ABSTRACT
Purpose
To analyze the cycle outcomes and the incidence of ovarian hyperstimulation syndrome (OHSS), when oocyte maturation was triggered by gonadotropin-releasing hormone agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation.
Methods
One hundred twenty-nine women aged ≤ 45 years, diagnosed with stage ≤ 3 breast cancer, with normal ovarian reserve who desired fertility preservation were included in the retrospective cohort study. Ovarian stimulation was achieved utilizing letrozole and gonadotropins. Oocyte maturation was triggered with GnRHa or hCG. Baseline AMH levels, number of oocytes, maturation and fertilization rates, number of embryos, and the incidence of OHSS was recorded.
Results
The serum AMH levels were similar between GnRHa and hCG groups (2.7 ± 1.9 vs. 2.1 ± 1.8; p = 0.327). There was one case of mild or moderate OHSS in the GnRHa group compared to 12 in the hCG group (2.1 % vs. 14.4 %, p = 0.032). The maturation and fertilization rates, and the number of cryopreserved embryos were significantly higher in the GnRHa group.
Conclusions
GnRHa trigger improved cycle outcomes as evidenced by the number of mature oocytes and cryopreserved embryos, while significantly reducing the risk of OHSS in breast cancer patients undergoing fertility preservation.
Keywords: Fertility preservation, Breast cancer, Trigger, GnRH agonist, hCG
Introduction
Breast cancer is the most common malignancy diagnosed in women worldwide. Early detection and improvements in screening have increased the number of premenopausal women diagnosed with breast cancer, while advances in treatment options have contributed to declining breast cancer mortality rates [1, 2]. With increasing numbers of breast cancer survivors, quality of life issues such as the delayed effects of cancer treatments and fertility preservation have gained prominence [3–6]. Embryo and mature oocyte cryopreservation are currently the most established techniques for fertility preservation [7, 8].
Since women with breast cancer typically have a window of approximately 6 weeks between surgery and the initiation of adjuvant chemotherapy, it is feasible to undergo controlled ovarian stimulation (COS) [9–11]. The supraphysiologic estrogen (E2) levels resulting from traditional stimulation protocols have precluded most oncologists and breast cancer patients from pursuing fertility preservation, fearing the high estrogenic state can promote cancer growth or recurrence [12, 13]. In an effort to mitigate the potential effects of elevated E2 levels, novel ovarian stimulation protocols utilizing aromatase inhibitors have been developed [14–16]. In one study, the risk of breast cancer recurrence in women who underwent COS utilizing aromatase inhibitors was similar to the control group who declined fertility preservation after 2 years of follow-up [17].
In the original aromatase inhibitor protocol, final oocyte maturation was triggered with hCG [18]. Accumulating evidence suggests hCG administration increases the risk of developing ovarian hyperstimulation syndrome (OHSS), a serious complication of COS. Given its longer half-life as compared to endogenous luteinizing hormone (LH), hCG induces a prolonged luteotropic effect, potentiating the recruitment of multiples follicles and resulting in supraphysiologic E2 levels [19, 20]. Recent data suggests the risk of OHSS may be reduced by utilizing GnRH agonist triggers [21, 22].
In our previously published pilot data of 74 breast cancer patients undergoing fertility preservation, we found reduced estrogen exposure and risk of OHSS when final oocyte maturation was triggered by GnRHa as compared to hCG [23]. The aims of our current study are to expand our previously published pilot data by further studying the ovarian reserve prior to initiating treatment and investigating the risk of OHSS following GnRH agonist trigger versus hCG trigger for final oocyte maturation in breast cancer patients undergoing fertility preservation.
Materials and methods
The study was approved by the New York Medical College Institutional Review Board and was registered at clinicaltrials.gov (NCT00504699). The study subjects were referred by their oncologists to discuss fertility preservation prior to initiating adjuvant chemotherapy between 2006 and 2011. The inclusion criterion were: histologically proven breast cancer, age 18–45 years, no prior exposure to chemotherapy, no prior infertility, the absence of hypothalamic dysfunction and adequate ovarian reserve as defined by the following baseline (cycle day 2 or 3) parameters: FSH level ≤ 13mIU/mL, estradiol level ≤ 75 pg/mL. Exclusion criteria included advanced stage breast cancer defined as greater than Stage IIIB, previous history of infertility or ovarian surgery. The study was conducted in 2013 and the retrospective data were generated through secondary analysis of a previously collected database investigating the long-term effects of controlled ovarian stimulation in women diagnosed with breast cancer undergoing fertility preservation cycles with letrozole between 2006–2011.
