Abstract
Purpose
To assess irradiance and total energy dose from different microscopes during the in-vitro embryonic developmental cycle in mouse and pig and to evaluate its effect on embryonic development and quality in pig.
Method
Spectral scalar irradiance (380–1050 nm) was measured by a fiber-optic microsensor in the focal plane of a dissection microscope, an inverted microscope and a time-lapse incubation system. Furthermore, the effect of three different red light levels was tested in the time-lapse system on mouse zygotes for 5 days, and on porcine zona-intact and zona-free parthenogenetically activated (PA) embryos for 6 days.
Results
The time-lapse system used red light centered at 625 nm and with a lower irradiance level as compared to the white light irradiance levels on the dissection and inverted microscopes, which included more energetic radiation <550 nm. Even after 1000 times higher total energy dose of red light exposure in the time-lapse system, no significant difference was found neither in blastocyst development of mouse zygotes nor in blastocyst rates and total cell number of blastocysts of porcine PA embryos.
Conclusions
Our results indicate that red light (625 nm, 0.34 W/m2) used in the time-lapse incubation system does not decrease the development and quality of blastocysts in both mouse zygotes and porcine PA embryos (both zona-intact and zona-free).
Keywords: Spectral irradiance, Microsensor, Dosimetry, Mouse, Pig, Time-lapse observation
Introduction
Mammalian oocytes and embryos are typically exposed to several kinds of light exposure during their in-vitro processing, which all can affect the subsequent embryonic development and quality [1, 2]. This can encompass fluorescent room light in the lab, halogen light on the microscopes and even daylight during ovary collection. Some types of light such as sunlight are lethal, but can easily be avoided [3], whereas some light exposure for observation during in-vitro manipulations is inevitable. There is e.g. a need for frequent observations to select the best in-vitro produced embryos for transfer, because better embryonic quality means higher chance for implantation and ultimately pregnancy [4–6]. In practice, the number of observations used in clinical IVF on human and animal embryos is therefore a compromise between a wish to keep the embryos undisturbed (with respect to light, temperature and pH) and to have as many observations as possible to facilitate optimal selection for transfer.
For decades, evaluation of embryonic quality has been based on simple morphological observations performed either a couple of times during culture or at the time of selection for transfer. The limitations in this approach have been known just as long, but there was not any realistic alternative until the introduction of time-lapse cinematography to observe mammalian embryos some 30 years ago [7, 8]. Since then, this type of frequent observation with images taken regularly for subsequent analyses has become more or less routine in several IVF set-ups, especially in the human field [9–12]. In the animal field, such additional measures have found less application except for experimental purposes where it is greatly valued [13, 14]. With the rapidly spreading use of time-lapse observations, especially on human embryos in fertility clinics, it is important to investigate, whether light exposure for image recording affects or even compromises the embryo’s developmental competence. Different measures based on studies of animal model embryos have already been implemented to minimize light exposure of visible [15] or longer wavelength light [2, 15, 16], and in combination with sensitive camera systems it is possible to obtain high quality images for evaluation without disadvantages on embryonic development and quality [17]. Microscopy typically works with visible light (380–700 nm), where wavelengths <500-550 nm are regarded as harmful to the development and quality of mammalian embryos [2, 15]. Increasing exposure time leads to higher total energy dose potentially causing more stress to the embryos. In mammalian cells, the function of fibroblast cells was disrupted when the total dose of blue light was increased to 10 kJ/m2 [18]. However, most studies only use light intensity to quantify illumination effect on embryos [3, 15, 16]. Light at longer wavelength carries lower energy and red light is recommended as a safe illumination source for embryo observation systems [15] and is now routinely applied in time-lapse incubation systems [11, 12, 19–21]. However, the maximum tolerance of embryos to red light is unknown, and no comparisons have yet been made of an eventual effect of maximizing light exposures to obtain even more morphological details over a full pre-implantation period in-vitro, i.e. for 5, 6 or 7 days.
