Abstract
This study aims to explore the genetic and environmental contributions to autistic-like behaviors in a general population sample of toddlers. In a classic twin study of 313 same-sex, 2-year-old twin pairs, autistic-like behaviors were assessed via parent ratings on the pervasive developmental problems subscale of the Child Behavior Checklist and observationally using tester ratings on the orientation/engagement subscale of the Behavior Rating Scale. Analyses show moderate, significant heritabilities for both measures of autistic-like behaviors, as well as modest, but significant shared environmental effects. These genetic and environmental influences overlap greatly between the two measures. Autistic-like behaviors in 2-year-old twins are largely genetic in etiology, but are also influenced by a shared environmental component at this age. This is the first study to examine the etiology of such behaviors in a sample of toddlers, thus providing novel information which could guide future research on genetic and environmental factors that affect these behaviors.
Keywords: Autistic disorder, Behavioral genetics, Child Behavior Checklist
Introduction
The broad category of autism spectrum disorders (ASD) comprises several disorders such as autism, Asperger’s syndrome, and pervasive developmental disorder not otherwise specified (PDD-NOS). These syndromes are marked by three core domains of impairments: unusual social interactions, impaired communication, and the presence of stereotyped or circumscribed behaviors and interests (American Psychiatric Association [DSM-IV-TR] 2000). While an autism diagnosis requires that all of the aforementioned categories of symptoms be present before age three, to be diagnosed with Asperger’s, there can be no language delay; and PDD-NOS is diagnosed when some autistic symptoms are present, but not enough to warrant a full autism diagnosis or with an onset after age three (DSMIV-TR 2000). This spectrum of disorders, once thought to be relatively rare, is now estimated to be as prevalent as 1 in every 166 births (Chakrabarti and Fombonne 2005).
The behaviors associated with ASD are thought to be the result of differences in the neuroanatomy of these individuals (DiCicco-Bloom et al. 2006), but the underlying etiology of these neural changes is largely unknown. There is a growing body of evidence showing strong heritability of diagnosable autism. The prevalence of autism has been estimated to be as high as 3–10% in siblings of those who are affected with the disorder, about five to fifteen times higher than in the general population; further, genetically identical monozygotic (MZ) twins are far more likely to be concordant for autism diagnosis than are dizygotic (DZ) twins who share ~50% of their segregating genes (for reviews, see Abrahams and Geschwind 2008; Folstein and Rosen-Sheidley 2001; Rutter 2000). Heritability estimates as high as 91% have been suggested for diagnosable autism (Bailey et al. 1995). However, because the concordance between MZ twins for autism diagnosis is much more than twice that of DZ twins (e.g., 69 vs. 0%, Bailey et al. 1995), it has been proposed that autism involves epistatic genetic factors, meaning that a certain combination of alleles must be present for the syndrome to emerge (Risch et al. 1999). Therefore, while several susceptibility genes have been proposed in recent years (Abrahams and Geschwind 2008), it is not surprising that researchers have not been able to identify a single gene for autism. Also, the lack of complete concordance between identical twins suggests modest environmental effects. Additionally, there may be gene-environment interactions which influence these behaviors.
Recent studies have suggested that autistic symptoms may be common in the general population, with a continuous distribution of severity, and that individuals with diagnosable autism represent those with symptoms at the extreme of the severity continuum (Constantino and Todd 2003; Hoekstra et al. 2007; Spiker et al. 2002). Family studies have shown that relatives of autistic probands show milder manifestations of autistic behaviors, often below diagnostic threshold levels (Piven et al. 1997). This is consistent with a trait perspective of autism (i.e., as a continuously distributed spectrum of autistic behaviors).
Given that autistic symptoms can be present in individuals without a diagnosable autism spectrum disorder and that these autistic-like behaviors show quantitative variation in the general population, it is reasonable to explore the etiology of these behaviors in non-clinical samples. Few twin studies have examined genetic and environmental influences on individual differences in autistic-like behaviors measured on a continuum in a general population sample. In the Twins Early Development Study (TEDS), genetic influences explained ~78–81% of the variance in autistic traits in a large population-based sample of 8-year-old twins (Ronald et al. 2006). Similar studies involving twins aged 7–15 (Constantino and Todd 2003) and 18-year-old twins and their siblings (Hoekstra et al. 2007) have also revealed substantial genetic influence, but with somewhat more moderate heritability estimates. To the best of our knowledge, no study yet has yet examined the etiology of such behaviors in younger children.
