Introduction
Fetal sex differentiation of the male involves complex processes and is dependent on the androgen production from fetal Leydig cells triggered by placental human chorionic gonadotropin (hCG) [1, 2]. It is later replaced by luteinizing hormone (LH) that is secreted by the fetal pituitary gland during gestation [3]. The hormones, such as antimullerian hormone (AMH) synthesized by Sertoli cells of the testes, testosterone produced by the Leydig cells and dihydrotestosterone, are also essential for the formation of male genitalia [4–6]. Because the action of both hCG and LH is through the luteinizing hormone/chorionic gonadotropin receptor (LHCGR), its presence and smooth function is crucial for male sex differentiation and characteristics in fetal and adult life. Any changes in the DNA or gene expression levels of LHCGR are associated with a variety of phenotypic as well as clinical symptoms and pathologies.
Human LHCGR gene is located on chromosome 2 and encodes 11 exons (NCBI GeneID 3,973; http://www.ncbi.nlm.nih.gov/). Exons 1–10 and a part of exon 11 encode the extracellular domain which is responsible for ligand binding, and the remaining of exon 11 encodes a transmembrane domain and intracellular C-terminal domain which is responsible for signal transduction [7].
Leydig cell hypoplasia (LCH) or Leydig cell agenesis is a very rare disease with an approximate incidence of 1:1,000,000 occurring with inactivating mutations in the LHCGR gene (6). These mutations mainly affect the amino acid sequence, leading to the alterations of receptor protein structure and function. Depending on the severity of the functional incompetence, a spectrum of phenotypes has been described [8]. The phenotype of males with LCH varies from micropenis, ambiguous genitalia, hypospadias to pseudohermaphroditism. In females, LH insensitivity is also presented with irregular menstrual cycles, infertility, recurrent miscarriage loss, polycystic ovarian syndrome (PCOS), and genital malformations [9–13]. The most severe form of LCH, type I, occurs due to luteinizing hormone/human chorionic gonadotropin receptor (LHCGR) mutations that completely prevent hCG/LH signal transduction [10, 14, 15]. On the other hand, patients with LCH type II have milder symptoms caused by LHCGR mutations that partially affect the responsiveness of hCG [9, 10, 16]. In the last decade, several mutations including homozygous and compound heterozygous mutations in the LHCGR gene have been reported in both familial and sporadic cases of LCH. A number of these studies showed an impaired or absent ligand binding and cAMP production in response to hCG stimulation, suggesting that some of these mutations have inactivating roles [11–13, 16]. In such cases, the basal hormone status, usually with normal FSH, elevated LH, and low testosterone, provides an initial diagnostic hint of the underlying defect. Diagnosis confirmed by testicular biopsy showed LCH and by techniques of molecular biology revealed mutations of the LH receptor gene [17].
To date, no standard treatment approaches have been suggested or developed in LCH. Here, we report our experience with a man with LCH that resulted in successful sperm recovery and live birth with IVF treatment.
Case report
The couple was referred to our in vitro fertilization (IVF) centre due to primary infertility and azoospermia. The male partner was 31 years old, born to consanguineous parents. His parents had three deceased siblings before the age of 2 years, for unknown/undetermined reasons. One of his sisters was also infertile for 24 years and even after several IVF treatments, pregnancy could not be established. Three of his other sisters and a brother conceived naturally.
The physical examination of the patient revealed a eunuchoid habitus and diminished facial and body hair. He was 1.93 m in height and his weight was 85 kg. Genital examination showed that this patient has a micropenis of 5 cm in length and enlarged testes of approximately 25 cm with Prader’s orchiodometer. The serum FSH and prolactin levels were normal (2.26 mIU/mL, reference 1–8 mIU/mL and 9.16 ng/mL, reference 1.6–18.7 ng/mL, respectively) but LH level was elevated (15.66 mIU/mL, reference 2–12 mIU/mL). The total testosterone and free testosterone levels were extremely low (0.32 ng/mL, reference 2.8–8 ng/mL and 0.015 nmol/L, reference 0.091–0.58 nmol/L, respectively). The karyotypes of both the patient (46, XY) and his wife (46, XX) were normal and Y chromosome microdeletion analysis was negative.