Ovarian reserve assessment
Serum anti- müllerian hormone (AMH) was measured in serum obtained on cycle day 2 or 3, immediately prior to initiating ovarian stimulation. The results were not available to the investigators at the time of ovarian stimulation and did not factor into FSH dosing. The frozen serum was assayed by quantitative three-step sandwich type immunoassay.
Stimulation protocol
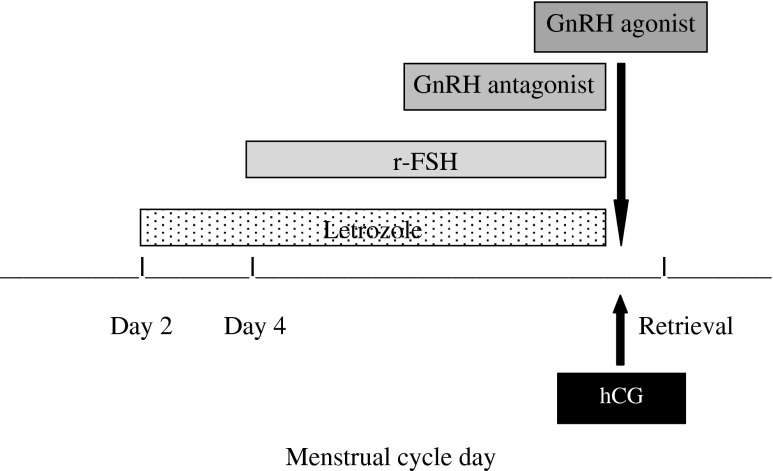
In the original aromatase inhibitor-gonadotropin protocol, an hCG trigger was utilized for final oocyte maturation. Over time the protocol was transitioned to a GnRHa trigger with the aim of reducing OHSS risks and further reducing post-hCG rise in E2 levels; therefore, this is a comparison of the initial hCG trigger with the later GnRHa trigger [15, 23]. All other aspects of the aromatase inhibitor-gonadotropin protocol remained the same (Fig. 1). Briefly, 5 mg of letrozole (Femara, Novartis Oncology, East Hanover, NJ) was administered on cycle day 2. Daily injections of recombinant FSH (Follistim, Organon, West Orange, NJ or Gonal-F, Serono, Rockville, MD) were added 2 days later. The initial dose of recombinant FSH ranged from 150 to 300 IU/day. Monitoring of ovarian response during stimulation consisted of hormonal levels and transvaginal ultrasound every 1–2 days until the day of oocyte retrieval. To prevent a premature surge of LH, a GnRH antagonist (Ganirelix 250 μg/d, Organon, West Orange, NJ) was administered when E2 levels exceeded 250 pg/mL or when the lead follicle reached 14 mm in diameter. When at least two follicles reached at least 19–20 mm in diameter, oocyte maturation was triggered with either hCG (Ovidrel, EMD Serono, Rockland, MA) or leuprolide acetate 1 mg (Lupron, Ferring Pharmaceuticals, Parsippany, NJ). Transvaginal oocyte retrieval was performed approximately 34–36 h later. Subsequently, IVF with intracytoplasmic sperm injection was performed and the embryos were cryopreserved at the 2-pronuclear stage. For women without partners, oocyte cryopreservation was performed. Letrozole 5 mg was restarted on the day of retrieval and continued until E2 levels fell below 50 pg/ml [14].
Fig. 1.
