Abstract
Background
Polycystic ovary syndrome (PCOS) is a condition with central feature of hyperandrogensism that affects 5-12 % of women worldwide. P450sec the cholesterol side chain cleavage enzyme encoded by CYP11A1 gene is instrumental in the synthesis of sex hormones. A promoter pentanucleotide repeat (tttta)n polymorphism of this gene is reported to be associated with several hormone related diseases including PCOS. Here we aimed to examine the involvement of CYP11A1 polymorphism with PCOS susceptibility in a case–control study conducted among South Indian women.
Methods
A total of 542 subjects comprised of 267 PCOS patients and 275 controls were recruited. DNA was extracted from blood and CYP11A1 (tttta)n polymorphism was genotyped by PCR-PAGE.
Results
Fifteen different alleles ranging between 2–16 repeats were identified in the studied group and the most frequent allele observed in controls was of 8 repeats. The presence of >8 repeat allele was common in patients (64 % vs. 38 %) and showed a three-fold risk for PCOS susceptibility than controls (OR = 2.93; p < 0.05). PCOS women with higher BMI were markedly elevated in early quartile (p < 0.05).
Conclusion
CYP11A1 (tttta)n repeat polymorphism appeared to be a potential molecular marker for PCOS risk in our population. Gene-gene and gene-environmental interactions with respect to obesity may play a role in the early onset of this multifactorial condition. This is the first report from South India; however, replicative studies considering other probable causative factors for PCOS risk are warranted.
Keywords: CYP11A1, (tttta)n polymorphism, Hyperandrogenism, BMI
Introduction
Polycystic Ovary Syndrome (PCOS) is one of the most common endocrine disorders affecting 5% to 12% of women of reproductive age worldwide [1, 2]. However, the estimated frequency may rise up to 25 % depending on the diagnostic criteria used. This disorder is characterized by clinical features such as hyperandrogenism, chronic anovulation or infrequent ovulation and presence of multiple follicular cysts in enlarged ovaries. Elevated levels of androgens seem to influence the process of follicular maturation leading to PCOS phenotype [3, 4]. This complex disorder with clinical features changes throughout the life span and is often associated with long term consequences such as obesity, type 2 diabetes, endometrial hyperplasia, thyroid dysfunction and cardiovascular diseases [1]. Clinical and genetic heterogeneity is observed in PCOS and invariably associated with hyperandrogenic condition indicating the possible involvement of abnormalities associated with steroidogenic pathway.
Steroidogenesis is a fundamental process for essential physiological functions such as blood salt balance, carbohydrate metabolism and reproduction. Major sites for steroid hormone biosynthesis include the adrenal cortex, testis, ovary and placenta. There are two broad categories of steroidogenic pathway enzymes i.e. involved in the hydroxysteroid dehydrogenases and cytochrome P450scc [5]. The cytochrome P450scc is a cholesterol side chain cleavage enzyme coded by CYP11A1 gene, located at 15q23-q24 and resides on mitochondrial inner membranes and catalyzes conversion of cholesterol to pregnenolone, the initial and rate-limiting step in steroid hormone synthesis [6]. CYP11A1 gene deletions in rabbits have been shown to eliminate steroidogenesis indicating that the entire steroid hormone biosynthesis is initiated through the action of this enzyme [7]. Genetic variants of CYP11A1 gene may alter its expression and result in certain hormone-related diseases such as breast cancer, prostate cancer, endometrial cancer including polycystic ovary syndrome [8–11]. It is postulated that polymorphisms in this gene may play an important role in the regulation of CYP11A1 expression by up or down regulation of transcription leading to increased or decreased androgen production. Microsatellite (tttta)n polymorphism of CYP11A1 gene was associated with altered expression [11–13].
The present study was aimed to investigate the association of this repeat polymorphism in PCOS and non-PCOS controls of South India.