In vitro fertilization
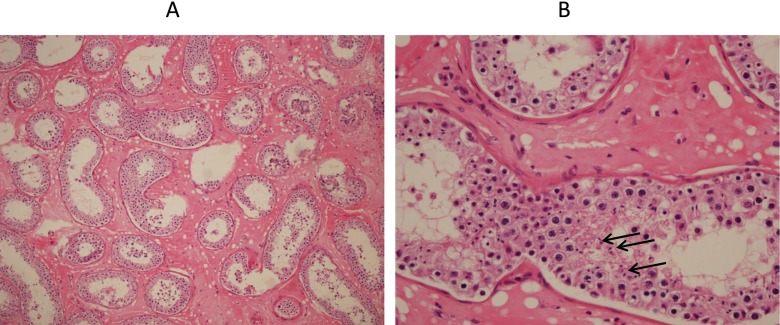
The patient was initially treated with weekly administration of 5,000 IU hCG for one month. At the end of this period, testosterone level was measured to be 0.47 ng/mL. Therefore, the hCG administration was increased to twice a week (5,000 IU) until the end of the hormonal therapy. The changes in testosterone levels were evaluated monthly; however, the highest serum testosterone concentration was only 1.28 ng/mL after 1 year of treatment (Table 1). At the end of the 14th month, the serum testosterone level dropped to 0.7 ng/mL. Semen analysis was performed monthly after 6 months of hCG therapy and all showed azoospermia. Therefore, micro-TESE procedure was performed to determine whether there was any sperm production. Pathological evaluation of the testicular biopsy was conducted. Motile spermatozoa were recovered by micro-TESE from the testicular tissue and were cryopreserved for the ICSI procedure. Histological analysis of the biopsied tissues obtained by micro-TESE showed seminiferous tubules with thickened basal lamina and the absence of Leydig cells (Fig. 1). The spermatocytes, a low number of spermatids, and spermatozoa were detected in both testes. Both the phenotype and the histological analysis of the biopsy suggested that this patient has LCH that may be due to lack of necessary LHCGR activity.
Table 1.
hCG injection scheme and total testosterone (ng/mL) follow up during hormonal treatment
| Follow-up months | Concentration of total testosterone (ng/mL) |
|---|---|
| 1 | 0.47 |
| 2 | 0.57 |
| 3 | 0.68 |
| 4 | 0.7 |
| 6 | 1.0 |
| 7 | 0.9 |
| 8 | 1.20 |
| 9 | 1.25 |
| 10 | 1.26 |
| 12 | 1.28 |
| 14 | 0.7 |
Fig. 1.
Testicular tissue of right testis with (A) haematoxylin and eosin staining in 40× magnification. No Leydig cells were present in the testicular tissue and (B) in 200× magnification. A tubule showed spermatocytes and spermatids and very few spermatozoa shown by arrows
Cryopreserved spermatozoa were later used in controlled ovarian hyperstimulation (COH) and ICSI procedure. Following the first fresh cycle, three embryos were obtained. A single embryo was transferred on Day 3 of embryo development and two embryos were cryopreserved. However, clinical pregnancy could not be established. The frozen embryos were thawed and a single embryo was subsequently transferred in a frozen cycle, resulting in a singleton pregnancy. There were no complications during the pregnancy period and a healthy girl was delivered at term.
LHCGR Mutation analysis
In order to investigate the possible LHCGR mutations present in the patient as well as in his relatives, the LHCGR exons were sequenced using primers shown in Table 2 (Macrogen, Korea). Genomic DNA was extracted from the peripheral blood of the patient, his daughter, sister, and mother by using RTA genomic DNA isolation kit from blood (RTA, Turkey). Exons 1–10 and overlapping fragments of exon 11 of the LHCGR gene were amplified by polymerase chain reaction (PCR). Sequencing was performed using Big Dye sequencing mix (ABI, UK) in both directions.
Table 2.