Protocol using GnRHa vs. hCG trigger for final oocyte maturation in fertility preservation cycles for women diagnosed with breast cancer
Outcome measures
Parameters examined per treatment cycle included: total gonadotropin dose, length of ovarian stimulation, total oocyte number, maturation rate, total mature oocyte number, fertilization rate, number of pronuclear stage embryos frozen, pre-trigger estradiol levels, and rates of OHSS. The signs and symptoms of OHSS were assessed in all patients 1 week after the procedure, at the time of the routine return visit, or earlier if the patient developed any symptoms. The evaluation consisted of a symptom assessment, physician examination, transvaginal ultrasound, and blood sampling. OHSS was classified based on standard criteria. Briefly, mild hyperstimulation was defined as abdominal distension or discomfort in the setting of bilateral ovarian enlargement up to 12 cm. Moderate hyperstimulation was defined as features of mild OHSS in the setting of sonographic evidence of ascites. Severe hyperstimulation was defined as features of moderate OHSS plus clinical evidence of ascites or hydrothorax or difficulty in breathing, change in blood volume, hemoconcentration, coagulation abnormalities and diminished renal function [24].
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (release 15.0; SPSS Inc., Chicago, IL). Continuous data were analyzed by student t test. The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov Simirnov/Shapiro-Wilks test) to determine whether or not they were normally distributed. Chi-square and Fischer’s exact test was used to compare proportions of different groups. Data were presented as mean ± standard deviation. A p-value less than 0.05 was considered statistically significant.
Results
One hundred twenty-nine women met inclusion criteria and returned for follow-up of which 46 women were in the GnRHa group and 83 women were in the hCG group. The mean age, body mass index and ovarian reserve assessment as determined by baseline FSH, E2, AMH and antral follicle count (AFC) were similar between both groups (Table 1). AMH levels were unavailable in approximately one third of patients, 16 in the GnRHa group and 29 in the hCG group. The total dose of gonadotropin, mean length of stimulation, and peak pre-trigger E2 levels were also similar between the two groups (Table 2).
Table 1.
Demographic characteristics and ovarian reserve assessment in breast cancer patients triggered with GnRHa vs. hCG
| Characteristic | GnRHa (N = 46) | hCG (N = 83) | P-value |
|---|---|---|---|
| Age (years) | 37.9 ± 4.9 | 39.2 ± 3.8 | 0.078 |
| Body Mass Index (kg/m2) | 22.5 ± 3.2 | 22.4 ± 3.4 | 0.894 |
| Baseline AMH (ng/ml)a | 2.7 ± 1.9 | 2.1 ± 1.8 | 0.327 |
| Baseline FSH (mIU/ml) | 8.9 ± 4.5 | 8.5 ± 3.8 | 0.587 |
| Baseline estradiol (pg/ml) | 46.0 ± 37.9 | 49.3 ± 38.7 | 0.624 |
| Baseline antral follicle count | 9.7 ± 5.5 | 9.2 ± 4.9 | 0.636 |
Values for continuous variables are mean ± SD. A p-value less than 0.05 was considered statistically significant
aAMH levels were unavailable in 16 women the GnRHa group and 29 women in the hCG group
Table 2.
Cycle outcomes in breast cancer patients triggered with GnRHa vs. hCG
| Outcome | GnRHa (N = 46) | hCG (N = 83) | P-value |
|---|---|---|---|
| Ovarian stimulation length (days) | 10.1 ± 1.0 | 10.5 ± 1.8 | 0.121 |
| Total gonadotropin dose (IU) | 1982.0 ± 902.2 | 1858.0 ± 668.3 | 0.376 |
| Peak estradiol pre-trigger (pg/ml) | 564.8 ± 313.2 | 529.7 ± 400.6 | 0.589 |
| Number of oocytes retrieved | 14.1 ± 6.6 | 12.4 ± 8.6 | 0.218 |
| Oocyte maturation rate (%) | 77.3 ± 18.5 | 67.6 ± 21.9 | 0.007 |
| Number of mature oocytes | 10.5 ± 5.1 | 7.7 ± 5.3 | 0.002 |
| Fertilization rate (%) | 82.6 ± 15.9 | 75.2 ± 22.9 | 0.041 |
| Number of cryopreserved embryosa | 7.7 ± 4.2 | 5.4 ± 73.8 | 0.002 |
| Mild or moderate OHSS (%) | 1/46 (2.1 %) | 12/83 (14.4 %) | 0.032 |
Values for continuous variables are mean ± SD. A p-value less than 0.05 was considered statistically significant
aEmbryo cryopreservation occurred in 37 women in the GnRHa group and 69 women in the hCG group
The total number of oocytes was similar between the two groups, however the maturation rate (77.3 ± 18.5 % vs. 67.6 ± 21.9 %, p = 0.007) and the number of mature oocytes (10.5 ± 5.1 vs. 7.7 ± 5.3, p = 0.002) were significantly higher in the GnRHa group when compared to the hCG group. The fertilization rate (82.6 ± 15.9 % vs. 75.2 ± 22.9 %; p = 0.041) and the number of cryopreserved embryos (7.7 ± 4.2 vs. 5.4 ± 3.8, p = 0.002) were also significantly higher in the GnRHa group when compared to the hCG group (Table 2). The majority of patients underwent embryo cryopreservation compared to oocyte cryopreservation. Specifically, 37 in the GnRHa group and 69 in the hCG underwent embryo cryopreservation.