Materials and methods
Study population
A total of 542 subjects comprised of 267 PCOS patients and 275 non-PCOS controls were enrolled for the present study. Patients were recruited based on Rotterdam criteria according to which a woman is said to have PCOS, if she has any two features out of three such as polycystic ovaries on ultrasound scan, menstrual irregularities and/or clinical signs or symptoms of hyperandrogenism. Hyperandrogenism was defined as the clinical presence of hirsutism (Ferriman–Gallwey score >8), acne, alopecia or premature pubarche and/or elevated androgen levels. The non-PCOS control group consisted of ultrasound scanned normal fertile women (for the exclusion of polycystic ovaries). These women had normal menstrual cycles (<32 days), with no signs of hyperandrogenism and some of them were with one child or more. All the subjects were recruited from Government Maternity Hospital, Petlaburz, Hyderabad, India. Written approval was taken from the ethics committee of Osmania University and informed consent was obtained from each study participant prior to sample collection. Detailed information on clinical and anthropometric measures was recorded through proforma.
Characteristics of the study population
Inter-individual variation is commonly observed with respect to clinical features of PCOS changing throughout the life span starting from adolescence to post-menopausal age. The clinical features represent the signs or symptoms of PCOS that had risen due to hyperandrogenism and hyperinsulinemia. The two markers; acanthosis nigricans for insulin resistance, acne, hirsutism, alopecia and premature pubarche for hyperandrogenism were considered as important clinical features of PCOS. The patients were invariably asked to record about the onset of menstrual dysfunction, development of clinical symptoms, either at adolescence or at adulthood and the age at onset (AAO) of the clinical symptoms.
The two anthropometric measures considered for the present study were Body Mass Index (BMI) and Waist to Hip ratio (WHR). Body weight, height, waist and hip circumferences were measured in all participants in order to calculate BMI and WHR. Their BMI was calculated by the formula: body weight in kg/ height in meters square (kg/m2) and waist circumference was measured between the lower rib and the iliac crest at the end of a normal expiration. A value ≥23 kg/m2 [1, 14] was considered as over-weight/obese and a value of ≥ 0.8 was regarded to have abdominal obesity.
Molecular analysis
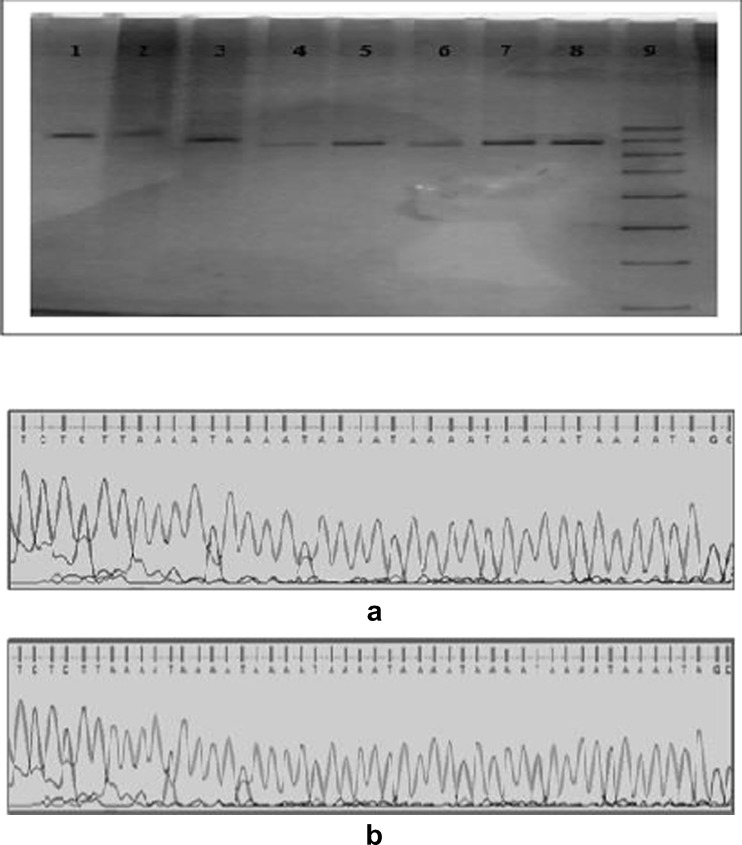
Genomic DNA was extracted from 5 ml of peripheral blood samples using salting out method and stored at -20ºc until use [4]. CYP11A1 (tttta)n genotyping was performed by PCR using forward and reverse primers [(FP: 5-GAGCTGACATCCTGAGTTCC-3, RP: 5- CTGGCAGCCATTGGTCAAGA-3)(Bioserve India)]. PCR amplification was carried out in a final volume of 10 μl containing 50ηg template DNA, 1.0 μl of 10× PCR buffer, 0.2 μl of dNTPS (2 mM) (Labpro, India), 0.2 μl of each primers (10 mM) and 0.2 μl of Taq polymerase (5U/μl) (Labpro, India). PCR was performed in a thermocycler (Eppendorf, Germany) with an initial denaturation step at 95 °C for 5 min, followed by 30 cycles consisting of denaturation at 94 °C for 30 sec, annealing at 57 °C for 45 sec, primer extension at 72 °C for 45 sec and final extension for 5 min at 72 °C. The amplified products were analyzed using 10 % polyacrylamide gel electrophoresis (PAGE) followed by silver staining and the number of repeats for each allele was determined. The number of (tttta)n repeats in every particular allele was identified by sequencing of representative samples (Fig. 1a and b ).