List of primers used for sequencing
| Primers | Primer sequence (3′–5′) |
|---|---|
| LhR exon1 Forward | AGGGAAGGGTGTGTGGAAG |
| LhR exon 1 Reverse | GCAAAGCGTTTTTCTCCAAG |
| LhR exon 2 Forward | TGAACTTAAAAGGCACCCTATCA |
| LhR exon 2 Reverse | TTCATTATTCCAATTACGAATGTCTT |
| LhR exon 3 Forward | TTGGGTCACACACATAGCTCA |
| LhR exon 3 Reverse | GGATGCTTGAATACAAATACCAA |
| LhR exon 4 Forward | AGCCAGCAACTTCTGGTGAC |
| LhR exon 4 Reverse | TCTTTCCAACCTTTTCCTTGTT |
| LhR exons 5/6 Forward | TTTGCAAAATGCCTTTGTACTC |
| LhR exons 5/6 Reverse | CCAGTGAGTGAGGAATGTGG |
| LhR exon 7 Forward | TTCCCATTCCTAAACCCTCTC |
| LhR exon 7 Reverse | CTGAGGGCTAGCCACTTGAA |
| LhR exon 8 Forward | CCTTTTTCCCCCTTTTAACC |
| LhR exon 8 Reverse | GGAGAGGCCTGGAATAGATG |
| LhR exon 9 Forward | TCCACTAAGGGCAATATCCTTC |
| LhR exon 9 Reverse | GGAGCAAGACTCCGTCTCAA |
| LhR exon 10 Forward | AAGTGACCCCATGTCTACGG |
| LhR exon 10 Reverse | GCAACAGCTCCGTAACCAAG |
| LhR exon 11.1 Forward | TGGCTTTGTTTCCTTTTGTTT |
| LhR exon 11.1 Reverse | CAGTGAAAAAGCCAGCAGTG |
| LhR exon 11.2 Forward | AGACTGGCAGACAGGGAGTG |
| LhR exon 11.2 Reverse | AGGCCACCACATTGAGAATC |
| LhR exon 11.3 Forward | CCATGGATGTGGAAACCACT |
| LhR exon 11.3 Reverse | GTGGACAACTTCAAGGTGGA |
| LhR exon 11.4 Forward | GGAAAGATTTTTCAGCTTACACC |
| LhR exon 11.4 Reverse | TTTTTAGTGTGGCAGTGGTCA |
| LhR exon 12 Forward | AATGTGGTGGCCTTCTTCAT |
| LhR exon 12 Reverse | GAAATCTCTTTGGAATGTCTTAGTGA |
| LhR exon 7 of transcript 3 Forward | TTCTTTATACCTAAAGCCCAGTGA |
| LhR exon 7 of transcript 3 Reverse | GGATACCAATTTTTAGAATCCAGTG |
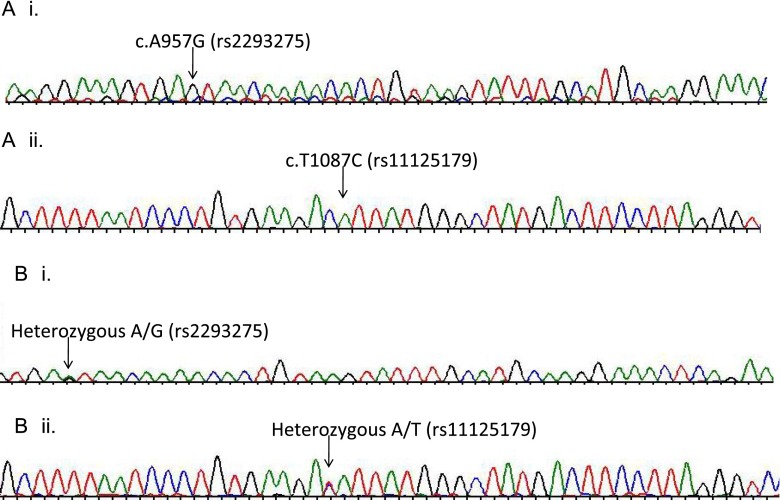
Sequencing analysis revealed two homozygous variations on the LHCGR gene of the patient. One of these variations, c.A957G (rs2293275), changed the codon 312 from AAT for aspargine to AGT for serine (Fig. 2). In exon 11, c.T1087C (rs11125179) variation changed the codon 355 from GAT to GCT. The latter variation did not result in any changes of the amino acid residue, hence, it was considered a polymorphic variation. The sequencing of these regions for the infertile sister of the patient also showed the same homozygous profile as his genotype. Sequencing of these regions in the patient’s mother DNA showed that she was heterozygous for both of these changes, rs2293275 A/G and rs11125179 A/T. The sequencing results of the patient’s daughter also showed the same genotype as his mother (Fig. 2). Since the father of the patient was deceased, sequencing analysis could not be performed.