In the present study, embryos were therefore exposed to three different levels of red light during the whole in-vitro culture period in a time-lapse system: no light, low exposure for normal image recording for time-lapse observation (75 ms/15 min and 75 ms/20 min), or extra exposure (50 s/20 min, 60 s/15 min and 100 s/20 min). Mouse embryos were chosen to represent a robust model that is fairly tolerant to light exposure [3], while porcine embryos were applied as a model more sensitive to in-vitro conditions [22, 23]. The specific purposes of our study were: (1) to compare the total light energy dose under different illumination scenarios during an in-vitro mammalian embryonic developmental cycle, as quantified by microscale spectral irradiance measurements; (2) to test the developmental rates and quality of both mouse and pig embryos after extra light exposure of red light in a time-lapse incubator.
Materials and methods
All chemicals were purchased from Sigma–Aldrich Corp. (St Louis, MO, USA) except otherwise indicated.
Light measurements
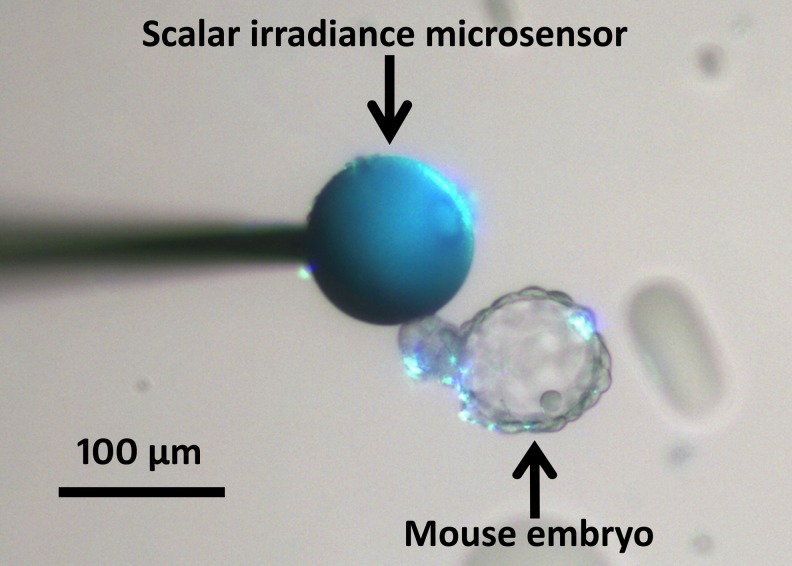
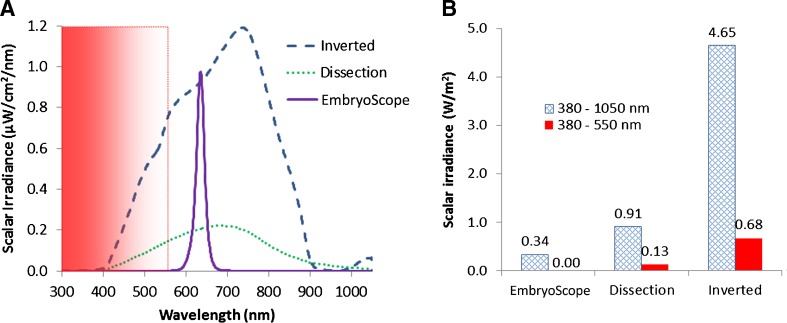
To quantify light exposure of embryos when observed on an inverted microscope, a dissection microscope and in a time-lapse incubation system (EmbryoScope®; Unisense FertiliTech A/S, Aarhus, Denmark), spectral scalar irradiance (380–1050 nm) was measured by a fiber-optic microsensor connected to a fiber-optic spectrometer calibrated for absolute irradiance measurement (USB2000, Ocean Optics, USA) [24, 25]. Briefly, a scalar irradiance micro-probe with a tip diameter of ~100 μm, i.e., similar dimensions as a mouse embryo, was used to measure the spectral light exposure (in units of μW/cm2/nm) in a culture dish (shown in Fig. 1) mounted on the two different microscope types and in the well of an EmbryoSlide® culture dish in the time-lapse incubator (Unisense FertiliTech A/S). Measurements were conducted in the focal plane on all three microscope platforms with typical light settings used for embryo inspection as adjusted by a trained person. Measurements were recorded in 9 different positions in the light exposed field of view. Spectral data were calculated as the means of these 9 values (Fig. 2a). Based on spectral integration, the total scalar irradiance was calculated for 380–1050 nm light, as well as for the harmful wavelength range 380–550 nm only (Fig. 2b).
