatt RA, Luke DA, Orleans CT, Ponkshe P, et al. Science, Prudence, and Politics: The Case of Smoke-Free Indoor Spaces. Annals of Epidemiology. 2010;20(6):428–35. doi: 10.1016/j.annepidem.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Samet JM, McMichael GH, II, Wilcox AJ. The Use of Epidemiological Evidence in the Compensation of Veterans. Annals of Epidemiology. 2010;20(6):421–7. doi: 10.1016/j.annepidem.2010.03.002. [DOI] [PubMed] [Google Scholar]