Fig. 2.
The chromatogram images for sequencing are shown for a the patient and b the mother of the patient. The bases of the variation/mutation are shown with an arrow
Discussion
In azoospermic men, testicular failure is the most common reason of the absence of sperm production, which is characterized by low serum testosterone and elevated serum FSH levels. In most of the men with non-obstructive azoospermia (NOA), there are still no specific medical treatments available before or after testicular sperm extraction. Therefore, some authors suggested using empirically hormonal agents such as clomiphene or aromatase inhibitors (testolactone or anastrazole) to increase the successful sperm recovery before testicular sperm extraction procedure [18, 19]. However, the effect of optimizing serum testosterone levels on spermatogenesis is still under debate. In a recent study, Reifsnyder et al. [20] reported that preoperative treatment for optimizing testosterone levels did not aid finding spermatozoa in NOA patients; however, Shinjo et al. [21] suggested that hCG-based hormonal therapy significantly raises intra testicular testosterone level and improves spermatogenesis in such patient. In our case, we were able to find spermatozoa in the testicular tissue of a man with a very low serum testosterone level who was unresponsive to long-term hCG therapy. This finding can indicate that unlike alternative hormonal therapy approaches that are primarily focused on increasing serum testosterone levels, even very low testosterone level can, in fact, be enough to produce spermatozoa in testicular tissue. The quantity and quality of the testicular spermatozoa were also enough to survive after freeze/thaw procedure and result in viable embryos leading to live birth.
It has been reported that in cases with LCH depending on the type of the LH receptor defect, hCG responses to testosterone production may vary from no response to pronounce rise [17]. In our case, the serum total testosterone level was slightly increased at the end of the one-year hormonal therapy. At the 14th month, the serum testosterone level dropped to below 1.0 ng/mL. LHCGR in regions other than Leydig cells of the testes and effects of LH and hCG on extragonadal organs such as the adrenal cortex are still under investigation. Further studies need to understand testosterone response to hCG treatment in patients with LCH [22].
Inactivating mutations in the LHCGR gene have been reported in both males and females, leading to LCH and infertility [10, 14, 16, 23–25]. Previous studies have shown that missense or nonsense mutations, as well as small deletions or insertions, may lead to structural or functional alterations of the LHCGR, disrupting the signalling of the LH receptor protein and receptor activation [4, 26, 27]. In our case, gene sequencing analysis of the male partner resulted in two novel genotypic variations. The missense LHCGR mutation c.G1065A caused a change in the amino acid sequence of codon 312 from Asp to Ser. This amino acid is located near the glycosylation site that is crucial in the stabilization and cell surface expression of G-protein-coupled receptor superfamily. Therefore, this mutation may increase the risk of defects in the sensitivity of LHCGR for LH or hCG that was also observed in our patient’s response to hCG treatment. Although this polymorphic change (Asn/Ser or Ser/Asn) was reported previously in independent studies [28–32], this study is the first report showing the inactivating role of the mutation in an LCH patient. Previous studies involved patients with impaired spermatogenesis [32], males with elevated FSH levels with eunuchoid phenotype [31], and patients with breast or testicular cancer susceptibility [28–30].
The second variation that was detected in exon 11 was c.T1087C (rs11125179). This variation did not change the encoded amino acid (aspartic acid), therefore, it does not seem to have an effect on the development of LCH. However, because this patient was homozygous for this polymorphism, a possible change in mRNA level and stability could not be ruled out.
In summary, we report here for the first time that spermatozoa were obtained from a man with a novel mutation (c.A957G, rs2293275) in the LHCGR gene causing LCH. Although this patient presented very low concentration levels of serum testosterone, IVF treatment resulted in a live birth.
Acknowledgments
Funding
This study received no funding and the authors do not have any competing interests.
Conflict of interest
All authors contributed to design, acquisition, analysis, data intrepretation, and manuscript writing and declare no conflict of interest.