Fig. 1.
Photograph of the spherical light collecting tip of a scalar irradiance microsensor positioned in a culture dish next to a mouse embryo
Fig. 2.
Spectral composition and intensity of observation light on different microscopes. A) Spectral scalar irradiance. The harmful wavelength range (380–550 nm) is shown in the gradient area; B) Integral scalar irradiance for total light (380–1050 nm, shown in outlined column) and light <550 nm (harmful part of light, shown in color column) on the different microscopes
Preparation of culture dish
All 12 wells in an EmbryoSlide® culture dish (Unisense FertiliTech A/S) were filled with 25 μl cultivation media (for mouse: Global culture medium (LifeGlobal, Guelph, Canada); for pig: porcine zygote medium-3 (PZM-3, [26])) at room temperature; any gas bubbles were carefully removed using a glass pipette. To cover the 12 wells, 1.2 ml mineral oil was filled into the common reservoir. The culture dish was left to equilibrate for at least 20 h either at 37 °C and 5 % CO2 in air for mouse embryos or at 38.5 °C in an atmosphere of 5 % CO2, 5 % O2, and 90 % N2 for porcine PA embryos.
Mouse embryo preparation
Cryopreserved 1-cell embryos from cross-bred mice were obtained from Vitrolife (Vitrolife Inc, San Diego, USA). The straw containing the embryos was removed from the liquid nitrogen tank and left to thaw horizontally for 2 min in air at room temperature. Subsequently, the holder was removed, and the straw was equilibrated for 3 min in a 37 °C water bath and for 2 min in a 20-25 °C water bath. After thawing, the embryos were washed twice in 4-well dishes (Nunc, Roskilde, Denmark) with GALW washing media (LifeGlobal) covered with LiteOil (LifeGlobal) to prevent evaporation. After washing, the embryos were transferred to 4-well dishes with Global culture media supplemented with 7.5 % LGPS (LifeGlobal,) and then transferred to the EmbryoSlide® culture dish. The mouse embryos from both light treatment and normal observation groups were cultured in Global culture media supplemented with 7.5 % LGPS (LifeGlobal) in EmbryoSlide® culture dishes at 37 °C with 5 % CO2 in air.
Porcine embryo production
Cumulus–oocyte complexes (COCs) were aspirated from 2 to 6 mm follicles in slaughterhouse-derived sow ovaries and matured as described earlier [14]. Briefly, groups of 50 COCs with compact and at least two layers of cumulus cells were selected and cultured for 42–44 h in 4-well dishes in 400 μl bicarbonate-buffered TCM-199 (supplemented with 10 % (v/v) cattle serum (CS; Danish Veterinary Institute, Frederiksberg, Denmark), 10 % (v/v) sow follicular fluid, 10 IU/ml pregnant mare serum gonadotrophin and 5 IU/ml human chorionic gonadotrophin (Suigonan Vet, Intervet, Boxmeer, Holland)) covered with 400 μl mineral oil at 38.5 °C in 5 % CO2 with 100 % humidity.