There were a total of 13 cases of mild or moderate OHSS in the study, one in the GnRHa group (1/46, 2.1 %) and 12 in the hCG group (8/83, 9.6 %) (Table 2). The only case of OHSS in the GnRHa was classified as mild. In the hCG group, 50 % of patients were classified as having moderate OHSS. There were no cases of severe OHSS in either group.
Discussion
In our study, we found GnRHa was effective in triggering final oocyte maturation as hCG in women with breast cancer undergoing fertility preservation using an aromatase inhibitor-gonadotropin protocol. Furthermore, there was a significant reduction in the rate of OHSS in the GnRHa group compared to the hCG group. Our current study builds on our pilot data and adds to the growing body of literature supporting the potential use of GnRHa triggers in certain high risk populations [23, 25]. Although we do not have the sufficient data on pregnancy outcomes at the present time, our preliminary analysis does not suggest a difference in pregnancy rates between the two triggers.
Although our study is the largest to date in breast cancer patients to test the efficacy of GnRHa triggers in fertility preservation cycles, the success of this approach has also been demonstrated in earlier studies, in particular in oocyte donor programs [25–27]. In a prospective randomized trial investigating oocyte triggering agents in donor cycles, oocyte donors were randomized to receive either GnRHa or hCG. There was no difference in the number of oocytes retrieved, fertilization rate, embryo quality, implantation or pregnancy rates between the two groups; however, the rate of OHSS was significantly lower in the GnRHa group as compared to the hCG group [25]. In a retrospective cohort study of 1,171 oocyte donors undergoing 2,077 cycles, donors were triggered with either GnRHa or hCG at the discretion of the treating physician. There was no difference in the number of oocytes retrieved, proportion of mature oocytes, and implantation or pregnancy rates between the two groups, while the rate of moderate or severe OHSS was significantly lower in the GnRHa group [27].
In a prospective randomized trial evaluating the effects of a GnRHa trigger on cycle outcomes in a non-cancer infertility population, the number of mature oocytes retrieved was significantly higher in the GnRHa group compared to the hCG group, which is consistent with our findings and those of Acevedo et al. [25, 28]. Erb et al. noted a significantly higher number of quality embryos in the GnRHa group than in the hCG group in a retrospective donor oocyte study [26]. A potential explanation for this observation is that GnRHa trigger a more physiologic approach, similar to that of the natural mid-cycle surge. Since hCG has a much longer half-life than LH, it can theoretically induce over-luteinization of recruited follicles and subsequently affect their quantity and or quality. GnRHa on the other hand induce an endogenous rise in both LH and FSH due to an initial flare effect, which appears to be more physiologic [29].
In a recent meta-analysis of GnRH antagonist fresh-autologous IVF cycles, women triggered with GnRHa had significantly lower pregnancy and live birth rates compared to women triggered with hCG, although the rate OHSS was significantly lower in the GnRHa group [30]. This finding suggests that GnRHa trigger may contribute to a luteal phase defect resulting in lower implantation and pregnancy rates [31]. However when adequate luteal phase hormonal support was provided, pregnancy rates significantly improved and were consistent with those triggered by hCG [32]. In a prospective observation study comparing GnRHa to hCG for final oocyte maturation, there was no difference in live birth rate after the transfer of frozen-thawed embryos [33]. Since the goal of fertility preservation cycles is to maximize the number of high quality embryos and oocytes available for cryopreservation and future conception, the immediate issue of implantation and pregnancy rates may not directly relevant.