In the present study, we explore genetic and environmental contributions to individual differences in autistic-like behaviors in a population sample of 2-year-old twins. Although previous studies have shown such behaviors to have genetic etiologies in older children (Constantino and Todd 2003; Hoekstra et al. 2007; Ronald et al. 2006; Ronald et al. 2005) genetic effects that influence a behavior at one age do not necessarily influence the same behavior at another age. Genes are dynamic in nature, changing in the quantity and quality of their effects across time (Plomin 1986), thus it is important to investigate the etiology of these behaviors in toddlers. The age of two is particularly salient because it is frequently acknowledged that this is the age when autism can first be reliably diagnosed (DiCicco-Bloom et al. 2006). Further, diagnoses assigned at this age have been shown to be stable into later preschool years (Chawarska et al. 2007). Clearly autistic-like behaviors are present and developmentally significant at this young age; at question then, is what accounts for the variability in such behaviors? Based on prior research, we predicted that these symptoms would be heritable, but given that other behavior problems in very early childhood tend to be influenced by shared environments as well as by genes (Derks et al. 2004; Saudino et al. 2008; Schmitz et al. 1994), we also expected to find shared environmental influences on autistic-like behaviors in our normative sample.
In the present study we use multivariate genetic methods to explore genetic and environmental sources of covariance between parent ratings of autistic-like behaviors and an observed measure of orientation/engagement behaviors in our sample of 2-year-old twins. The observational measure was not originally intended to be used to assess autistic symptoms, but rather as a measure of social orientation and task engagement, however, the items included in the measure (listed in Table 1) assess social skills and behaviors towards objects that tend to be impaired in children with autism spectrum disorders. Thus, in the current study, we suggest that the orientation/engagement measure may be an observational measure of normative development that is sensitive to autistic-like behaviors. A major component of this measure is the child’s observed social behaviors. Social behavior is affected by both genetic and environmental factors in early childhood with shared environmental influences decreasing and genetic influences increasing with age (Knafo and Plomin 2006; Saudino et al.2008; Scourfield et al. 2004). Similarly, social impairment and social responsiveness, as assessed on autism screening measures, show substantial heritability (i.e., over 70%) in middle to late childhood (Constantino et al. 2003; Ronald et al. 2005). Given that autistic symptoms include unusual social interactions and behaviors towards objects, we predicted that autistic-like behaviors and orientation/engagement would be related; however, because our measure of orientation/engagement assesses skills rather than symptoms, we expected that the relation would be in the negative direction. Moreover, because these two behavioral domains appear to tap behaviors related to ASD, we also expected that common genetic factors would contribute to the correlation between the two phenotypes.
Table 1.
BRS orientation/engagement scale
| Item | Description | Behavior type |
|---|---|---|
| 5 | Positive affect | Social |
| 9 | Energy | Other |
| 11 | Interest in test materials and stimuli | Interest in objects |
| 12 | Initiative with tasks | Other |
| 13 | Exploration of objects and/or surroundings | Interest in objects |
| 16 | Enthusiasm toward tasks | Other |
| 17 | Fearfulness | Social |
| 19 | Orientation to examiner | Social |
| 20 | Social engagement | Social |
Methods
Sample
The Boston University Twin Project sample was recruited from birth records supplied by the Massachusetts Registry of Vital Records. Twins were selected preferentially for higher birth weight and gestational age. No twins with birth weights below 1,750 g or with gestational ages less than 34 weeks were included in the study. One twin was excluded due to a chromosomal abnormality. The present analyses include 313 same-sex pairs of twins (165 male: 74 MZ, 91 DZ; 148 female: 71 MZ, 77 DZ), mean age 2.07 years (SD = 0.05). Although the sample was predominately Caucasian (85.4%), ethnicity was generally representative of the Massachusetts population (3.2% Black, 2% Asian, 7.3% Mixed, 2.2% Other). Socioeconomic status according to the Hollingshead Four Factor Index (Hollingshead 1975) ranged from low to upper middle class (range = 20.5–66; M = 50.9, SD = 14.1).
Zygosity was determined via DNA analyses using DNA obtained from cheek swab samples. In the cases where DNA was not available (n = 3), zygosity was determined using parents’ responses on physical similarity questionnaires which have been shown to be more than 95% accurate when compared to DNA markers (Price et al. 2000).
Procedure overview
Twins were assessed within ~2 weeks of their second birthday. The procedure consisted of two visits, 48 h apart, to the laboratory. At the initial visit, informed consent from parents was obtained. One twin was assessed within a standardized test situation, while the other twin was assessed within a laboratory play situation. The test situation involved administration of the Mental Scale of the Bayley Scales of Infant Development–Second Edition (BSID-II; Bayley 1993). The play situation comprised various episodes from the Laboratory Temperament Assessment Battery–Preschool Version (Goldsmith et al. 1995). At the second visit, situations were reversed for each twin. Cheek scrapings were then collected for genetic analyses. Twins were assessed by different testers; however, for each twin, the tester was the same across the two laboratory situations. All procedures were approved by the Boston University Institutional Review Board.