Fig. 1.
Gel picture showing different (tttta)n genotypes of CYP11A1 gene. Results of sequencing analysis on two different representative samples showing (a) 6 repeats and (b) 8 repeats. Note: W1. 10 repeats, W2. 10, 11 repeats, W3. 8 repeats, W4. 6 repeats W5, W7, W8. 7 repeats and W6. 6, 7 repeats and W9. 50 bp ladder
Quartile data analysis
In order to see whether AAO of the condition is being associated with genotypes / anthropometric measures and/or clinical features, the whole patient data was categorized into quartiles based at age at onset. Quartile analysis was carried out by arranging the whole data in ascending order of AAO and was categorized into 1, 2, 3 and 4 quartiles.
Statistical analysis
The data was analyzed using SPSS 17 version. Continuous data were expressed as mean ± SD. The demographic characteristics of patients and controls were compared by using Student’s t test for unpaired data. The association between genotypes and PCOS risk was evaluated by calculating the odds ratios (OR) at 95 % confidence interval. Allele and genotype frequencies were determined from observed genotype counts. Hardy Weinberg equilibrium was estimated by the χ2 test. A two tailed p-value of <0.05 was considered to be statistically significant.
Results
Characteristics of study population
The characteristics of the study group are presented in Table 1. In our study over 90 % of the PCOS cases belonged to the age group of 20 to 35 years and 10 % were below 20 years of age. Nearly 75 % of cases were infertile and are under treatment. Of the total patient group 34 % of probands revealed hyperandrogenic clinical symptoms, 22 % insulin resistance, while 13 % both hyperandrogenism and acanthosis .
Table 1.
Anthropometric measurements and clinical features of the study group
| Variables | Total patients (267) | Total controls (275) | p-value |
|---|---|---|---|
| Age | 24.24 ± 4.42 | 25.02 ± 4.85 | 0.05 |
| BMI (kg/m2) | 25.71 ± 5.05 | 22.11 ± 3.39 | <0.05 |
| WHR | 0.78 ± 0.05 | 0.75 ± 0.04 | <0.05 |
| AAM | 12.60 ± 1.80 | 12.18 ± 0.80 | <0.05 |
| AAO | 16.49 ± 4.91 | - | - |
| Hyperandrogenism | 92(34) | 0(0) | - |
| Acnathosis | 60(22) | 0(0) | - |
| Both | 35(13) | 0(0) | - |
| FHCD | 184(69) | 41(31) |
Quantitative data are presented as X ± SD
The mean age at onset (AAO) of the PCOS clinical symptoms in patients was 16.49 ± 4.91 years. The mean age, age at menarche (AAM) of women with PCOS and controls at the time of sample collection was 24.24 ± 4.42, 25.02 ± 4.85 and 12.60 ± 1.80, 12.18 ± 0.80 years respectively. Sixty two percent of PCOS probands were overweight/ obese having BMI >23 kg/m2 and 37 % had WHR ≥0.80. With respect to mean BMI, WHR and AAM a significant difference between patients and controls was observed (p < 0.05).