After maturation, oocytes were parthenogenetically activated (PA) as described earlier [14]. Briefly, oocytes were equilibrated for 10–15 s in drops of activation medium (0.3 M mannitol, 0.1 mM MgSO4, 0.1 mM CaCl2 and 0.01 % polyvinyl alcohol). Under a 0.12 kV/cm alternating current, oocytes were aligned to the wire of a fusion chamber (Microslide 0.5-mm fusion chamber, model 450; BTX, San Diego, CA, USA). Meanwhile, a single direct current pulse (1.26 kV/cm, 80 μs) was applied. The time of activation by electricity was defined as Day 0. After washing twice in drops of TCM-199 (supplemented with 10 % CS), groups of 100 oocytes were incubated for 4 h in 400 μl PZM-3 (supplemented with 4 mg/ml bovine serum albumin, 5 μg/ml cytochalasin B and 10 μg/ml cycloheximide) covered with 400 μl mineral oil at 38.5 °C in an atmosphere of 5 % CO2, 5 % O2 and 90 % N2 with 100 % humidity. PA embryos were then washed three times in culture medium prior to culture. To acquire zona-free PA embryos, the zona pellucida (ZP) was removed as described earlier [14]. In brief, the embryos were placed in 0.3 % (w/v) pronase for 30 s followed by immediate washing 2–3 times in culture medium, and then the remaining ZP was removed mechanically by repeated pipetting by a glass pipette (diameter: 200–300 μm).
The porcine embryos from both extra light treatment and normal observation groups were cultured in PZM-3 in EmbryoSlide® culture dishes at 38.5 °C in an atmosphere of 5 % CO2, 5 % O2 and 90 % N2. As control, embryos were cultured in groups of 20–25 per well in 4-well dishes in 400 μl culture medium covered with 400 μl oil at 38.5 °C in an atmosphere of 5 % CO2, 5 % O2 and 90 % N2 with 100 % humidity.
Time-lapse observation of embryo development
The embryos were cultured in the EmbryoScope® time-lapse incubator in the EmbryoSlide® culture dishes. Mouse embryos were cultured for 5 days at 37 °C in 5 % CO2 in air, and porcine PA embryos were cultured for 6 days at 38.5 °C in an atmosphere of 5 % CO2, 5 % O2 and 90 % N2. All embryos from the control groups were cultured without light in standard incubator for 5 days (mouse) or 6 days (pig). Digital images of the cultured embryos were obtained at each recording interval (15/20 min) with a red light emitting diode (LED, R42182, Seoul Semiconductor, Korea) that was only turned on during image acquisition at 75 ms intervals (15 ms for each image capture x 5 different focus planes = 75 ms/15-20 min). The images were collected and analyzed using the EmbryoViewer® software (Unisense Fertilitech A/S).
The low light exposure treatment used the standard light settings for image recording on the instrument. For the extra light exposure treatment, a modified EmbryoScope® time-lapse system was used to keep the light on for 60 s/15 min or 100 s/20 min.
Evaluation of the embryonic development and quality
Embryonic development was checked on Day 5 for mouse and Day 6 for pig embryos. For mouse embryos, only blastocyst formation was assessed and defined as the formation of a blastocoel cavity. All porcine blastocysts were evaluated by dividing the quality into 4 grades according to their morphology as previously described [14]: (1) Excellent: Spherical, regular border, symmetrical with cells of uniform size, even distribution of color and texture in trophectoderm; (2) Good: Fragmentations (<10 %), irregular shape of blastocoel cavity; (3) Fair: Fragmentations (10-30 %), vesiculation of cavity; (4) Poor: Fragmentations (>30 %), varying sizes of cells, numerous vesicles.
The quality of porcine blastocysts was evaluated by the total cell number on Day 6. Briefly, the blastocysts were stained for 20 min with 1 μg/ml Hoechst 33342 (using 4 % paraformaldehyde as base medium) and were then mounted in glycerol on a microscope glass slide. Stained embryos were examined and images were taken on fluorescence microscopy (360 ± 20 nm excitation, ebq 100 Filter, Leica, Germany).