Measures
Parent reports of autistic-like behaviors
The Pervasive Developmental Problems subscale from the Child Behavior Checklist for Ages 1 ½–5 (CBCL/1 ½–5; Achenbach and Rescorla 2000) provided a parent-rating measure of autistic-like behaviors. This subscale consists of 13 statements regarding the child’s social, communicative, and repetitive behaviors (e.g., “Avoids looking others in the eye.”; “Repeatedly rocks head or body.”; full listing in Table 2). Parents are asked to indicate how well each statement describes their child’s behavior as observed within the past 2 months on a three-point Likert scale (0 = Not True to 2 = Very True or Often True). Previous research (Sikora et al. 2008) has shown this subscale, along with the withdrawn subscale, to be a sensitive and useful screening device for autism spectrum disorders in toddlers, and strongly correlated with other autism screening measures such as the Gilliam Autism Rating Scale (GARS). In the present sample, internal consistency was 0.64 as estimated by Cronbach’s alpha.
Table 2.
CBCL PDP subscale
| Item | Description | Behavior type |
|---|---|---|
| 3 | Afraid to try new things | Restricted/repetitive behavior |
| 4 | Avoids looking others in the eye | Social |
| 7 | Can’t stand having things out of place | Restricted/repetitive behavior |
| 21 | Disturbed by any change in routine | Restricted/repetitive behavior |
| 23 | Doesn’t answer when people talk to him/her | Communicative |
| 25 | Doesn’t get along with other children | Social |
| 63 | Repeatedly rocks head or body | Restricted/repetitive behavior |
| 67 | Seems unresponsive to affection | Social |
| 70 | Shows little affection toward people | Social |
| 76 | Speech problems | Communicative |
| 80 | Strange behavior | Restricted/repetitive behavior |
| 92 | Upset by new people or situations | Restricted/repetitive behavior |
| 98 | Withdrawn, doesn’t get involved with others | Social |
Observer reports of orientation/engagement
The orientation/engagement (OE) subscale of the Behavior Rating Scale (BRS) from the BSID-II (Bayley 1993) was used to provide an observational measure of autistic-like behaviors in the laboratory. The OE subscale for 2-year-olds consists of nine items (listed in Table 1), rated on five-point scales evaluating infant behavior including social engagement, orientation to examiner, positive affect, enthusiasm, initiative, interest and exploration of surroundings and experimental tasks, energy, and fearfulness. Testers completed the BRS following both visits to the lab. The standardized, unweighted items were aggregated and averaged across visits. This composite score displayed good reliability in terms of internal consistency (α = 0.92). For 20% of the sample, a second observer rated the behavior of the target child. Inter-rater agreement for composite scores based on these ratings was high (r = 0.80, P < 0.01).
Data transformation
The PDP subscale score from the CBCL showed a moderate positive skew (1.3); consequently, this variable was square-root transformed to create a more normal distribution. Similarly, the OE subscale scores on the BRS showed a modest negative skew (−0.8); thus, this variable was power-transformed. Because twin covariances can be inflated by variance due to sex, all scores were residualized for sex effects (see McGue and Bouchard 1984). These residualized scores were used in all behavioral genetic analyses.
Correlational analyses
Genetic influences are implied when cotwin similarity covaries with the degree of genetic relatedness. Intraclass correlations typically serve as indices of cotwin similarity. An MZ correlation that is greater than the DZ correlation suggests genetic influences on the phenotype. Shared environmental influences are evidenced when the DZ correlation is greater than half of the value of the MZ correlation; non-shared environmental effects are present when the MZ correlation is less than unity.
To evaluate genetic and environmental sources of covariance across variables, cross-twin cross-variable correlations were calculated. Cross-twin correlations are the essence of a multivariate analysis of covariance. For the present analyses, the cross-twin cross-variable correlation involved correlating Twin 1’s score for autistic-like behaviors as measured on the CBCL with Twin 2’s score for orientation/engagement behaviors and vice versa. Genetic contributions to the covariance between two measures are implied when the MZ cross-twin correlation is greater than the DZ cross-twin correlation. As with the intraclass correlations, a DZ cross-correlation that is greater than half the MZ correlation suggests shared environmental influences and an MZ cross-correlation of less than one indicates the presence of non-shared environmental effects.
Model-fitting analyses
Although the major results of a multivariate twin analysis can be gleaned from twin cross correlations, model-fitting procedures analyze all of the data simultaneously, provide tests of the fit of models, yield confidence intervals for parameter estimates, and test the fit of alternative models (Plomin et al. 2001). Multivariate genetic models decompose the variance of each phenotype and the covariances between phenotypes into additive genetic effects (A), shared environmental effects (C), and nonshared environmental effects (E). Additive genetic influences refer to the sum of the average effect of all genes that influence a trait. Based on the degree of genetic relatedness, the A factors correlate 1.0 and 0.5 for MZ and DZ twins, respectively. The C factors refer to the influence of shared rearing environments on twin resemblance. Because all twins in this study were reared in the same family, shared environments correlate 1.0 for both MZ and DZ twins. Finally, the E factors reflect nonshared environmental influences that are unique to each member of a twin pair, including measurement error.