OHSS is a serious complication of COS and measures should be taken to avoid it in cancer patients because it may delay or even complicate the initiation of life-saving adjuvant treatment. Cancer patients in general are hypercoagulable and OHSS in that setting we presume may be even more thrombogenic. Since cancer patients presenting for fertility preservation typically have a limited window, it is important to balance the risk of OHSS with optimizing the number of retrieved high quality oocytes and embryos. Predicting which patients are at risk for developing OHSS can be challenging, and several parameters have been investigated such as elevated E2 levels and the number of follicles recruited [34]. However, the use of aromatase inhibitors may limit the predictive value of E2 levels given their profound suppression in fertility preservation cycles [16]. Despite the significantly lower absolute peak E2 levels, OHSS was still diagnosed in 13 of the 129 participants in our study. This suggests that elevated E2 levels are not the only contributing factor to the pathogenesis of OHSS. Furthermore, alternative parameters are needed to better identify women at risk for OHSS, particularly in fertility preservations cycles using aromatase inhibitors. It may be prudent to use the rate of rise of E2s levels rather than the absolute value in combination with follicular pattern to accurately identify at risk patients.
One limitation of our study is that it was not a randomized trial. Despite the lack of prospective randomization, the two groups were very similar and did not differ by age, body mass index or initial markers of ovarian reserve. It was a secondary analysis of a database of women with breast cancer presenting for consultation of fertility preservations options. Thus, the study cohort may have an over-representation of healthier women. In addition, fertility preservation options are not universally covered by insurance; therefore, the women who elected to proceed with embryo or oocyte cryopreservation may be different from those women who declined. The relatively small sample size precludes use from making broad generalization regarding the optimal triggering agent.
Conclusion
In conclusion, our study is the largest to date to demonstrate that the risk of OHSS can be significantly reduced with the administration of a GnRHa trigger compared to hCG, without adversely affecting cycle outcomes in breast cancer patients undergoing COS utilizing aromatase inhibitors. Given that cancer patients presenting for fertility preservation often only have time for one cycle, it is important to balance the risk of OHSS with optimizing the number of high quality oocytes and embryos. Since E2 levels are suppressed in aromatase inhibitor cycles, alternative predictors are needed to accurately identify patients at risk of OHSS. Larger studies and data on pregnancy outcomes will be needed to verify the safely and efficacy our approach.
Acknowledgments
We thank all patients and staff involved in our clinical study.
Disclosures
None.
Details of Ethics Approval
The study was approved by the New York Medical College Institutional Review Board and was registered at clinicaltrials.gov (NCT00504699).
Funding
This work is partially funded by NICHD grants R01 HD053112 and R21 HD061259.
Footnotes
Capsule GnRHa trigger appears to be superior to hCG trigger in fertility preservation cycles as it improves oocyte and embryo yield and minimizes OHSS risks.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2012;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 4.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: american society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists Committee on Practice Bulletins 126: Management of gynecological issues in women with breast cancer. Obstet Gynecol. 2012;119:666–82. doi: 10.1097/AOG.0b013e31824e12ce. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol. 2010;28:4683–6. doi: 10.1200/JCO.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedoschi G, Oktay K. Current approach to fertility preservation by embryo cryopreservation. Fertil Steril. 2013;99:1496–502. doi: 10.1016/j.fertnstert.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Practice Committee of the American Society of Reproductive Medicine Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Baynosa J, Westphal LM, Madrigrano A, Wapnir I. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg. 2009;209:603–607. doi: 10.1016/j.jamcollsurg.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Madrigrano A, Westphal L, Wapnir I. Egg retrieval with cryopreservation does not delay breast cancer treatment. Am J Surg. 2007;194:477–81. doi: 10.1016/j.amjsurg.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Mitwally MF, Bhakoo HS, Crickard K, Sullivan MW, Batt RE, Yeh J. Estradiol production during controlled ovarian hyperstimulation correlates with treatment outcome in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2006;86:588–96. doi: 10.1016/j.fertnstert.2006.02.086. [DOI] [PubMed] [Google Scholar]