Statistical analysis
A Chi-square test was used to analyze the development rate of mouse embryos. For the porcine PA embryos, 3 replicates were performed for each experiment, and one-way ANOVA, Tukey’s Honestly Significant Difference (TukeyHSD) test was used to analyze the blastocyst rates and total cell number. All statistical analyses were performed with software of R (version 2.14.2). A value of P < 0.05 was considered to be statistically significant.
Experimental design
Experiment 1: Comparison of the spectral scalar irradiance in three systems: inverted microscope (used for intracytoplasmic sperm injection), dissection microscope (used for embryo handling and checking) and EmbryoScope® time-lapse system.
Experiment 2: Testing the effect of the light source on mouse embryos.
Experiment 3: Comparison of the embryonic development and quality of porcine PA embryos (zona-intact) using three levels of light exposure with red LED light.
Experiment 4: Comparison of the embryonic development and quality of zona-free porcine PA embryos under three levels of light exposure with red LED light.
Results
Experiment 1: The spectral scalar irradiance in the three microscope systems (Fig. 2).
There was no light at <550 nm in the time-lapse system (Fig. 2a), and the system illuminated specimens with an integrated scalar irradiance (380–1050 nm) of 0.34 W/m2 when the red LED light was turned on (Fig. 2b). In comparison, the two microscopes illuminated specimens with higher scalar irradiance, including the potentially harmful 380–550 nm range (Fig. 2b).
Experiment 2: The effect of red light in the time-lapse system on mouse embryos (Table 1).
Table 1.
Total energy dose in 3 levels of light exposure for 5 days observation and the effect on the blastocyst rate of mouse embryos in the time-lapse system
| Exposure time/interval | 75 ms/20 min | 50 s/20 min | 100 s/20 min |
|---|---|---|---|
| Total dose (J/m2) | 10 | 6120 | 12240 |
| Blastocyst % (Blastocysts/zygotes) | 96 (27/28) | 100 (11/11) | 95 (20/21) |
| Replicates | 3 | 1 | 2 |
In 60 incubated mouse zygotes, no significant difference was found in the blastocyst rates, even when the total light dose was increased up to 1000 times (>10 kJ/m2).
Experiment 3: The development and quality of zona-intact porcine PA embryos after 3 levels of red light exposure in the time-lapse system (Table 2).
Table 2.
Effect of extra light exposure (60 s/15 min) on development and quality of zona intact porcine PA embryos
| Exposure time/interval | Total time (s) | Total energy (J/m2) | Activated oocytes (Replicates) | Blastocyst%* | Grade1 & 2 blastocyst%* | Total cell number** |
|---|---|---|---|---|---|---|
| 60 s/15 min | 34560 | 11750 | 36 (3) | 74.7 ± 8.3 (27) | 55.7 ± 5.6 (20) | 55.2 ± 2.7 (27/3) |
| 75 ms/15 min | 44 | 15 | 36 (3) | 88.7 ± 5.7 (32) | 72.3 ± 2.8 (26) | 51.1 ± 3.2 (32/3) |
| Control | 0 | 0 | 58 (3) | 77.7 ± 2.7 (45) | 62.3 ± 3.6 (36) | 57.6 ± 3.1 (25/2) |
*mean of replicates ± SEM (No. of blastocysts), **mean of blastocyst ± SEM (No. of blastocysts/replicates). There is no significant difference in each column
In 130 activated oocytes, there were no significant differences in the blastocyst rates and quality between control and extra light exposure groups.
Experiment 4: The development and quality of zona-free porcine PA embryos after 3 levels of red light exposure in time-lapse system (Table 3).
Table 3.