In the present study, a Cholesky decomposition model was used to investigate the sources of covariance between autistic-like behaviors and orientation/engagement. In a Cholesky decomposition, the number of latent genetic, shared environmental, and nonshared environmental variables each equal the number of phenotypes under study. Thus, a model that includes a measure of autistic-like behaviors and one of orientation/engagement behavior has two latent factors for each source of variance (i.e., genetic and environmental effects); the first factor loads on both measures, and the second factor loads only on the second measure. As illustrated in Fig. 1, the latent variables A1, C1, and E1 represent genetic and environmental effects that are common to both the orientation/engagement and autistic-like behavior measures; A2, C2, and E2 represent effects that are unique to the autistic-like behavior measure. This model fully parameterizes the covariances structure and consequently, imposes no underlying genetic or environmental structure on the data. The factor loadings on the genetic and environmental latent variables simply inform about the etiology of the covariation between the measured phenotypes (Martin and Eaves 1977). The phenotypes were ordered in such a manner to explore additional influences that might be acting on the autistic-like behaviors beyond those which also act on orientation/ engagement; a reversal of the ordering would yield identical results with regard to genetic and environmental interrelations between the measures, and would provide information about genetic and environmental influences unique to orientation/engagement behaviors. A series of reduced models were fit to the data to test the significance of genetic and environmental covariance between the two behaviors. For example, significant genetic covariance is suggested when the path leading from the common A1 factor to the autistic-like behaviors phenotype cannot be eliminated from the model without a decrement in fit. The same logic applies to shared and nonshared environmental covariances.
Fig. 1.
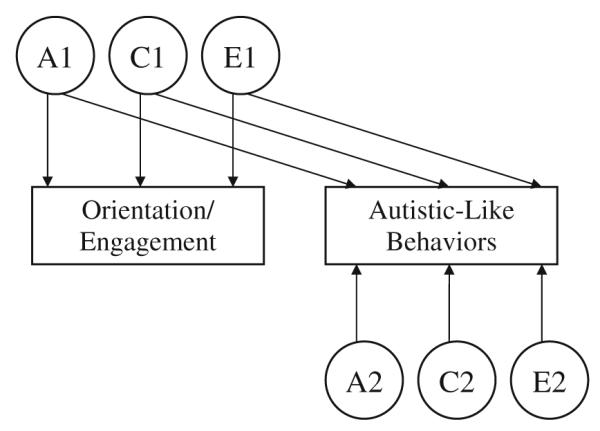
Cholesky Model
Models were fit to raw data using a maximum likelihood pedigree approach implemented in Mx structural equation modeling software (Neale et al. 2006). This approach does not yield a χ2 for assessing the fit of the model, however, the fit of a model can be assessed by calculating twice the difference between the negative log-likelihood (−2LL) of the model and that of a saturated model (i.e., a model in which the variance/covariance structure is not estimated and all variances and covariances for MZ and DZ twins are estimated). The difference in −2LL is asymptotically distributed as χ2 with degrees of freedom equal to the difference in the number of parameters in the full model and that in the saturated model.
Results
Descriptive statistics
Table 3 lists the means and standard deviations by sex and zygosity. Mean differences were evaluated using generalized estimating equations (GEE) implemented in the SAS GENMOD procedure to account for dependence in the data due to the fact that our sample comprised pairs of twins. GEE are an extension of the standard generalized linear models that allow modeling of correlated data (Liang and Zeger 1986; Zeger and Liang 1986). The data show no significant differences in autistic-like behaviors or orientation/engagement behaviors between sexes or zygosity groups.
Table 3.
Means (and standard deviations) for measures of autistic-like behaviors and orientation/engagement behaviors by sex and zygosity
| Measure | Males |
Females |
Effect size |
|||
|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | Sex | Zygosity | |
| CBCL: PDP subscale | 0.45 (0.31) | 0.47 (0.30) | 0.42 (0.28) | 0.48 (0.30) | −0.01 | 0.03 |
| BRS: OE subscale | 11.58 (3.83) | 11.59 (3.88) | 11.61 (3.94) | 11.82 (3.65) | 0.14 | 0.10 |
Effect size estimated as Cohen’s d, which expresses group differences in standard deviation units
MZ monozygotic twins, DZ dizygotic twins
Correlational analyses
As predicted, children whose parents rated them as having more autistic-like behaviors tended to have lower ratings of orientation/engagement in the laboratory (r = −0.21, P < 0.001). For both measures, the intraclass correlations for MZ twins exceeded those for DZ twins, suggesting genetic influences on autistic-like behaviors and orientation/engagement (Table 4). Cross-twin cross-measure correlations for MZ twins were also greater in magnitude than those for DZ twins, indicating that genetic factors contribute to the phenotypic correlation between autistic-like behaviors and social behaviors. As the DZ correlations were greater than half of the MZ correlations, we can expect that there may be some shared environmental effects on these phenotypes. The MZ correlation was substantially less than unity, suggesting the presence of non-shared environmental influences as well.
Table 4.