Sizing analysis of CYP11A1 (tttta)n repeat polymorphisms in patients and controls revealed 15 different alleles ranging between 2–16 , however 14–16 repeats were identified only in patients (Fig. 2). The most frequent allele observed in non-PCOS controls was 8 repeats (32 % vs.20 %). Based on this information, 8 repeat allele was considered as the referral allele (mode) to categorize the data into three subgroups i) Women with <8 repeats ii) Women with 8 repeats in single or double dose and iii) women with >8 repeats. When the frequencies of various genotypes were compared between the groups, <8 and =8 repeats were more frequent in controls while women carrying >8 repeats were extremely elevated in patients.
Fig. 2.
Distribution of CYP11A1 (tttta)n alleles in PCOS cases and non PCOS controls
Table 2 represents the distribution of clinical and anthropometric measures with respect to the genotypes (<8, =8 and >8) were 21 %, 15 %, 64 % in patients while 38 %, 24 %, and 38 % in controls respectively. The genotype and allelic frequencies between the patients and controls differed significantly (p < 0.01). Individuals with >8 repeats predominated in patients with an OR of 2.93 (CI = 2.06-4.15; p < 0.01) and <8 in controls [OR = 0.42(0.29 - 0.62), p < 0.01; 0.55(0.35 - 0.85, p < 0.001) (Table 2).
Table 2.
Distribution of CYP11A1 repeat polymorphism in patients and controls
| Category | < 8 N (%) | =8 N (%) | >8 N (%) | Comparison of groups | OR(95 % CI) | p-value |
|---|---|---|---|---|---|---|
| Patients (267) | 56(21) | 39(15) | 172(64) | <8 vs. Others | 0.42 (0.29-0.62) | <0.0001 |
| Controls (275) | 105(38) | 65(24) | 105(38) | 8 vs. Others | 0.55 (0.35-0.85) | 0.008 |
| χ2 (p-value) | 35.96(0.00) | >8 vs. Others | 2.93 (2.06-4.15) | <0.001 | ||
| HWE χ2 (p-value) | Cases | 109.32(0.00) | ||||
| Control | 76.45(0.00) | |||||
HWE Hardy Weinberg Equilibrium, OR Odds Ratio, CI Confidence interval
Distribution of clinical and anthropometric measures with respect to the genotypes (<8, =8 and >8) did not reveal any significant involvement (Table 3). Quartile analysis showed no involvement of (tttta)n polymorphism on AAO of the condition. However, a significantly increased frequency of women with higher BMI and elevated frequency of women with hyperandrogenic clinical features was observed in the first two quartiles (p < 0.05) (Fig. 3).
Table 3.
Distribution of various clinical factors with respective with three genotypes
| Characteristics | <8 | 8 | >8 | χ2 (p value) | |
|---|---|---|---|---|---|
| Hyperandrogenism | Yes | 23(41) | 13(33) | 56(32) | 1.00(0.60) |
| No | 33(59) | 26(66) | 116(68) | ||
| Acanthosis | Yes | 15(27) | 11(28) | 34(20) | 1.39(0.49) |
| No | 41(73) | 28(72) | 138(80) | ||
| Both | Yes | 10(18) | 4(11) | 21(22) | 0.87(0.64) |
| No | 46(82) | 35(89) | 151(88) | ||
| FHCD | Yes | 39(70) | 28(72) | 117(68) | 0.07(0.96) |
| No | 17(30) | 11(28) | 55(32) | ||
| Early AAO | Yes | 31(55) | 17(44) | 96(56) | 1.47(0.47) |
| No | 25(45) | 22(64) | 76(44) | ||
| Higher BMI | Yes | 34(61) | 24(62) | 109(63) | 0.04(0.97) |
| No | 22(39) | 15(38) | 63(37) | ||
| Higher WHR | Yes | 19(34) | 11(28) | 68(40) | 1.43(0.48) |
| No | 37(66) | 28(72) | 104(60) | ||
Fig. 3.