Effect of extra light exposure (60 s/15 min and 100 s/20 min) on development and quality of zona free porcine PA embryos
| Exposure time/interval | Total time (s) | Total energy (J/m2) | Activated oocytes (Replicates) | Blastocyst%* | Grade1 & 2 blastocyst%* | Total cell number** |
|---|---|---|---|---|---|---|
| 60 s/15 min | 34560 | 11750 | 36 (3) | 69.3 ± 7.3 (25) | 52.7 ± 12.2 (19) | 34.6 ± 2.1 (15/2) |
| 75 ms/15 min | 44 | 15 | 36 (3) | 75 ± 4.6 (27) | 52.7 ± 5.3 (18) | 41.4 ± 3.2 (18/2) |
| Control | 0 | 0 | 59 (3) | 81.3 ± 6.8 (49) | 66 ± 9.5 (38) | 41.9 ± 2.8 (29/2) |
| 100 s/20 min | 43200 | 14690 | 36 (3) | 72.3 ± 5.3 (26) | 44.6 ± 2.7 (16) | 51.5 ± 5.1 (8/1) |
| 75 ms/20 min | 33 | 10 | 36 (3) | 69.4 ± 10.0 (25) | 47.3 ± 2.7 (17) | 46.8 ± 2.2 (6/1) |
| Control | 0 | 0 | 64 (3) | 65.3 ± 15.6 (43) | 52.7 ± 15.2 (35) | 59.3 ± 4.6 (8/1) |
*mean of replicates ± SEM (No. of blastocysts), **mean of blastocyst ± SEM (No. of blastocysts/replicates). There is no significant difference in each column
Of 267 activated oocytes, 131 were used in one experiment with a light exposure of 60 s/15 min, while 136 were used in an experiment with a higher light exposure of 100 s/20 min. There was no decrease in the blastocyst rates and quality between the control and extra light exposure groups.
Discussion
Light is a common stress factor for embryos during their in-vitro processes, especially when being handled and observed for evaluation and selection. The type [2, 3, 15], intensity [15] and exposure time [27] of light can affect the subsequent development of embryos. In the present study, the light source in the time-lapse system was a red LED light emitting within a narrow wavelength range peaking at 625 nm. The scalar irradiance and therefore light exposure in this system was found to be much lower than in dissection and inverted microscopes in both tested wavelength areas. Furthermore, the blastocyst rates and total cell numbers in both mouse and porcine embryos were not affected even after a considerably increased exposure to red light.
A total dose of 10 kJ/m2 blue light was found to damage the function of mammalian fibroblast cells [18]. In the present study, we used a maximum energy dose of red light in the time-lapse system of 10–15 kJ/m2 after extra 100 s/20 min light exposure over 5 to 6 days. This caused no significant difference in the blastocyst rates and quality of incubated embryos from both mouse and pig. Possible explanations for this result can be:
(i) The red light used in the EmbryoScope® time-lapse system is not harmful. Light exposure to the wavelength range of 380–550 nm has been shown to induce harmful effects on embryonic quality and survival under in-vitro manipulation and monitoring [2, 15]. This spectral range can increase heat shock proteins (Hsp70) gene expression and the formation of reactive oxygen species, which result in more apoptotic cells appearing at the blastocyst stage [2, 15].
(ii) The exposure to red LED light in the time-lapse system was low. Under visible light intensities up to 1200 lux, the development and quality of hamster embryos can be significantly decreased after only 30 min exposure [3]. Moreover, the development and quality of embryos will gradually decrease with increasing light intensity when the intensity was over 200 lux [15]. The light intensity in the EmbryoScope® time-lapse system was much lower, and our results indicate that even under a dose of 10–15 kJ/m2 of red light, i.e., the maximal inducible light level during observation in the EmbryoScope® time-lapse system, there was apparently no harmful effect on the development and quality of mammalian embryos.
Over a 5 to 7 day normal observation period in the time-lapse system, we showed that the total exposure time was maximally 50 s resulting in a total energy dose of 10–20 J/m2. This is a very short exposure time and represents a much lower total energy dose as compared to light exposure under different traditional manipulation steps such as a typical 10–15 min common evaluation of embryo quality under a dissection microscope, or e.g. a typical time frame of 30 min for performing an intracytoplasmic sperm injection on the inverted microscope. It is thus important to reduce the light exposure during such in-vitro manipulations, either by decreasing exposure time and irradiance [15, 27] or by avoiding harmful wavelengths by the use of filters or LED’s with a more narrow spectral emission [2, 28].