Twin intraclass correlations, cross-measure correlations (and 95% confidence intervals)
| Measure | Intraclass correlations |
Cross correlations |
||
|---|---|---|---|---|
| MZ | DZ | MZ | DZ | |
| CBCL: PDP subscale | 0.58 (0.46; 0.68) | 0.38 (0.24; 0.50) | −0.17 (−0.33; −0.01) | 0.05 (−0.10; 0.20) |
| BRS: OE subscale | 0.40 (0.26; 0.53) | 0.22 (0.07; 0.36) | ||
MZ monozygotic twins, DZ dizygotic twins
Model-fitting analyses
Table 5 presents the fit statistics for the model-fitting analyses. In addition to the full model, a series of reduced models were fit to the data to evaluate sources of covariance between measures. For our measures, the full model fit the data well; however, it was possible to drop the nonshared environmental covariance (E cov model) from the model without a significant decrease in fit. In contrast, dropping the genetic covariance (A cov model) or the shared environmental covariance (C cov model) resulted in significantly worse fits. Therefore, in the best fitting model, both genetics and shared environmental factors significantly contributed to the covariance between the parent ratings of autistic-like behaviors and the observer ratings of orientation/engagement behaviors.
Table 5.
Model-fitting statistics: autistic-like behaviors-orientation/engagement
| Variable and model | Overall fit of modela |
Relative fit of modelb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| −2LL | df | χ 2 | df | P | AIC | Δ χ 2 | df | P | |
| Saturated | 3536.845 | 1219 | |||||||
| Full | 3553.722 | 1230 | 16.877 | 11 | 0.11 | −5.123 | |||
| Drop A-cov | 3562.722 | 1231 | 25.877 | 12 | 0.01 | 1.877 | 9.00 | 1 | 0.003 |
| Drop C-cov | 3558.185 | 1231 | 21.340 | 12 | 0.04 | −2.660 | 4.46 | 1 | 0.035 |
| Drop E-cov | 3556.782 | 1231 | 19.937 | 12 | 0.07 | −4.063 | 3.06 | 1 | 0.080 |
−2LL likelihood statistic; χ2 Chi-square fit statistic; df degrees of freedom; AIC Akaike’s information criterion; A cov genetic covariate; C cov shared environmental covariate; E cov nonshared environmental covariate
Overall fit of the model is determined by the difference in −2LL of the model and that of a saturated model
Relative fit of the model determined by the χ2 difference (Δχ2) between full bivariate ACE model and reduced model
Estimates of genetic and environmental variances and correlations from the full- and best-fitting models are presented in Table 6. In both models, both the autistic-like behavior and orientation/engagement measures were found to be heritable (with genes accounting for ~40 and 30% of the variance, respectively), and each had a significant shared environmental variance component as well (~20 and 12% of variance explained, respectively). The remaining variance in each measure is accounted for by nonshared environmental influences, which include measurement error. There were no genetic or shared environmental effects that were unique to autistic-like behaviors. That is, all of the genetic and shared environmental factors that influenced the autistic-like behavior measure were common to the orientation/engagement measure.
Table 6.
Estimates of genetic and environmental variances and genetic and environmental correlations (and 95% confidence intervals) from full- and best-fitting models
| Model | Variance components |
Covariances | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orientation/engagement | Autistic-like behavior |
|||||||||||
|
|
Common |
Unique |
|
|||||||||
| a 2 | c 2 | e 2 | a 2 | c 2 | e 2 | a 2 | c 2 | e 2 | r g | r c | r e | |
| Full | 0.26 (0.06; 0.46) |
0.13 (0.01; 0.30) |
0.61 (0.50; 0.73) |
0.38 (0.14; 59) |
0.21 (0.04; 0.40) |
0.01 (0.00; 0.03) |
0.00 (−0.43; 0.43) |
0.00 (−0.34; 0.34) |
0.40 (0.32; 0.51) |
−1.0 (−1.0; −0.39) |
1.0 (0.07; 1.0) |
−0.12 (−0.26; 0.01) |
| Reduced | 0.30 (0.14; 0.45) |
0.11 (0.02; 0.25) |
0.59 (0.49; 0.70) |
0.39 (0.19; 0.58) |
0.21 (0.05; 0.38) |
– | 0.00 (−0.30; 0.30) |
0.00 (−0.30; 0.30) |
0.40 (0.30; 0.50) |
−1.0 (−1.0; −0.62) |
1.0 (0.23; 1.0) |
– |
a2 genetic variance, c2 shared environmental variance, e2 nonshared environmental variance, rg genetic correlation, rc shared environmental correlation, re nonshared environmental correlation
With a genetic correlation of −1.0, we again see this pattern of completely overlapping genetic effects between the two measures. As expected, this genetic covariance between the autistic-like behavior and the orientation/ engagement variable was in the negative direction, suggesting an inverse relationship between these measures, with the genetic factors that influence individuals to have more autistic-like behaviors also influencing them to perform worse on the orientation/engagement measure. An unexpected finding was that the shared environmental covariance, again showing complete overlap between measures, was in the positive direction (rc = 1), indicating that the shared environmental factors that resulted in more autistic-like behavior problems also influenced better orientation/engagement behaviors in the laboratory setting. It should be noted that the confidence interval around this estimate is rather wide, so it is possible that this correlation might be as low as 0.23, suggesting a much more modest degree of overlap.