Analysis of genotypes, clinical features and epidemiological factors in quartiles with respect to AAO in patient group. Note: Each bar represents the distribution of parameters with respect to presence or absence of a specific trait. BMI—Body mass index, WHR—Waist to hip ratio, HA—Hyperandrogenism, AC—Acanthosis
Discussion
Adrenal and ovarian steroidogenesis begins with the conversion of cholesterol into pregnenolone that is catalyzed by P450scc enzyme, an initial and rate-limiting step at the start of steroid hormone biosynthetic pathway [15]. In-vitro studies have shown that theca cells from polycystic ovaries produce excess progesterone, 17-α hydroxyprogesterone and androstenedione levels than normal theca cells [16]. Several SNPs of CYP11A1 have been reported to be involved in the aetiopathogenesis of PCOS that may alter testosterone levels [15, 17]. Microsatellite allelic variation (tttta)n of this gene, a region containing multiple cAMP-regulated elements that control it’s expression has been reported to be associated with hyperandrogenic state in PCOS [18, 19]. This is the first investigation aimed to establish the role of CYP11A1 polymorphism in susceptibility to PCOS of South Indian women.
Our study revealed a significant relation of CYP11A1 penta nucleated repeat polymorphism with PCOS risk. Similar to our findings, Colhoun HM et al., (2003), Wang et al., (2005) and Wenjing et al., (2014) proposed a noteworthy association of CYP11A1 repeat polymorphism with PCOS in UK, Han Chinese and Caucasian population correspondingly [19–21]. Considering the two studies from UK and Finnish populations which were showing contradictory results, Gassenbeck et al., (2002) in a meta analysis concluded inconsistency in the association of this marker with either PCOS status or serum testosterone levels [22]. However, studies conducted by Millan et al., (2001) in Spanish women and Taponen et al.,(2003) in Finnish women reported no role of this polymorphism in the pathogenesis of PCOS [23, 24].
A wide variation has been shown in the distribution of (tttta)n repeat alleles in various ethnic groups including ours. Studies from Caucasian and Asian population reported an association of 4 and 6 repeat alleles correspondingly with that of PCOS [18]. Further, (tttta)6 allele was shown to be linked with breast cancer and prostate cancer in Chinese and Slovanian population respectively [9, 25]. Daneshmand et al., (2002) observed an over-representation of 9 repeat allele in PCOS patients.
Our results showed an increased frequency of women with >8 repeats alleles demonstrating a three-fold increased risk to develop PCOS than other genotypes (<8 and =8 repeats). This polymorphism not seems to influence the anthropometric measures and hyperandrogenic clinical features of PCOS in our population. Our results were in accordance to Tan et al., (2005) findings exhibiting no correlation between CYP11A1 and serum testosterone levels in patients and controls [11]. Millan et al., (2001) in Spanish PCOS women reported lack of association of this marker with hirsutism / hyperandrogenism [23].
The clinical features of PCOS may change throughout the lifespan including age at onset. The consideration of AAO in the present study did not show any influence of this polymorphism. However, earlier we reported a strong association of AAO with ACE I/D polymorphism in the similar study population [4]. This is suggestive of AAO to be considered as one of the clinical markers for this type of studies.
The salient findings of the present study are; irrespective of the age at onset of the condition, more than 8 repeats predisposes the women to PCOS and can be considered as a general marker of disease susceptibility in South Indian women. Further, the appearance of increased frequency of individuals with higher BMI in early quartiles may suggest gene-gene interactions with respect to obesity related and other unknown factors in the early onset of this multifactorial condition.
We conclude that generation of wide variation in repeat number in diverse populations could be due to different extrinsic and intrinsic factors. Hence, knowing the threshold repeat number associated with various diseases in different populations may help in understanding the gene-environment interaction. In order to realize the significance of this marker, cell based studies should be carried out with various repeat numbers in relation to expression to translate the generated information and comprehend the susceptibility of different population to hormone related diseases.
Acknowledgments
We thank all the subjects who have participated in this study and ICMR, India for providing financial assistance to Deepika M.L.N.
Declaration of interest
The authors report no declaration of interest.
Footnotes
Capsule Women with >8 repeats of (tttta)n polymorphism are predisposed to PCOS. Hence may serve as a potential marker in South Indian women. Appearance of patients with higher BMI at early ages suggested the interaction of this polymorphism with obesity related genes and environment.