Conclusion
The red light (625 nm, 0.34 W/m2) applied in the EmbryoScope® time-lapse system does not compromise the blastocyst rates and quality in either mouse zygotes or porcine PA embryos (both zona-intact and zona-free).
Acknowledgments
The authors thank Anette M. Pedersen, Janne Adamsen, Klaus Villemoes and Ruth Kristensen for excellent technical assistance. Part of this work was supported by grants from the Danish National Advanced Technology Foundation and the Danish Council of Independent Research.
Footnotes
Capsule Our results indicate that red light (625 nm, 0.34 W/m2) used in the time-lapse incubation system does not decrease the development and quality of blastocysts in both mouse zygotes and porcine PA embryos (both zona-intact and zona-free).
References
- 1.Schultz RM. Of light and mouse embryos: Less is more. Proceedings of the National Academy of Sciences of the United States of America 2007; 104(37):14547–14548 [DOI] [PMC free article] [PubMed]
- 2.Korhonen K, Sjovall S, Viitanen J, Ketoja E, Makarevich A, Peippo J. Viability of bovine embryos following exposure to the green filtered or wider bandwidth light during in vitro embryo production. Human Reproduction. 2009;24(2):308–314. doi: 10.1093/humrep/den432. [DOI] [PubMed] [Google Scholar]
- 3.Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(36):14289–14293. doi: 10.1073/pnas.0706687104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puissant F, Vanrysselberge M, Barlow P, Deweze J, Leroy F. Embryo Scoring As A Prognostic Tool in Ivf Treatment. Human Reproduction. 1987;2(8):705–708. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 5.Fisch JD, Rodriguez H, Ross R, Overby G, Sher G. The Graduated Embryo Score (GES) predicts blastocyst formation and pregnancy rate from cleavage-stage embryos. Human Reproduction. 2001;16(9):1970–1975. doi: 10.1093/humrep/16.9.1970. [DOI] [PubMed] [Google Scholar]
- 6.Vernon M, Stern JE, Ball GD, Wininger D, Mayer J, Racowsky C. Utility of the national embryo morphology data collection by the Society for Assisted Reproductive Technologies (SART): correlation between day-3 morphology grade and live-birth outcome. Fertility and Sterility. 2011;95(8):2761–2763. doi: 10.1016/j.fertnstert.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Massip A, Mulnard J. Time-Lapse Cinematographic Analysis of Hatching of Normal and Frozen-Thawed Cow Blastocysts. Journal of Reproduction and Fertility 1980; 58 (2):475-&. [DOI] [PubMed]
- 8.Massip A, Mulnard J, Vanderzwalmen P, Hanzen C, Ectors F. The Behavior of Cow Blastocyst Invitro - Cinematographic and Morphometric Analysis. Journal of Anatomy 1982; 134 (MAR):399–405. [PMC free article] [PubMed]
- 9.Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Human Reproduction. 1997;12(3):532–541. doi: 10.1093/humrep/12.3.532. [DOI] [PubMed] [Google Scholar]
- 10.Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nature Biotechnology. 2010;28(10):1115–1199. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 11.Kirkegaard K, Hindkjaer JJ, Grondahl ML, Kesmodel US, Ingerslev HJ. A randomized clinical trial comparing embryo culture in a conventional incubator with a time-lapse incubator. Journal of Assisted Reproduction and Genetics. 2012;29(6):565–572. doi: 10.1007/s10815-012-9750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Human Reproduction. 2011;26(10):2658–2671. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 13.Holm P, Shukri NN, Vajta G, Booth P, Bendixen C, Callesen H. Developmental kinetics of the first cell cycles of bovine in vitro produced embryos in relation to their in vitro viability and sex. Theriogenology. 