Discussion
The goal of this study was twofold: first, to explore the etiology of autistic-like behaviors in a general-population sample of 2-year-old twins, and secondly, to examine the relationship between parent-reports of autistic-like behaviors and an observed measure of orientation/ engagement within the lab situation. We were curious to test whether these two conceptually-related measures (albeit in opposite directions with one assessing deficits and the other assessing skills in the same domains) were influenced by common genetic and environmental factors. While earlier studies have investigated genetic and environmental factors that influence autistic-like behaviors (Constantino and Todd 2003; Hoekstra et al. 2007; Ronald et al. 2006), the current study included a much younger sample than had any of the previous studies. Moreover, no prior studies have included observational measures that may be sensitive to autistic-like behaviors.
This study confirms previous claims both that subclinical autistic-like behaviors exist in the general population and that they are moderately heritable; however, the heritability estimate in the current study, at ~0.4, was substantially smaller in magnitude than in previous studies (Hoekstra et al. 2007; Ronald et al. 2006). One possible explanation for a finding of lower genetic variance is that it might be more difficult to reliably assess autistic-like behaviors in younger children (Zwaigenbaum et al. 2007), which would make it harder to detect genetic effects and would result in greater nonshared environmental variance. However, our estimate of nonshared environmental variance does not differ substantially from that reported by Hoekstra et al. so this does not appear to be the case. Instead, the difference in the relative proportion of genetic variance seems to be due to the increased influence of shared environmental effects in this younger age group, suggesting that there are different factors influencing variability in toddlers than in older children. While half of the children from our sample who were later diagnosed with autism received higher scores on the PDP subscale (above 80th percentile), very few scored high enough to be in the clinical range at this young age.
As hypothesized, there were genetic influences that contributed significantly to both the autistic-like behavior and orientation/engagement measures. Furthermore, the genetic covariance followed the expected pattern of negative correlation in which genetic factors related to higher incidence of autistic-like behaviors were also those which impaired orientation/engagement behavior. This suggests that at the level of genetic etiology these two measures are both tapping similar types of behaviors, and that perhaps lab-based observational measures of normative behaviors such as the OE subscale of the BRS might be sensitive to autistic-like behavior.
Previous studies with older children have been inconsistent in their findings with respect to shared environmental influences on autistic-like behaviors, with some reporting no significant shared environmental effects (Constantino et al. 2003; Ronald et al. 2006) and others finding substantial influences (Constantino and Todd 2003). In the present study, shared environmental factors accounted for 21% of the variance in our autistic-like behavior measure. This discrepancy in findings may be explained by the difference in age between our toddler-aged sample and those of previous studies and/or differences in measures used to assess autistic traits. The finding that shared environmental influences have larger effects in a younger population is consistent with previous research on the etiology of autism and other behavior problems in children (Eley and Stevenson 1999; Rice et al. 2002; Saudino et al. 2008). Assortative mating for autistic symptoms could cause an inflated “shared environmental” effect in such analyses; some previous studies have shown no evidence of passive or active assortative mating for autistic behavior (Hoekstra et al. 2007), but others have estimated that as much as 30% of variance in some measures of autistic-like traits in parents can be accounted for by assortative mating (Constantino and Todd 2005). Assortative mating though, would be expected to operate across all age levels, and thus cannot explain why we find higher shared environmental influences in our young sample than those with older samples.
Shared environment also played a small role (12%) in explaining the variance in our orientation/engagement measure. This, too, is consistent with previous work on the conceptually similar phenotype of affect-extraversion in toddlers (Saudino et al. 1996). Based on prior research studying behavior problems and prosocial behaviors (Eley and Stevenson 1999; Knafo and Plomin 2006; Rice et al. 2002), we predict that shared environmental influences on these two phenotypes will decrease with age as a result of the child’s increasing interactions with others outside the family. To that end, we hope to follow-up with this sample when they are in school.
The finding of a positive shared environmental correlation between autistic-like behaviors and orientation/engagement behaviors was somewhat puzzling. It is difficult to conceptualize family-wide environmental influences that would increase autistic-like behaviors while also increasing social engagement in the lab. It might be easier to consider shared environmental factors that might decrease both behaviors. For example, negative reactions to the child’s quirky mannerisms (i.e., autistic-like behaviors) might both discourage these problem behaviors and make the child more socially reticent in the novel laboratory situation.
A strength of this study lies in the fact that we have different sources of information for assessing these behaviors. Because both autistic-like behaviors and orientation/engagement behaviors were assessed via different methods (i.e., parent report and trained observer) and in different contexts (i.e., in the home and in the laboratory), the genetic and shared environmental covariance between the two phenotypes do not simply reflect rater or situation effects. The finding of common genetic and shared environmental influences across different methods of assessment provides strong evidence of the shared etiology of these two conceptually-related phenotypes, one assessing strengths in orientation/engagement, the other, the lack of these abilities in autistic-like behaviors.