Contributor Information
K. Ranjith Reddy, Phone: +91-9000120345, Email: kranjithreddy@yahoo.com.
M. L. N. Deepika, Phone: +91-9885617014, Email: mlndeepika@gmail.com
K. Supriya, Phone: +91-9908214718, Email: sprya111@gmail.com
K. Prasanna Latha, Phone: +91-9493389288, Email: p_komaravalli@yahoo.com.
S. S. Lakshmana Rao, Phone: +91-9704183313, Email: s_lrao@yahoo.com.
V. Usha Rani, Phone: +91-9848318181, Email: drvusharani@rediffmail.com.
P. Jahan, Phone: +91-40-27682335, FAX: +91-40-27095178, Email: dr.pjahan@gmail.com
References
- 1.Deepika MLN, Ranjith K, Yashwanth A, Usha Rani V, Prasanna Latha K, Jahan P. TNF-α haplotype association with Polycystic ovary syndrome- a South Indian study. J Assist Reprod Genet. 2013 Nov;30(11):1493–503. [DOI] [PMC free article] [PubMed]
- 2.Ranjith Reddy K, Deepika MLN, Ishaq M, Jahan P. Haptoglobin: a pleiotropicmarkerin polycysticovary syndrome-astudyfromSouth India. Am J Biochem Mol Biol. 2011;1:399–404. doi: 10.3923/ajbmb.2011.399.404. [DOI] [Google Scholar]
- 3.Deepika MLN, Ranjith K, Usha Rani V, Ishaq M, Jahan P. Familial background of complex diseases in PCOS probands of South Indian population. Asian J Epidemiol. 2012;5(2):50–5. doi: 10.3923/aje.2012.50.55. [DOI] [Google Scholar]
- 4.Deepika MLN, Ranjith Reddy K, Usha Rani V, Balakrishna N, Prasana Latha K, Jahan P. Do ACE I/D gene polymorphism serve as a predictive marker for age at onset in PCOS? J Assist Reprod Genet. 2013;30(1):125–30. doi: 10.1007/s10815-012-9906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 6.Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Iwamoto K, Wang M, Artwohl J, Mason JI, Pang S. Inherited congenital adrenal hyperplasia in the rabbit is caused by a deletion in the gene encoding cytochrome P450 cholesterol side-chain cleavage enzyme. Endocrinology. 1993;132:1977–1982. doi: 10.1210/endo.132.5.7682938. [DOI] [PubMed] [Google Scholar]
- 8.Setiawan VW, Cheng I, Stram DO, Giorgi E, Pike MC, Van den Berg D, Pooler L, Burtt NP, Le Marchand L, Altshuler D. A systematic assessment of common genetic variation in CYP11A and risk of breast cancer. Cancer Res. 2006;66:12019–12025. doi: 10.1158/0008-5472.CAN-06-1101. [DOI] [PubMed] [Google Scholar]
- 9.Celhar T, Gersak K, Ovcak Z, Sedmak B, Mlinaric-Rascan I. The presence of the CYP11A1 (TTTTA)6 allele increases the risk of biochemical relapse in organ confined and low-grade prostate cancer. Cancer Genet. Cytogenet. 2008;187:28–33. doi: 10.1016/j.cancergencyto.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Kathryn T, Monica MG, IMin L, Julie Buring I, De V. Genetic variation in CYP11A1 and StAR in relation to endometrial cancer risk. Gynecol Oncol. 2010;117:255–259. doi: 10.1016/j.ygyno.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Guijin Z. Role of Pentanucletide (tttta)n polymorphism of CYP11α gene in the pathogenesis of Hyperandrogenism in Chinese Women with Polycystic Ovary Syndrome. J Huazhong Univ Sci Technolog Med Sci. 2005;25:212–214. doi: 10.1007/BF02873580. [DOI] [PubMed] [Google Scholar]
- 12.Diamanti Kandarakis E, Bartis M, Bergiele AT. Microsatellite polymorphism (tttta)n at -528 base pairs of gene CYP11A influences hypernadrogenemia in patients with poly cystic ovary syndrome. Fertil Steril. 2000;73:123. doi: 10.1016/S0015-0282(99)00454-9. [DOI] [PubMed] [Google Scholar]