Footnotes
Capsule Successful testicular sperm recovery and IVF outcome from a man with Leydig cell hypoplasia carrying a novel LHCGR mutation
References
- 1.Jost A. Hormonal factors in the sex differentiation of the mammalian foetus. Philos Trans R Soc Lond B Biol Sci. 1970;259:119–30. doi: 10.1098/rstb.1970.0052. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab. 2006;20:91–110. doi: 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Fowler PA, Bhattacharya S, Gromoll J, Monteiro A, O’Shaughnessy PJ. Maternal smoking and developmental changes in luteinizing hormone (LH) and the LH receptor in the fetal testis. J Clin Endocrinol Metab. 2009;94:4688–95. doi: 10.1210/jc.2009-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huhtaniemi I, Alevizaki M. Gonadotrophin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:561–76. doi: 10.1016/j.beem.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Josso N, Picard JY, Tran D. The anti-mullerian hormone. Birth Defects Orig Artic Ser. 1977;13:59–84. [PubMed] [Google Scholar]
- 6.Siiteri PK, Wilson JD. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metab. 1974;38:113–25. doi: 10.1210/jcem-38-1-113. [DOI] [PubMed] [Google Scholar]
- 7.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocrine reviews. 2002;23:141–74. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 8.Martens JW, Verhoef-Post M, Abelin N, Ezabella M, Toledo SP, Brunner HG, et al. A homozygous mutation in the luteinizing hormone receptor causes partial Leydig cell hypoplasia: correlation between receptor activity and phenotype. Mol Endocrinol. 1998;12:775–84. doi: 10.1210/mend.12.6.0124. [DOI] [PubMed] [Google Scholar]
- 9.Bentov Y, Kenigsberg S, Casper RF. A novel luteinizing hormone/chorionic gonadotropin receptor mutation associated with amenorrhea, low oocyte yield, and recurrent pregnancy loss. Fertil Steril. 2012;97:1165–8. doi: 10.1016/j.fertnstert.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Latronico AC, Anasti J, Arnhold IJ, Rapaport R, Mendonca BB, Bloise W, et al. Brief report: testicular and ovarian resistance to luteinizing hormone caused by inactivating mutations of the luteinizing hormone-receptor gene. N Engl J Med. 1996;334:507–12. doi: 10.1056/NEJM199602223340805. [DOI] [PubMed] [Google Scholar]
- 11.Latronico AC, Chai Y, Arnhold IJ, Liu X, Mendonca BB, Segaloff DL. A homozygous microdeletion in helix 7 of the luteinizing hormone receptor associated with familial testicular and ovarian resistance is due to both decreased cell surface expression and impaired effector activation by the cell surface receptor. Mol Endocrinol. 1998;12:442–50. doi: 10.1210/mend.12.3.0077. [DOI] [PubMed] [Google Scholar]
- 12.Stavrou SS, Zhu YS, Cai LQ, Katz MD, Herrera C, Defillo-Ricart M, et al. A novel mutation of the human luteinizing hormone receptor in 46XY and 46XX sisters. J Clin Endocrinol Metab. 1998;83:2091–8. doi: 10.1210/jcem.83.6.4855. [DOI] [PubMed] [Google Scholar]
- 13.Toledo SP, Brunner HG, Kraaij R, Post M, Dahia PL, Hayashida CY, et al. An inactivating mutation of the luteinizing hormone receptor causes amenorrhea in a 46, XX female. J Clin Endocrinol Metab. 1996;81:3850–4. doi: 10.1210/jcem.81.11.8923827. [DOI] [PubMed] [Google Scholar]
- 14.Kremer H, Kraaij R, Toledo SP, Post M, Fridman JB, Hayashida CY, et al. Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat Genet. 1995;9:160–4. doi: 10.1038/ng0295-160. [DOI] [PubMed] [Google Scholar]
- 15.Laue L, Wu SM, Kudo M, Hsueh AJ, Cutler GB, Jr, Griffin JE, et al. A nonsense mutation of the human luteinizing hormone receptor gene in Leydig cell hypoplasia. Hum Mol Genet. 1995;4:1429–33. doi: 10.1093/hmg/4.8.1429. [DOI] [PubMed] [Google Scholar]
- 16.Laue LL, Wu SM, Kudo M, Bourdony CJ, Cutler GB, Jr, Hsueh AJ, et al. Compound heterozygous mutations of the luteinizing hormone receptor gene in Leydig cell hypoplasia. Mol Endocrinol. 1996;10:987–97. doi: 10.1210/mend.10.8.8843415. [DOI] [PubMed] [Google Scholar]
- 17.Nieschlag E, Behre HM, Nieschlag S. Male Reproductive Health and Dysfunction 2010.