1998;50(8):1285–1299. doi: 10.1016/S0093-691X(98)00227-1. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Liu Y, Pedersen H, Kragh P, Callesen H. Development and quality of porcine parthenogenetically activated embryos after removal of zona pellucida. Theriogenology. 2013;80(1):58–64. doi: 10.1016/j.theriogenology.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Oh SJ, Gong SP, Lee ST, Lee EJ, Lim JM. Light intensity and wavelength during embryo manipulation are important factors for maintaining viability of preimplantation embryos in vitro. Fertility and Sterility. 2007;88:1150–1157. doi: 10.1016/j.fertnstert.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M, Saka N, Takahashi H, Kanai Y, Schultz RM, Okano A. Assessment of DNA damage in individual hamster embryos by comet assay. Molecular Reproduction and Development. 1999;54(1):1–7. doi: 10.1002/(SICI)1098-2795(199909)54:1<1::AID-MRD1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara T, Iwase A, Goto M, Harata T, Suzuki M, Ienaga M, et al. Evaluation of the safety of time-lapse observations for human embryos. Journal of Assisted Reproduction and Genetics. 2010;27(2–3):93–96. doi: 10.1007/s10815-010-9385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wataha JC, Lockwood PE, Lewis JB, Rueggeberg FA, Messer RLW. Biological effects of blue light from dental curing units. Dental Materials. 2004;20(2):150–157. doi: 10.1016/S0109-5641(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 19.Cruz M, Garrido N, Herrero J, Perez-Cano I, Munoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reproductive Biomedicine Online. 2012;25(4):371–381. doi: 10.1016/j.rbmo.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Li R, Li J, Liu Y, Kragh PM, Hyttel P, Schmidt M, et al. Optimal developmental stage for vitrification of parthenogenetically activated porcine embryos. Cryobiology. 2012;64(1):60–4. [DOI] [PubMed]
- 21.Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reproductive Biomedicine Online. 2013;27(2):140–146. doi: 10.1016/j.rbmo.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Niemann H. Sensitivity of Pig Morulae to Dmso/Pvp Or Glycerol Treatment and Cooling to 10-Degrees-C. Theriogenology. 1985;23(1):213. doi: 10.1016/0093-691X(85)90119-0. [DOI] [Google Scholar]
- 23.Wilmut I. Low-Temperature Preservation of Mammalian Embryos. Journal of Reproduction and Fertility. 1972;31(3):513–514. doi: 10.1530/jrf.0.0310513. [DOI] [PubMed] [Google Scholar]
- 24.Kühl M. Optical microsensors for analysis of microbial communities. Methods in Enzymology. 2005;397:166–199. doi: 10.1016/S0076-6879(05)97010-9. [DOI] [PubMed] [Google Scholar]
- 25.Exposure to light during image acquisition in the EmbryoScope. http://www.fertilitech.com/Admin/Public/DWSDownload.aspx?File=%2FFiles%2FFiles%2Fdownloads%2FLight+exposure+in+EmbryoScope+v4.pdf. 2012. Ref Type: Online Source
- 26.Yoshioka K, Suzuki C, Tanaka A, Anas IMK, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biology of Reproduction. 2002;66(1):112–119. doi: 10.1095/biolreprod66.1.112. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JQ, Li XL, Peng YZ, Guo XR, Heng BC, Tong GQ. Reduction in exposure of human embryos outside the incubator enhances embryo quality and blastulation rate. Reproductive Biomedicine Online. 2010;20(4):510–515. doi: 10.1016/j.rbmo.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Ottosen LDM, Hindkjaer J, Ingerslev J. Light exposure of the ovum and preimplantation embryo during ART procedures. Journal of Assisted Reproduction and Genetics. 2007;24(2–3):99–103. doi: 10.1007/s10815-006-9081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]