- 13.Gharani N, Dawn M, Waterworth SB, White D, Gilling Smith C, Conway GS, et al. Association of the steroid synthesis gene CYP11A with polycystic ovary syndrome and hyperandrogenism. Hum Mol Genet. 1997;6:397–402. [DOI] [PubMed]
- 14.Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in Asian Indian adults. Diabetes Care. 2003;26(5):1380–4. doi: 10.2337/diacare.26.5.1380. [DOI] [PubMed] [Google Scholar]
- 15.Gao GH, Cao YX, Yi L, Wei ZL, Xu YP, Yang C. Polymorphism of CYP11A1 gene in Chinese patients with polycystic ovarian syndrome. Zhonghua Fu Chan Ke Za Zhi. 2010 Mar;45(3):191–6 [PubMed]
- 16.Daneshmand S, Weirsman SR, Navab A. Over expression of theca cell messenger RNA in polycystic ovary syndrome does not correlate and 17α-hydroxylase/ C 17–20 lyase promoters. Fertil Steril. 2002;77:274. doi: 10.1016/S0015-0282(01)02999-5. [DOI] [PubMed] [Google Scholar]
- 17.Cheng-wei Z, Xin-lin Z, Yan-jie X, Yun-xia C, Wmen-jun W, Pei X, Ye-na C, Xiao-ke W, Long Y, Qian G, Yong W. Association between polymorphisms of the CYP11A1 gene and polycystic ovary syndrome in Chinese women. Mol BioL Rep. 2012;39:8379–8385. doi: 10.1007/s11033-012-1688-7. [DOI] [PubMed] [Google Scholar]
- 18.Hao CF, Bao HC, Zhang N, Gu HF, Chen ZJ. Evaluation of association between the CYP11alpha promoter pentannucleotide (TTTTA)n polymorphism and polycystic ovarian syndrome among Han Chinese women. Neuro Endocrinol Lett. 2009;30(1):56–60. [PubMed] [Google Scholar]
- 19.Wang Y, XiaoKe W, Cao Y, Yi L, Chen J. A microsatellite polymorphism (tttta)n in the promoter of the CYP11a gene in Chinese women with polycystic ovary syndrome. Fertil Steril. 2006;86(1):223–226. doi: 10.1016/j.fertnstert.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Colhoun HM, McKeigue PM, Davey SG. Problems of reporting genetic associations with complex outcomes. Lancet. 2003;361:865–872. doi: 10.1016/S0140-6736(03)12715-8. [DOI] [PubMed] [Google Scholar]
- 21.Shen W, Li T, Yanjie H, Liu H, Song M. Common polymorphisms in the CYP1A1 and CYP11A1 genes and polycystic ovary syndrome risk: a meta-analysis and meta-regression. Arch Gynecol Obestet. 2014;289:107–118. doi: 10.1007/s00404-013-2939-0. [DOI] [PubMed] [Google Scholar]
- 22.Gaasenbeek M, Powell BL, Sovio U, Haddad L. Large-Scale Analysis of the Relationship between CYP11A Promoter Variation, Polycystic Ovarian Syndrome, and Serum Testosterone. J Clin Endocrinol Metab. 2004;89:2408–2413. doi: 10.1210/jc.2003-031640. [DOI] [PubMed] [Google Scholar]
- 23.San Millán JL, Sancho J, Calvo RM, Escobar-Morreale HF. Role of the pentanucleotide (tttta)n polymorphism in the promoter of the CYP11a gene in the pathogenesis of hirsutism. Fertil Steril. 2001;75(4):797–802. doi: 10.1016/S0015-0282(01)01677-6. [DOI] [PubMed] [Google Scholar]
- 24.Taponen S, Martikainen H, Jarvelin M-R, Laitinen J, Pouta A, Hartikainen A-L, Sovio U, McCarthy MI, Franks S, Ruokonen A. The hormonal profile of women with self- reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. J Clin Endocrinol Metab. 2003;88:141–147. doi: 10.1210/jc.2002-020982. [DOI] [PubMed] [Google Scholar]
- 25.Sakoda LC, Blackston C, Doherty JA, Ray RM, Lin MG, Stalsberg H, et al. Polymorphisms in steroid hormone biosynthesis genes and risk of breast cancer and fibrocystic breast conditions in Chinese women. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1066–73. [DOI] [PMC free article] [PubMed]