One limitation to the current study was the absence of children with severe autism in our sample. Although it was a population-based study, families with severely impaired children typically do not choose to participate in this normative type of research. That said, we did have several children in our sample that later received ASD diagnoses. Eight individuals out of our sample of 626 were formally diagnosed with an ASD at age 3—a rate that is remarkably close to the estimated national prevalence of one in 166 (Chakrabarti and Fombonne 2006). Thus, our sample does appear to be representative of the general population in terms of our phenotype of interest. The children with diagnoses all scored above the 50th percentile, with half above the 80th on the parent-report measure of autistic-like behaviors (mean raw score for children with PDD: 4.9, compared with mean of 2.5 for entire twin sample); similarly, all but one of these participants scored below the mean for orientation/engagement (mean PDD: 25.3, vs. entire sample: 33.5), thus showing that our measures do seem to be sensitive to behaviors that are present in children with ASD. An additional benefit of including these children at this early stage, prior to diagnosis, was that they were not yet receiving any form of formal intervention for autistic symptoms at the time of testing.
Although previous studies with older twins have found sex differences in continuous measures of autistic-like behaviors, our finding of no sex differences on the CBCL PDP scale in toddlers matches findings by Rescorla et al. (2007) using the same measure in early childhood, again providing evidence to suggest that our sample is not atypical. Of note, only one out of the eight individuals later diagnosed with an ASD was female; therefore, despite the fact that there is no sex difference in continuously measured autistic-like behaviors in our young sample, the sex ratio of male to female children later identified as having ASD is reflective of the higher prevalence of these disorders in males. The fact that there were no mean sex differences in autistic-like behaviors, does not, however, mean that there is no sex difference at the level of etiology. Unfortunately, our relatively small sample lacked sufficient power to test for sex differences in the magnitude of genetic and environmental influences (i.e., power was <0.40). Additional studies with much larger samples and the inclusion of opposite sex DZ twins are needed to inform about possible sex limitation genetic effects.
Although the orientation/engagement scale assesses behaviors that tend to be impaired in children with autism (i.e., social behavior and object interest) it should be noted that all items on this measure are scored in the same direction and that higher scores indicate children who are more engaged. This potentially poses a problem in that a high interest in test stimuli on the observed measure is viewed positively, whereas from the perspective of autistic-like behaviors, it would be viewed negatively. Factor analyses of the orientation/engagement items confirmed the positive loadings of all items. Nonetheless, to create an observational measure that might be more conceptually consistent with autistic-like behaviors we reversed the scoring for the two object-orientation questions and reran our analyses. This non-traditional scoring of BRS orientation/engagement yielded results that were almost identical to those reported above, both in terms of the magnitude of the correlation between the rescored observed measure and the CBCL PDP (−0.21) and conclusions about genetic and environmental variances and covariances. One reason for this is likely due to the fact that the CBCL PDP scale does not include items reflecting object orientation, so these items do not substantially influence the covariance. Moreover, we suspect that either high or low scores on these two object-orientation items might be sensitive to autistic-like behavior within the lab setting.
In sum, as hypothesized, we found that both autistic-like behaviors and orientation/engagement behaviors are heritable and influenced by shared environmental factors. Further, our results show that these measures share the same influential factors in these domains. This finding is consistent with the concept of autism as the extreme end of a continuum of behaviors. It would be valuable to replicate this study with other standardized measures that are more specific to autism spectrum disorders. Another possible research direction might be to explore the relationships between the various clusters of autistic symptoms (i.e., social impairments, communication deficits, and repetitive/ restricted behaviors) in toddlers to determine whether all symptoms share the same etiology.