- 12.Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril. 2013;100:1673–80. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 13.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 14.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–53. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–90. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 16.Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–9. doi: 10.1016/j.fertnstert.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–5. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 18.Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18:90–5. doi: 10.1093/humrep/deg045. [DOI] [PubMed] [Google Scholar]
- 19.Haning RV, Jr, Austin CW, Carlson IH, Kuzma DL, Shapiro SS, Zweibel WJ. Plasma estradiol is superior to ultrasound and urinary estriol glucuronide as a predictor of ovarian hyperstimulation during induction of ovulation with menotropins. Fertil Steril. 1983;40:31–6. doi: 10.1016/s0015-0282(16)47173-6. [DOI] [PubMed] [Google Scholar]
- 20.Kol S, Itskovitz-Eldor J. Gonadotropin-releasing hormone agonist trigger: the way to eliminate ovarian hyperstimulation syndrome–a 20-year experience. Semin Reprod Med. 2010;28:500–5. doi: 10.1055/s-0030-1265677. [DOI] [PubMed] [Google Scholar]
- 21.Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertil Steril. 1992;58:249–61. doi: 10.1016/s0015-0282(16)55188-7. [DOI] [PubMed] [Google Scholar]
- 22.Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12:159–68. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- 23.Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Repro Biomed Online. 2010;20:783–8. doi: 10.1016/j.rbmo.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whelan JG, Vlahos NF. The ovarian hyperstimulation syndrome. Fertil Steril. 2000;73:883–96. doi: 10.1016/S0015-0282(00)00491-X. [DOI] [PubMed] [Google Scholar]
- 25.Acevedo B, Gomez-Palomares JL, Ricciarelli E, Hernandez ER. Triggering ovulation with gonadotropin-releasing hormone agonist does not compromise embryo implantation rates. Fertil Steril. 2006;76:1682–7. doi: 10.1016/j.fertnstert.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 26.Erb TM, Vitek W, Wakim AN. Gonadotropin-releasing hormone agonist or human chorionic gonadotropin for final oocyte maturation in an oocyte donor program. Fertil Steril. 2010;93:374–8. doi: 10.1016/j.fertnstert.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Bodri D, Guillén JJ, Galindo A, Mataró D, Pujol A, Coll O. Triggering with human chorionic gonadotropin or a gonadotropin-releasing hormone agonist in gonadotropin-releasing hormone antagonist-treated oocyte donor cycles: findings of a large retrospective cohort study. Fertil Steril. 2009;91:365–71. doi: 10.1016/j.fertnstert.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 28.Humaidan P, Bredkjaer HE, Bungum L, Bungum M, Grøndahl ML, Westergaard L, et al. 2005. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod. 2005;20:1213–20. doi: 10.1093/humrep/deh765. [DOI] [PubMed] [Google Scholar]
- 29.Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:709–15. doi: 10.1210/jcem.87.2.8197. [DOI] [PubMed] [Google Scholar]
- 30.Youssef MA, Van der Veen F, Al-Inany HG, Griesinger G, Mochtar MH, Aboulfoutouh I, et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles. Cochrane Database Syst Rev. 2011;1 doi: 10.1002/14651858.CD008046.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Kolibianakis EM, Schultze-Mosgau A, Schroer A, van Steirteghem A, Devroey P, Diedrich K, et al. A lower ongoing pregnancy rate can be expected when GnRH agonist is used for triggering final oocyte maturation instead of HCG in patients undergoing IVF with GnRH antagonists. Hum Reprod. 2005;20:2887–92. doi: 10.1093/humrep/dei150. [DOI] [PubMed] [Google Scholar]
- 32.Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril. 2008;89:84–91. doi: 10.1016/j.fertnstert.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Griesinger G, Kolibianakis E, Papanikolaou E, Diedrich K, Van Steirteghem A, Devroey P, et al. Triggering of final oocyte maturation with gonadotropin-releasing hormone agonist or human chorionic gonadotropin. Live birth after frozen-thawed embryo replacement cycles. Fertil Steril. 2007;88:616–21. doi: 10.1016/j.fertnstert.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006;85:112–20. doi: 10.1016/j.fertnstert.2005.07.1292. [DOI] [PubMed] [Google Scholar]