- 18.Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU international. 2013;111:E110–4. doi: 10.1111/j.1464-410X.2012.11485.x. [DOI] [PubMed] [Google Scholar]
- 19.Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167:624–9. doi: 10.1016/S0022-5347(01)69099-2. [DOI] [PubMed] [Google Scholar]
- 20.Reifsnyder JE, Ramasamy R, Husseini J, Schlegel PN. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. Journal of Urology. 2012;188:532–6. doi: 10.1016/j.juro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Shinjo E, Shiraishi K, Matsuyama H. The effect of human chorionic gonadotropin-based hormonal therapy on intratesticular testosterone levels and spermatogonial DNA synthesis in men with non-obstructive azoospermia. Andrology. 2013;1:929–35. doi: 10.1111/j.2047-2927.2013.00141.x. [DOI] [PubMed] [Google Scholar]
- 22.Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: origins of difference. Mol Cell Endocrinol. 2014;383:203–13. doi: 10.1016/j.mce.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Gromoll J, Eiholzer U, Nieschlag E, Simoni M. Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: differential action of human chorionic gonadotropin and LH. J Clin Endocrinol Metab. 2000;85:2281–6. doi: 10.1210/jcem.85.6.6636. [DOI] [PubMed] [Google Scholar]
- 24.Han B, Wang ZQ, Xue LQ, Ma JH, Liu W, Liu BL et al. Functional study of an aberrant splicing variant of the human luteinizing hormone (LH) receptor. Mol Hum Reprod;18:129-35. [DOI] [PMC free article] [PubMed]
- 25.Qiao J, Han B, Liu BL, Chen X, Ru Y, Cheng KX, et al. A splice site mutation combined with a novel missense mutation of LHCGR cause male pseudohermaphroditism. Hum Mutat. 2009;30:E855–65. doi: 10.1002/humu.21072. [DOI] [PubMed] [Google Scholar]
- 26.Suganuma N, Furui K, Furuhashi M, Asada Y, Kikkawa F, Tomoda Y. Screening of the mutations in luteinizing hormone beta-subunit in patients with menstrual disorders. Fertil Steril. 1995;63:989–95. doi: 10.1016/s0015-0282(16)57535-9. [DOI] [PubMed] [Google Scholar]
- 27.Segaloff DL. Diseases associated with mutations of the human lutropin receptor. Progress in molecular biology and translational science. 2009;89:97–114. doi: 10.1016/S1877-1173(09)89004-2. [DOI] [PubMed] [Google Scholar]
- 28.Piersma D, Verhoef-Post M, Look MP, Uitterlinden AG, Pols HA, Berns EM, et al. Polymorphic variations in exon 10 of the luteinizing hormone receptor: functional consequences and associations with breast cancer. Mol Cell Endocrinol. 2007;276:63–70. doi: 10.1016/j.mce.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Kristiansen W, Aschim EL, Andersen JM, Witczak O, Fossa SD, Haugen TB. Variations in testosterone pathway genes and susceptibility to testicular cancer in Norwegian men. Int J Androl. 2012;35:819–27. doi: 10.1111/j.1365-2605.2012.01297.x. [DOI] [PubMed] [Google Scholar]
- 30.Valkenburg O, Uitterlinden AG, Piersma D, Hofman A, Themmen AP, de Jong FH, et al. Genetic polymorphisms of GnRH and gonadotrophic hormone receptors affect the phenotype of polycystic ovary syndrome. Hum Reprod. 2009;24:2014–22. doi: 10.1093/humrep/dep113. [DOI] [PubMed] [Google Scholar]
- 31.Mongan NP, Hughes IA, Lim HN. Evidence that luteinising hormone receptor polymorphisms may contribute to male undermasculinisation. Eur J Endocrinol. 2002;147:103–7. doi: 10.1530/eje.0.1470103. [DOI] [PubMed] [Google Scholar]
- 32.Simoni M, Tuttelmann F, Michel C, Bockenfeld Y, Nieschlag E, Gromoll J. Polymorphisms of the luteinizing hormone/chorionic gonadotropin receptor gene: association with maldescended testes and male infertility. Pharmacogenet Genomics. 2008;18:193–200. doi: 10.1097/FPC.0b013e3282f4e98c. [DOI] [PubMed] [Google Scholar]