Acknowledgments
The Boston University Twin Project (BUTP) is supported by grant MH062375 from the National Institute of Mental Health. This work was also supported by a predoctoral fellowship from NAAR/Autism Speaks. The authors gratefully acknowledge the parents and twins in the BUTP.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. doi:10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA preschool forms and profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington: 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders DSM-IV-TR Text revision. APA; Washington: 2000. [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant development. 2nd edn The Psychological Corporation; San Antonio: 1993. [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162:1133–1141. doi: 10.1176/appi.ajp.162.6.1133. doi:10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: stability and change in syndrome expression. J Child Psychol Psychiatry. 2007;48:128–138. doi: 10.1111/j.1469-7610.2006.01685.x. doi:10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. doi:10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57:655–660. doi: 10.1016/j.biopsych.2004.12.014. doi:10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. J Am Acad Child Adolesc Psychiatry. 2003;42:458–467. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- Derks EM, Hudziak J, van Beijsterveldt C, Dolan C, Boomsma D. A study of genetic and environmental influences on maternal and paternal cbcl syndrome scores in a large sample of 3-year-old Dutch twins. Behav Genet. 2004;34:571–583. doi: 10.1007/s10519-004-5585-2. doi:10.1007/s10519-004-5585-2. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. doi:10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Stevenson J. Exploring the covariation between anxiety and depression symptoms: a genetic analysis of the effects of age and sex. J Child Psychol Psychiatry. 1999;40:1273–1284. doi:10.1111/1469-7610.00543. [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. doi:10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley J, Prescott A. The laboratory temperament assessment battery-preschool version: description of procedures. University of Wisconsin; Madison: 1995. [Google Scholar]
- Hoekstra RA, Bartels M, Verweij CJH, Boomsma D. Heritability of autistic traits in the general population. Arch Pediatr Adolesc Med. 2007;161:372–377. doi: 10.1001/archpedi.161.4.372. doi:10.1001/archpedi.161.4.372. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four-factor index of social status. Yale University; New Haven: 1975. [Google Scholar]
- Knafo A, Plomin R. Prosocial behavior from early to middle childhood: genetic and environmental influences on stability and change. Dev Psychol. 2006;42:771–786. doi: 10.1037/0012-1649.42.5.771. doi:10.1037/0012-1649.42.5.771. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi:10.1093/biomet/73.1.13. [Google Scholar]
- Martin NG, Eaves LJ. The genetical analysis of covariance structure. Heredity. 1977;38:79–95. doi: 10.1038/hdy.1977.9. doi:10.1038/hdy.1977.9. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behav Genet. 1984;14:325–343. doi: 10.1007/BF01080045. doi:10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. 7th edn Virginia Commonwealth University; Richmond: 2006. [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Plomin R. Development, genetics, and psychology. Erlbaum; Hillsdale: 1986. [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral genetics. 4th edn Worth Publishing; NY: 2001. [Google Scholar]
- Price TS, Freeman B, Craig I, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Res. 2000;3:129–133. doi: 10.1375/136905200320565391. doi:10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Rescorla L, Ross GS, McClure S. Language delay and behavioral/emotional problems in toddlers: finding from two developmental clinics. J Speech Lang Hear Res. 2007;50:1063–1078. doi: 10.1044/1092-4388(2007/074). doi:10.1044/1092-4388(2007/074) [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A. Assessing the effects of age, sex and shared environment on the genetic aetiology of depression in childhood and adolescence. J Child Psychol Psychiatry. 2002;43:1039–1051. doi: 10.1111/1469-7610.00231. doi:10.1111/1469-7610.00231. [DOI] [PubMed] [Google Scholar]
- Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J, et al. A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet. 1999;65:493–507. doi: 10.1086/302497. doi:10.1086/302497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Happé F, Plomin R. The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism. Dev Sci. 2005;8:444–458. doi: 10.1111/j.1467-7687.2005.00433.x. doi:10.1111/j.1467-7687.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happé F, Bolton P, et al. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006;45:691–699. doi: 10.1097/01.chi.0000215325.13058.9d. doi:10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- Rutter M. Genetic studies of autism: from the 1970s into the millennium. J Abnorm Child Psychol. 2000;28:3–14. doi: 10.1023/a:1005113900068. doi:10.1023/A:1005113900068. [DOI] [PubMed] [Google Scholar]
- Saudino KJ, Plomin R, DeFries JC. Tester-related temperament at 14, 20, and 24 months: environmental change and genetic continuity. Br J Dev Psychol. 1996;14:129–144. [Google Scholar]
- Saudino KJ, Carter AS, Purper-Ouakil D, Gorwood P. The etiology of behavioral problems and competences in very young twins. J Abnorm Psychol. 2008;117:48–62. doi: 10.1037/0021-843X.117.1.48. doi:10.1037/0021-843X.117.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz S, Cherny S, Fulker D, Mrazek D. Genetic and environmental influences on early childhood behavior. Behav Genet. 1994;24:25–34. doi: 10.1007/BF01067926. doi:10.1007/BF01067926. [DOI] [PubMed] [Google Scholar]
- Scourfield J, John B, Martin N, McGuffin P. The development of prosocial behaviour in children and adolescents: a twin study. J Child Psychol Psychiatry. 2004;45:927–935. doi: 10.1111/j.1469-7610.2004.t01-1-00286.x. doi:10.1111/j.1469-7610.2004.t01-1-00286.x. [DOI] [PubMed] [Google Scholar]
- Sikora DM, Hall TA, Hartley SL, Gerrard-Morris AE, Cagle S. Does parent report of behavior differ across ADOS-G classifications: analysis of scores from the CBCL and GARS. J Autism Dev Disord. 2008;38:440–448. doi: 10.1007/s10803-007-0407-z. doi:10.1007/s10803-007-0407-z. [DOI] [PubMed] [Google Scholar]
- Spiker D, Lotspeich LJ, Dimiceli S, Myers RM, Risch N. Behavioral phenotypic variation in autism multiplex families: evidence for a continuous severity gradient. Am J Med Genet. 2002;114:129–136. doi: 10.1002/ajmg.10188. doi:10.1002/ajmg.10188. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. doi:10.2307/2531248. [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, et al. Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. J Autism Dev Disord. 2007;37:466–480. doi: 10.1007/s10803-006-0179-x. doi:10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]


